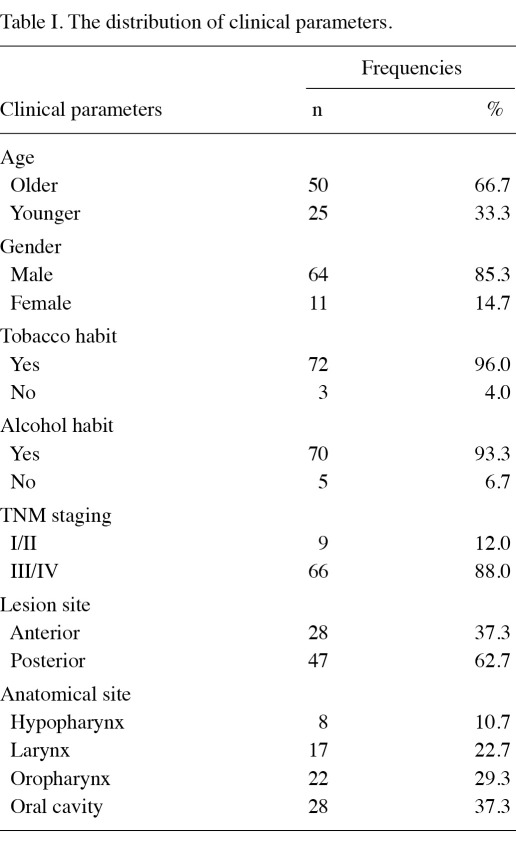Abstract
Recently, high-risk human papillomavirus (HPV) has emerged as a possible agent associated with head and neck squamous cell carcinoma (HNSCC) in younger patients. Therefore, the purpose of the present study was to assess the effect of age on the distribution of HPV-16/18 in HNSCC, together with the impact of the virus on patient prognosis. A longitudinal prospective study was used adjusted for age, gender, TNM staging, smoking status and alcohol consumption. HPV was detected by PCR with consensus primers. Results showed there was no difference in the frequency of HPV-16/18 positivity when younger patients were compared to the older patients. No association was found among high-risk HPV positivity, gender, smoking habit and anatomical site. High-risk HPV was associated with advanced TNM in bivariate analyses; however, it did not impact on survival. Only TNM staging was associated with risk of mortality. Our study supports the theory that age does not affect the presence of HPV-16/18 in HNSCC and has no impact on patient prognosis. The incidence of HNSCC among patients under the age of 45 years is reportedly on the increase worldwide. The factors associated with HNSCC in younger adults are not well established. Findings of this study indicate that HPV-16/18 may not play a role in HNSCC patients under the age of 45 years.
Keywords: head and neck cancer, HPV, younger patients, squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common type of cancer and causes 350,000 cancer mortalities worldwide each year (1,2). HNSCC comprises malignant epithelial neoplasms that arise in the paranasal sinuses, nasal cavity, oral cavity, pharynx and larynx, and generally affects males between the sixth and ninth decades of life following long-term exposure to smoking and alcohol consumption (3). However, an increase in the incidence of head and neck cancer among patients under the age of 45 years has been reported worldwide (4-10). Some studies suggest that in younger patients, HNSCC arises regardless of the classical risk factors (4-8,10). HPV infection has been suggested as a possible etiologic factor for such cases (11-16). HPV was detected in a variety of HNSCCs (11-15,17) and it was suggested to be associated with lesions of potential malignisation in the head and neck (18,19); however, divergent results are found in the literature regarding the role of HPV presence in HNSCC (20,21).
Taking these facts into consideration, we investigated whether there is any change in the distribution of HPV-16/18 in younger patients compared to older ones. In addition, we assessed the effect of the virus on patient prognosis. To test these hypotheses we performed a longitudinal prospective study.
Patients and methods
Patients. In total, 75 patients diagnosed with HNSCC recruited from a database of head and neck surgeries that occurred between 1996 and 2007 in Montes Claros, Brazil, were included in the current study (4). Patients younger than 45 years old were selected (n=25). The older patients (n=50) were randomly selected in a proportion of 2:1 adjusted for gender, TNM staging, anatomical site, smoking and alcohol intake. The patients were from the same geographical area and evaluated/treated by the same practitioner.
