Summary
Background and objectives
The uremic solutes p-cresol sulfate (PCS) and indoxyl sulfate (IS) are generated by colon bacteria acting on food components that escape absorption in the small bowel. The production of these potentially toxic compounds may thus be influenced by diet. This study examined whether production of PCS and IS is different in vegetarians and omnivores.
Design, setting, participants, & measurements
The production of PCS and IS was assessed by measuring their urinary excretion rates in participants with normal kidney function. Studies were carried out in 15 vegetarians and 11 individuals consuming an unrestricted diet. Participants recorded food intake over 4 days and collected urine over the final 2 days of each of two study periods, which were 1 month apart.
Results
Average PCS excretion was 62% lower (95% confidence interval [95% CI], 15–83) and average IS excretion was 58% lower (95% CI, 39–71) in vegetarians than in participants consuming an unrestricted diet. Food records revealed that lower excretion of PCS and IS in vegetarians was associated with a 69% higher (95% CI, 20–139) fiber intake and a 25% lower (95% CI, 3–42) protein intake. PCS and IS excretion rates varied widely among individual participants and were not closely correlated with each other but tended to remain stable in individual participants over 1 month.
Conclusions
PCS and IS production rates are markedly lower in vegetarians than in individuals consuming an unrestricted diet.
Introduction
Numerous organic solutes that are normally cleared by the kidneys accumulate in the plasma when the kidneys fail (1). Some of these “uremic” solutes are produced in the colon by bacteria acting on food components that escape digestion in the small bowel (2,3). The colon-derived solutes include p-cresol sulfate (PCS) and indoxyl sulfate (IS), which are produced by bacterial metabolism of the amino acids tyrosine and tryptophan, respectively (4,5). There is considerable, albeit inconclusive, evidence that these compounds are toxic (4,6–10).
Treatments that limit solute production could in theory be combined with dialysis to reduce uremic solute levels in patients with renal failure. Because they are made in an isolated compartment by processes that are foreign to those of mammalian cells, the production of colon-derived solutes such as PCS and IS might prove particularly susceptible to manipulation. Recent studies have explored the effect of dietary maneuvers designed to reduce the production of PCS in normal participants as well as hemodialysis patients (2). Promising results have been obtained, but the reductions achieved in solute production have been modest. While measuring PCS and IS production in normal participants to obtain control data for a study of dialysis patients, we observed that urinary excretion rates for PCS and IS were much lower in vegetarians than in participants consuming unrestricted diets. This study compared PCS and IS production in a larger number of participants consuming vegetarian and unrestricted diets and examined the variability in the production rates for these solutes.
Materials and Methods
Studies were carried out in 26 healthy participants with no known kidney disease. Fifteen participants were vegetarians, whereas the remaining 11 participants consumed an unrestricted diet including meat and meat products. The vegetarian participants ate egg and dairy products but no meat or fish. Vegetarian participants’ dietary habits were confirmed by interview. All participants had no history of gastrointestinal surgery or diarrheal illness and had not used antibiotics for at least 1 month before enrollment. Thirteen of the 15 vegetarian participants and 6 of the 11 nonvegetarian participants were of Asian ancestry. The study was performed in accordance with the Declaration of Helsinki and was approved by the Stanford University institutional review board.
Samples were collected during two study periods 1 month apart, referred to below as period 1 and period 2. During each period, participants recorded their food intake over 4 consecutive days in a food diary. Two consecutive 24-hour urine samples were collected during the last 2 days of each 4-day period. Urinary PCS and IS were measured by HPLC as previously described (11). Blinded analysis of split urine samples (four aliquots of each of four samples) revealed an average coefficient of variation of 5% (range, 1%–6%) for PCS and 2% (range, 1%–4%) for IS. Urine urea nitrogen was measured using a commercial kit (1770–500; Thermo Electron Corp, Melbourne, Australia). Urinary sulfate was measured by reaction with TCA and barium chloride and was read spectrophotometrically at 360 nm (12). Urinary phosphate levels were measured using a commercial kit (P7516; Pointe Scientific Inc, Canton, MI). Urinary sodium, potassium, and creatinine concentrations were measured in the clinical laboratory. Daily solute excretion was calculated as the product of the urine solute concentration and the 24-hour urine volume. Food records were analyzed using Food Processor SQL Edition software (version 10.3.0; ESHA Research, Salem, Oregon). Thirteen of the 15 vegetarian participants and all 11 unrestricted participants who provided an initial food record and urine samples (period 1) repeated the study after 1 month (period 2). One vegetarian participant provided urine but did not complete the food record for period 2.
