Summary
Background and objectives
Vascular calcification is associated with increased cardiovascular mortality in chronic hemodialysis patients. This prospective study investigated the relationship between serum osteoprotegerin, receptor activator of NF-κB ligand, inflammatory markers, and progression of coronary artery calcification score.
Design, setting, participants, & measurements
Seventy-eight hemodialysis patients were enrolled. Serum IL-1β, IL-6, TNF-α, osteoprotegerin, receptor activator of NF-κB, fetuin A, and bone alkaline phosphatase were measured by ELISA. Coronary artery calcification score was measured two times with 1-year intervals, and patients were classified as progressive or nonprogressive.
Results
Baseline and first-year serum osteoprotegerin levels were significantly higher in the progressive than nonprogressive group (17.39±9.67 versus 12.90±6.59 pmol/L, P=0.02; 35.17±18.35 versus 24±11.65 pmol/L, P=0.002, respectively). The ratio of serum osteoprotegerin to receptor activator of NF-κB ligand at 1 year was significantly higher in the progressive group (0.26 [0.15–0.46] versus 0.18 [0.12–0.28], P=0.004). Serum osteoprotegerin levels were significantly correlated with coronary artery calcification score at both baseline (r=0.36, P=0.001) and 1 year (r=0.36, P=0.001). Importantly, progression in coronary artery calcification score significantly correlated with change in serum osteoprotegerin levels (r=0.39, P=0.001). In addition, serum receptor activator of NF-κB ligand levels were significantly inversely correlated with coronary artery calcification scores at both baseline (r=−0.29, P=0.01) and 1 year (r=−0.29, P=0.001). In linear regression analysis for predicting coronary artery calcification score progression, only baseline coronary artery calcification score and change in osteoprotegerin were retained as significant factors in the model.
Conclusions
Baseline coronary artery calcification score and serum osteoprotegerin levels were significantly associated with progression of coronary artery calcification score in hemodialysis patients.
Introduction
Vascular calcification (VC) is associated with increased cardiovascular morbidity and mortality in chronic hemodialysis (HD) patients (1–3). Disordered mineral metabolism was implicated as the major culprit for the development of VC. However, recent studies have suggested that the calcification process is an active process in which osteoblast-like cells that transdifferentiated from vascular smooth muscle cells play major active roles (4,5). Newly discovered molecules such as osteoprotegerin (OPG) and receptor activator of NF-κB ligand (RANKL) are considered to be the main regulators of this active process (6–8). OPG, belonging to the TNF receptor superfamily, functions as a soluble decoy receptor that binds to RANKL and inhibits osteoclastogenesis and osteoclast activation (7). OPG and RANKL have opposing effects on bone resorption, and the OPG/RANKL ratio may be considered to be the major determinant of bone mass and turnover (9). Indeed, high OPG/RANKL ratio may represent a state of low bone turnover, which is known to be related to progression of VC.
Several epidemiologic studies strongly suggested that elevated levels of OPG are associated with increased cardiovascular risk and mortality in both the nonuremic (8,10–12) and uremic population (13). In cross-sectional observational studies, it was reported that high-serum OPG levels were associated with increased coronary artery calcification score (CACS) in patients with CKD (14–16). In two similar studies performed on renal transplant recipients, findings suggested a possible role of serum OPG on the progression of VC (17,18). However, prospective and longitudinal studies investigating the effects of OPG/RANKL axis on progression of VC are scarce in the literature. In a prospective study by Nitta et al. (19), serum OPG levels were correlated with progression of aortic calcification in a small number of HD patients. Low levels of RANKL were revealed to be associated with increased cardiovascular risk in studies performed on patients with ischemic heart disease (20,21), but a direct role of serum RANKL on VC has not been reported.
In this prospective study performed on HD patients, we aimed to investigate the relationship between serum OPG, RANKL, inflammatory markers, bone mineral density (BMD), and progression of CACS.
Materials and Methods
Seventy-eight HD patients (38 male and 40 female; mean age=52±14.5 years) were used in this study; 42 healthy volunteers (20 male and 22 female; mean age=54±8 years) and 44 CKD stage 4 patients (23 male and 21 female; mean age=56±13 years) as control groups were enrolled. There was no difference in age and gender distribution among the study groups.
Control groups were evaluated in terms of serum OPG, RANKL, and OPG/RANKL ratio.
Mean time on dialysis was 53 (23–96) months. All patients have been receiving dialysis more than 6 months. Information on age, sex, weight, duration of HD treatment, and the etiology of CKD was gathered by review of medical records.
