Summary
Background and objectives
Increased inflammation and oxidative stress may be caused by proteins and lipids modified by cytotoxic advanced glycation end products (AGEs) in food. Restricting food containing elevated AGEs improves these risk factors in diabetic CKD. Because diet adherence can be problematic, this study aimed to remove cytotoxic AGEs from food already ingested and to determine whether sevelamer carbonate sequesters cytotoxic AGEs in the gut, preventing their uptake and thereby reducing AGE-induced abnormalities.
Design, setting, participants, & measurements
This single-center, randomized, 2-month, open-label, intention-to-treat, crossover study compared sevelamer carbonate with calcium carbonate treatment in stage 2–4 diabetic CKD. Participants received 2 months of treatment with one drug, had a 1-week washout, and then received the opposite drug for 2 months.
Results
Sevelamer carbonate reduced HbA1c, serum methylglyoxal, serum εN-carboxymethyl-lysine, triglycerides, and 8-isoprostanes. Total cholesterol and fibroblast growth factor 23 were reduced by sevelamer carbonate, relative to calcium carbonate. AGE receptor 1 and sirtuin 1 mRNA were increased and PMNC TNFα levels were decreased by sevelamer carbonate, but not calcium carbonate. Medications and caloric and AGE intake remained unchanged. Sevelamer carbonate reversibly bound AGE-BSA at intestinal, but not stomach, pH.
Conclusions
Sevelamer carbonate significantly reduces HbA1c, fibroblast growth factor 23, lipids, and markers of inflammation and oxidative stress, and markedly increases antioxidant markers, independently of phosphorus in patients with diabetes and early kidney disease. These novel actions of sevelamer carbonate on metabolic and inflammatory abnormalities in type 2 diabetes mellitus may affect progression of early diabetic CKD.
Introduction
Patients with type 1 and type 2 diabetes and CKD remain at high risk for ESRD despite currently available interventions (1,2). A recent meta-analysis suggests the need for new agents with a better safety profile (3).
Because proteins and lipids modified by cytotoxic advanced glycation end products (AGEs) elevate levels of inflammatory markers and oxidative stress, AGEs may be important in the development and progression of CKD in type 2 diabetes (4–6). Type 2 diabetes mellitus patients with increased levels of AGEs have a three-fold increased risk for kidney disease and a seven-fold increased risk for any complication (7). A drug that blocks AGE formation blunts the increase in serum creatinine in patients with type 2 diabetes mellitus and progressive CKD, confirming the beneficial effects of AGE reduction (8,9). The recent recognition that AGEs present in food are a major risk factor for cardiovascular disease (CVD) in diabetes was based on the fact that dietary AGE restriction prevents renal and cardiovascular complications in diabetic animals and that AGE restriction rapidly reduces markers of inflammation and oxidative stress in patients with CKD as well as in patients with type 2 diabetes mellitus with CKD (10,11). AGE restriction also reduces insulin levels in type 2 diabetes, without a change in fasting blood glucose, a change compatible with decreased insulin resistance (10). This has also been shown in mouse models (4). Finally, a study of monozygotic and dizygotic twins and northern European children revealed that environmental AGEs, likely from the diet, are important in the development of type 1 diabetes mellitus (D. Leslie, unpublished observations) (12).
Abnormalities in calcium and phosphate intake and blood levels are associated with vascular calcification, which increases the risk and/or severity of CVD in CKD patients with type 2 diabetes mellitus (13). Fibroblast growth factor 23 (FGF-23), serum calcium, and phosphate are reduced by sevelamer carbonate, a nonabsorbed phosphate binding polymer often used in stage 4–5 CKD treatment (14–17). Although sevelamer carbonate also decreases LDL cholesterol and improves insulin resistance (18), it is not known whether these effects on CVD risk factors are mediated by reduced inflammation and oxidative stress. Calcium carbonate and sevelamer carbonate both lower dietary phosphate uptake (19). Whereas high serum phosphorus levels are associated with increased cardiovascular mortality, reduction of serum phosphorus levels has not modified mortality (19,20). Sevelamer carbonate reduces coronary artery calcification in hemodialysis patients (15,21). Finally, FGF-23 is associated independently and strongly with all-cause mortality CVD and initiation of dialysis, and predicts renal outcomes in diabetes (17,22).
Because compliance with dietary manipulations can be difficult for some patients to follow, we sought another method of reducing the amount of AGEs available for uptake in the small intestine, namely by sequestering AGEs from ingested food and eliminating them in the stool. The chemical structure of sevelamer carbonate and its effects on lipid metabolism led us to postulate that it could bind AGEs and make them unavailable for uptake. This study was designed to determine if sevelamer carbonate bound AGEs and if administration of this nonabsorbable drug led to metabolic, anti-inflammatory and antioxidative stress actions that are independent of its effect on phosphate uptake in patients with type 2 diabetes and stage 2–4 CKD.
