Abstract
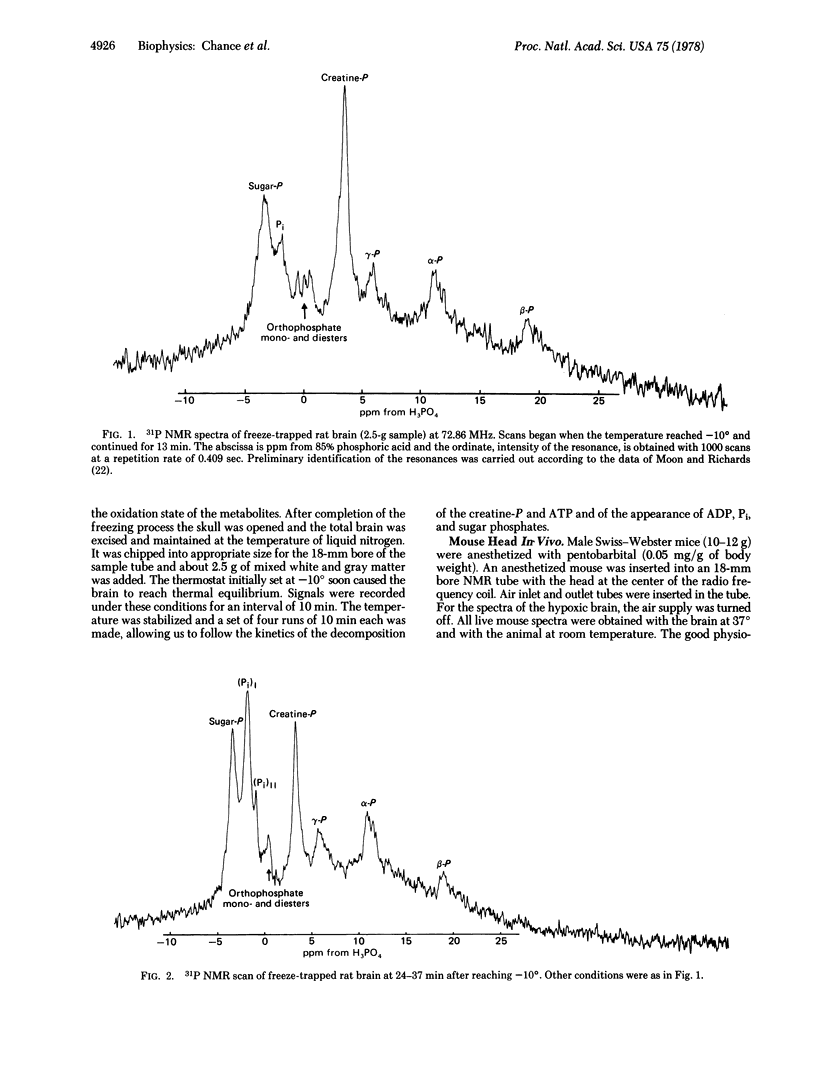
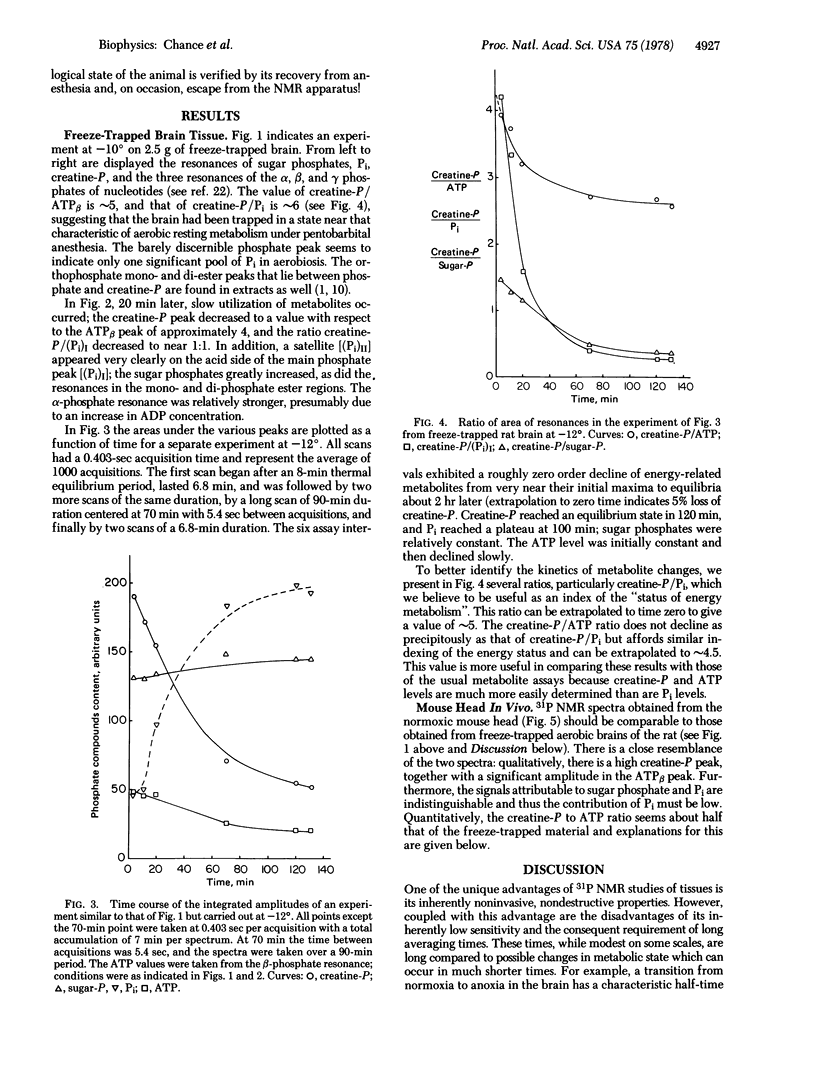
The 31P NMR spectrum of energy-related metabolites under strictly aerobic conditions in rapidly respiring tissues under physiological conditions has been approached by the study of the 31P NMR signals in vivo and in freeze-trapped organs. Freezing the head of the anesthetized animal by liquid N2, excision of the brain tissue (white and gray matter) at -196°, and transfer to the NMR tube occurs without alteration of the metabolite concentrations. The sample is warmed to the region -15° to -10°, at which temperatures there is sufficient mobility for recording 31P NMR at concentrations characteristic of brain tissues (∼5 mM) with an adequate signal to noise ratio in 10 min but insufficient mobility for significant enzymatic activity. A ∼0.4-sec acquisition time is adequate for nuclear relaxation and a 10-min scan gives an adequate signal to noise ratio. Metabolism of creatine phosphate, Pi, and sugar phosphates occurs by 1 hr at -10° and 2 hr at -12°. Extrapolation of the approximately zero order kinetics of disappearance of creatine phosphate and appearance of Pi suggests that <10% of these two metabolites has been altered in the time of the first measurement.
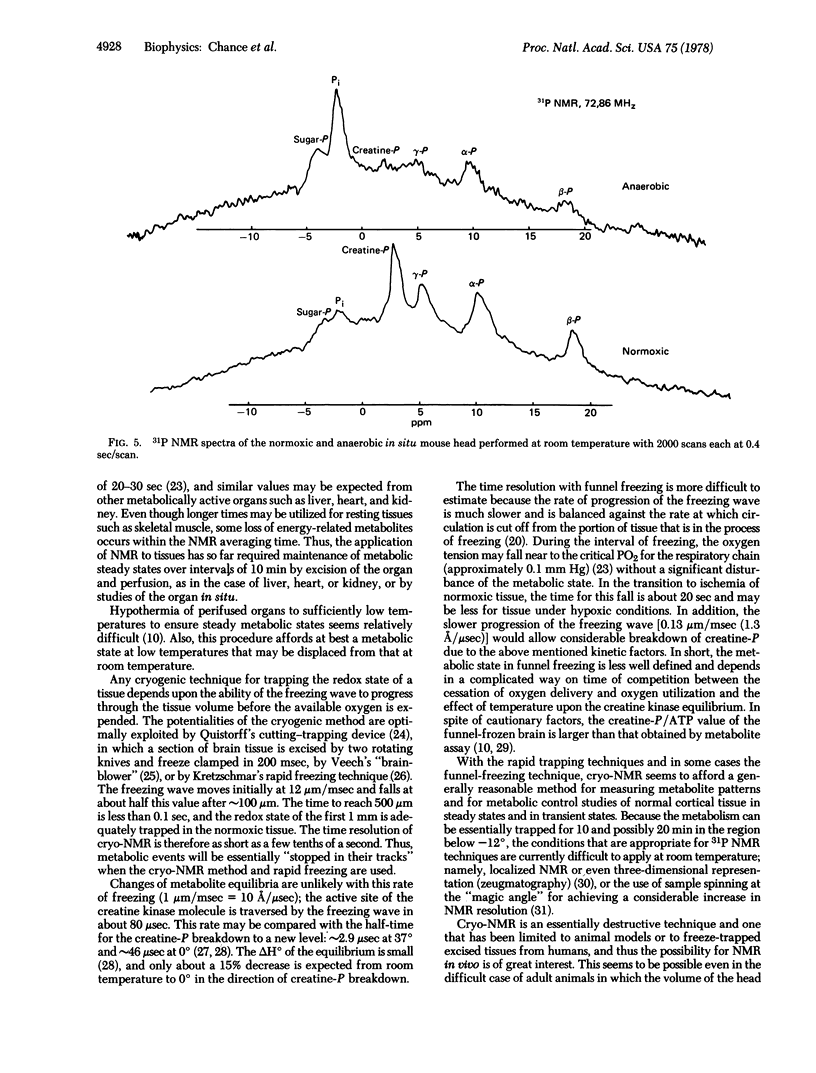