In the HD study group, patients received dialysis three times per week for a 4-h period with a standard bicarbonate-containing dialysate bath using biocompatible HD membrane (Polysulphone, FX-80 series; Fresenius, Germany). Blood flow rates ranged from 350 to 400 ml/min, whereas dialysate flow rate was kept constant at 500 ml/min. Adequacy of dialysis received was calculated with double-pool Kt/V, and Kt/V≥1.4 was considered as the target. All patients were on calcium-based phosphate binder treatment as needed according to Kidney Disease Outcomes Quality Initiative guidelines. Aluminum hydroxide was used as a rescue treatment for short period.
Etiology of CKD included hypertension in 20 patients, diabetes mellitus in 10 patients, tubulointerstitial nephritis in 9 patients, glomerulonephritis in 4 patients, others in 11 patients, and unknown in 24 patients.
All biochemical blood samples were collected before the midweek HD session. Laboratory values including complete blood cell counts, and serum levels of urea nitrogen, creatinine, electrolytes, calcium, phosphorus, total protein, albumin, total cholesterol, triglycerides, and intact parathyroid hormone (PTH) were measured. Serum IL-1β (Invitrogen, CA) and TNF-α (Invitrogen, CA) were analyzed only at baseline. Serum OPG (BioVendor-Laboratorni Medicina, Brno, Czech Republic), RANKL (BioVendor-Laboratorni Medicina, Brno, Czech Republic), fetuin A (BioVendor-Laboratorni Medicina, Brno, Czech Republic), bone alkaline phosphatase (BAP; Immundiagnostic Systems, Bensheim, Germany), and IL-6 (Invitrogen, CA) concentrations were determined two times at least 1 year apart. These parameters were analyzed with ELISA.
Computed Tomography Examination
CACS was measured two times with a 1-year interval by computed tomography. A scan run consisted of acquisition of 40 contiguous transverse two-dimensional images of 3-mm-thick sections at the level above the coronary artery origins to the cardiac apex. Exposure duration was 0.1 s per tomographic level, and other parameters were 130 kVp and 630 mA. Images were acquired with electrocardiogram triggering at 71% of the R–R interval during diastole and were obtained using a 26-cm2 field of view and a 512×512 reconstruction matrix. Contrast agents were not used. A calcification was defined as a minimum of two adjacent pixels (>0.52 mm2) with a density over 130 Hounsfield units. The peak intensity (in Hounsfield units) and area (in square millimeters) of the individual calcifications were calculated. As described in the work by Agatston et al. (22), calcium scores were obtained by multiplying each area of interest by a factor indicating peak density within the individual area. Image quality and scoring accuracy were assessed by one radiologist, who carefully made vessel by vessel and calcific focus by calcific focus inspections of each image. The radiologist was blinded to the clinical and laboratory results of the patients.
BMD Examination
BMD measurements were performed with a standard dual-energy x-ray absorptiometry. To avoid the potential interfering effects of aortic calcificaton on BMD measurements, only femur scores were evaluated. Patients with t scores less than −2.5 were considered to have low BMD.
Our examinations of the patients conformed to good medical and laboratory practices and the recommendations of the Declaration of Helsinki on Biomedical Research Involving Human Subjects. This study was approved by the Ethical Committee of Istanbul School of Medicine.
Statistical Analyses
For statistical analyses, we used the Statistical Package for Social Sciences version 16.0 (SPSS Inc, Chicago, IL). Between-group comparisons of continuous data for two groups were performed using the t test or Mann–Whitney U test when appropriate. The chi-squared test with Yates correction and Fisher exact test were used for 2×2 contingency tables for non-numerical data when appropriate. Correlations between numerical parameters with non-normal distribution were analyzed with Spearman’s ρ correlation test. To predict the CACS progression, linear regression analysis with backward likelihood ratio elimination was performed, and age, time on dialysis, serum phosphorus and calcium phosphorus products, baseline CACS, ∆OPG, ∆RANKL, and serum albumin levels were included as independent variables. Results are expressed as mean ± SD, and median (interquartile range) was used for the variables that are not normally distributed. All tests of significance were two-sided, and differences were considered statistically significant when the P value was <0.05.
Results
The mean serum OPG level of HD patients (15.11±8.50 pmol/L) was significantly higher than mean serum OPG levels in both CKD stage 4 patients (9.33±4.29 pmol/L, P<0.001)) and healthy controls (5.82±1.39 pmol/L, P<0.001). Serum OPG levels in HD patients were also significantly higher than in patients with stage 4 CKD (P=0.02). The mean serum RANKL level of HD patients (145 [110–235] pmol/L) was significantly lower than in patients with CKD stage 4 (205 [153–365] pmol/L, P=0.01) and the healthy controls (222 [141–364] pmol/L, P=0.02). However, no difference was found between patients with CKD and controls. The mean serum OPG/RANKL ratio of HD patients was also significantly higher than in both patients with CKD and healthy controls. Comparison results of the control and patient groups are presented in Table 1.