Materials and Methods
Patients who were treated for diabetes with at least one medication and who had proteinuria (milligrams of urinary protein/milligrams of creatinine >0.2) on ≥2 occasions within the previous 18 months were recruited from the Mount Sinai Medical Center Clinics. Of 600 clinic records screened, 80 participants were identified and 20 were randomized, the number calculated to give an 80% chance of finding a 20% difference in AGEs at the study end. Exclusion criteria included current treatment for hyperphosphatemia, biopsy-proven renal disease other than diabetic kidney disease, hypophosphatemia, and hypercalcemia. Anthropometric parameters, 3-day food records, 24-hour urine collections (except for three participants who were incontinent), and fasting blood samples were obtained at the beginning and at the end of each phase of the 8-week intervention.
The Mount Sinai Medical Center Institutional Review Board approved this study (NCT01493050) and all patients signed informed consent forms, consistent with the Declaration of Helsinki.
Study Design
This was a single-center, randomized, crossover, open-label, intention-to-treat study. The participants continued the medical care prescribed by their primary physicians. Medications were as follows: insulin or oral antidiabetic drugs (n=13), metformin (n=3), glipizide (n=2), glyburide (n=2), repaglinide (n=2), pioglitazone (n=4), statins (n=15), aspirin (n=14), vitamin D (n=7), activated vitamin D (n=3), furosemide (n=13), or hydrochlorothiazide (n=6). All participants received ≥1 antihypertensive drugs, and 15 received angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Daily multivitamin supplements containing 400 IU of cholecalciferol (Natures Bounty Inc, Bohemia, NY) were provided.
Participants were randomized to either sevelamer carbonate (1600 mg three times per day with meals) or calcium carbonate (1200 mg three times per day with meals) for 8 weeks. After a 1-week washout period, participants were followed for 8 additional weeks on the alternate drug. Compliance, defined as returning <20% of the prescribed drugs, was estimated to be 70% in the calcium carbonate arm and 75% in the sevelamer carbonate arm.
A study dietitian followed participants at monthly clinic visits to ensure stability of dietary intake of calcium, inorganic phosphates, and nutrients.
Dietary Intake
Nutrient, mineral content, and AGE intake were estimated from food records using a nutrient software program (Food Processor version 10.1; ESHA Research, Salem, OR) and from a food-AGE database (23).
Routine Blood Tests
The hospital laboratory performed routine blood and urine measurements. GFR was estimated using the Modified Diet in Renal Disease study formula (13).
Measurement of AGEs and Circulating Biomarkers
εN-carboxymethyl-lysine (CML) and methylglyoxal derivatives in serum and urine were quantified by ELISA using two non–cross-reactive mAbs that recognize lipids and proteins modified by AGEs, but not unbound AGEs. Interassay coefficients of variation were 2.8% for CML and 5.2% for methylglyoxal, whereas intra-assay coefficients of variation were 2.6% for CML and 4.1% for methylglyoxal) (24). Leptin, adiponectin, and TNF receptor 1 were tested by commercial ELISA kits. Cystatin C and FGF-23 were measured by the Mount Sinai Clinical Laboratories and Genzyme Diagnostics Laboratories (Boston, MA), respectively.
PMNCs
PMNCs were separated by Ficoll-Hypaque Plus gradient (American Biosciences, Uppsala, Sweden) (25). Proteins were extracted from cell lysates. Total RNA was extracted using TRIzol (Molecular Probes Inc). The extracted RNA OD 280/260 ratio was 1.8/2.0. Total RNA was reverse transcribed using Superscript III RT (Invitrogen).
Quantitative Real-Time PCR Assay
mRNA expression of AGE receptor 1 (AGER1) and sirtuin 1 (SIRT1) were analyzed by quantitative SYBR Green real-time PCR, as previously described (26,27). The transcript copy number of target genes was determined based on cycle threshold values.
Western Blot Analyses
Cell lysates were prepared by sonication in 500 μl of lysis buffer (New England Biolabs), and cell proteins were separated on 8% SDS-PAGE gels, transferred onto nitrocellulose membranes, and visualized by chemiluminescence (Roche) (28). Bound immune complexes in RIPA lysis buffer were used for immunoblotting after SDS-PAGE and nitrocellulose membrane transfer (28).
Binding of AGEs to Sevelamer Carbonate
Sevelamer hydrochloride (25 mg) (Genzyme Corporation, Boston, MA), an insoluble resin, was washed in N,N-Bis(hydroxyethyl)-2-aminoethanesulfonic acid (BES) (Sigma Chemical Company, St. Louis, MO) and suspended in 30 ml of BES (29). BSA and LPS-free AGE-BSA were prepared (30). The insoluble resin was added to BES buffer, and after adjusting the buffer to pH 7.0, 5 µg of 125I-AGE-BSA or 125I-BSA was added, stirred at 28°C for 6 hours, and washed with BES until the supernatant was free of radioactivity. The sevelamer resin was suspended in BES buffer, and the pH was adjusted to 1.0 with 1N HCl. After incubation for 6 hours at 28°C, the resin was washed with BES and sevelamer-bound radioactivity was re-measured. The experiments were repeated using the reverse protocol to determine if AGE-BSA or BSA released at pH 1.0 was rebound at pH 7.0.