A comparison of the freeze-trapped state and the in vivo metabolite pattern is afforded in preliminary experiments on the head of the living mouse (brain and skeletal tissue) in aerobic and anaerobic states. Longer relaxation times and mild hypoxia due to the restricted diameter of the NMR tube gives significantly lower creatine phosphate/ATP values for this condition. Both direct in vivo and freeze-trapped assays of energy-related metabolites afford excellent approaches to the detection of anoxia and to the evaluation of metabolic control in hypoxic conditions.
Keywords: cryo-nuclear magnetic resonance, live-animal nuclear magnetic resonance, cerebral hypoxia, creatine phosphate
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belton P. S., Jackson R. R., Packer K. J. Pulsed NMR studies of water in striated muscle. I. Transverse nuclear spin relaxation times and freezing effects. Biochim Biophys Acta. 1972 Nov 24;286(1):16–25. doi: 10.1016/0304-4165(72)90084-0. [DOI] [PubMed] [Google Scholar]
- Belton P. S., Packer K. J., Sellwood T. C. Pulsed NMR studies of water in striated muscle. II. Spin-lattice relaxation times and the dynamics of non-freezing fraction of water. Biochim Biophys Acta. 1973 Mar 30;304(1):56–64. doi: 10.1016/0304-4165(73)90114-1. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Chance B., Nishimura M. ON THE MECHANISM OF CHLOROPHYLL-CYTOCHROME INTERACTION: THE TEMPERATURE INSENSITIVITY OF LIGHT-INDUCED CYTOCHROME OXIDATION IN CHROMATIUM. Proc Natl Acad Sci U S A. 1960 Jan;46(1):19–24. doi: 10.1073/pnas.46.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch E. D., Harmon J. F., Muller B. H. Pulsed NMR measurements of the diffusion constant of water in muscle. Arch Biochem Biophys. 1971 Nov;147(1):299–310. doi: 10.1016/0003-9861(71)90337-7. [DOI] [PubMed] [Google Scholar]