Clinical data. The mean age was 42.1 years (SD 3.17; range, 33-45) for younger HNSCC patients and 62.2 years (SD 8.0; range, 49-82) for older HNSCC patients. Skin colour was not used as a physical descriptor since it is a poor predictor of genomic ancestry in Brazil (22,23). The current investigation was approved by the local Ethics Committee. Information on age, gender, tobacco smoking history, alcohol consumption history, medical history, tumour site, TNM clinical staging and survival was obtained from medical files.
All patients were staged according to the UICC TNM classification of malignant tumours (1997) (24). HNSCC lesions were classified according to the primary site as described in the international classification of diseases (ICD-10) for oncology. The anatomical sites reviewed in this study included: i) 28 (37.3%) mouth and perioral region sites (C00, C01, C02, C04, C05, C06.0 and C06.2); ii) 22 (29.3%) oropharynx (C09-C10) sites; and iii) 25 (23.4%) hypopharynx-larynx sites (C12, C13 and C32). The sites were classified according to anatomical site (anterior: Oral mucosa, tongue, retromolar trigon, mouth floor, jugal mucosa and gengival; border/posterior: Base tongue, oropharynx and hypopharynx-larynx). The patients had histologically confirmed HNSCC based on the World Health Organisation criteria (WHO, 1997) (25,26). Patients with a diagnosis of carcinoma in situ or multiple head and neck carcinomas were excluded. Ethics approval for this study was obtained from the local ethics committee (468/06).
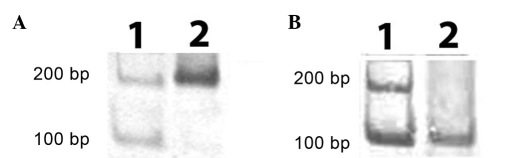
HPV identification. HPV-DNA sequences were first polymerase chain reaction (PCR)-amplified by L1 (F: 5'-GCMCAGGGWCATAAYAATGG-3' and R: 5'-CGTCCMAARGGAWACTGATC-3', where, M=A or C, R=A or G, W=A or T, Y=C or T) and then by HPV-16 (F: 5'-AAGGCCAACTAAATGTCA-C-3' and R: 5'-CTGCTTTTATACTAACCGG-3') and HPV-18 (F: 5'-ACCTTAATGAAAAACCACGA-3' and R: 5'-CGTCGTTTAGAGTCGTTC-3'). β-globin gene primers were used as an internal control. The primer sequences were described by Katiyar et al (27). PCR was performed in a total volume of 25 µl containing approximately 100 ng genomic DNA as a template, 0.5 µl of each primer (20 pmol/µl), 2.5 µl dNTP-mix (25 mM of each, Amresco, Ohio, CA, USA), 2.5 µl 10X PCR buffer, 1.25 µl magnesium chloride (50 mM) and 2.5 units of Platinum Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA, USA) (Fig. 1). Reactions were performed using positive (cultured virus) and negative (PCR without DNA) controls.
Figure 1.
A) PCR for HPV-16. Lane 1, 100-bp molecular marker. Lane 2, HPV-16-positive (217 bp). (B) PCR for HPV-18. Lane 1, 100-bp molecular marker. Lane 2, HPV-18-positive (100 bp). PCR, polymerase chain reaction; HPV, human papillomavirus.
Electrophoresis. The PCR products were verified on a 6.5% polyacrylamide gel that was electrophoresed at a constant voltage of 120 V for 1.5 h and stained with silver nitrate. Electrophoresis results were estimated against a 100-bp ladder.
Statistical analysis. The statistical significance of differences between case and control group distributions for HPV positivity was evaluated using Fisher's test or the Chi-squared test. Survival time was calculated from the date of diagnosis to the time of the last follow-up visit or to the time of mortality. Using these criteria, the records of each patient were reviewed from 0 to 2500 days. Mortalities were the result of locoregional and/or metastatic disease. Mortalities that occurred without evidence of recurrence were excluded from analysis. Survival time was presented by the means of the Kaplan-Meier method for the variables. Variables were included in the Cox proportional hazards multivariate model. In accordance with the literature, categorical variables considered as references were those associated with a reduced risk of mortality. Analyses were assessed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was set at p<0.05.
