Abstract
To validate the recently reported European Treatment and Outcomes Study (EUTOS) score, we applied it to 465 patients with early chronic phase chronic myeloid leukemia treated with standard-dose imatinib (n = 71), high-dose imatinib (n = 208), or second-generation tyrosine kinase inhibitors (n = 186), and assessed its ability to predict event-free survival (EFS), transformation-free survival (TFS), and overall survival (OS). The median follow-up was 69 months. The overall complete cytogenetic response and major molecular response rates were 92% and 85%, respectively. The 3-year EFS, TFS, and OS rates were 86%, 95%, and 97%, respectively. Of the 465 patients, 427 (92%) were in low EUTOS score category. There was no difference in the major molecular response, TFS, EFS, and OS rates between patients with low and high EUTOS score, overall and within specific therapies. In conclusion, 8% of patients with chronic phase chronic myeloid leukemia treated at our institution are in the high EUTOS score; in this population, the EUTOS score was not predictive for outcome.
Introduction
The introduction of the tyrosine kinase inhibitors (TKIs), which suppress the molecular processes driving chronic myeloid leukemia (CML), has revolutionized the management and prognosis in CML.1 Imatinib therapy induced high rates of complete cytogenetic response (CCyR) and major molecular response (MMR), and improved survival in CML.2–6 Second-generation TKIs (dasatinib, nilotinib) are more potent BCR-ABL inhibitors with demonstrated efficacy in patients resistant to or intolerant of imatinib.7,8 Dasatinib and nilotinib were first approved for patients resistant to or intolerant of prior imatinib therapy, are active against most BCR-ABL mutations with the exception of T315I, and have well-established safety profiles.9,10 Single-arm phase 2 studies11–13 suggested, and phase 3 randomized trials later confirmed, that dasatinib and nilotinib were superior to imatinib, inducing faster and higher rates of CCyR and molecular responses. Therefore, both drugs were granted FDA approval as initial therapy for patients with newly diagnosed CML in chronic phase (CML-CP).14,15
Until recently, the prognosis of patients with CML treated with TKI was based on scores developed in the chemotherapy and interferon era.16,17 The European LeukemiaNet has developed a new scoring system (European Treatment and Outcome Study [EUTOS] score) using data from 2060 patients with newly diagnosed CML-CP treated with imatinib-based regimens. The EUTOS score was reported to have superior prognostic power compared with the Sokal score.18 The EUTOS score using the percentage of basophils and spleen size divided patients in 2 groups of low- and high-risk patients with significant correlations with the achievement of an 18-month CCyR and progression-free survival.
The aims of this study were to validate the EUTOS score in an independent cohort of patients with early CML-CP referred to our institution and treated with TKIs, and to assess its ability to predict event-free survival (EFS), transformation-free survival (TFS), and overall survival (OS).
Methods
A total of 465 consecutive patients with newly diagnosed CML-CP were treated with imatinib 400 mg daily (n = 71), imatinib 800 mg daily (n = 208), and second-generation TKIs (n = 186: dasatinib n = 88, nilotinib n = 98) in sequential phase 2 trials. Entry criteria were similar for all trials. CML-CP was as previously defined.19 Patients were treated on University of Texas MD Anderson Cancer Center Institutional Review Board–approved protocols. Informed consent was obtained in accordance with the Declaration of Helsinki. Response criteria were as previously described.2 Conventional cytogenetic analysis was done in bone marrow cells using a G-banding technique. At least 20 metaphases were analyzed, and marrow specimens were examined on direct or short-term (24-hour) cultures. MMR was defined as a BCR-ABL/ABL transcript ratio of less than or equal to 0.1% (international scale). A complete molecular response was defined as undetectable transcripts with an assay with sensitivity of at least 4.5-log.5
The EUTOS score was defined by (7 × basophils) plus (4 × spleen size) where the spleen size was measured in centimeters below the costal margin and basophils as a percentage at baseline. A EUTOS score of more than 87 indicates high risk, and less than or equal to 87 low risk.18
EFS was measured from the start of treatment to the date of any of the following events: death from any cause at any time, loss of complete hematologic response, loss of major cytogenetic response, or progression to accelerated or blast phases. TFS was measured from the start of treatment to the date progression to accelerated or blast phases at any time, last follow-up, or death from any cause. Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test. Differences among variables were evaluated by the χ2 test and Mann-Whitney U test for categorical and continuous variables, respectively.