Reported consumption of calories, protein, fiber, carbohydrate, and fat was averaged over the 4 days of the food record. Solute excretion and nutrient consumption rates were corrected for body surface area calculated using the Mosteller formula (13). Values for vegetarian participants and unrestricted participants were compared using the unpaired t test. Values for PCS and IS excretion were log transformed for comparison. The average percentage differences in PCS and IS excretion in vegetarian and unrestricted participants were calculated based on mean excretion rates over all days of urine collection in each participant. Potential relationships between the excretion of PCS and IS and other parameters were examined using linear regression. Statistical calculations were performed using SPSS software (version 19; IBM, Chicago, IL).
Results
Characteristics of the participants are summarized in Table 1. Vegetarian participants were slightly older than those eating an unrestricted diet but had the same average body size. Excretion rates for PCS and IS are summarized in Table 2. Participants eating a vegetarian diet excreted less of both solutes than participants eating an unrestricted diet. During the first collection period, daily PCS excretion averaged 30 mg/d per 1.73 m2 (interquartile range [IQR], 11–74) in vegetarian participants compared with 100 mg/d per 1.73 m2 (IQR, 47–124) in unrestricted participants. IS excretion averaged 24 mg/d per 1.73 m2 (IQR, 12–42) and 47 mg/d per 1.73 m2 (IQR, 40–59) in the two groups. Repeat values obtained after a 1-month interval were similar. Excretion rates for PCS and IS in individual participants who were studied at both intervals are further depicted in Figure 1. There was wide variation among individual participants; however, it was notable that for each solute and within each group, participants with lower than average excretion rates on initial evaluation tended to have lower than average excretion rates when studied again 1 month later. The average ratios of the period 2/period 1 excretion rates were 0.93±0.46 and 0.91±0.37 in the vegetarian and unrestricted groups, respectively, for PCS, and 0.98±0.34 and 1.07±0.34, respectively, for IS. Urinary excretion rates for urea nitrogen and other diet-derived solutes are summarized in Tables 2 and 3. Average urea nitrogen excretion was approximately 30% lower in the vegetarian participants than in those eating an unrestricted diet. Excretion rates for creatinine were also significantly lower in vegetarian participants, although to a lesser degree. Sulfate excretion in the vegetarian participants was lower by approximately the same proportion as urea nitrogen excretion. Average rates of sodium and potassium excretion were not different, whereas the average rate of urinary phosphate excretion was significantly lower in the vegetarian participants.
Table 1.
Participant characteristics
| Characteristic | Vegetarian (n=15) | Unrestricted (n=11) |
|---|---|---|
| Age (yr) | 49±15a | 39±11 |
| Vegetarian (yr) | 26±12 | – |
| Female/male | 6:9 | 6:5 |
| Weight (kg) | 68±14 | 67±11 |
| Height (m) | 1.67±0.11 | 1.68±0.11 |
| Body surface area (m2) | 1.77±0.23 | 1.77±0.19 |
Values mean ± SD.
P=0.02, vegetarian versus unrestricted.
Table 2.
Urinary excretion of PCS and IS
| Vegetarian | Unrestricted | P Value (Vegetarian versus Unrestricted) | ||||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Period 1 | Period 2 | |
| PCS (mg/d per 1.73 m2) | 30 (11–74) | 23 (17–47) | 100 (47–124) | 95 (20–124) | 0.03 | 0.03 |
| IS (mg/d per 1.73 m2) | 24 (12–42) | 19 ( 17–26) | 47 (40–59) | 52 (39–58) | 0.001 | <0.001 |
Values are median (interquartile range). PCS, p-cresol sulfate; IS, indoxyl sulfate.
Figure 1.