Table 1.
Comparison of the groups in terms of serum osteoprotegerin, receptor activator of NF-κB ligand, and osteoprotegerin/receptor activator of NF-κB ligand ratio
| HD Patients (n=78) | CKD Stage 4 Patients (n=44) | Control Patients (n=42) | P Values | |
|---|---|---|---|---|
| OPG (pmol/L; range) | 15.11±8.50a,b (4.94–38.38) | 9.33±4.29c (4.62–19.61) | 5.82±1.39 (3.58–9.35) | <0.001a |
| <0.001b | ||||
| 0.03c | ||||
| RANKL (pmol/L; IQR) | 145 (110–235)a,b | 205 (153–365) | 222 (141–364) | 0.007a |
| 0.02b | ||||
| OPG/RANKL (IQR) | 0.07 (0.04–0.21)a,b | 0.05 (0.02–0.07) | 0.02 (0.01–0.04) | <0.001a |
| <0.001b |
For variables that are not normally distributed, median (interquartile range) was used. HD, hemodialysis; OPG, osteoprotegerin; RANKL, receptor activator of NF-κB ligand; IQR, interquartile range.
HD patients are different from patients with CKD stage 4.
HD patients are different from controls.
Patients with CKD stage 4 are different from controls.
Patients with increase of CACS more than 10% and 50 units were classified as the progressive group (PG). According to these criteria, 39 patients (50%) were in the PG group. Comparison of PG and nonprogressive group (NPG) in terms of baseline laboratory results is given in Table 2.
Table 2.
Baseline demographic and biochemical results of the study patients
| Progressive (n=39) | Nonprogressive (n=39) | P Value | |
|---|---|---|---|
| Age (years) | 56±13 | 48±15 | 0.02 |
| BMI (kg/m2) | 24.5 ±3.6 | 23.9±3.9 | 0.51 |
| Time on dialysis (months) | 56 (23–97) | 47 (23–83) | 0.32 |
| Systolic BP (mmHg) | 125±17 | 126±21 | 0.95 |
| Diastolic BP (mmHg) | 75±10 | 75±10 | 0.98 |
| Calcium (mg/dl) | 8.8±0.6 | 8.8±0.5 | 0.66 |
| Phosphorus (mg/dl) | 4.8±1.3 | 5.0±1.5 | 0.63 |
| Calcium × phosphorous (mg2/dl2) | 43.0±12.6 | 44.2±13.9 | 0.69 |
| ALP (U/L) | 129 (87–203) | 124 (92–160) | 0.26 |
| PTH (pg/ml) | 276 (121–464) | 239 (96–434) | 0.48 |
| Uric acid (mg/dl) | 6.5±1.1 | 6.4±1.2 | 0.83 |
| Albumin (g/dl) | 3.8±0.3 | 3.9±0.2 | 0.15 |
| CRP (mg/L) | 7.90 (3.65–22.15) | 6.65 (2.35–18.55) | 0.84 |
| Triglyceride (mg/dl) | 146 (104–219) | 153 (105–225) | 0.60 |
| Cholesterol (mg/dl) | 158.7±33.2 | 174.5±44.3 | 0.08 |
| Ferritin (ng/ml) | 660 (343–893) | 548 (307–913) | 0.81 |
| Hemoglobin (g/dl) | 11.0±1.4 | 10.8±1.6 | 0.57 |
| Kt/V | 1.8±0.3 | 2.0±0.5 | 0.07 |
For variables that are not normally distributed, median (interquartile range) was used. BMI, body mass index; ALP, alkaline phosphatase; PTH, parathyroid hormone; CRP, C-reactive protein.