- Folbergrová J., Ljunggren B., Norberg K., Siesjö B. K. Influence of complete ischemia on glycolytic metabolites, citric acid cycle intermediates, and associated amino acids in the rat cerebral cortex. Brain Res. 1974 Nov 15;80(2):265–279. doi: 10.1016/0006-8993(74)90690-8. [DOI] [PubMed] [Google Scholar]
- Fung B. M., McGaughy T. W. The state of water in muscle as studied by pulsed NMR. Biochim Biophys Acta. 1974 May 24;343(3):663–673. doi: 10.1016/0304-4165(74)90287-6. [DOI] [PubMed] [Google Scholar]
- Gadian D. G., Hoult D. I., Radda G. K., Seeley P. J., Chance B., Barlow C. Phosphorus nuclear magnetic resonance studies on normoxic and ischemic cardiac tissue. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4446–4448. doi: 10.1073/pnas.73.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Chemical change and energy output during muscular contraction. J Physiol. 1971 Oct;218(1):163–193. doi: 10.1113/jphysiol.1971.sp009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lipton P. Effects of membrane depolarization on light scattering by cerebral cortical slices. J Physiol. 1973 Jun;231(2):365–383. doi: 10.1113/jphysiol.1973.sp010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Quistorff B. A mechanical device for the rapid removal and freezing of liver or brain tissue from unanaesthetized and nonparalyzed rats. Anal Biochem. 1975 Sep;68(1):102–118. doi: 10.1016/0003-2697(75)90684-3. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]


