Abstract
Acetylcholine receptors (AChRs) are pentameric ligand-gated ion channels involved in the neurotransmission of both vertebrates and invertebrates. A number of anthelmintic compounds like levamisole and pyrantel target the AChRs of nematodes producing spastic paralysis of the worms. The muscle AChRs of nematode parasites fall into three pharmacological classes that are preferentially activated by the cholinergic agonists levamisole (L-type), nicotine (N-type) and bephenium (B-type), respectively. Despite a number of studies of the B-type AChR in parasitic species, this receptor remains to be characterized at the molecular level. Recently, we have reconstituted and functionally characterized two distinct L-AChR subtypes of the gastro-intestinal parasitic nematode Haemonchus contortus in the Xenopus laevis oocyte expression system by providing the cRNAs encoding the receptor subunits and three ancillary proteins (Boulin et al. in Br J Pharmacol 164(5):1421–1432, 2011). In the present study, the effect of the bephenium drug on Hco-L-AChR1 and Hco-L-AChR2 subtypes was examined using the two microelectrode voltage-clamp technique. We demonstrate that bephenium selectively activates the Hco-L-AChR1 subtype made of Hco-UNC-29.1, Hco-UNC-38, Hco-UNC-63, Hco-ACR-8 subunits that is more sensitive to levamisole than acetylcholine. Removing the Hco-ACR-8 subunit produced the Hco-L-AChR2 subtype that is more sensitive to pyrantel than acetylcholine and partially activated by levamisole, but which was bephenium-insensitive indicating that the bephenium-binding site involves Hco-ACR-8. Attempts were made to modify the subunit stoichiometry of the Hco-L-AChR1 subtype by injecting five fold more cRNA of individual subunits. Increased Hco-unc-29.1 cRNA produced no functional receptor. Increasing Hco-unc-63, Hco-unc-38 or Hco-acr-8 cRNAs did not affect the pharmacological characteristics of Hco-L-AChR1 but reduced the currents elicited by acetylcholine and the other agonists. Here, we provide the first description of the molecular composition and functional characteristics of any invertebrate bephenium-sensitive receptor.
Keywords: Bephenium, Levamisole-sensitive acetylcholine receptor, Oocyte expression system, Electrophysiology, Haemonchus contortus
Introduction
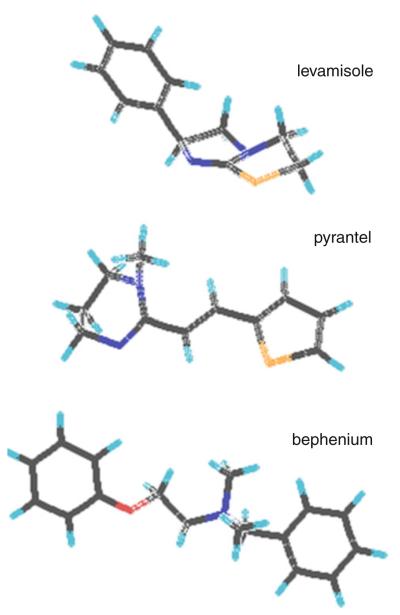
The control of gastro-intestinal nematodes of veterinary importance is largely based on anthelmintic chemotherapy (McKellar and Jackson 2004). Among the most widely used drugs, the cholinergic agonists such as levamisole (Lev), pyrantel (Pyr) and bephenium (Beph), which have very heterogeneous chemical structures (Fig. 1), specifically target acetylcholine receptors (AChRs) located muscle cells of the nematodes (Martin et al. 2005). The prolonged activation of these ligand-gated ion channels causes muscle hypercontraction that produces spastic paralysis of the worm that is then swept away out of the host (Aceves et al. 1970; Aubry et al. 1970; Colquhoun et al. 1991). However, the efficacy of these anthelmintic compounds has been compromised by the emergence of resistant nematode isolates, especially in the blood feeding nematode Haemonchus contortus that is responsible for substantial loss of production in sheep and goat industry (Kaplan 2004; Peter and Chandrawathani 2005). In that respect, the identification and the pharmacological characterization of muscle AChRs of parasitic nematodes is of major importance to optimize their use and provide rational design for future new drug discovery. Historically, bephenium was introduced on the market as an anthelmintic compound in 1959 for its activity against human and dog hookworms and gastrointestinal parasitic nematodes in sheep, but it was not broad spectrum like other drugs (Burrows 1958; Copp et al. 1958; Young et al. 1958). Bephenium is a member of the quaternary ammonium drug class that are highly ionized (Fig. 1) and unlike levamisole and pyrantel, the mode of action of bephenium is poorly understood.
Fig. 1.