Urinary PCS and IS excretion. Average daily excretion rates for PCS (left panel) and IS (right panel). Values obtained for individual vegetarian participants in period 1 and period 2 are represented by triangles connected by broken lines. Values obtained for unrestricted participants are represented by circles connected by solid lines. PCS, p-cresol sulfate; IS, indoxyl sulfate.
Table 3.
Urinary excretion of diet-derived solutes
| Diet-Derived Solute | Vegetarian | Unrestricted | P Value (Vegetarian versus Unrestricted) | |||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Period 1 | Period 2 | |
| Urea nitrogen (g/d per 1.73 m2) | 8.3±1.6 | 8.1±1.6 | 11.4±3.0 | 12.1±1.9 | 0.007 | <0.001 |
| Creatinine (g/d per 1.73 m2) | 1.16±0.23 | 1.15±0.25 | 1.45±0.25 | 1.50±0.25 | 0.007 | 0.001 |
| Sulfate (mmol/d per 1.73 m2) | 18±4 | 19±7 | 26±6 | 27±4 | 0.001 | 0.01 |
| Sodium (mEq/d per 1.73 m2) | 134±58 | 125±58 | 146±58 | 144±49 | 0.59 | 0.38 |
| Potassium (mEq/d per 1.73 m2) | 75±21 | 73±27 | 68±16 | 71±19 | 0.40 | 0.77 |
| Phosphate (mg/d per 1.73 m2) | 741±184 | 763±172 | 913±181 | 934±134 | 0.03 | 0.01 |
Values are mean ± SD.
Consumption rates for food components as determined from food records are summarized in Table 4. Caloric intake was not different in the two groups. Consistent with their lower urea nitrogen excretion, vegetarian participants reported lower protein intake than unrestricted participants. The estimated intake of fiber was significantly higher in vegetarian participants as was the intake of carbohydrate, whereas estimated fat intake was not different.
Table 4.
Nutrient intake as assessed by food record
| Nutrient | Vegetarian | Unrestricted | P Value (Vegetarian versus Unrestricted) | |||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Period 1 | Period 2 | |
| Calories (kcal/d per 1.73 m2) | 1806±461 | 1775±448 | 1887±291 | 1718±387 | 0.59 | 0.74 |
| Protein (g/d per 1.73 m2) | 62±18 | 62±18 | 82±25 | 84±23 | 0.04 | 0.02 |
| Fiber (g/d per 1.73 m2) | 33±14 | 32±11 | 19±7 | 19±10 | 0.002 | 0.005 |
| Carbohydrate (g/d per 1.73 m2) | 276±72 | 271±82 | 228±37 | 202±52 | 0.04 | 0.02 |
| Fat (g/d per 1.73 m2) | 56±30 | 55±18 | 66±18 | 57±19 | 0.32 | 0.89 |
Values mean ± SD.
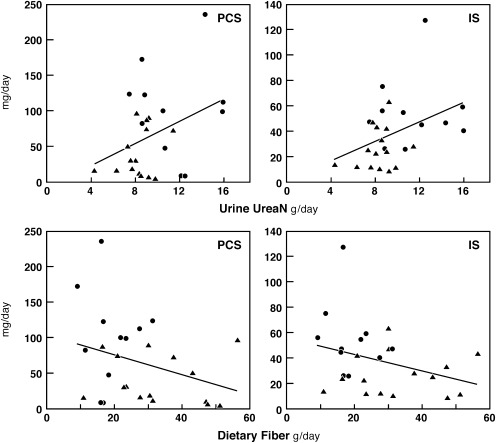
There was little relation between the rates of PCS and IS production in individual participants, as illustrated in Figure 2. The production rates for both solutes tended to increase with protein intake as reflected by urea nitrogen excretion and to decrease with fiber intake; however, these correlations were not strong, as illustrated in Figure 3. The production for both solute also tended to rise with protein intake as recorded in the food record, but the correlations were weaker than those with urea nitrogen excretion (not shown).
Figure 2.
The relation of PCS and IS excretion in individual participants. PCS and IS excretion rates were not closely correlated. Values are for period 1 in vegetarian (triangles) and unrestricted (circles) participants (r2=0.06; P=0.21). Results for period 2 were similar (not shown). PCS, p-cresol sulfate; IS, indoxyl sulfate.