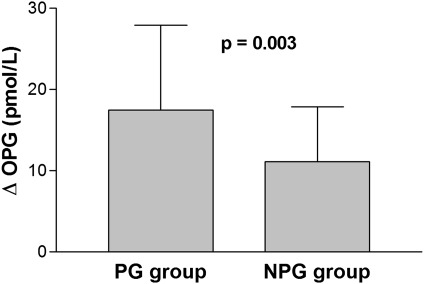
Age was significantly higher in the PG group compared with the NPG group (56±13 versus 48±15 years, P=0.02). Routine biochemical analysis was similar in both groups. Serum IL-1β, IL-6, TNF-α, and high-sensitivity (hs)–C-reactive protein (CRP) were also similar between the groups. Baseline and first-year serum levels of BAP, fetuin-A, and RANKL were similar between the groups (Table 3). Both baseline and first-year serum OPG levels were significantly higher in the PG group compared with the NPG group (17.39±9.67 versus 12.90±6.59 pmol/L, P=0.02; 35.17±18.35 versus 24±11.65 pmol/L, P=0.002, respectively). Furthermore, change in OPG levels (∆OPG) was also higher in the PG group (17.49±10.45 versus 11.10±6.74 pmol/L, P=0.003) (Figure 1).
Table 3.
Comparison of inflammatory cytokines, osteoprotegerin/receptor activator of NF-κB ligand axis, fetuin-A, and coronary artery calcification score between the progressive and nonprogressive groups
| Progressive (n=39) | Nonprogressive (n=39) | P Value | |
|---|---|---|---|
| IL-1β (baseline; pg/ml) | 0.90 (0.84–0.96) | 0.91 (0.86–1.00) | 0.36 |
| IL-6 (baseline; pg/ml) | 6.93 (3.38–10.38) | 6.93 (2.35–14.78) | 0.28 |
| TNF-α (baseline; pg/ml) | 33.1±10.6 | 32.6±9.3 | 0.83 |
| BAP (baseline; µg/L) | 22 (13–34) | 24 (12–42) | 0.65 |
| BAP (first year; µg/L) | 29 (18–52) | 25 (18–44) | 0.14 |
| RANKL (baseline; pmol/L) | 145 (104–244) | 147 (112–232) | 0.77 |
| RANKL (first year; pmol/L) | 100 (90–137) | 103 (97–148) | 0.65 |
| ∆RANKL (pmol/L) | 29 (−20–82) | 52 (5–82) | 0.70 |
| OPG (baseline; pmol/L) | 17.39±9.67 | 12.9±6.59 | 0.02 |
| OPG (first year; pmol/L) | 35.17±18.35 | 24±11.65 | 0.002 |
| ∆OPG (pmol/L) | 17.49±10.45 | 11.1±6.74 | 0.003 |
| OPG/RANKL (baseline) | 0.10 (0.04–0.26) | 0.06 (0.04–0.12) | 0.11 |
| OPG/RANKL (first year) | 0.26 (0.15–0.46) | 0.18 (0.12–0.28) | 0.004 |
| Fetuin A (baseline; g/L) | 1.34±0.34 | 1.31±0.36 | 0.72 |
| Fetuin A (first year; g/L) | 0.60±0.23 | 0.57±0.38 | 0.65 |
| CACS (baseline) | 178 (52–858) | 4 (0.3–200) | 0.18 |
| CACS (first year) | 495 (127–1,038) | 2.15 (0.82–72.8) | <0.001 |
For variables that are not normally distributed, median (interquartile range) was used. BAP, bone alkaline phosphatase; RANKL, receptor activator of NF-κB ligand; ∆RANKL, change in RANKL; OPG, osteoprotegerin; ∆OPG, change in OPG; CACS, coronary artery calcification score.
Figure 1.
Comparison of the progressive (PG) and nonprogressive (NPG) groups in terms of change in osteoprotegerin (∆OPG).
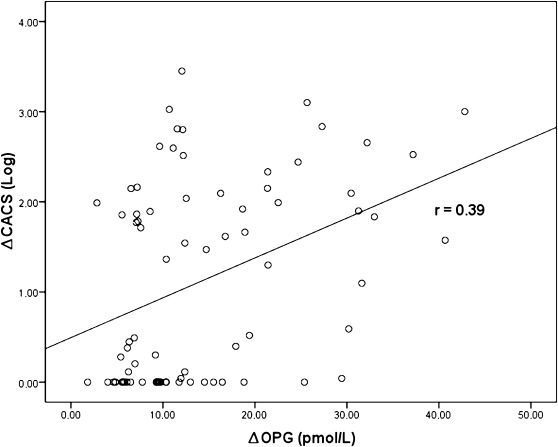
Serum OPG levels were significantly correlated with CACS at baseline (r=0.36, P=0.001) and 1 year (r=0.36, P=0.001). Serum OPG levels were significantly correlated with age (r=0.69, P<0.001) and duration of dialysis (r=0.28, P=0.01) and negatively correlated with serum albumin levels (r=−0.31, P=0.01). Similar to baseline correlations, serum 1-year OPG levels were also significantly correlated with age (r=0.52, P<0.001) and duration of dialysis (r=0.31, P=0.01) and negatively correlated with serum albumin levels (r=−0.37, P=0.001). However, no association was found between serum OPG levels and inflammatory markers such as serum hs-CRP, IL-1β, IL-6, and TNF-α concentrations. More importantly, progression of CACS (∆CACS) significantly correlated with OPG baseline (r=0.27, P=0.01) and ∆OPG levels (r=0.39, P=0.001) (Figure 2). When patients were grouped according to age (<40 and ≥40 years), ∆OPG levels were significantly associated with progression of CACS in younger patients (n=17, r=0.64, P=0.01).