Stick diagram of the chemical structures of the cholinergic agonists levamisole, pyrantel and bephenium that are representative drugs from imidazothiazole, tetrahydropyrimidine and quaternary ammonium salt anthelmintic groups, respectively
Previous electrophysiological analysis conducted on the parasitic nematode species Ascaris suum have revealed multiple pharmacological subtypes of muscle AChRs: the L-subtype preferentially activated by levamisole; the N-subtype preferentially activated by nicotine; and the B-subtype preferentially activated by bephenium (Martin et al. 1997; Robertson et al. 2002). The molecular composition of the L-AChR and N-AChR was first deciphered in the free-living nematode Caenorhabditis elegans where there is currently no evidence for a B-subtype AChR. The C. elegans L-AChR is a heteropentameric receptor composed the UNC-38, UNC-63, LEV-8, UNC-29 and LEV-1 subunits (Boulin et al. 2008; Culetto et al. 2004; Fleming et al. 1997; Towers et al. 2005). This receptor was robustly expressed in the Xenopus laevis oocyte heterologous system by co-injection of the cRNAs encoding these 5 subunits together with 3 additional C. elegans ancillary proteins involved in AChR assembly (RIC-3), folding (UNC-74) and trafficking (UNC-50) (Eimer et al. 2007; Halevi et al. 2002; Haugstetter et al. 2005). The recombinant C. elegans L-AChR was found to be sensitive to levamisole but insensitive to nicotine. The C. elegans N-AChR is a homopentameric receptor made of 5 ACR-16 subunits and only required RIC-3 ancillary protein to enhance its functional expression in Xenopus oocyte (Boulin et al. 2008; Touroutine et al. 2005). In contrast with the L-AChR subtype, the recombinant C. elegans N-AChR was found to be only responsive to acetylcholine and nicotine. The reconstitution of parasitic ion channels in the Xenopus oocytes is a tractable approach for the fundamental and functional study of AChRs composition, pharmacological and electrophysiological properties, previously impossible to investigate in the absence of parasitic nematode transgenesis approach. Interestingly, in the parasitic nematode A. suum, only two subunits homologous to UNC-29 and UNC-38 were sufficient to reconstitute functional L- or N-subtype AChRs in Xenopus oocyte, depending on the ratio of the two subunits (Williamson et al. 2009). In H. contortus and other trichostrongylid species such as Teladorsagia circumcincta and Trichostrongylus colubriformis, the orthologues of unc-29, unc-63, unc-38 and lev-1 have been identified (Hoekstra et al. 1997; Neveu et al. 2010; Walker et al. 2001; Wiley et al. 1996). No orthologous gene for C. elegans lev-8 could be detected so far in trichostrongylid species except the Hco-ACR-8 subunit that is closely related to the C. elegans LEV-8 and ACR-8 subunit. Furthermore, using a transcriptomic approach, we have highlighted Hco-acr-8 as a potential candidate gene involved in levamisole resistance (Fauvin et al. 2010). We have recently demonstrated that two L-AChRs of H. contortus can be functionally reconstituted in Xenopus oocytes by co-expressing the receptor subunits and the conserved ancillary factors (Boulin et al. 2011). The first one referred as Hco-L-ACHR1 is made of UNC-29.1, UNC-63, UNC-38 and ACR-8 subunits and the second one referred as Hco-L-AChR2 is made of UNC-29.1, UNC-63, UNC-38. Although differing by only one subunit, these two H. contortus L-AChR subtypes harbored dramatic differences in their pharmacological properties. Hco-L-AChR1 was found to be more sensitive to levamisole than acetylcholine, poorly responsive to pyrantel and nicotine insensitive. Hco-L-AChR2 was ten fold less responsive to acetylcholine but more sensitive to pyrantel and nicotine than levamisole underlining the critical role of the Hco-ACR-8 subunit in selective sensitivity to levamisole. In H. contortus, the modes of action of cholinergic agonists have been investigated using whole worm cannulation and force transduction (Sangster et al. 1991). Bephenium was shown to induce muscular contraction in addition to levamisole, pyrantel and nicotine. Despite extensive electrophysiological study of the B-subtype receptors in parasitic species, the molecular composition of the nematode bephenium-sensitive receptor has not yet been determined.
Here, we investigate the effect of bephenium on the two recombinant L-AChR subtypes of H. contortus. We report that bephenium is able to act on the Hco-L-AChR1 subtype in a dose-dependant manner, whereas the Hco-L-AChR2 subtype missing Hco-ACR-8 is insensitive to bephenium. This report is the first molecular evidence for an AChR sensitive to bephenium in a nematode species. It further opens the way for an extended characterization of muscle AChR subtypes in parasitic nematodes.
Materials and methods
Accession numbers
The accession numbers for cDNA and protein sequences mentioned in this article are: H. contortus: Hco-unc-29.1 GU060980, Hco-unc-38 GU060984, Hco-unc-63a GU060 985, Hco-acr-8 EU006785, Hco-unc-50 HQ116822, Hco-unc-74 HQ116821, Hco-ric-3.1 HQ116823.
Materials
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich® (USA).