Results
The descriptive data of the population used in the current study are shown in Table I. The majority of patients (96%) in the study population were smokers and consumed alcohol (93.3%). Moreover, 88% presented with advanced TNM staging.
Table I.
The distribution of clinical parameters.
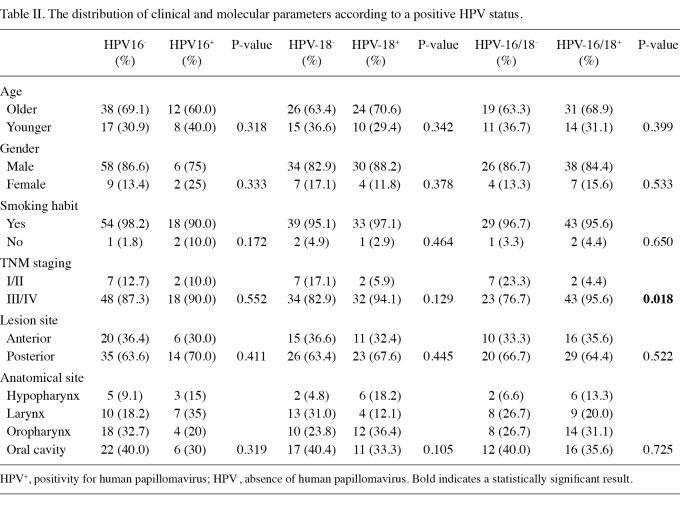
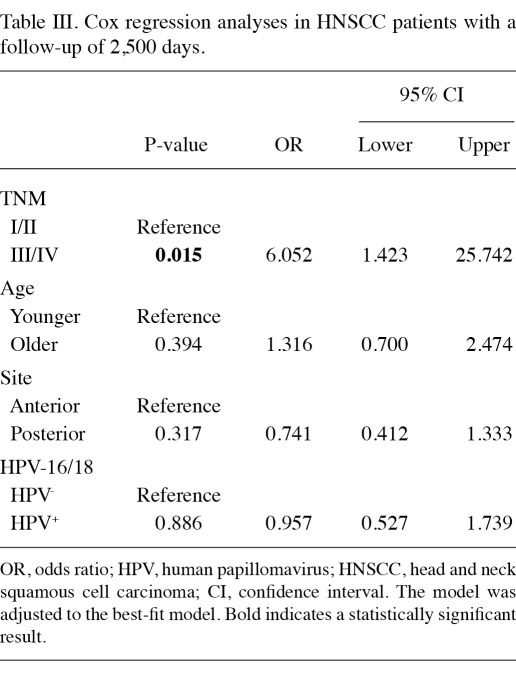
Table II shows the distribution of clinical and molecular parameters according to HPV status. There is no change in the frequency of HPV-16/18 related to patient age. No association was found among high-risk HPV positivity, gender, smoking habit and anatomical site. In combination, high-risk HPV was associated with advanced TNM in bivariate analyses (Table II); however, it did not impact on the survival (Table III). Anatomical site and age did not interfere with the survival. Only TNM staging was associated with risk of mortality (Table III).
Table II.
The distribution of clinical and molecular parameters according to a positive HPV status.
Table III.
Cox regression analyses in HNSCC patients with a follow-up of 2,500 days.
Discussion
Currently, there are no biological mechanisms that could justify the high-risk HPV predilection for a specific anatomical site in the head and neck. The role of high-risk HPV in HNSCC has recently been highlighted in the oropharynx (13), oral cavity (14), larynx (15), hypopharynx (11,12) and in HNSCC metastasis (17). The relevance of the high-risk HPV-E6/E7 protein for the carcinogenesis of multiple head and neck sites was demonstrated in transgenic mice (28-30). In addition, there is no consensus in the literature to detect HPV infection (20). In agreement with these facts, we did not observe high-risk HPV predilection for anatomical sites. The absence of consensus is justified by the differences in the study sample. It is well known that there are limitations for paraffin-embedded tissues, particularly as the fixation makes it difficult to purify RNA from paraffin-embedded tissues (17,31,32). Although non-quantitative PCR-based methods do not allow for the determination of infectious activity, these methods are beneficial as they provide greater sensitivity and specificity than in situ hybridisation techniques (20). Evidence suggests that the p16 immunohistochemistry could be used as a survival biomarker in head and neck cancer (33) and aid in high-risk HPV detection (18).