Statistical Analyses
Baseline data were sometimes skewed; Spearman rank correlations were thus used to summarize associations. For each variable, the effect of sevelamer carbonate was defined as the last recorded value minus the first recorded value under sevelamer carbonate, similarly for calcium carbonate. To account for highly skewed variables, within-treatment changes were tested using signed rank and Wilcoxon tests. The difference between the effect for sevelamer carbonate and the effect for calcium carbonate was taken as the effect of sevelamer carbonate relative to calcium carbonate. Generalized estimating equations, with an unstructured correlation structure and robust variance estimation, were used to test for carryover effects (i.e., treatment by period interactions). When this test was significant, similar models were used to estimate and test the effect of sevelamer carbonate relative to calcium carbonate, adjusting for the period in which sevelamer carbonate was administered. The only evidence of a period by treatment interaction was for cystatin C. For this variable, the relative treatment effect was computed with a t test using only the data from the first period of the observation (31). Tests were considered significant if the two-sided P value was <0.05. Analyses were carried out using SAS (version 9.2) and Stata (version 11) software.
Results
Baseline Demographics
The patient demographics are as follows. Twenty patients had stage 2–4 CKD (stage 2, n=4; stage 3a, n=2; stage 3ab, n=4; and stage 4, n=10). Fifty percent of patients were male and 50% were female. The average age was 61 years, mean estimated GFR (eGFR) was 38 ml/min per 1.73 m2, and 41% of patients white, 45% were African American, and 14% were Asian (Table 1). Baseline serum CML and methylglyoxal levels were increased approximately three-fold in diabetic CKD; PMNC CML (intracellular CML), methylglyoxal (intracellular methylglyoxal), and TNF-α levels were increased; and AGER1 and SIRT1 mRNA levels were markedly decreased in patients with diabetes compared with healthy controls (25). In addition, although 15 of 20 participants were receiving lipid-lowering therapy, HDL levels were low and LDL levels were decreased. TNFα, leptin, and 8-isoprostane levels were two-fold to five-fold higher and HbA1c levels were significantly more elevated in study participants than in healthy participants (25). Urinary protein excretion was increased and eGFR was decreased compared with normal volunteers (25).
Table 1.
Baseline summary statistics
| Variable | Mean | SD | Median | First Quartile | Third Quartile |
|---|---|---|---|---|---|
| Age (yr) | 61.1 | 11.5 | 60.5 | 54.5 | 69 |
| Systolic BP (mmHg) | 143.4 | 17.51 | 143 | 130 | 154.5 |
| Diastolic BP (mmHg) | 75 | 10.1 | 71.5 | 66.5 | 84.5 |
| Body mass index (kg/m2) | 30.7 | 6.7 | 29.8 | 26.2 | 34.5 |
| HbA1c (%) | 8.3 | 2.2 | 7.6 | 6.8 | 9.7 |
| Glucose (mg/dl) | 135 | 76 | 109 | 92 | 161 |
| Total cholesterol (mg/dl) | 178 | 38 | 168 | 156 | 202 |
| HDL cholesterol (mg/dl) | 49 | 15 | 46 | 40 | 54 |
| LDL cholesterol (mg/dl) | 96 | 37 | 89 | 71 | 117 |
| Triglycerides (mg/dl) | 160 | 108 | 125 | 94 | 212 |
| Adiponectin (μg/ml) | 19.15 | 9.97 | 15.5 | 13 | 22.5 |
| Leptin (ng/ml) | 44.1 | 19.7 | 38.2 | 30.8 | 51.2 |
| High-sensitivity C-reactive protein (mg/L) | 10.1 | 12.2 | 6.95 | 1.8 | 10.4 |
| Intracellular TNFα (ng/mg protein) | 26.4 | 8.9 | 26.7 | 19.6 | 33.3 |
| 8-isoprostanes (pg/ml) | 288.7 | 99.6 | 249.7 | 223.6 | 316.7 |
| Serum carboxymethyllysine (U/ml) | 36.68 | 9.92 | 36.07 | 28.39 | 43.93 |
| Serum methylglyoxal (nmol/ml) | 3.21 | 0.91 | 3.33 | 2.26 | 3.87 |
| Intracellular carboxymethyllysine (U/mg protein) | 9.99 | 2.21 | 9.49 | 8.5 | 11.23 |
| Intracellular methylglyoxal (nmol/mg protein) | 1.61 | 0.56 | 1.52 | 1.14 | 1.78 |
| AGER1 (mRNA) | 86.8 | 34.5 | 84.5 | 64 | 110 |
| SIRT1 (mRNA) | 159.8 | 62.4 | 163.5 | 104.5 | 211 |
| Cystatin C (mg/L) | 1.86 | 0.74 | 1.76 | 1.1 | 2.63 |
| Estimated GFR (ml/min per 1.73 m2) | 38.1 | 22.1 | 30.1 | 21.7 | 49.9 |
| Serum potassium (mmol/L) | 4.42 | 0.71 | 4.5 | 3.5 | 5.05 |
| Serum phosphate (mg/dl) | 4.02 | 0.74 | 3.8 | 3.45 | 4.55 |
| Serum chloride (mmol/L) | 101.45 | 3.14 | 101.5 | 99 | 103.5 |
| Serum bicarbonate (mmol/L) | 24.9 | 2.9 | 24.5 | 23.2 | 26.8 |
| Fibroblast growth factor 23 (μg/ml) | 94.95 | 98.18 | 52.45 | 32.25 | 101.65 |
| Urine phosphate (g/d) | 0.58 | 0.31 | 0.6 | 0.36 | 0.8 |
| Urine phosphate/creatinine (g/g) | 0.51 | 0.21 | 0.49 | 0.38 | 0.58 |
| Urine protein/creatinine (mg/mg) | 1.77 | 2.65 | 0.8 | 0.44 | 1.61 |
| Dietary phosphate (mg/d) | 967 | 479 | 863 | 651 | 1326 |
AGER1, advanced glycation end product receptor 1; SIRT1, sirtuin 1.