Molecular biology
The AChR cDNAs of interest were cloned into the pTB207 expression vector containing the 3′-UTR of X. laevis β-globin gene that is suitable for in vitro transcription (Boulin et al. 2011). The resulting pTB207 recombining constructions were sequence-checked by GATC biotech (Konstanz, Germany). The PTB207 vectors containing the different subunits and ancillary proteins were linearized with NheI restriction enzyme and used as templates for in vitro cRNA synthesis with the mMessage mMachine T7 Kit (Ambion) according to the manufacturer’s instructions. Neosynthesized-capped cRNAs were lithium chlorideprecipitated and resuspended in RNAse-free water. cRNA concentration was quantified using a Nanodrop spectrophotometer (Thermo Scientific®). cRNA samples were subjected to electrophoresis through a 1 % denaturing gel stained with ethidium bromide to assess their purity and integrity before storing them at −80 °C.
Electrophysiological characterization of H. contortus nAChR in X. laevis oocytes
Xenopus laevis ovaries were obtained from NASCO (Fort Atkinson, Wisconsin, USA) and defolliculated using 1–2 mg/ml collagenase type II and calcium-free OR2 (composition in mM: NaCl 100, KCl 2.5, HEPES 5, pH 7.5 with NaOH) or alternatively defolliculated oocytes were obtained from Ecocyte Bioscience (Austin, Texas, USA). Defolliculated oocytes were kept overnight in incubation solution (composition in mM: NaCl 100, KCl 2, CaCl2 1.8, MgCl2 1, HEPES 5, pH 7.5, supplemented with sodium pyruvate 2.5, penicillin 100 U/ml and streptomycin 100 μg/ml) to select for the healthiest oocytes for microinjection. The oocytes were injected into the animal pole with a total volume of approximately 36 nL of a cRNA mix containing 50 ng/μL of each cRNA species in RNAse-free water using the Drummond nanoject II microinjector. Several mixes of RNA coding for one to 4 different H. contortus AChR subunits with and without the H. contortus ancillary proteins were tested to characterize the functional receptors and the requirement for ancillary factors. The microinjected oocytes were kept at 19 °C in the incubation solution for 3–5 days to allow the receptor expression. Prior to recording, the oocytes were incubated 4 h in the incubation solution supplemented with the calcium chelator BAPTA-AM 100 μM to prevent the activation of endogenous calcium-activated chloride conductances. Two-electrode voltage-clamp recordings were carried out using an Axoclamp 2B amplifier (Warner Instruments) with oocytes being voltage-clamped at −60 mV. Agonist dilutions were prepared in recording solution. Unless otherwise stated, the recording solution had the following composition in mM: NaCl 100, KCl 2.5, CaCl2 1, HEPES 5, pH 7.3). Solutions were applied to oocytes by gravity flow using perfusion pinch valve control systems (Harvard Apparatus, MA, USA). All data were acquired on a desktop computer with Clampex 9.2 and analyzed with Clampfit 9.2 (Molecular Devices, Sunnyvale, CA, USA). Results are shown as means ± SD. Graphpad Prism 5.0 software (San Diego, CA, USA) was used to fit sigmoid dose–response curves as described previously (Boulin et al. 2008).
Results
Expression/characterization of recombinant H. contortus L-AChR in Xenopus oocytes
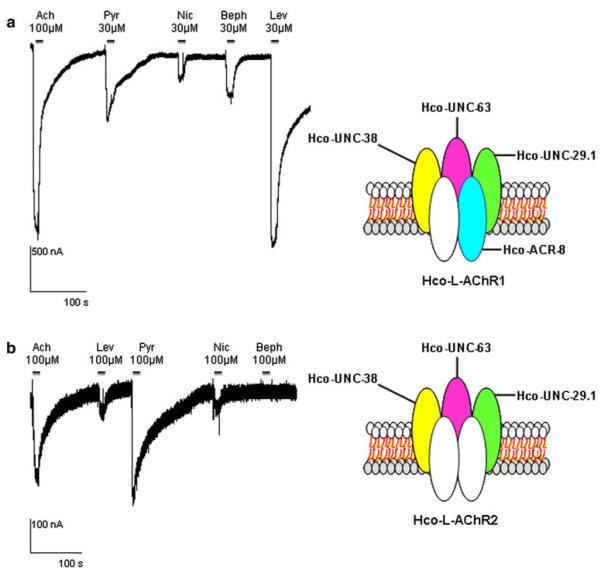
The functional expression of parasitic nematode AChRs in Xenopus laevis oocytes is a straightforward approach to decipher their subunit composition and perform detailed pharmacological characterization with the use of the two microelectrode voltage-clamp technique. By injecting the cRNAs corresponding to the 4 subunit genes: Hco-unc-29.1, Hco-unc-63, Hco-unc-38, and Hco-acr-8 and the 3 ancillary factors Hco-ric-3.1, Hco-unc-50, and Hco-unc-74, we recorded robust and immediate acetylcholine elicited inward currents in the μA range corresponding to the Hco-L-AChR1 subtype previously described (Fig. 2a). Average peak current was 1730 ± 943 nA for 100 μM acetylcholine and 1789 ± 915 for 30 μM levamisole. Missing out the Hco-acr-8 cRNAs led to the expression of another receptor that was ten fold less sensitive to acetylcholine corresponding to the Hco-L-AChR2 subtype (Fig. 2b). The experiments were performed at a holding potential of −60 mV and with 1 mM external CaCl2. Under voltage-clamp, the response is measured as the maximum current induced by the drug. To further characterize both receptors, we have added various commonly used cholinergic agonist compounds. Our data confirm that the previously observed rank of potency of the cholinergic drugs was: levamisole > acetylcholine > pyrantel > nicotine for the Hco-L-AChR1 (Figs. 2a, 4). In contrast, the Hco-L-AChR2 potency series was: pyrantel > acetylcholine > nicotine > levamisole (Fig. 2b). Taken together, these data show that we were able to reproduce independently the pharmacological pattern of the H. contortus L-AChR subtypes expressed in Xenopus oocytes and previously characterized (Boulin et al. 2008).