A possible association between HPV infection and the development of HNSCC in younger patients has been proposed (16). In addition, it has been postulated that HPV-positive tumours are a subgroup of HNSCC that is distinct from tobacco- and alcohol-induced carcinomas (19). An absence of p16CDKN2A mutations as well as the expression of p16 is the characteristic feature of high-risk HPV-positive tumours, in contrast to the inactivation of the p16CDKN2A gene in HPV-negative tumours (34). p16 protein is important in the regulation of the G1/S phase cell-cycle checkpoint (33,35,36) and it has been reported that p16-positive tumours have a better prognosis than p16-negative tumours (33). Therefore, p16 expression may explain the reason for HPV-positive tumours showing a favourable prognosis as compared to HPV-negative tumours. In the current study, HPV-16/18 positivity was not associated with age or survival. The explanation for these findings may be the rigorous selection criteria (age, gender, TNM staging and anatomical site) or that the simultaneous presence of some of these independent variables alters the evolution of cancer in a different way. Moreover, the majority of our patients were smokers, thus our data are in agreement with previous studies (21). Genetic and epigenetic factors are associated with a susceptibility to cancer development at a young age (4,37). Alternatively, genetic and epigenetics factors may play a stronger role than HPV-16/18 infection in the development of HNSSC at an early age (38-42). In the current study, we did not observe differences in survival associated with age, in agreement with previous studies (4,37).
The mechanisms underlying the association between high-risk HPV and HNSCC have been unclear. Studies using SCC samples shed light on the role of high-risk HPV infection in epigenetic regulation (43). For example, it was suggested that HPV-16 induces the methylation of p16CDKN2A (44); however, as oncogenic HPV neutralises phosphorylated Rb (PRb)-mediated control of the cell cycle, there would be no advantage for the HPV-infected host cell to block the same signalling pathway at another checkpoint by downregulating p16CDKN2A (45-47).
In conclusion, our study supports the theory that there is no change in the frequency of HPV-16/18 positivity in younger patients when compared to the older ones. In addition, HPV-16/18 presence did not change the HNSCC prognosis.
Acknowledgments
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG). Dr Guimarães and Dr Gomez are research fellows of CNPq. Dr De Paula is a research fellow of FAPEMIG.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Argiris A, Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res. 2003;114:15–60. doi: 10.1007/0-306-48060-3_2. [DOI] [PubMed] [Google Scholar]
- 4.De Paula AM, Souza LR, Farias LC. et al. Analysis of 724 cases of primary head and neck squamous cell carcinoma (HNSCC) with a focus on young patients and p53 immunolocalization. Oral Oncol. 2009;45:777–782. doi: 10.1016/j.oraloncology.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Garavello W, Spreafico R, Gaini RM. Oral tongue cancer in young patients: a matched analysis. Oral Oncol. 2007;43:894–897. doi: 10.1016/j.oraloncology.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Gawecki W, Kostrzewska-Poczekaj M, Gajecka M, Milecki P, Szyfter K, Szyfter W. The role of genetic factor in etiopathogenesis of squamous cell carcinoma of the head and neck in young adults. Eur Arch Otorhinolaryngol. 2007;264:1459–1465. doi: 10.1007/s00405-007-0386-x. [DOI] [PubMed] [Google Scholar]
- 7.Llewellyn CD, Johnson NW, Warnakulasuriya S. Factors associated with delay in presentation among younger patients with oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:707–713. doi: 10.1016/j.tripleo.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Manuel S, Raghavan SK, Pandey M, Sebastian P. Survival in patients under 45 years with squamous cell carcinoma of the oral tongue. Int J Oral Maxillofac Surg. 2003;32:167–173. doi: 10.1054/ijom.2002.0271. [DOI] [PubMed] [Google Scholar]