Circulating Metabolic and Inflammatory Parameters
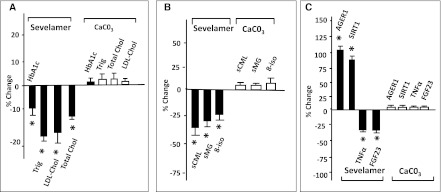
HbA1c, total cholesterol, and triglycerides were reduced by sevelamer carbonate, compared with calcium carbonate (Figure 1 and Tables 2 and 3). These changes were associated with reduced serum levels of CML, methylglyoxal, and 8-isoprostanes. However, 8-isoprostanes were increased after 8 weeks of calcium carbonate treatment, as previously noted in type 2 diabetes mellitus (10). Blood glucose, AGE intake, adiponectin, leptin, or C-reactive protein did not differ between groups at any point.
Figure 1.
Effect of sevelamer on metabolic, AGE, oxidant, and antioxidant factors. The differential effects of calcium carbonate and sevelamer carbonate on (A) metabolic factors, (B) AGEs and oxidized lipids, and (C) antioxidant status and markers of inflammation. Changes are expressed as the mean percentage change from baseline to the end of the 8-week treatment period for individual patients. An asterisk denotes a significant change (P<0.05). AGE, advanced glycation end product; trig, triglycerides; LDL-Chol, LDL cholesterol; total chol, total cholesterol; sCML, serum carboxymethyllysine; sMG, serum methylglyoxal; 8-iso, 8-isoprostanes; AGER1, AGE receptor 1; SIRT1, sirtuin 1; FGF23, fibroblast growth factor 23.
Table 2.
Summary statistics for deltas (last minus first visit), by treatment
| Calcium Carbonate | Sevelamer Carbonate | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | P Value | n | Mean | SD | P Value | |
| Body mass index (kg/m2) | 20 | −0.12 | 0.9 | 0.73 | 20 | −0.08 | 1.12 | 0.73 |
| HbA1c (%) | 20 | −0.07 | 0.81 | 0.99 | 18 | −0.74 | 0.89 | 0.002 |
| Glucose (mg/dl) | 20 | 14.8 | 48.79 | 0.23 | 20 | −20.55 | 85.53 | 0.34 |
| Total cholesterol (mg/dl) | 20 | 2.85 | 31.19 | 0.81 | 19 | −13.84 | 34.34 | 0.08 |
| HDL cholesterol (mg/dl) | 20 | −0.6 | 6.37 | 0.73 | 19 | 1.79 | 10.87 | 0.19 |
| LDL cholesterol (mg/dl) | 20 | 2.8 | 28.4 | 0.85 | 19 | −8.74 | 28.82 | 0.21 |
| Triglycerides (mg/dl) | 20 | 21.7 | 75.4 | 0.59 | 19 | −32.95 | 61.3 | 0.02 |
| Adiponectin (µg/ml) | 20 | −1.4 | 3.5 | 0.16 | 20 | −0.85 | 2.06 | 0.10 |
| Leptin (ng/ml) | 20 | 0.6 | 6.71 | 0.41 | 20 | −4.56 | 12.14 | 0.02 |
| High-sensitivity C-reactive protein (mg/ml) | 20 | 0.29 | 3.88 | 0.81 | 20 | −1.68 | 15.04 | 0.28 |
| Intracellular TNFα (ng/mg protein) | 20 | 0.11 | 4.79 | 0.93 | 20 | −7.08 | 8.3 | 0.002 |
| 8-isoprostanes (pg/ml) | 20 | 34.1 | 69 | 0.04 | 20 | −69.75 | 109.92 | 0.003 |
| Serum carboxymethyllysine (U/ml) | 20 | 2.87 | 9.15 | 0.41 | 20 | −8.24 | 10.86 | 0.006 |
| Serum methylglyoxal (nmol/ml) | 20 | 0.11 | 0.5 | 0.56 | 20 | −0.44 | 0.91 | 0.02 |
| Intracellular carboxymethyllysine (U/mg protein) | 17 | 0.41 | 1.95 | 0.52 | 17 | −1.96 | 2.92 | 0.03 |
| Intracellular methylglyoxal (nmol/mg protein) | 17 | 0.1 | 0.38 | 0.33 | 17 | −0.35 | 0.67 | 0.02 |
| AGER1 (mRNA) | 20 | −6.65 | 41.04 | 0.96 | 20 | 114.00 | 126.60 | 0.001 |
| SIRT1 (mRNA) | 20 | −3.5 | 56.78 | 0.58 | 20 | 89.45 | 104.51 | <0.001 |
| Cystatin C (mg/L) | 18 | 0.02 | 0.26 | 0.93 | 19 | 0.013 | 0.56 | 0.89 |
| Estimated GFR (ml/min per 1.73 m2) | 20 | −0.32 | 10.73 | 0.98 | 19 | 1.45 | 5.39 | 0.39 |
| Serum potassium (mmol/L) | 20 | −0.22 | 0.60 | 0.16 | 20 | 0.24 | 0.57 | 0.07 |
| Serum phosphate (mg/dl) | 20 | −0.26 | 0.7 | 0.10 | 19 | 0.02 | 0.86 | 0.62 |