Fig. 2.
Pharmacological profiling of H. contortus levamisole-sensitive receptors reveals a selective sensitivity of Hco-L-AChR1 subtype to bephenium. Two-microelectrode voltage-clamp recording traces of inward currents elicited by a series of cholinergic agonists in X. laevis oocytes (Ach acetylcholine, Pyr pyrantel, Nic nicotine, Beph bephenium and Lev levamisole). Horizontal bars indicate when agonists are applied. All oocytes were treated with 100 μM BAPTA-AM for 4 h prior to recording. All recordings were performed with 1 mM external CaCl2. a (Left panel) Hco-L-AChR1 reconstituted by coinjection of an equimolar amount of 7 cRNAs (Hco-unc-29.1, Hco-unc-38, Hco-unc-63, Hco-acr-8, Hco-ric-3.1, Hco-unc-50, Hco-unc-74) displayed inward currents elicited by the 5 cholinergic agonist drugs. (Right panel) Schematic representation of the Hco-L-AChR1 possible subunit composition. The subunit in white can be any of the 4 others. b (Left panel) Hco-L-AChR2 reconstituted by coinjection of an equimolar amount of 6 cRNAs (Hco-unc-29.1, Hco-unc-38, Hco-unc-63, Hco-ric-3.1, Hco-unc-50, Hco-unc-74) omitting Hco-acr-8 is not activated by bephenium. (Right panel) Schematic representation of the Hco-L-AChR2 possible subunit composition. The subunits in white can be any of the 3 others
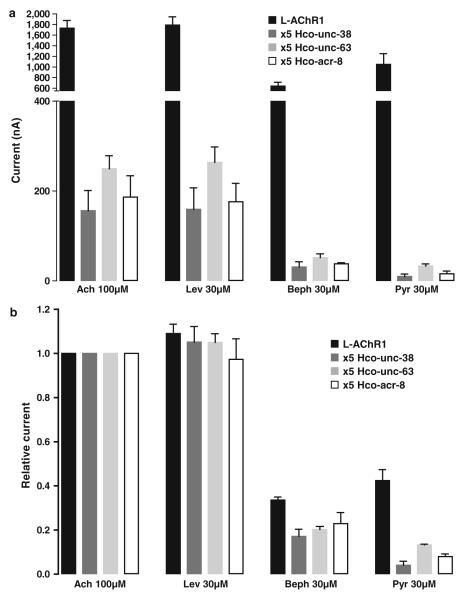
Fig. 4.
Effect of changing the subunit stoichiometry of Hco-L-AChR1 on cholinergic agonist responses. a Amplitude of inward currents elicited by Ach (100 μM), levamisole (Lev, 30 μM), bephenium (Beph, 30 μM) and pyrantel (Pyr, 30 μM) on oocytes injected with Hco-L-AChR1 encoding mix or cRNA mixes containing five fold of Hco-unc-38, Hco-unc-63, Hco-acr-8 cRNAs, respectively. The number of oocytes recorded for each conditions is ranging from 3 to 42 displayed on 3 batches of oocytes. b Relative efficacy of cholinergic agonists on oocytes injected with L-AChR1 encoding mix or cRNA mixes containing five fold of Hco-unc-38, Hco-unc-63, Hco-acr-8 cRNAs, respectively. All values are normalized to the current elicited by application of 100 μM Ach
Selective effect of bephenium on H. contortus L-AChRs
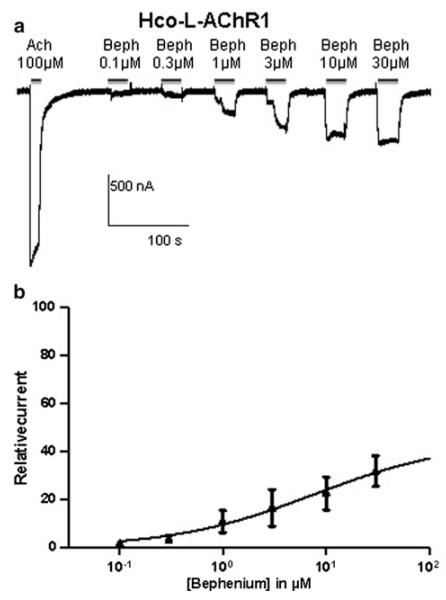
Bephenium was applied to study its effect on both Hco-L-AChRs and further compare their respective pharmacological properties. Hco-L-AChR1 displayed robust and reproducible bephenium elicited currents (33.5 ± 9 % of acetylcholine response) (Figs. 2a, 4). Interestingly, this bephenium effect was not significantly different from the pyrantel evoked response. Strikingly, Hco-L-AChR2 that is more sensitive to pyrantel than acetylcholine (Fig. 2b) failed to respond significantly to bephenium (<1 % of the acetylcholine response), suggesting that at least one bephenium-binding site is created in the presence of Hco-ACR8 (Fig. 2a). In order to determine the concentration– response relationships of Hco-L-AChR1 for bephenium, we established full dose–response curves and nonlinear regression was used to estimate EC50 value for bephenium. An example of the dose effect responses of a single oocyte to increasing concentrations of bephenium is shown in Fig. 3. EC50 value for bephenium was 7.7 ± 0.6 μM compared with 5.8 ± 0.5 μM for acetylcholine and 6.08 ± 0.3 μM for levamisole indicating that Hco-L-AChR1 has a similar affinity for these three agonists. These results represent a first step toward uncovering the detailed mode of action of bephenium as they demonstrate a previously undescribed bephenium response on H. contortus L-AChR1 subtype and a bephenium-insensitive Hco-L-AChR2 subtype that is more sensitive to pyrantel.
