Abstract
The purpose of this study was to confirm the correlation between serum levels of cystatin C (Cys-C) and those of creatinine (Cre), the most widely used index of renal function in clinical practice, and to evaluate serum levels of Cys-C, with a particular focus on the effects of aging, body mass index (BMI), C-reactive protein (CRP) and disease extent in patients with lung cancer. Serum Cys-C levels in a total of 39 patients (median 72 years), who were diagnosed as having lung cancer were analyzed retrospectively. The serum Cys-C was determined using colloidal gold particles coated with anti-Cys C antibodies. A significant correlation coefficient was found between serum levels of Cys-C and those of Cre (P=0.001), but only moderate agreement. Serum Cys-C levels increased in an age-dependent manner with significant differences among 3 age groups: patients younger than 65 years, those aged 65 to 74 years and those aged 75 years or older (P=0.001). However, no correlation was observed between Cys-C and BMI, Cys-C and CRP, or Cys-C and disease extent of lung cancer. Estimation of renal function is important since renal insufficiency is directly correlated to increased chemotherapeutic complications in oncological practice. Thus, estimation of serum Cys-C levels may be important, but adjustment of Cys-C for age should be taken into consideration.
Keywords: cystatin C, elderly, lung cancer
Introduction
Cystatin C (Cys-C) is a non-glycosylated, low molecular weight basic protein containing 120 amino acids (1). Human Cys-C is a housekeeping gene, and serum Cys-C is steadily produced by all human nucleated cells (1). In earlier studies, Cys-C was evaluated to be independent of age, muscle mass or body mass index (BMI) in healthy individuals (2,3). This finding is in contrast to serum creatinine (Cre), the most widely used index of renal function in clinical practice, which is not accurate in the elderly since Cre production decreases as muscle mass decreases with age. The accuracy of urinary Cre clearance (CrCl) is affected by the problem of incomplete urine collections, particularly in older individuals (4,5). In a recent review by Van Pottelbergh et al serum levels of Cys-C appear to be a promising method for estimating the glomerular filtration rate (GFR); however, estimating the GFR on the basis of serum Cys-C levels has not been extensively studied in the elderly (6). Earlier reports have indicated that the production rate of Cys-C is stable and does not change in inflammatory conditions (7,8). However, findings of recent reports have shown an association between serum Cys-C levels and inflammatory biomarkers such as C-reactive protein (CRP) (9,10). For example, Keller et al have shown that Cys-C has a linear association with inflammatory biomarkers in an ambulatory elderly cohort with estimated GFRs of more than 60 ml/min (9). Singh et al reported that Cys-C concentrations have moderate associations with CRP and fibrinogen that are not dependent on CrCl (10). In addition, results of recent studies have shown a significant correlation between serum Cys-C levels and malignant progression in colorectal cancer, melanoma and ovarian cancer (11–13), although earlier studies reported that serum Cys-C levels were not affected by the presence of malignancies (14,15). With regard to serum Cys-C levels in patients with lung cancer, there is only one report by Naumnik et al (16). These authors reported that no correlation exists between serum Cys-C levels and staging of the disease (16).
The purpose of this study was to confirm a correlation between serum Cys-C levels and those of Cre, and to evaluate serum Cys-C levels with a particular focus on the effects of aging, BMI, CRP and disease extent in patients with lung cancer.
Patients and methods
Patients
A total of 39 patients with newly diagnosed lung cancer who were admitted to the Division of Respiratory Medicine, Mito Medical Center, Mito Kyodo General Hospital, University of Tsukuba, Japan, between April 2010 and March 2011 were analyzed retrospectively. For each patient, the diagnosis of lung cancer was confirmed with pathological and/or cytological specimens. Pathological and/or cytological diagnosis was defined by the World Health Organization (WHO) classification, and patients were staged according to the 2010 UICC TNM system (17). Age, height, actual body weight and gender were recorded at the initial visit.
Given that lung cancer rates, or the need to undergo chemotherapy, increase proportionately to increasing age (18), we evaluated serum levels of Cys-C, which are not necessarily studied in sufficient detail, particularly in these patients. Therefore, in this study, patients were divided into 3 age groups: patients <65 years, those aged 65–74 years and those aged ≥75 years. For the analysis of serum Cys-C levels and the extent of lung cancer, we divided the patients into 2 groups: advanced and non-advanced groups. The advanced group included patients with carcinomatous pleuritis/pericarditis and/or distant metastasis. The non-advanced group included patients with neither carcinomatous pleuritis/pericarditis nor distant metastasis.
This study was approved by the institutional ethics committee of Mito Medical Center, University of Tsukuba. Written informed consent was obtained from all the patients.