Results and discussion
A total of 465 patients were treated. The median (range) age was 47 years (15-85 years). The median (range) time from diagnosis to TKI therapy was 1 month (0-6 months), with 119 (25%) receiving previous cytoreduction therapy. The median (range) follow-up was 117 months (16-130 months) for the patients receiving standard-dose imatinib (n = 71), 88 months (4-118 months) for those receiving high-dose imatinib (n = 208), and 30 months (3-69 months) for those receiving second-generation TKI (n = 186). The median (range) basophil percentage at baseline was 3% (0%-19%), and the median (range) splenomegaly size was 0 cm (0-30 cm). A total of 319 patients (69%), 112 (24%), and 34 (7%) were in low, intermediate, and high Sokal score category, respectively.
The overall CCyR and MMR rates for the whole study group were 92% and 85%, respectively. The overall CCyR rates were 87%, 91%, and 95%, for patients treated with standard-dose imatinib, high-dose imatinib, and second-generation TKIs, respectively. The overall MMR rates were 78%, 86%, and 86%, respectively. The 3-year EFS, TFS, and OS rates for the whole group were 86%, 95%, and 97%, respectively. The 3-year EFS rates were 80%, 85%, and 91% for patients treated with standard-dose imatinib, high-dose imatinib, and second-generation TKIs, respectively. The 3-year TFS rates were 89%, 95%, and 99%, respectively. The 3-year OS rates were 93%, 97%, and 99%, respectively.
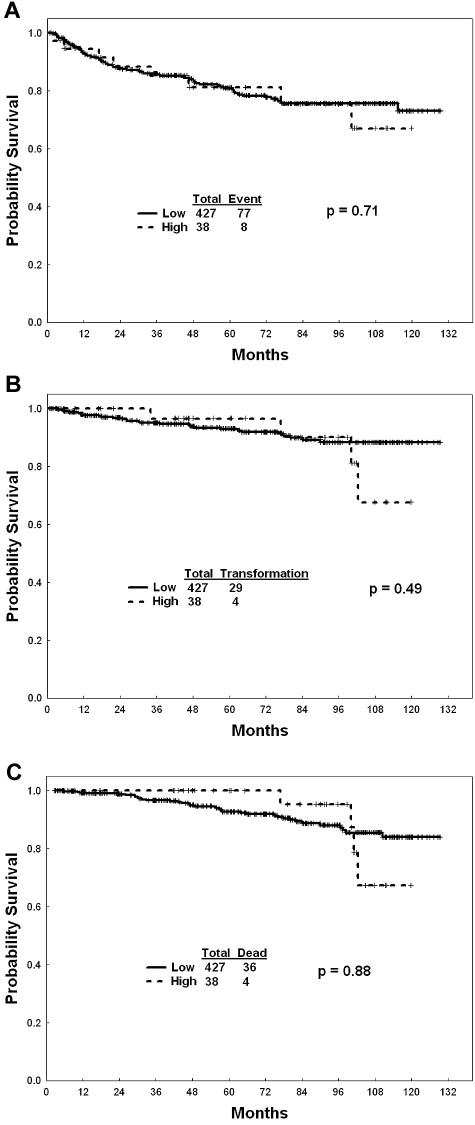
Overall, of the 465 patients, 427 (92%; 67 [94%] receiving standard-dose imatinib, 189 (91%) high-dose imatinib, 171 [92%] second-generation TKI) were in the low EUTOS score category (Table 1). Patients with a low EUTOS score had higher rates of CCyR at any time compared with patients with high EUTOS score (93% vs 81%, P = .02). This difference was mainly significant among patients receiving second-generation TKIs (P = .03) whereas it was not different among patients receiving imatinib (P = .27). There was no difference in the rates of MMR (85% vs 81%, P = .48) between patients with low and high EUTOS score. There was no difference in EFS, TFS, and OS rates between patients with low and high EUTOS score, overall and within specific therapies (Figure 1). The lack of difference was consistent whether patients were treated with imatinib or second-generation TKI. Similarly, there was no difference in overall outcome when applying the Sokal score (data not shown).
Table 1.