Fig. 3.
Bephenium dose–response relationship for Hco-L-AChR1. a Representative recording traces from a single oocyte expressing Hco-L-AChR1 challenged with 100 μM Ach and increasing concentrations of bephenium. Concentrations are indicated above each application. b Bephenium dose–response curve for Hco-LAChR1. Current is normalized to 100 μM Ach (n = 8–19). The values are mean ± SD. EC50 for bephenium is 7.7 ± 0.6 μM
Effect of changing the stochiometry of Hco-L-AChR1
The subunit stoichiometry may change the pharmacological properties of Hco-L-AChR1, including bephenium sensitivity, so we injected subunit cRNAs and varied their relative concentrations in Xenopus oocytes. Using the equimolar ratio of 1:1:1:1 (Hco-UNC-29.1, Hco-UNC-38, Hco-UNC-63 and Hco-ACR-8) as a positive control, we have tested 5:1:1:1, 1:5:1:1, 1:1:5:1 and 1:1:1:5 ratios, respectively (Fig. 4). Providing five fold more Hco-unc-63, Hco-unc-38 or Hco-acr-8 cRNAs significantly reduced the currents evoked by acetylcholine and the other agonists (Fig. 4a) but did not alter the relative pharmacological rank of potency compared to Hco-L-AChR1, except for pyrantel which seemed to be even less effective (Fig. 4b). Changing the subunit stoichiometry of Hco-L-AChR1 by injecting five fold more of Hco-unc-29.1 cRNA produced no recordable currents. This unexpected dramatic effect at the 5:1:1:1 ratio of Hco-unc-29.1 is suggestive either of a dominant-negative effect due to the saturation of the expression machinery (no receptors produced) or of the expression of non-functional receptors.
Discussion
In the present study, we have functionally expressed two distinct H. contortus L-AChR subtypes (Hco-L-AChR1 and Hco-L-AChR2) in Xenopus oocyte to test the effect of the anthelmintic bephenium. Our experiments clearly demonstrated that bephenium selectively activates the Hco-L-AChR1 subtype that is very sensitive to levamisole but weakly responsive to pyrantel and insensitive to nicotine. In contrast, the Hco-L-AChR2 subtype (more related to a P-subtype) was more responsive to pyrantel and nicotine than levamisole and showed bephenium insensitivity. These results provide new insights into the pharmacological properties of the AChR subtypes that could be expressed in muscle of parasitic nematodes.