Figure 2.
Progression of coronary artery calcification score (∆CACS) was significantly associated with change in osteoprotegerin (∆OPG).
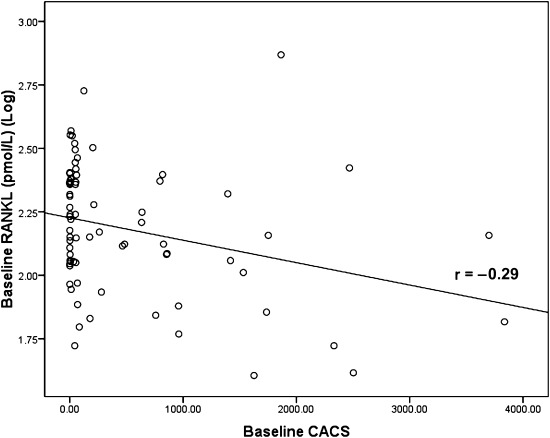
Serum RANKL levels were significantly inversely associated with CACS at baseline (r=−0.29, P=0.01) (Figure 3). However, there was no correlation between changes in CACS and RANKL baseline, RANKL at 1 year, and changes in RANKL.
Figure 3.
Baseline serum receptor activator of NF-κB ligand (RANKL) was significantly inversely related to baseline coronary artery calcification score (CACS).
Ratio of serum OPG/RANKL was calculated as an indicator of bone turnover. The patients were divided into two groups according to the median values of serum BAP (<23.3 and ≥23.3 μg/L) and PTH (<256 and ≥256 pg/ml) levels as high and low BAP and PTH groups. Baseline OPG/RANKL ratio was found to be significantly lower in the high BAP group (0.06 [0.03–0.17] versus 0.10 [0.05–0.30], P=0.04). Similarly, patients with high PTH had significantly lower baseline OPG/RANKL ratio (0.07 [0.05–0.15] versus 0.08 [0.04–0.24], P=0.04). OPG/RANKL ratios measured at baseline and 1 year after were significantly correlated with baseline CACS and 1-year CACS (r=0.32, P=0.003; r=0.41, P<0.001, respectively). Comparing PG with NPG, the ratio of serum OPG/RANKL at 1 year was significantly higher in PG than NPG (0.26 [0.15–0.46] versus 0.18 [0.12–0.28], P=0.004).
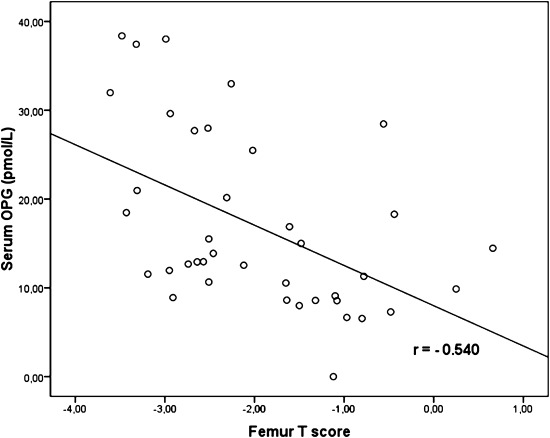
Twenty-nine patients (37%) according to femur T score have low BMD. Patients with low BMD were older (57±16 versus 49±13 years, P=0.01). Body mass index (23±3.13 versus 24.96±3.98 kg/m2, P=0.02) and serum albumin (3.81±0.30 versus 3.99±0.27 g/dl, P=0.01) levels were significantly lower in the low BMD group. Serum OPG levels were higher in the low BMD group compared with the other group (18.77±9.55 versus 12.91±7.04 pmol/L, P=0.01). BMD measurements were not significantly different between PG and NPG. Femur T scores were significantly correlated with age (r=−0.37, P=0.001) and serum OPG levels (r=−0.41, P<0.001). These correlations were stronger in the PG group for serum OPG levels (with femur T score; r=−0.54, P<0.001) (Figure 4). However, there was no significant difference in RANKL, baseline CACS, and ΔCACS in patients with low BMD compared with the other group.