EUTOS score and outcome
| Parameter | Category | Overall (N = 465) | Low EUTOS score, 427 (92%) | High EUTOS score, 38 (8%) | P |
|---|---|---|---|---|---|
| CCyR, % | Overall (evaluable = 454) | 92 | 93 | 81 | .02 |
| Imatinib | 90 | 91 | 82 | .27 | |
| 400 mg | 87 | 86 | 100 | 1.0 | |
| 800 mg | 91 | 92 | 79 | .08 | |
| 2nd-TKI | 95 | 96 | 80 | .03 | |
| MMR, % | Overall (evaluable = 452) | 85 | 85 | 81 | .48 |
| Imatinib | 84 | 85 | 82 | .76 | |
| 400 mg | 78 | 77 | 100 | 1.0 | |
| 800 mg | 86 | 87 | 79 | .30 | |
| 2nd-TKI | 86 | 86 | 80 | .46 | |
| 3-year EFS, % | Overall | 86 | 86 | 86 | .71 |
| Imatinib | 84 | 84 | 77 | .20 | |
| 400 mg | 80 | 81 | 50 | .34 | |
| 800 mg | 85 | 85 | 83 | .29 | |
| 2nd-TKI | 91 | 90 | 100 | .16 | |
| 3-year TFS, % | Overall | 95 | 95 | 97 | .51 |
| Imatinib | 94 | 93 | 94 | .33 | |
| 400 mg | 89 | 90 | 67 | .47 | |
| 800 mg | 95 | 95 | 100 | .46 | |
| 2nd-TKI | 99 | 99 | 100 | .53 | |
| 3-year OS, % | Overall | 97 | 97 | 100 | .88 |
| Imatinib | 96 | 96 | 100 | .75 | |
| 400 mg | 93 | 92 | 100 | .34 | |
| 800 mg | 97 | 97 | 100 | .32 | |
| 2nd-TKI | 99 | 97 | 100 | .64 |
Sixty-seven patients (94%) receiving standard-dose imatinib, 189 (91%) receiving high-dose imatinib, and 171 (92%) receiving second-generation TKIs were in the low EUTOS score category.
2nd-TKI indicates second-generation TKI.
Figure 1.
EUTOS score and outcome. (A) EFS among patients with high- and low-risk EUTOS score. (B) TFS among patients with high- and low-risk EUTOS score. (C) OS among patients with high- and low-risk EUTOS score.
In our study groups, the EUTOS score was not predictive for overall MMR, TFS, EFS, and OS among patients in early chronic phase treated with imatinib and second-generation TKIs. Using the EUTOS scoring system, the proportion of high-risk patients in our study was similar to that recently reported in 2 studies18,20 where the rate of high-risk patients was approximately 10%. Compared with the previous studies, our analysis has the advantage of assessing, additionally, the impact of the EUTOS score among patients receiving second-generation TKI as frontline therapy. Unlike the report by Hasford et al,18 but similar to the report by Marin et al,20 the EUTOS score in our analysis did not predict for outcome of patients. This difference may be in part the result of different CML populations and possibly the size of our study population where, in the setting of treatment with high efficacy that potentially may overcome the prognostic impact of the disease burden at baseline, a larger number of patients may be needed to show significant difference. In addition, the EUTOS score, like the Sokal score, measures at least in part the disease burden and may not reflect the dynamic of the disease in response to TKI therapy. New prognostics models reflecting the disease biology and factors affecting response to TKI therapy are being assessed and may help better tailoring of upfront therapy.21
In conclusion, the EUTOS score did not predict for outcome in an independent cohort of patients with early chronic phase treated at our institution with imatinib and second-generation TKIs. New prognostic models are warranted in the modern era of TKI therapy.
Acknowledgments
This work was supported in part by the National Cancer Institute (grant P01CA049639).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J. designed the concept, analyzed data, and wrote and approved the manuscript; H.K. and J.C. designed the concept and wrote and approved the manuscript; S.O., A.Q.-C., A.N., and G.G.-M. provided materials and approved the manuscript; and S.P. analyzed data and approved the manuscript.
Conflict-of-interest disclosure: E.J. received honoraria from BMS and Novartis. H.K. and J.C. received research grants from Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Elias Jabbour, Department of Leukemia, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: ejabbour@mdanderson.org.
References
- 1.Jabbour E, Cortes JE, Giles FJ, O'Brien S, Kantarjian HM. Current and emerging treatment options in chronic myeloid leukemia. Cancer. 2007;109(11):2171–2181. doi: 10.1002/cncr.22661. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 4.Deininger M, O'Brien SG, Guilhot F, et al. International randomized study of interferon vs. STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract]. Blood. 2009;114 Abstract 1126. [Google Scholar]
- 5.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11(9):3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108(6):1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 7.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 9.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 11.Cortes JE, Jones D, O'Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes JE, Jones D, O'Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114(24):4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 14.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 16.Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa: Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90(11):850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 17.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–799. [PubMed] [Google Scholar]
- 18.Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118(3):686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Talpaz M, O'Brien S, et al. Imatinib mesylate for Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-alpha. Clin Cancer Res. 2002;8(7):2177–2187. [PubMed] [Google Scholar]
- 20.Marin D, Ibrahim AR, Goldman JM. European Treatment and Outcome Study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol. 2011;29(29):3944–3945. doi: 10.1200/JCO.2011.37.6962. [DOI] [PubMed] [Google Scholar]
- 21.White DL, Hughes TP. Predicting the response of CML patients to tyrosine kinase inhibitor therapy. Curr Hematol Malig Rep. 2011;6(2):88–95. doi: 10.1007/s11899-011-0087-9. [DOI] [PubMed] [Google Scholar]