Muscle L-subtype AChRs have been investigated at the single-channel level in the pig worms A. suum and O. dentatum as well as in the soil nematode C. elegans highlighting some major differences between the parasitic and free-living species, respectively. In A. suum and O. dentatum, levamisole was found to activate up to three common populations of channels that were clearly separated by their conductance (Qian et al. 2006; Robertson et al. 1999). They were designated as G25 (small conductance: 22 pS), G35 (intermediate conductance: 33 pS) and G45 (large conductance: 45 pS). In addition to these three channel populations, in the strongyle species O. dentatum belonging to the same phylogenetic clade as H. contortus and C. elegans, a forth levamisole activated AChR subtype harboring a conductance of 40 pS has also been observed (Robertson et al. 1999). In sharp contrast, levamisole application revealed only one conductance of approximately 30 pS in C. elegans (Qian et al. 2008). Therefore, it is tempting to hypothesize that L-AChR diversity could be associated with parasitic life-style. In accordance with this hypothesis, we have previously demonstrated that at least two distinct recombinant H. contortus L-AChR subtypes can be robustly expressed in Xenopus oocyte (Boulin et al. 2011). Our experiments emphasized the existence of a levamisole-sensitive AChR (L-AChR) and pyrantel-sensitive AChR (P-AChR). Although levamisole and pyrantel have been basically assumed to act at the same sites in nematode parasites, there may be significant differences between roundworm species that are evolutionary distant. In A. suum, the cholinergic agonist bephenium was shown to be a potent activator of G35 and G45 levamisole-activated channels indicating that levamisole and bephenium could act on common AChR subtypes (Qian et al. 2006). Our present results are consistent with this observation as we demonstrate that bephenium activates in a dose–response manner the recombinant H. contortus L-AChR1 expressed in Xenopus oocyte. In contrast, bephenium had no effect on the recombinant H. contortus L-AChR2 providing significant pharmacological evidence of the separation of the levamisole-sensitive and pyrantel-sensitive receptor subtypes. Hence, bephenium may share common binding sites with levamisole involving the Hco-ACR-8 subunit. This observation confirms that a L-AChR and a P-AChR may be separated in vitro, but this is not necessarily the case in vivo at the worm level. Nevertheless, this result supports the idea that bephenium could selectively target some, but not all levamisole-sensitive AChR subtypes in H. contortus worms. This hypothesis is indeed supported by previous data obtained with force transduction assays carried out on levamisole-susceptible and levamisole-resistant H. contortus isolates (Sangster et al. 1991). In these experiments, bephenium was shown to have an equivalent efficacy in both levamisole-susceptible and levamisole-resistant worms further indicating that different levamisole-sensitive AChR subtypes that have different sensitivities to bephenium might be expressed on muscle cells of H. contortus. In the dog hookworm Ancylostoma caninum that is closely related to H. contortus (Clade V), a high level of resistance to pyrantel was shown to be associated with a reduced levamisole sensitivity and an increased sensitivity to bephenium (Kopp et al. 2008). The inverse relationship between sensitivities to levamisole or pyrantel and bephenium could suggest that the reduction in some L-AChR subtypes may induce a compensatory increase in bephenium-sensitive AChRs. The functional basis for maintaining multiple L-AChR subtypes on the muscle of parasitic nematodes remains elusive but even though it may have a significant role in the development of resistance, such diversity could also be exploited for the development of novel anthelmintic compounds.
At the molecular level, differences between the Hco-L-AChR1 and Hco-L-AChR2 subtypes are responsible for their differential sensitivity to bephenium. Hco-L-ACHR1 and Hco-L-AChR2 both contain absolutely required Hco-UNC-38, Hco-UNC-63 and Hco-UNC-29.1 subunits and only differ by the presence or absence of an additional Hco-ACR-8 subunit, respectively. Therefore, it is tempting to hypothesize that Hco-ACR-8 plays a pivotal role in the bephenium sensitivity of Hco-L-AChR1. Although information on the ordering and arrangement of the subunits is unknown, Hco-L-AChR1 subunit composition allows for only 4 putative combinations depending on which subunit is present twice in the receptor: (Hco-UNC-29.1)2:Hco-UNC38:Hco-UNC-63:Hco-ACR-8, Hco-UNC-29.1: (Hco-UNC38)2:Hco-UNC-63:Hco-ACR-8, Hco-UNC-29.1: Hco-UNC38:(Hco-UNC-63)2:Hco-ACR-8 and Hco-UNC-29.1:Hco-UNC38:Hco-UNC-63:(Hco-ACR-8)2. Further experiments using concatamers of specific subunit stochiometries will help to decipher the exact stoichiometry and the handedness of the circular subunit arrangement of the Hco-L-AChRs. In vitro experiments with A. suum levam-isole receptors have postulated that different subunit stoichiometries could explain differences in pharmacology (Williamson et al. 2009). Thus, in the present study, we have injected in the Xenopus oocytes, Hco-L-AChR1 subunit cRNAs with different ratio in order to investigate a potential effect on its pharmacological properties including bephenium sensitivity. Using the equimolar subunit ratio of 1:1:1:1 for Hco-UNC-29.1:Hco-UNC-38:Hco-UNC-63:Hco-ACR-8, respectively, as a positive control, we have tested 5:1:1:1, 1:5:1:1, 1:1:5:1 and 1:1:1:5 ratios, respectively. For Hco-UNC-38, Hco-UNC-63 and Hco-ACR-8, the relative excess amount of cRNAs resulted in a simultaneous decrease of sensitivity to acetylcholine, levamisole, pyrantel and bephenium but did not reveal striking differences in the pharmacological profile compared to Hco-L-AChR1. This result suggests an overall reduction of receptor expression rather than different pharmacological properties. This effect was even more drastic when Hco-UNC-29.1 cRNA was added in excess to the co-injection mix as it resulted in the complete loss of recordable current after application of acetylcholine and any other agonists. Clearly, further functional experiments using intermediate amounts of Hco-UNC-29.1 cRNA will be required to investigate the dominant-negative effect exerted by this subunit on Hco-L-AChR1 expression. Interestingly, coinjection in Xenopus oocyte of in 5:1 or 1:5 ratio of A. suum UNC-38/UNC-29 subunits led to functional receptors harboring an inversed sensitivity to levamisole and to nicotine, whereas response to acetylcholine was mostly unchanged (Williamson et al. 2009). Therefore, our results underline major differences between H. contortus and A. suum subunit assembly and receptor properties. Unfortunately, the definitive assignment of specific subunit combination to in vivo channels would require nematode transgenesis experiments that are not yet feasible in both of these parasitic nematode species.