| Serum chloride (mmol/L) | 20 | −1.45 | 3.52 | 0.08 | 20 | 1.45 | 4.31 | 0.19 |
| Serum bicarbonate (mmol/L) | 20 | 1.45 | 3.72 | 0.09 | 20 | −0.05 | 3.65 | 0.85 |
| Fibroblast growth factor 23 (µg/ml) | 20 | 30 | 95 | 0.17 | 20 | −30 | 64 | 0.08 |
| Urine phosphate (g/d) | 20 | −0.18 | 0.23 | 0.002 | 20 | −0.1 | 0.23 | 0.03 |
| Urine phosphate/creatinine (g/g) | 19 | −0.13 | 0.19 | 0.01 | 19 | −0.14 | 0.35 | 0.18 |
| Urine protein/creatinine (mg/mg) | 19 | 0.02 | 0.84 | 0.48 | 19 | 0.04 | 1.01 | 0.84 |
| Dietary phosphate (mg/d) | 18 | −112 | 582 | 0.13 | 20 | −143 | 489 | 0.55 |
AGER1, advanced glycation end product receptor 1; SIRT1, sirtuin 1.
Table 3.
Estimates of the effect of sevelamer carbonate relative to calcium carbonate, adjusted for period of treatment
| Variable | Estimate | 95% Confidence Interval | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| HbA1c | −0.67 | −1.25 | −0.10 | 0.02 |
| Glucose | −35.4 | −78.6 | 7.9 | 0.11 |
| Total cholesterol | −14.58 | −27.22 | −1.94 | 0.02 |
| HDL cholesterol | 2.34 | −3.00 | 7.68 | 0.39 |
| LDL cholesterol | −9.96 | −20.2 | 0.29 | 0.06 |
| Triglycerides | −56.2 | −107.7 | −4.70 | 0.03 |
| TNFα | −7.2 | −11.01 | −3.38 | <0.001 |
| 8-isoprostanes | −103.8 | −161.7 | −46.0 | <0.001 |
| Serum carboxymethyllysine | −11.11 | −16.22 | −6.00 | <0.001 |
| Serum methylglyoxal | −0.55 | −0.99 | −0.11 | 0.01 |
| Intracellular carboxymethyllysine | −2.43 | −4.05 | −0.82 | 0.003 |
| Intracellular methylglyoxal | −0.48 | −0.85 | −0.11 | 0.01 |
| AGER1 | 120.7 | 64.4 | 176.9 | <0.001 |
| SIRT1 | 93.0 | 35.2 | 150.7 | 0.002 |
| Cystatin Ca | 0.29 | −0.02 | 0.60 | 0.07 |
| Serum potassium | 0.46 | 0.008 | 0.84 | 0.02 |
| Serum chloride | 2.90 | 1.10 | 4.70 | 0.002 |
| Serum bicarbonate | −1.51 | −3.83 | 0.82 | 0.21 |
| Fibroblast growth factor 23 | −59.74 | −116.35 | −3.12 | 0.04 |
| Urine phosphate | 0.07 | −0.02 | 0.170 | 0.12 |
| Urine protein/creatinine | −0.02 | −0.45 | 0.41 | 0.92 |
| Dietary phosphate | −20.62 | −344.87 | 386.11 | 0.91 |
AGER1, advanced glycation end product receptor 1; SIRT1, sirtuin 1.
First period of observation analyzed only due to evidence of a period by treatment interaction.
FGF-23, Calcium, and Phosphorus
Urinary phosphorus excretion was decreased by both treatments in continent participants (Table 2). There were no changes in serum phosphate or calcium levels due to either treatment.
Overall, FGF-23 was decreased by sevelamer carbonate and was increased substantially by calcium carbonate (Table 2), although neither change was formally significant (P=0.0.07 and 0.08, respectively). The difference between the two changes was significant (P=0.04) (Table 3). For those participants with baseline values >70 pg/ml, sevelamer carbonate treatment led to a significant decrease in FGF-23 (median first minus final difference = −85.55 μg/ml; interquartile range, −12.05 to −128.30; P=0.02), whereas treatment with calcium carbonate resulted in a nonsignificant median increase of +38 μg/ml (−21.85 to +73.45; P=0.20). The P value for a difference between the two subgroups was 0.002. Those with a baseline level <70 pg/ml showed no significant differences over time for either sevelamer carbonate or calcium carbonate.
Blood Lipids
Plasma triglycerides and total cholesterol levels were further decreased by sevelamer carbonate compared with calcium carbonate (Tables 2 and 3).