Figure 3.
The relation of PCS and IS excretion to urea nitrogen excretion and to fiber intake. PCS and IS excretion rates were only weakly correlated with protein intake as reflected by urine urea nitrogen excretion (upper panels) and with fiber intake as obtained by dietary history (lower panels). Values are for period 1 for vegetarian (triangles) and unrestricted (circles) participants with the following correlations: PCS versus urine urea nitrogen (r2=0.14; P=0.06); IS versus urine urea nitrogen (r2=0.16; P=0.04); PCS versus dietary fiber, (r2=0.10; P=0.12); and IS versus dietary fiber (r2=0.11; P=0.10). PCS, p-cresol sulfate; IS, indoxyl sulfate; UreaN, urea nitrogen.
Discussion
Treatment of ESRD is now focused on removing uremic solutes by dialysis. In theory, treatments that reduce solute production could also reduce solute levels and ameliorate uremic illness. One barrier to the development of such treatments has been limited knowledge of the factors that influence solute production.
This study focused on the production of two uremic solutes, PCS and IS, which have recently been the subject of intense study (4,5). There is substantial, albeit inconclusive evidence, that both PCS and IS contribute to illness in dialysis patients (4,6–10). Their dialytic clearance is greatly restricted by protein binding, which could make maneuvers that lower their production particularly valuable. PCS and IS are also the best known members of the class of uremic solutes derived from the action of colonic microbes (2,3,14). Because such solutes are made in an isolated compartment by nonmammalian metabolism, their production could prove simpler to suppress than the production of other uremic solutes.
One obvious means to influence the production of colon-derived solutes is manipulation of the diet. This study showed that average excretion of PCS and IS was approximately 60% lower in vegetarians than in individuals eating an unrestricted diet. Urinary excretion is considered to reflect solute production in normal participants, there being no other known route of removal from the plasma except dialysis (15,16).
Previous studies, however, identified mechanisms that could account for reduced production of these solutes in vegetarians (2). One potential mechanism by which a vegetarian diet could reduce PCS and IS production is by increasing the dietary intake of fiber. Reported average fiber intake in our study was approximately 70% higher in vegetarians than in participants consuming an unrestricted diet. This difference is similar to that observed in a large study of US vegetarians (17). The term fiber includes various forms of carbohydrate that escape digestion in the small intestine. Fermentation of some of this carbohydrate by colon microbes provides energy to the host in the form of short-chain fatty acids. Colon microbes are also supplied with amino acids in the form of incompletely digested proteins, sloughed intestinal cells, and secretions (18,19). PCS is the sulfate conjugate of p-cresol, which is formed by microbes from phenylalanine and tyrosine; IS is the sulfate conjugate of indoxyl, which is formed by microbes from tryptophan. The portion of these amino acids converted to PCS and IS may be reduced by increasing fiber intake (2). Increasing fiber intake provides increased substrate for microbial fermentation so that amino acids are consumed in microbial growth rather than broken down to waste solutes. In addition, a reduced colon transit time may limit conversion of amino acids to waste solutes when fiber intake is high (20).
A second mechanism by which a vegetarian diet could reduce PCS and IS production is by reducing protein intake. In this study, average reported protein intake was approximately 25% lower in vegetarians than in participants consuming an unrestricted diet. This difference, which was confirmed by measurement of urine urea nitrogen excretion, is again similar to that observed in a large study of US vegetarians (17). Lowering dietary protein can reduce delivery of amino acids to the colon and thereby limit their availability for conversion to PCS and IS (18).
The extent to which a higher fiber intake and a lower protein intake accounted for lower PCS and IS production in vegetarians cannot be determined from our results. The differences we observed, however, are larger than might be expected based on short-term studies in which fiber and protein intake have been manipulated. Ingestion of 20 g/d of the indigestible carbohydrate oligo-fructose inulin for 4 weeks was found to reduce PCS production by 20%–30% in normal volunteers (21). The same treatment was subsequently shown to decrease plasma PCS levels by about 20% without altering IS levels in hemodialysis patients (7). Available studies of the effect of protein intake are of shorter duration and have documented changes in PCS production, assessed as urinary p-cresol excretion, only with a reduction of protein intake to 20 g/d or an increase of protein intake to approximately 125 g/d (22,23).