In the present study, we have investigated the mode of action of bephenium on two recombinant H. contortus L-AChR subtypes and in that respect we cannot rule out the possibility that bephenium could act on other AChR subtypes in vivo. Interestingly, for some of the subunits involved in H. contortus L-AChR composition, we have previously reported the identification of multiple paralogues (Neveu et al. 2010). It is tempting to suggest that some of these subunits could contribute to Hco-L-AChR subtype diversity and might also represent potential targets for bephenium. Ideally single-channel experiments in H. contortus would be the best way first to investigate L-AChR diversity at the worm level, second to determine which L-AChR subtype could be preferentially activated by bephenium and third to determine which recombinant receptor subtypes have the same single-channel properties of the native receptors recorded in vivo.
In summary, we have demonstrated that the H. contortus L-AChR1 subtype expressed in xenopus oocytes is selectively targeted by the cholinergic anthelmintic bephenium. This report represents the first description of the molecular composition of an invertebrate bephenium-sensitive receptor.
Acknowledgments
This work was supported by the Institut National de la Recherche Agronomique (INRA). Claude Charvet, via a 2011 fellowship award under the OECD Co-operative Research Programme: Biological Resource Management for Sustainable Agricultural Systems to perform the present work at Richard Martin’s laboratory at Iowa State University. RJM and APR were funded by Grant Number R R56 AI047194–11 and R21AI092185-01, respectively, from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. We wish to acknowledge Samuel K. Buxton for technical assistance and training at electrophysiology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Footnotes
Conflict of interest The authors state no conflict of interest.
Contributor Information
Claude L. Charvet, INRA, UMR1282 Infectiologie et Santé Publique, Centre de Recherches de Tours, 37380 Nouzilly, France; Université François Rabelais de Tours, UMR1282 Infectiologie et Santé Publique, 37000 Tours, France
Alan P. Robertson, Department of Biomedical Sciences, Iowa State University, Ames, IA 50011-1250, USA
Jacques Cabaret, INRA, UMR1282 Infectiologie et Santé Publique, Centre de Recherches de Tours, 37380 Nouzilly, France; Université François Rabelais de Tours, UMR1282 Infectiologie et Santé Publique, 37000 Tours, France.
Richard J. Martin, Department of Biomedical Sciences, Iowa State University, Ames, IA 50011-1250, USA
Cédric Neveu, INRA, UMR1282 Infectiologie et Santé Publique, Centre de Recherches de Tours, 37380 Nouzilly, France; Université François Rabelais de Tours, UMR1282 Infectiologie et Santé Publique, 37000 Tours, France.
References
- Aceves J, Erlij D, Martinez-Maranon R. The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br J Pharmacol. 1970;38(3):602–607. doi: 10.1111/j.1476-5381.1970.tb10601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry ML, Cowell P, Davey MJ, Shevde S. Aspects of the pharmacology of a new anthelmintic: pyrantel. Br J Pharmacol. 1970;38(2):332–344. doi: 10.1111/j.1476-5381.1970.tb08521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau JL. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci USA. 2008;105(47):18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T, Fauvin A, Charvet CL, Cortet J, Cabaret J, Bessereau JL, et al. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br J Pharmacol. 2011;164(5):1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows RB. The anthelmintic effect of bephenium on Ancylostoma caninum. J Parasitol. 1958;44(6):607–610. [PubMed] [Google Scholar]
- Colquhoun L, Holden-Dye L, Walker RJ. The pharmacology of cholinoceptors on the somatic muscle cells of the parasitic nematode Ascaris suum. J Exp Biol. 1991;158:509–530. doi: 10.1242/jeb.158.1.509. [DOI] [PubMed] [Google Scholar]
- Copp FC, Standen OD, Scarnell J, Rawes DA, Burrows RB. A new series of anthelmintics. Nature. 1958;181(4603):183. doi: 10.1038/181183a0. [DOI] [PubMed] [Google Scholar]
- Culetto E, Baylis HA, Richmond JE, Jones AK, Fleming JT, Squire MD, et al. The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J Biol Chem. 2004;279(41):42476–42483. doi: 10.1074/jbc.M404370200. [DOI] [PubMed] [Google Scholar]
- Eimer S, Gottschalk A, Hengartner M, Horvitz HR, Richmond J, Schafer WR, et al. Regulation of nicotinic receptor trafficking by the transmembrane Golgi protein UNC-50. EMBO J. 2007;26(20):4313–4323. doi: 10.1038/sj.emboj.7601858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvin A, Charvet C, Issouf M, Cortet J, Cabaret J, Neveu C. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Mol Biochem Parasitol. 2010;170(2):105–107. doi: 10.1016/j.molbiopara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Fleming JT, Squire MD, Barnes TM, Tornoe C, Matsuda K, Ahnn J, et al. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci. 1997;17(15):5843–5857. doi: 10.1523/JNEUROSCI.17-15-05843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, et al. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002;21(5):1012–1020. doi: 10.1093/emboj/21.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugstetter J, Blicher T, Ellgaard L. Identification and characterization of a novel thioredoxin-related transmembrane protein of the endoplasmic reticulum. J Biol Chem. 2005;280(9):8371–8380. doi: 10.1074/jbc.M413924200. [DOI] [PubMed] [Google Scholar]