Renal Function
Serum creatinine levels, estimated GFR, and urinary protein excretion were unchanged after 8 weeks with either treatment or at the study end. There was a trend for decreased cystatin C levels in the sevelamer carbonate arm compared with calcium carbonate. Serum potassium and chloride, while remaining in the normal range, were increased by sevelamer carbonate compared with calcium carbonate. Proteinuria correlated positively with serum TNFR1 (r=0.66; P=0.014) and inversely with eGFR (r=−0.51; P=0.02).
Intracellular (PMNC) Changes
Intracellular TNFα, CML, and methylglyoxal levels were decreased in PMNC by sevelamer carbonate, but not by calcium carbonate. AGER1 and SIRT1 mRNA, indicators of antioxidant status, were increased by sevelamer carbonate but not by calcium carbonate. Changes in AGER1 mRNA correlated with AGER1 protein levels, as previously noted (data not shown) (4).
Binding of AGEs by Sevelamer Carbonate In Vitro
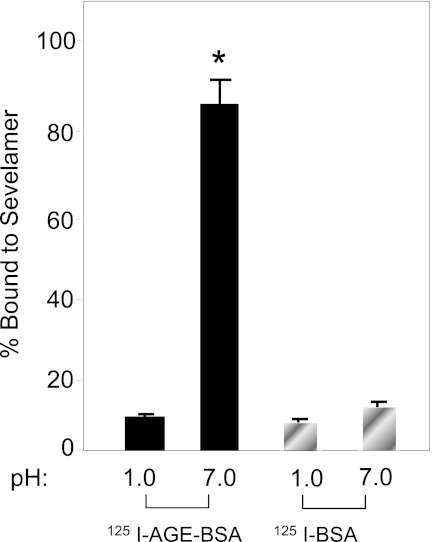
Binding of AGE-modified BSA to sevelamer carbonate was pH dependent and reversible (Figure 2). Less than 5% of 125I-AGE-BSA or 125I-BSA bound to sevelamer carbonate at pH 1.0, the approximate stomach pH. Whereas >80% of 125IAGE-BSA bound to sevelamer carbonate at pH 7.0, <1% of 125I-BSA bound. Lowering the pH from 7.0 to 1.0 resulted in release of 125I-AGE-BSA and 125I-BSA. The released 125I-AGE-BSA quantitatively rebound to sevelamer carbonate when the pH was returned to 7.0.
Figure 2.
The percentage of 125I-AGE-BSA or 125I-AGE-BSA at either pH 1.0 or 7.0 is shown. Sevelamer carbonate beads (25 mg) were incubated with 5 µg of 125I-AGE-BSA or 125I-AGE-BSA at pH 1.0 or 7.0 at room temperature for 12 hours. The radioactivity bound to the beads was determined and expressed as the percentage of the added amount of radioactivity that was retained on the beads. 125I-AGE-BSA was bound to sevelamer carbonate beads at pH 7.0 but not at pH 1.0. The binding was reversible. Less than 5% of 125I-AGE-BSA bound to sevelamer carbonate beads at either pH 1.0 or 7.0. AGE, advanced glycation end product.
Discussion
We report that sevelamer carbonate reduced HbA1c and improved several lipid abnormalities and FGF-23, risk factors for diabetic cardiorenal disease, in type 2 diabetes mellitus participants with stage 2–4 CKD. Because sevelamer carbonate and calcium carbonate both bind phosphates, these changes seemed to be independent of reduced phosphorus uptake. The reduction of FGF-23 by sevelamer carbonate could be clinically important because high FGF-23 levels predict progression in CKD patients and are strongly associated with CVD and all-cause mortality as well as with initiation of dialysis (17,22).
The marked reductions in circulating and cellular AGEs, certain lipids, and lipid 8-isoprostanes were associated with restoration of suppressed antioxidant and anti-inflammation defenses noted at baseline (10). Similar results have been found by restricting AGEs in food (10). Therefore, we hypothesized that these new effects of sevelamer carbonate may be attributable to binding and removal of AGEs from the intestinal contents, a mode of action not previously described for this drug.
The proinflammatory state, characteristic of CKD and diabetes, correlates with high circulating levels of AGEs due to the combined effects of increased glucose, impaired renal function, excessive dietary AGE intake, and suppressed anti-AGE AGER1 activity (4,10,26). The current data that reducing AGE levels either by anti-AGE drugs or by AGE restriction results in significantly decreased oxidative stress and inflammation support the hypothesis that dietary AGEs are an important source of AGEs in type 2 diabetes (32,33). These effects might be responsible for an improvement in glucose utilization and insulin resistance in participants with diabetes who are placed on an AGE-restricted diet (32). Furthermore, serum CML levels were recently identified as additional potent diabetes risk determinants in type 1 diabetes mellitus (D. Leslie, unpublished observations). HbA1c levels decreased in this study, suggesting that overall glucose metabolism was improved by sevelamer carbonate even though fasting blood glucose levels were not changed, as has been previously noted with dietary AGE restriction (10). This change may reflect reduced overall glycation of proteins because HbA1c is a glycated molecule (34). This could be important because consideration of HbA1c levels was recently shown to improve the prediction of cardiovascular risk in patients with type 2 diabetes (35).