Figure 4.
Serum osteoprotegerin (OPG) levels were inversely correlated with femur T score in the progressive group.
Baseline CACS was positively correlated with age (r=0.45, P<0.001), Ca × P product (r=0.24, P=0.03), and serum TNF-α levels (r=0.24, P=0.03). In PG, there was a stronger association between baseline CACS and serum TNF-α levels (r=0.30, P=0.05). Baseline CACS was also associated with ∆CACS (r=0.45, P<0.001). No significant relationship was found between CACS and serum fetuin-A, BAP, IL-6, and IL-1β.
In linear regression analysis with backward likelihood ratio elimination for predicting ∆CACS, age, time on dialysis, serum phosphorus and calcium phosphorus products, albumin, baseline CACS, ∆OPG, and ∆RANKL were included as independent variables. Only baseline CACS and ∆OPG were retained as significant in the model (Table 4). Interaction analysis of age on change in CACS was performed, and no significant interaction was found (P=0.66).
Table 4.
Model of linear regression with backward likelihood ratio elimination for predicting increase in coronary artery calcification
| Difference in CACS (95% CI) | P Value | |
|---|---|---|
| Model adjusted r2 | +0.25 | |
| Model P | <0.001 | |
| Constant | 0.12 | |
| Baseline CACS (log) | 0.38 (0.17–0.54) | <0.001 |
| ΔOPG (log) | 0.28 (0.32–1.92) | 0.006 |
CACS, coronary artery calcification score; CI, confidence interval; ∆OPG, change in osteoprotegerin.
Discussion
Vascular calcification is common in chronic HD patients and associated with increased cardiovascular mortality (1–3). Recently, OPG/RANKL axis has been implicated in the pathogenesis of VC, endothelial dysfunction, and atherosclerosis (23,24). OPG and RANKL play important roles in formation and regulation of osteoclasts and osteoblasts (7,25). OPG and RANKL have opposing roles; RANKL increases the number of active osteoclasts, thus increasing bone resorption, whereas OPG, which neutralizes RANKL, also decreases bone resorption. Thus, the OPG/RANKL ratio might be considered to be the major determinant of bone mass and turnover (9). Indeed, high OPG/RANKL ratio may represent a state of low bone turnover. In chronic HD patients with adynamic bone disease, additional inhibition of bone resorption by means of high serum OPG levels results in the inability of accumulation of calcium and phosphorus in the bone followed by metastatic calcification of vascular tree and thus, progression of VC. This theory elucidates the importance of the OPG/RANKL axis in close association between bone and cardiovascular disease in CKD patients. In the bone histomorphometry study by Barreto et al. (26), the OPG/RANKL ratio was found to be an independent determinant of trabecular bone volume in HD patients. Moreover, OPG/RANKL ratio might be associated with adynamic bone disease, which was evidenced by a trend to a negative correlation between OPG/RANKL ratio and the eroded surface. Also in our study, the consideration that the OPG/RANKL ratio reflects a state of low bone turnover was supported by the finding that this ratio was found to be significantly higher in the low BAP and low PTH groups, which are known to represent bone disease with low turnover.
Serum OPG levels were reported to be associated with VC and cardiovascular events. In the study of Anand et al. (27), 510 diabetic patients had CAC scans and were followed over a period of 18±5 months. OPG levels were associated with VC and future cardiovascular events during the follow-up. Similar findings have been reported also in other cross-sectional and observational studies on HD patients (14,15). Additionally, the work by Moreno et al. (16) showed that, among all risk factors examined, serum OPG level is the strongest predictor of CAC, and values of OPG>757.7 pg/ml (equivalent to 6.31 pmol/L) may predict the presence of CAC in CKD patients. Prospective and longitudinal studies investigating the effects of OPG/RANKL axis on the progression of VC are scarce in the literature. In the prospective study by Nitta et al. (19), aortic calcification index was examined two times in 26 HD patients. Rapid progression of VC was found to be associated with serum OPG concentration, which was consistent with our study results. In a very recent study performed on 47 HD patients, CACS and serum OPG levels were assessed at baseline and after 30 months (28). In this study, ∆CACS was found to be correlated with age, dialysis vintage, carotid intima–media thickness, and baseline serum OPG levels. Similar to this study, we examined serum OPG together with RANKL and CACS measurements two times with a 1-year interval and observed that the increase in serum OPG levels was significantly correlated with progression of CACS. Change in serum OPG concentration might be important in the follow-up of HD patients and might predict CAC progression.