Cys-C, Cre and CRP
Serum levels of Cys-C in patients with lung cancer were evaluated using an automated analyzer, JCA-BM 8020 (JEOL, Tokyo, Japan) using colloidal gold particles coated with anti-Cys-C antibodies (Alfresa Pharma, Osaka, Japan) (19). Serum Cre and CRP were measured using the enzymatic method.
Statistical analysis
The Mann-Whitney U test or Kruskal- Wallis test was applied to elucidate the difference between 2 or more independent groups. To evaluate the correlation between 2 independent groups, the Spearman’s rank correlation was used. Statistical analyses were performed using SPSS 10.1 for Windows (SPSS, Chicago, IL, USA), and a probability value <0.05 was considered to be statistically significant.
Results
Patient characteristics
The clinicopathological characteristics of the lung cancer patients are shown in Table I. Of the 39 patients, 66.7% (n=26) were male. The median age was 72 years (range 53–86). Of the 39 patients, 17 (43.6%) patients were ≥75 years of age. The lung cancers comprised 31 non-small cell carcinomas and 8 small cell carcinomas. Seventeen patients were at an advanced clinical stage, and 22 patients were at a non-advanced stage.
Table I.
Characteristics of 39 patients with lung cancer.
| Age (years) | Median 72, range 53–86 |
| <65 | 10 (25.6%) |
| 65–74 | 12 (30.8%) |
| ≥75 | 17 (43.6%) |
| Gender | |
| Male | 26 (66.7%) |
| Female | 13 (33.3%) |
| Performance status | |
| 0–1 | 31 (79.5%) |
| 2–4 | 8 (20.5%) |
| Histology | |
| Adenocarcinoma | 21 (53.8%) |
| Small cell carcinoma | 8 (20.5%) |
| Squamous cell carcinoma | 7 (17.9%) |
| Large cell carcinoma | 2 (5.1%) |
| Adenosquamous cell carcinoma | 1 (2.6%) |
| Clinical stage | |
| Non-advanced (stage IA-IIIB) | 22 (56.4%) |
| Advanced (stage IV) | 17 (43.6%) |
Cys-C and Cre
A significant correlation coefficient was found between serum levels of Cys-C and those of Cre (P=0.001). However, a Spearman’s ρ of 0.693 indicated only a moderate agreement between these levels.
Cys-C correlated with age
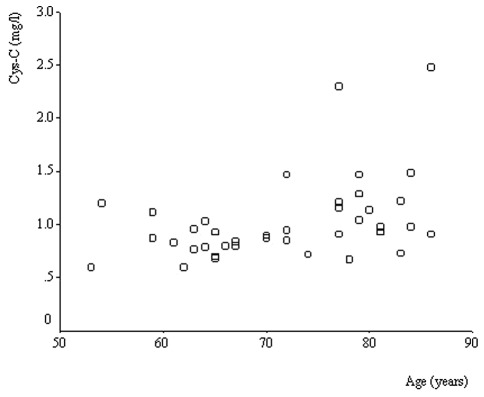
Serum Cys-C levels increased in an age-dependent manner, with significant differences among the 3 age groups (P=0.001, Kruskal-Wallis test; Fig. 1). In the 39 lung cancer patients, a significant correlation coefficient was found between serum Cys-C levels and age (Spearman’s ρ=0.452, P=0.004; Fig. 2).
Figure 1.
Serum levels of Cys-C and age. Serum levels of Cys-C increased in an age-dependent manner with significant differences (P=0.007, Kruskal-Wallis test). Cys-C, cystatin C.
Figure 2.
Correlation between the serum levels of Cys-C and age in 39 lung cancer patients. The correlation coefficient between them was significant (Spearman’s ρ=0.452, P=0.004). Cys-C, cystatin C.
Cys-C and BMI, and CRP
No significant correlation coefficient was found between the serum Cys-C levels and BMI (Spearman’s ρ=0.075), nor between serum Cys-C levels and CRP (Spearman’s ρ=0.370).
Cys-C and disease extent
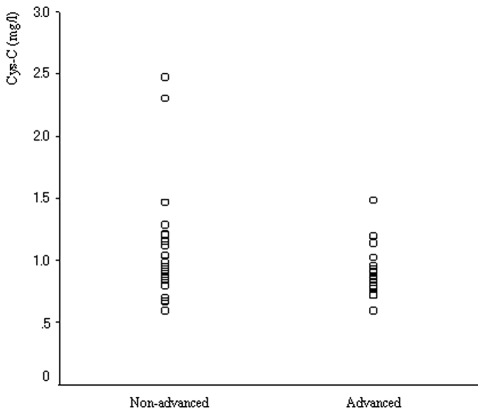
No statistical difference was found between Cys-C serum levels in the 17 patients of the advanced group and those in the 22 patients of the non-advanced group (P=0.123; Fig. 3).