- Hoekstra R, Borgsteede FH, Boersema JH, Roos MH. Selection for high levamisole resistance in Haemonchus contortus monitored with an egg-hatch assay. Int J Parasitol. 1997;27(11):1395–1400. doi: 10.1016/s0020-7519(97)00126-4. [DOI] [PubMed] [Google Scholar]
- Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20(10):477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kopp SR, Coleman GT, McCarthy JS, Kotze AC. Phenotypic characterization of two Ancylostoma caninum isolates with different susceptibilities to the anthelmintic pyrantel. Antimicrob Agents Chemother. 2008;52(11):3980–3986. doi: 10.1128/AAC.00523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, Robertson AP, Bjorn H, Sangster NC. Heterogeneous levamisole receptors: a single-channel study of nicotinic acetylcholine receptors from Oesophagostomum dentatum. Eur J Pharmacol. 1997;322(2–3):249–257. doi: 10.1016/s0014-2999(96)00996-x. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Verma S, Levandoski M, Clark CL, Qian H, Stewart M, et al. Drug resistance and neurotransmitter receptors of nematodes: recent studies on the mode of action of levamisole. Parasitology. 2005;131(Suppl):S71–S84. doi: 10.1017/S0031182005008668. [DOI] [PubMed] [Google Scholar]
- McKellar QA, Jackson F. Veterinary anthelmintics: old and new. Trends Parasitol. 2004;20(10):456–461. doi: 10.1016/j.pt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Neveu C, Charvet CL, Fauvin A, Cortet J, Beech RN, Cabaret J. Genetic diversity of levamisole receptor subunits in parasitic nematode species and abbreviated transcripts associated with resistance. Pharmacogenet Genomics. 2010;20(7):414–425. doi: 10.1097/FPC.0b013e328338ac8c. [DOI] [PubMed] [Google Scholar]
- Peter JW, Chandrawathani P. Haemonchus contortus: parasite problem No. 1 from tropics - Polar Circle. Problems andprospects for control based on epidemiology. Trop Biomed. 2005;22(2):131–137. [PubMed] [Google Scholar]
- Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the singlechannel level in Ascaris suum. FASEB J. 2006;20(14):2606–2608. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- Qian H, Robertson AP, Powell-Coffman JA, Martin RJ. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB J. 2008;22(9):3247–3254. doi: 10.1096/fj.08-110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AP, Bjorn HE, Martin RJ. Resistance to levamisole resolved at the single-channel level. FASEB J. 1999;13(6):749–760. doi: 10.1096/fasebj.13.6.749. [DOI] [PubMed] [Google Scholar]
- Robertson AP, Clark CL, Burns TA, Thompson DP, Geary TG, Trailovic SM, et al. Paraherquamide and 2-deoxyparaherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J Pharmacol Exp Ther. 2002;302(3):853–860. doi: 10.1124/jpet.102.034272. [DOI] [PubMed] [Google Scholar]
- Sangster NC, Davis CW, Collins GH. Effects of cholinergic drugs on longitudinal contraction in levamisole-susceptible and- resistant Haemonchus contortus. Int J Parasitol. 1991;21(6):689–695. doi: 10.1016/0020-7519(91)90080-q. [DOI] [PubMed] [Google Scholar]
- Touroutine D, Fox RM, Von Stetina SE, Burdina A, Miller DM, 3rd, Richmond JE. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem. 2005;280(29):27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- Towers PR, Edwards B, Richmond JE, Sattelle DB. The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J Neurochem. 2005;93(1):1–9. doi: 10.1111/j.1471-4159.2004.02951.x. [DOI] [PubMed] [Google Scholar]
- Walker J, Hoekstra R, Roos MH, Wiley LJ, Weiss AS, Sangster NC, et al. Cloning and structural analysis of partial acetylcholine receptor subunit genes from the parasitic nematode Teladorsagia circumcincta. Vet Parasitol. 2001;97(4):329–335. doi: 10.1016/s0304-4017(01)00416-2. [DOI] [PubMed] [Google Scholar]
- Wiley LJ, Weiss AS, Sangster NC, Li Q. Cloning and sequence analysis of the candidate nicotinic acetylcholine receptor alpha subunit gene tar-1 from Trichostrongylus colubriformis. Gene. 1996;182(1–2):97–100. doi: 10.1016/s0378-1119(96)00520-3. [DOI] [PubMed] [Google Scholar]
- Williamson SM, Robertson AP, Brown L, Williams T, Woods DJ, Martin RJ, et al. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog. 2009;5(7):e1000517. doi: 10.1371/journal.ppat.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Jeffery GM, Freed JE, Morehouse WG. Bephenium, a new drug active against human hookworm. J Parasitol. 1958;44(6):611–612. [PubMed] [Google Scholar]