A drug structurally resembling sevelamer chloride (colesevelam) produces changes in HbA1c in type 2 diabetes without CKD, similar to this study (36). Triglyceride levels were elevated in another colesevelam study, in contrast to this study (37).
Reductions in HbA1c, lipids, coronary artery calcification, and fetuin-A levels have been reported in hemodialysis patients receiving sevelamer chloride (38), and a number of studies of sevelamer chloride in animal models of CKD showed reduction of cardiac/renal calcification and prevention of renal function loss (39,40).
A link between metabolic abnormalities and CKD was demonstrated in a recent meta-analysis of longitudinal studies showing that the incidence of CKD was directly related to the number of metabolic syndrome components (41). Because sevelamer carbonate improved components of the metabolic syndrome in this study of type 2 diabetes mellitus patients with CKD, it may also reduce early loss of renal function. However, this must be examined in a larger and longer study.
Because sevelamer carbonate and calcium carbonate both bind inorganic phosphates in vitro, the changes associated with sevelamer carbonate in this study, other than urinary phosphate excretion, may not be directly attributable to a reduced phosphate load. Because sevelamer carbonate binds AGEs at a pH found in the intestine distal to the stomach, the beneficial effects of sevelamer carbonate could be related to sequestration of food AGEs in the gut. The effects of sevelamer carbonate are similar to those found by AGE restriction in either normal participants (15,25,26), CKD patients without diabetes (42), or patients with diabetes (32). Notably, sevelamer carbonate reduced intracellular serum AGEs, well established proinflammatory compounds (4). The fact that sevelamer carbonate, but not calcium carbonate, reproduced the findings of dietary AGE restriction constitutes a potentially important new finding that could have therapeutic implications for the metabolic syndrome and other chronic conditions associated with increased oxidant stress and inflammation (43,44). The fact that reducing AGEs by two independent methods results in improvements in glucose metabolism and reactive oxygen species/inflammation adds credibility to the hypothesis that this action of sevelamer carbonate was due to the binding of AGEs in the intestinal contents and elimination of AGEs, rather than an effect on bile-acid acids and phosphate uptake. The mechanism of binding of negatively charged AGEs to sevelamer may be due to formation of imidazolium adduct formation between amino groups and methylglyoxal. Calcium carbonate cannot participate in these reactions.
Elevated oxidative stress and inflammation are associated with increased risk of cardiorenal disease, especially in diabetes (45) For instance, elevated HbA1c levels in CKD further increase the risk for CVD (46,47), and the risk of death due to cardiovascular disease in stage 2–3 CKD patients exceeds the risk of progression to ESRD (13,48). Thus, the reduction of these risk factors by sevelamer carbonate suggests that it could become a valuable adjunct in the general management of type 2 diabetes mellitus and both early and late stages of kidney disease.
The limitations of this study include the small number of patients and the short duration. Many significance tests were performed; thus, the chance of a false positive result is high and the P values need to be interpreted with caution. Given the exploratory nature of this trial, it was judged inappropriate to adjust for multiple significance testing. In addition, the exact AGEs bound to sevelamer carbonate remain to be identified.
In summary, sevelamer carbonate is a promising drug for the management of early diabetic CKD because it reduces HbA1c, lipids, FGF-23, AGEs, and the proinflammatory/oxidative stress state characteristic of diabetes and CKD. These results, must be validated in a larger trial, but suggest that sevelamer carbonate may reduce cardiovascular risk factors by binding and removing dietary AGEs from the gastrointestinal tract.
Disclosures
None.
Acknowledgments
We acknowledge the many residents and fellows in the Division of Nephrology at Mount Sinai School of medicine who assisted in the conduct of this study.