Several factors may have contributed to our vegetarian participants having lower PCS and IS production rates than would be expected based on available studies in which fiber and protein intake were manipulated. Higher fiber and lower protein intakes were combined in vegetarian participants. This could cause larger changes in microbial metabolism than would be achieved by separate manipulation of either fiber or protein alone. The types of fiber consumed by the vegetarian participants may have been particularly effective in promoting microbial growth and PCS and IS production may also have been influenced by other differences in vegetarian and unrestricted diets that we did not explore, including differences in the type of protein ingested (17,24). Finally, our vegetarian participants had adhered to meat- and fish-free diets for many years, whereas experimental studies of dietary manipulation have necessarily been of limited duration.
Over the long term, vegetarian and unrestricted diets may have influenced PCS and IS production by altering the colon microbial flora, or microbiome, as well as by providing different nutrients to colon microbes on a day-to-day basis. DNA sequencing technology has greatly increased our knowledge of the microbiome, which dwarfs its human host in cell number and even more in gene number (25,26). Recent studies have documented major differences in the composition of the colon microbiome in individuals consuming different diets (27). Other studies have shown that there is enormous dissimilarity in the microbiome between individuals but that the composition of the microbiome in an individual tends to remain stable over long periods. These findings, coupled with knowledge that the enzymes that produce p-cresol and indole are irregularly present among the myriad types of colon microbes, could account for many features of PCS and IS production (28,29). As observed in prior studies, we found that PCS and IS production rates were poorly correlated in individual participants, which could be explained by differences in the microbial flora of different individuals (30). In addition, the variation in PCS and IS production among individuals was much larger than the variation in urea production, and the correlations between PCS and IS production and protein and fiber intake were weak. Although there were wide individual variations in PCS and IS production among individual participants, the rates of PCS and IS production tended to remain stable in each individual.
Some other differences were noted in the two groups. Vegetarian participants had lower urinary sulfate excretion, which can be accounted for by lesser intake of sulfur-containing amino acids associated with lesser protein intake. Vegetarian participants also had a lower urinary creatinine excretion, which can be accounted for by lesser intake of creatinine in the form of cooked meat and also by a slightly higher percentage of women among the vegetarian participants (31). A particularly interesting finding was that average phosphate excretion was lower in vegetarian participants. Moe et al. (32) recently found that plasma phosphate was lower and urinary phosphate tended to be lower in patients with chronic renal insufficiency on a vegetarian diet than on a meat diet with the same phosphorous content. This may reflect lesser absorption of phosphate from plant foods, in which a large portion of phosphate is in the form of phytate, than from meat to which phosphate salts are now often added as preservatives (33). Dietary recommendations for patients with renal insufficiency may require modification in light of these findings (32,34).
Our study has notable limitations. PCS and IS are only two of what is likely a very large number of uremic solutes made by colon microbes, and we do not know the extent to which these solutes are toxic (14). Our study was performed in participants with normal renal function, and we cannot be sure that a vegetarian diet has a similar effect on PCS and IS production in patients with renal failure. We did not assess fecal solute excretion, and thus could have failed to detect differences in colonic absorption of unconjugated PCS or IS precursors or intestinal secretion of the conjugates. Most importantly, our study does not reveal what feature(s) of the vegetarian participants' diets contributed most to their lower levels of PCS and IS production.
In conclusion, we found that in individuals with normal renal function production of the uremic solutes PCS and IS averaged approximately 60% lower in long-time vegetarians than in participants eating an unrestricted diet. This finding supports hope that production of these solutes could be reduced in dialysis patients. Dialysis patients are already subject to burdensome dietary restrictions, and few of them would likely chose to become vegetarians. Further study could, however, allow the effects of the vegetarian diet to be replicated by selective prebiotic, probiotic, or pharmacologic therapies.
Disclosures
None.
Acknowledgments
Support was provided by grants from the National Institutes of Health (R21 AT005123 and RO1 DK80123 to T.W.M. and RO1 DK80123 to T.H.H.) and by a National Kidney Foundation Fellowship award (to F.J.G.L.).