- 9.Myers JN, Elkins T, Roberts D, Byers RM. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg. 2000;122:44–51. doi: 10.1016/S0194-5998(00)70142-2. [DOI] [PubMed] [Google Scholar]
- 10.Siriwardena BS, Tilakaratne A, Amaratunga EA. et al. Analysis of histopathological and immunohistochemical differences of oral squamous cell carcinoma in young and old patients in Sri Lanka. J Oral Pathol Med. 2007;36:357–362. doi: 10.1111/j.1600-0714.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 11.Baumann JL, Cohen S, Evjen AN. et al. Human papillomavirus in early laryngeal carcinoma. Laryngoscope. 2009;119:1531–1537. doi: 10.1002/lary.20509. [DOI] [PubMed] [Google Scholar]
- 12.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978-2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;129:733–741. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 13.D'Souza G, Kreimer AR, Viscidi R. et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 14.Saini R, Tang TH, Zain RB. et al. Significant association of high-risk human papillomavirus (HPV) but not of p53 polymorphisms with oral squamous cell carcinomas in Malaysia. J Cancer Res Clin Oncol. 2011;137:311–320. doi: 10.1007/s00432-010-0886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torrente MC, Rodrigo JP, Haigentz M Jr. et al. Human papillomavirus infections in laryngeal cancer. Head Neck. 2011;33:581–586. doi: 10.1002/hed.21421. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZY, Sdek P, Cao J, Chen WT. Human papillomavirus type 16 and 18 DNA in oral squamous cell carcinoma and normal mucosa. Int J Oral Maxillofac Surg. 2004;33:71–74. doi: 10.1054/ijom.2002.0443. [DOI] [PubMed] [Google Scholar]
- 17.Barwad A, Sood S, Gupta N, Rajwanshi A, Panda N, Srinivasan R. Human papilloma virus associated head and neck cancer: A PCR based study. [Epub 2011 Apr 6];Diagn Cytopathol. 2011 doi: 10.1002/dc.21667. [DOI] [PubMed] [Google Scholar]
- 18.Angiero F, Gatta LB, Seramondi R. et al. Frequency and role of HPV in the progression of epithelial dysplasia to oral cancer. Anticancer Res. 2010;30:3435–3440. [PubMed] [Google Scholar]
- 19.Hoffmann M, Gorogh T, Gottschlich S. et al. Human papillomaviruses in head and neck cancer: 8 year-survival-analysis of 73 patients. Cancer Lett. 2005;218:199–206. doi: 10.1016/j.canlet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem A. Dismissing links between HPV and aggressive tongue cancer in young patients. Ann Oncol. 2010;21:13–17. doi: 10.1093/annonc/mdp380. [DOI] [PubMed] [Google Scholar]
- 22.Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA. 2003;100:177–182. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pimenta JR, Zuccherato LW, Debes AA. et al. Color and genomic ancestry in Brazilians: a study with forensic microsatellites. Hum Hered. 2006;62:190–195. doi: 10.1159/000096872. [DOI] [PubMed] [Google Scholar]
- 24.Sobin LH. TNM: evolution and relation to other prognostic factors. Semin Surg Oncol. 2003;21:3–7. doi: 10.1002/ssu.10014. [DOI] [PubMed] [Google Scholar]
- 25.Broders AC. Squamous-cell epithelioma of the skin: a study of 256 cases. Ann Surg. 1921;73:141–160. doi: 10.1097/00000658-192102000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryne M, Nielsen K, Koppang HS, Dabelsteen E. Reproducibility of two malignancy grading systems with reportedly prognostic value for oral cancer patients. J Oral Pathol Med. 1991;20:369–372. doi: 10.1111/j.1600-0714.1991.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 27.Katiyar S, Hedau S, Jain N. et al. p53 gene mutation and human papillomavirus (HPV) infection in esophageal carcinoma from three different endemic geographic regions of India. Cancer Lett. 2005;218:69–79. doi: 10.1016/j.canlet.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Jabbar S, Strati K, Shin MK, Pitot HC, Lambert PF. Human papillomavirus type 16 E6 and E7 oncoproteins act synergistically to cause head and neck cancer in mice. Virology. 2010;407:60–67. doi: 10.1016/j.virol.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ocadiz-Delgado R, Marroquin-Chavira A, Hernandez-Mote R. et al. Induction of focal epithelial hyperplasia in tongue of young bk6-E6/E7 HPV16 transgenic mice. Transgenic Res. 2009;18:513–527. doi: 10.1007/s11248-009-9243-6. [DOI] [PubMed] [Google Scholar]