Figure 3.
Serum levels of Cys-C in 17 advanced lung cancer patients with carcinomatous pleuritis and/or pericarditis, and/or metastatic disease were identical to those in 22 lung cancer patients without carcinomatous pleuritis and/or pericarditis or distant metastases. Cys-C, cystatin C.
Discussion
Cys-C is a non-glycosylated, low-molecular-weight (13 kDa) basic protein, which is produced by all nucleated cells. This protein is a member of the cystatin superfamily of cysteine protease inhibitors. The structure of the Cys-C gene and its promoter is of the ‘housekeeping type’, which is compatible with stable production of Cys-C (1). Serum Cys-C levels have been proposed to be an ideal marker of the GFR (3), and these levels are not dependent on muscle mass (20). Therefore, the serum level of Cys-C may be a better marker of GFR than serum Cre (2,3). Ognibene et al proposed 2 different age-related reference intervals for Cys-C: below and over the age of 45 (21). Their results revealed that more studies are required for the evaluation of serum Cys-C in the elderly. Van Den Noortgate et al evaluated and compared the concentration of serum Cys-C with markers of GFR in 48 elderly patients, including 25 patients aged 85 years or older. The results of these authors revealed that serum Cys-C, serum Cre, urinary CrCl, the Cockcroft-Gault formula and the MDRD formula were comparable markers of renal function in the overall older population (22). In a recent review, Van Pottelbergh et al indicated that evidence regarding the use of serum levels of Cys-C in elderly patients is limited (6). In addition, other investigators have reported the possibility of Cys-C as a marker of inflammation due to the significant association between serum Cys-C and CRP (23,24). Knight et al investigated factors other than renal function that have an impact on serum Cys-C levels and observed that certain factors, including high CRP levels, were independently associated with serum Cys-C levels (25). These investigators suggested that serum Cys-C is affected by factors other than renal function alone (25). By contrast, a recent report by Grubb et al evaluating CRP in patients with scheduled elective surgery, revealed that Cys-C was not affected by inflammation (26). In the present study, serum Cys-C levels were significantly correlated with age in very elderly lung cancer patients. Our results have also shown that serum Cys-C levels were correlated with Cre serum levels, although a Spearman’s ρ of 0.693 indicated only moderate agreement between these levels. In addition, no correlation was found between serum Cys-C levels and BMI, nor between serum Cys-C levels and CRP. We hypothesized that the correlation between serum Cys-C levels and age may be due to not only a physiological change, but also to other factors such as effects of comorbid diseases. The reason for the increase in serum Cys-C levels and age remains to be clarified.
Whether serum Cys-C is elevated in patients with malignant disease and whether serum Cys-C is correlated to tumor extent are also controversial aspects (13). Kos et al reported that serum Cys-C levels were significantly higher in patients with advanced melanoma and colorectal cancer than in patients with primary melanoma and healthy controls (11,12). Other investigators, however, reported that Cys-C is not influenced by tumor extent (13). In malignancy, an imbalance between cysteine proteases and their inhibitors, associated with metastatic tumor cell phenotype, is thought to facilitate tumor cell invasion and metastasis (27). Thus, the extent of malignancy may be thought to affect Cys-C levels. In the present study, no significant difference was found in the serum levels of Cys-C between advanced and non-advanced lung cancer patients, which was consistent with the findings in 40 non-small cell lung cancer patients reported by Naumnik et al (16). Whether the significant correlation between serum Cys-C levels and tumor extent is dependent on the original site of the cancer is beyond our current knowledge. Further large-sized prospective studies are required to clarify the reason for the significant correlation.
Despite its novel findings, the current study has a number of limitations. First, this was a retrospective study, and our findings, obtained from a limited number of patients, were neither population-based nor case-controlled. Second, the majority of the lung cancer patients included in this study were very elderly, albeit unselected and consecutive. Nevertheless, we believe that it is of note to report serum levels of Cys-C in unselected groups of elderly lung cancer patients. In the present study, we confirmed the importance of age as a factor in influencing serum Cys-C levels in patients with lung cancer.
In oncological practice, estimation of renal function at the bedside is crucial since renal insufficiency is directly correlated with increased chemotherapeutic complications. Estimation of the serum Cys-C levels may be important, but adjustment of Cys-C for age should be taken into consideration.