Footnotes
K.P.P. and F.J.G.L. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J, European Uremic Toxin Work Group : A bench to bedside view of uremic toxins. J Am Soc Nephrol 19: 863–870, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Evenepoel P, Meijers BK, Bammens BR, Verbeke K: Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 114: S12–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Schepers E, Glorieux G, Vanholder R: The gut: The forgotten organ in uremia? Blood Purif 29: 130–136, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Niwa T: Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr 20[Suppl]: S2–S6, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Vanholder R, Bammens B, de Loor H, Glorieux G, Meijers B, Schepers E, Massy Z, Evenepoel P: Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol Dial Transplant 26: 1464–1467, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R: P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 22: 592–596, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Sirich T, Meyer TW: Indoxyl sulfate: Long suspected but not yet proven guilty. Clin J Am Soc Nephrol 6: 3–4, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Martinez AW, Recht NS, Hostetter TH, Meyer TW: Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol 16: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Krijgsheld KR, Frankena H, Scholtens E, Zweens J, Mulder GJ: Absorption, serum levels and urinary excretion of inorganic sulfate after oral administration of sodium sulfate in the conscious rat. Biochim Biophys Acta 586: 492–500, 1979 [DOI] [PubMed] [Google Scholar]
- 13.Mosteller RD: Simplified calculation of body-surface area. N Engl J Med 317: 1098, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P: Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int 64: 2196–2203, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Niwa T: Organic acids and the uremic syndrome: Protein metabolite hypothesis in the progression of chronic renal failure. Semin Nephrol 16: 167–182, 1996 [PubMed] [Google Scholar]
- 17.Haddad EH, Tanzman JS: What do vegetarians in the United States eat? Am J Clin Nutr 78[Suppl]: 626S–632S, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Chacko A, Cummings JH: Nitrogen losses from the human small bowel: Obligatory losses and the effect of physical form of food. Gut 29: 809–815, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordgaard I, Mortensen PB: Digestive processes in the human colon. Nutrition 11: 37–45, 1995 [PubMed] [Google Scholar]
- 20.Stephen AM, Wiggins HS, Cummings JH: Effect of changing transit time on colonic microbial metabolism in man. Gut 28: 601–609, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Preter V, Vanhoutte T, Huys G, Swings J, De Vuyst L, Rutgeerts P, Verbeke K: Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am J Physiol Gastrointest Liver Physiol 292: G358–G368, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Wengle B, Hellström K: Volatile phenols in serum of uraemic patients. Clin Sci 43: 493–498, 1972 [DOI] [PubMed] [Google Scholar]
- 23.Geypens B, Claus D, Evenepoel P, Hiele M, Maes B, Peeters M, Rutgeerts P, Ghoos Y: Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 41: 70–76, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Key TJ, Appleby PN, Rosell MS: Health effects of vegetarian and vegan diets. Proc Nutr Soc 65: 35–41, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Dethlefsen L, McFall-Ngai M, Relman DA: An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449: 811–818, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman AL, Gordon JI: Our unindicted coconspirators: Human metabolism from a microbial perspective. Cell Metab 12: 111–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gophna U: Microbiology. The guts of dietary habits. Science 334: 45–46, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Blaser M, Andrei PI, Pierik AJ, Selmer T: 4-Hydroxyphenylacetate decarboxylases: Properties of a novel subclass of glycyl radical enzyme systems. Biochemistry 45: 9584–9592, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Yanofsky C: RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. RNA 13: 1141–1154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijers BK, De Loor H, Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin J Am Soc Nephrol 4: 1932–1938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preiss DJ, Godber IM, Lamb EJ, Dalton RN, Gunn IR: The influence of a cooked-meat meal on estimated glomerular filtration rate. Ann Clin Biochem 44: 35–42, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan C, Sayre SS, Leon JB, Machekano R, Love TE, Porter D, Marbury M, Sehgal AR: Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 301: 629–635, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Fukagawa M, Komaba H, Miyamoto K: Source matters: From phosphorus load to bioavailability. Clin J Am Soc Nephrol 6: 239–240, 2011 [DOI] [PubMed] [Google Scholar]