This independent investigator-initiated trial was funded by a contract from Genzyme Corporation to H.V.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS: Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 22: 545–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrows NR, Li Y, Geiss LS: Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 33: 73–77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett WL, Wilson LM, Bolen S, Maruthur N, Singh S, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Nicholson WK, Block L, Odelola O, Dalal DS, Ogbeche GE, Chandrasekhar A, Hutfless S, Bass EB, Segal JB: Oral diabetes medications for adults with type 2 diabetes: An update. In: Comparative Effectiveness Review, No. 27, Rockville, MD, Agency for Healthcare Research and Quality, 2011 [PubMed] [Google Scholar]
- 4.Vlassara H, Striker GE: AGE restriction in diabetes mellitus: A paradigm shift. Nat Rev Endocrinol 7: 526–539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A: Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89: 27–71, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Chao PC, Huang CN, Hsu CC, Yin MC, Guo YR: Association of dietary AGEs with circulating AGEs, glycated LDL, IL-1α and MCP-1 levels in type 2 diabetic patients. Eur J Nutr 49: 429–434, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL: Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: The Joslin 50-year medalist study. Diabetes Care 34: 968–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB: Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am J Nephrol 27: 605–614, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y, Yagil Y, Herskovits T, Atkins RC, Reutens AT, Packham DK, Lewis JB, Collaborative Study Group : Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol 23: 131–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H: Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: Potential role of AGER1 and SIRT1. Diabetes Care 34: 1610–1616, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, Vlassara H: Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol 173: 327–336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie RD, Beyan H, Sawtell P, Boehm BO, Spector TD, Snieder H: Level of an advanced glycated end product is genetically determined: A study of normal twins. Diabetes 52: 2441–2444, 2003 [DOI] [PubMed] [Google Scholar]
- 13.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P: Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J 149: 820–825, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Kakuta T, Tanaka R, Hyodo T, Suzuki H, Kanai G, Nagaoka M, Takahashi H, Hirawa N, Oogushi Y, Miyata T, Kobayashi H, Fukagawa M, Saito A: Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis 57: 422–431, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Ketteler M, Rix M, Fan S, Pritchard N, Oestergaard O, Chasan-Taber S, Heaton J, Duggal A, Kalra PA: Efficacy and tolerability of sevelamer carbonate in hyperphosphatemic patients who have chronic kidney disease and are not on dialysis. Clin J Am Soc Nephrol 3: 1125–1130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, Moysés RM: FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol 6: 241–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shantouf R, Budoff MJ, Ahmadi N, Tiano J, Flores F, Kalantar-Zadeh K: Effects of sevelamer and calcium-based phosphate binders on lipid and inflammatory markers in hemodialysis patients. Am J Nephrol 28: 275–279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raggi P, Vukicevic S, Moysés RM, Wesseling K, Spiegel DM: Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol 5[Suppl 1]: S31–S40, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE: The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H: Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 104: 1287–1291, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H: Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA 94: 6474–6479, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H: Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci 62: 427–433, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J: Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab 94: 4483–4491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai W, He JC, Zhu L, Lu C, Vlassara H: Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci USA 103: 13801–13806, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai W, He JC, Zhu L, Chen X, Striker GE, Vlassara H: AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am J Physiol Cell Physiol 294: C145–C152, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Swearingen RA, Chen X, Petersen JS, Riley KS, Wang D, Zhorov E: Determination of the binding parameter constants of Renagel capsules and tablets utilizing the Langmuir approximation at various pH by ion chromatography. J Pharm Biomed Anal 29: 195–201, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Mitsuhashi T, Li YM, Fishbane S, Vlassara H: Depletion of reactive advanced glycation endproducts from diabetic uremic sera using a lysozyme-linked matrix. J Clin Invest 100: 847–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward M: Epidemiology: Study Design and Data Analysis, Boca Raton, FL, CRC Press, 2005 [Google Scholar]
- 32.Urribarri J, CC, Ramdas M, Goodman S, Pyzik R, Chen L, Zu L, Striker GE, Vlassara H: Improved innate defenses after restricting AGE intake reduces insulin resistance in type 2 diabetes: Role of AGER1 and SIRT1. Diabetes Care 34: 1610–1616, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He C, Sabol J, Mitsuhashi T, Vlassara H: Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 48: 1308–1315, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A: Correlation of glucose regulation and hemoglobin A1C. J Biochem 252: 2992–2997, 1977 [DOI] [PubMed] [Google Scholar]
- 35.Paynter NP, Mazer NA, Pradhan AD, Gaziano JM, Ridker PM, Cook NR: Cardiovascular risk prediction in diabetic men and women using hemoglobin A1c vs diabetes as a high-risk equivalent. Arch Intern Med 171: 1712–1718, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bays HE, Goldberg RB, Truitt KE, Jones MR: Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: Glucose and lipid effects. Arch Intern Med 168: 1975–1983, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Vega GL, Dunn FL, Grundy SM: Effect of colesevelam hydrochloride on glycemia and insulin sensitivity in men with the metabolic syndrome. Am J Cardiol 108: 1129–1135, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group : Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Phan O, Ivanovski O, Nguyen-Khoa T, Mothu N, Angulo J, Westenfeld R, Ketteler M, Meert N, Maizel J, Nikolov IG, Vanholder R, Lacour B, Drüeke TB, Massy ZA: Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation 112: 2875–2882, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Cozzolino M, Staniforth ME, Liapis H, Finch J, Burke SK, Dusso AS, Slatopolsky E: Sevelamer hydrochloride attenuates kidney and cardiovascular calcifications in long-term experimental uremia. Kidney Int 64: 1653–1661, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD: Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol 6: 2364–2373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, He C, Vlassara H: Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol 14: 728–731, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Mirza MA, Alsiö J, Hammarstedt A, Erben RG, Michaëlsson K, Tivesten A, Marsell R, Orwoll E, Karlsson MK, Ljunggren O, Mellström D, Lind L, Ohlsson C, Larsson TE: Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol 31: 219–227, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Bili A, Sartorius JA, Kirchner HL, Morris SJ, Ledwich LJ, Antohe JL, Dancea S, Newman ED, Wasko MC: Hydroxychloroquine use and decreased risk of diabetes in rheumatoid arthritis patients. J Clin Rheumatol 17: 115–120, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R: Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111: 1448–1454, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Agrawal V, Marinescu V, Agarwal M, McCullough PA: Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat Rev Cardiol 6: 301–311, 2009 [DOI] [PubMed] [Google Scholar]
- 47.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group : Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 342: 381–389, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L: Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 26: 379–385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]