Baseline level of vascular calcification has been proven to be one of the most important determinants of the progression of vascular calcification. In the study by Block et al. (29), evidence of coronary artery calcification at baseline showed a significant increase in CACS within 6 months of starting dialysis, despite control of laboratory parameters of mineral metabolism. In another study by Sigrist et al. (30), patients with vascular calcification of the superficial femoral artery at baseline exhibited significantly increased calcification over 24 months. Consistent with these studies, baseline CACS was also confirmed to be a significant factor predicting the progression of CACS in the present study.
Clinical significance of the CACS progression has been reported in a number of studies in nonuremic population as higher cardiac event rates in patients with CACS progression during follow-up (31,32). However, outcome studies about the CACS progression are very scarce in the CKD population. The work by Sigrist et al. (30) reported that every 10 units of CACS progression were associated with a 3% increase in mortality per year in CKD patients.
In several studies, serum OPG concentrations were found to be associated with inflammatory markers (13,33,34). In the study by Morena et al. (13), OPG levels were the strongest predictors of all-cause mortality in HD patients, particularly in patients who had high CRP levels. Role of inflammation in the pathogenesis of VC has been reported previously. In a study by Tintut et al. (35), TNF-α was shown to enhance in vitro VC by promoting osteoblastic differentiation of vascular cells. However, in our study, no association was found between serum OPG levels and inflammation markers, such as serum hs-CRP, IL-1β, IL-6, and TNF-α concentrations. However, baseline CACS was positively correlated with serum TNF-α levels. Inflammation might be related to VC, but OPG might not be the link in this association.
The role of serum RANKL concentrations in VC and cardiovascular disease pathogenesis is still unclear. RANKL has been reported to be related to cardiovascular events in several studies (20,21,36). However, there is no data in the literature about the direct relationship between serum RANKL levels and CACS except a recent study performed on Framingham Study participants. In this study, serum RANKL concentrations were not related to CACS (37). In contrast to this study, we have found a significant negative correlation between serum RANKL values and CACS. Changes in bone metabolism in HD patients are much more prominent than in the nonuremic population. Recent bone histomorphometry and arterial calcification studies showed that there is a clear link between bone metabolism and arterial (coronary or aortic) calcification in HD patients (38). However, this link may not be very prominent in the nonuremic population, and it may explain the lack of association between RANKL and CACS in nonuremic patients. Regarding this finding, RANKL may be considered to have an important role in the pathogenesis of VC similar to OPG but in the opposite direction in HD patients.
One of the limitations of the study was that the time interval between the CACS measurements may be short, and a longer follow-up period would have yielded more informative data in terms of progression of CACS. However, similarly to the present study, previous reports also showed significant progression of vascular calcification in CKD patients at 1 year (29,30).
In conclusion, baseline CACS and serum OPG levels were significantly associated with progression of CACS. The OPG/RANKL axis might be considered to be the link between bone and cardiovascular disease in HD patients. To investigate the association of OPG/RANKL ratio with bone turnover in HD patients, there is need for additional studies with bone biopsies.
Disclosures
None.
Acknowledgments
This study was supported by the Istanbul University Research Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Caliskan Y, Demirturk M, Ozkok A, Yelken B, Sakaci T, Oflaz H, Unsal A, Yildiz A: Coronary artery calcification and coronary flow velocity in haemodialysis patients. Nephrol Dial Transplant 25: 2685–2690, 2010 [DOI] [PubMed] [Google Scholar]
- 3.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Tintut Y, Parhami F, Boström K, Jackson SM, Demer LL: cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem 273: 7547–7553, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Tintut Y, Demer LL: Recent advances in multifactorial regulation of vascular calcification. Curr Opin Lipidol 12: 555–560, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Collin-Osdoby P: Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 95: 1046–1057, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ: Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89: 309–319, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J: Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 109: 2175–2180, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Persy V, D’Haese P: Vascular calcification and bone disease: The calcification paradox. Trends Mol Med 15: 405–416, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, Squire IB, Gullestad L, Bollerslev J, Dickstein K, Aukrust P: Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol 44: 1970–1976, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC: Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab 88: 1024–1028, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Browner WS, Lui LY, Cummings SR: Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab 86: 