References
- 1.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function - a review. Clin Chem Lab Med. 1999;37:389–395. doi: 10.1515/CCLM.1999.064. [DOI] [PubMed] [Google Scholar]
- 2.Finney H, Bates CJ, Price CP. Plasma cystatin C determinations in healthy elderly population. Arch Gerontol Geriatr. 1999;29:75–94. doi: 10.1016/s0167-4943(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 3.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg TH, Finkelstein MS. Difficulties in estimating glomerular filtration rate in the elderly. Arch Intern Med. 1987;147:1430–1433. [PubMed] [Google Scholar]
- 5.Friedman JR, Norman DC, Yoshikawa TT. Correlation of estimated renal function parameters versus 24-hour creatinine clearance in ambulatory elderly. J Am Geriatr Soc. 1989;37:145–149. doi: 10.1111/j.1532-5415.1989.tb05873.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Pottelbergh G, Van Heden L, Matheï C, Degryse J. Methods to evaluate renal function in elderly patients: a systematic literature review. Age Ageing. 2010;39:542–548. doi: 10.1093/ageing/afq091. [DOI] [PubMed] [Google Scholar]
- 7.Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (c trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985;45:97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 8.Grubb A, Simonsen O, Sturtfelt G, Truedson L, Thysell H. Serum concentration of cystatin C, factor D and β2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand. 1985;218:499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 9.Keller CR, Odden MC, Fried LF, et al. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int. 2007;71:239–244. doi: 10.1038/sj.ki.5002042. [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Whooley MA, Ix JH, Ali S, Shlipak MG. Association of cystatin C and estimated GFR with inflammatory biomarkers: the Heart and Soul Study. Nephrol Dial Transplant. 2007;22:1087–1092. doi: 10.1093/ndt/gfl744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kos J, Krasovec M, Cimerman N, Nielsen HJ, Christensen IJ, Brünner N. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000;6:505–511. [PubMed] [Google Scholar]
- 12.Kos J, Stabuc B, Schweigen A, et al. Cathepsins B, H, and L and their inhibitors stefin A and cystatin C in sera of melanoma patients. Clin Cancer Res. 1997;3:1815–1822. [PubMed] [Google Scholar]
- 13.Bodnar L, Wcislo GB, Smoter M, et al. Cystatin C as a parameter of glomerular filtration rate in patients with ovarian cancer. Kidney Blood Press Res. 2010;33:360–367. doi: 10.1159/000319097. [DOI] [PubMed] [Google Scholar]
- 14.Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 15.Stabuc B, Vrhovec L, Stabuc-Silih M, Cizej TE. Improved prediction of decreased creatinine clearance by serum cystatin C: use in cancer patients before and during chemotherapy. Clin Chem. 2000;46:193–197. [PubMed] [Google Scholar]
- 16.Naumnik W, Niklińska W, Ossolińska M, Chyczewska E. Serum cathepsin K and cystatin C concentration in patients with advanced non-small-cell lung cancer during chemotherapy. Folia Histochem Cytobiol. 2009;47:207–213. doi: 10.2478/v10042-009-0024-0. [DOI] [PubMed] [Google Scholar]
- 17.Lababede O, Meziane M, Rice T. Seventh edition of the cancer staging manual and stage grouping of lung cancer: quick reference chart and diagrams. Chest. 2011;139:183–189. doi: 10.1378/chest.10-1099. [DOI] [PubMed] [Google Scholar]
- 18.Satoh H, Kurishima K, Nakamura R, et al. Lung cancer in patients aged 80 years and over. Lung Cancer. 2009;65:112–118. doi: 10.1016/j.lungcan.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Matsuo K, Enomoto M, Mizuno K. A sol particle homogeneous immunoassay for measuring serum cystatin C. Clin Biochem. 2004;37:27–35. doi: 10.1016/j.clinbiochem.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive measurement of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 21.Ognibene A, Mannucci E, Caldini A, et al. Cystatin C reference values and aging. Clin Biochem. 2006;39:658–661. doi: 10.1016/j.clinbiochem.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Van Den Noortgate NJ, Janssens WH, Delanghe JR, Afschrift MB, Lameire NH. Serum cystatin C concentration compared with other markers of glomerular filtration rate in the old old. J Am Geriatr Soc. 2002;50:1278–1282. doi: 10.1046/j.1532-5415.2002.50317.x. [DOI] [PubMed] [Google Scholar]
- 23.Reed CH. Diagnostic applications of cystatin C. Br J Biomed Sci. 2000;57:323–329. [PubMed] [Google Scholar]
- 24.Curhan G. Cystatin C: a marker of renal function or something more. Clin Chem. 2005;51:293–294. doi: 10.1373/clinchem.2004.044388. [DOI] [PubMed] [Google Scholar]
- 25.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 26.Grubb A, Björk J, Nyman U, et al. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest. 2011;71:145–149. doi: 10.3109/00365513.2010.546879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sloane BF. Suicidal tumor proteases. Nat Biotechnol. 1996;14:826–827. doi: 10.1038/nbt0796-826b. [DOI] [PubMed] [Google Scholar]