- 30.Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci USA. 2006;103:14152–14157. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coombs NJ, Gough AC, Primrose JN. Optimisation of DNA and RNA extraction from archival formalin-fixed tissue. Nucleic Acids Res. 1999;27:e12. doi: 10.1093/nar/27.16.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann U. MicroRNA-profiling in formalin-fixed paraffin-embedded specimens. Methods Mol Biol. 2010;667:113–125. doi: 10.1007/978-1-60761-811-9_8. [DOI] [PubMed] [Google Scholar]
- 33.Fischer CA, Zlobec I, Green E. et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Ordonez B, Beauchemin M, Jordan RC. Molecular biology of squamous cell carcinoma of the head and neck. J Clin Pathol. 2006;59:445–453. doi: 10.1136/jcp.2003.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakahara Y, Shintani S, Mihara M, Ueyama Y, Matsumura T. High frequency of homozygous deletion and methylation of p16(INK4A) gene in oral squamous cell carcinomas. Cancer Lett. 2001;163:221–228. doi: 10.1016/s0304-3835(00)00699-6. [DOI] [PubMed] [Google Scholar]
- 36.Reed AL, Califano J, Cairns P. et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630–3633. [PubMed] [Google Scholar]
- 37.Farias LC, Fraga CA, De Oliveira MV. et al. Effect of age on the association between p16CDKN2A methylation and DNMT3B polymorphism in head and neck carcinoma and patient survival. Int J Oncol. 2010;37:167–176. doi: 10.3892/ijo_00000664. [DOI] [PubMed] [Google Scholar]
- 38.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diniz MG, Borges ER, Guimaraes AL. et al. PTCH1 isoforms in odontogenic keratocysts. Oral Oncol. 2009;45:291–295. doi: 10.1016/j.oraloncology.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Gomes CC, Drummond SN, Guimaraes AL, Andrade CI, Mesquita RA, Gomez RS. P21/ WAF1 and cyclin D1 variants and oral squamous cell carcinoma. J Oral Pathol Med. 2008;37:151–156. doi: 10.1111/j.1600-0714.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Wang L, Wang LE, Sturgis EM, Wei Q. Polymorphisms of the DNMT3B gene and risk of squamous cell carcinoma of the head and neck: a case-control study. Cancer Lett. 2008;268:158–165. doi: 10.1016/j.canlet.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreira PR, Guimaraes MM, Guimaraes AL. et al. Methylation of P16, P21, P27, RB1 and P53 genes in odontogenic keratocysts. J Oral Pathol Med. 2009;38:99–103. doi: 10.1111/j.1600-0714.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 43.Wu MF, Cheng YW, Lai JC. et al. Frequent p16INK4a promoter hypermethylation in human papillomavirus-infected female lung cancer in Taiwan. Int J Cancer. 2005;113:440–445. doi: 10.1002/ijc.20597. [DOI] [PubMed] [Google Scholar]
- 44.Lin TS, Lee H, Chen RA. et al. An association of DNMT3b protein expression with P16INK4a promoter hypermethylation in non-smoking female lung cancer with human papillomavirus infection. Cancer Lett. 2005;226:77–84. doi: 10.1016/j.canlet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536–2545. doi: 10.1158/1055-9965.EPI-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munger K, Basile JR, Duensing S. et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 47.Nemes JA, Deli L, Nemes Z, Marton IJ. Expression of p16(INK4A), p53, and Rb proteins are independent from the presence of human papillomavirus genes in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:344–352. doi: 10.1016/j.tripleo.2005.10.069. [DOI] [PubMed] [Google Scholar]