631–637, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Morena M, Terrier N, Jaussent I, Leray-Moragues H, Chalabi L, Rivory JP, Maurice F, Delcourt C, Cristol JP, Canaud B, Dupuy AM: Plasma osteoprotegerin is associated with mortality in hemodialysis patients. J Am Soc Nephrol 17: 262–270, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Moe SM, Reslerova M, Ketteler M, O’neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX: Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 67: 2295–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Barreto DV, Barreto FC, Carvalho AB, Cuppari L, Cendoroglo M, Draibe SA, Moyses RM, Neves KR, Jorgetti V, Blair A, Guiberteau R, Fernandes Canziani ME: Coronary calcification in hemodialysis patients: The contribution of traditional and uremia-related risk factors. Kidney Int 67: 1576–1582, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Morena M, Dupuy AM, Jaussent I, Vernhet H, Gahide G, Klouche K, Bargnoux AS, Delcourt C, Canaud B, Cristol JP: A cut-off value of plasma osteoprotegerin level may predict the presence of coronary artery calcifications in chronic kidney disease patients. Nephrol Dial Transplant 24: 3389–3397, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Bargnoux AS, Dupuy AM, Garrigue V, Jaussent I, Gahide G, Badiou S, Szwarc I, Deleuze S, Vernhet H, Cristol JP, Mourad G: Evolution of coronary artery calcifications following kidney transplantation: Relationship with osteoprotegerin levels. Am J Transplant 9: 2571–2579, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferro S, Pasquali M, Taggi F, Baldinelli M, Conte C, Muci ML, Pirozzi N, Carbone I, Francone M, Pugliese F: Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol 4: 685–690, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitta K, Akiba T, Uchida K, Kawashima A, Yumura W, Kabaya T, Nihei H: The progression of vascular calcification and serum osteoprotegerin levels in patients on long-term hemodialysis. Am J Kidney Dis 42: 303–309, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Crisafulli A, Micari A, Altavilla D, Saporito F, Sardella A, Passaniti M, Raffa S, D’anneo G, Lucà F, Mioni C, Arrigo F, Squadrito F: Serum levels of osteoprotegerin and RANKL in patients with ST elevation acute myocardial infarction. Clin Sci (Lond) 109: 389–395, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Schoppet M, Schaefer JR, Hofbauer LC: Low serum levels of soluble RANK ligand are associated with the presence of coronary artery disease in men. Circulation 107: e76, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Shin JY, Shin YG, Chung CH: Elevated serum osteoprotegerin levels are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 29: 1664–1666, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sandberg WJ, Yndestad A, Øie E, Smith C, Ueland T, Ovchinnikova O, Robertson AK, Müller F, Semb AG, Scholz H, Andreassen AK, Gullestad L, Damås JK, Frøland SS, Hansson GK, Halvorsen B, Aukrust P: Enhanced T-cell expression of RANK ligand in acute coronary syndrome: Possible role in plaque destabilization. Arterioscler Thromb Vasc Biol 26: 857–863, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Barreto FC, Barreto DV, Moyses RM, Neves CL, Jorgetti V, Draibe SA, Canziani ME, Carvalho AB: Osteoporosis in hemodialysis patients revisited by bone histomorphometry: A new insight into an old problem. Kidney Int 69: 1852–1857, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Anand DV, Lahiri A, Lim E, Hopkins D, Corder R: The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J Am Coll Cardiol 47: 1850–1857, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kurnatowska I, Grzelak P, Kaczmarska M, Stefańczyk L, Nowicki M: Serum osteoprotegerin is a predictor of progression of atherosclerosis and coronary calcification in hemodialysis patients. Nephron Clin Pract 117: c297–c304, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Raggi P, Callister TQ, Shaw LJ: Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol 24: 1272–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Raggi P, Cooil B, Shaw LJ, Aboulhson J, Takasu J, Budoff M, Callister TQ: Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol 92:827–829, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Asanuma Y, Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, Raggi P, Sokka T, Pincus T, Stein CM: Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis 195: e135–e141, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SM, Lee J, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM: Serum osteoprotegerin levels are associated with inflammation and pulse wave velocity. Clin Endocrinol (Oxf) 63: 594–598, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Tintut Y, Patel J, Parhami F, Demer LL: Tumor necrosis factor-α promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 102: 2636–2642, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, Santer P, Mayr A, Xu Q, Willeit J: Soluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular disease. Circulation 116: 385–391, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Lieb W, Gona P, Larson MG, Massaro JM, Lipinska I, Keaney JF, Jr, Rong J, Corey D, Hoffmann U, Fox CS, Vasan RS, Benjamin EJ, O’Donnell CJ, Kathiresan S: Biomarkers of the osteoprotegerin pathway: Clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler Thromb Vasc Biol 30: 1849–1854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.London GM, Marchais SJ, Guérin AP, Boutouyrie P, Métivier F, de Vernejoul MC: Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 19: 1827–1835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]