Abstract
We report naturally occurring, systematic variations in synaptic strength at neuromuscular junctions along the dorsal-ventral (D-V) axis of the Drosophila larval body wall. These gradual changes were correlated with differences in presynaptic neurotransmitter release regulated by nerve terminal excitability and in postsynaptic receptor composition influencing miniature excitatory junctional potential (mEJP) amplitude. Surprisingly, synaptic strength and D-V differentials at physiological Ca2+ levels were not significantly altered in slowpoke (slo) and Shaker (Sh) mutants, despite their defects in two major repolarizing forces, Ca2+-activated Slo (BK) and voltage-activated Sh currents, respectively. However, lowering [Ca2+]o levels revealed greatly altered synaptic mechanisms in these mutants, indicated by drastically enhanced EJPs in Sh but paradoxically reduced EJPs in slo. Removal of Sh current in slo mutants by 4-aminopyridine blockade or by combining slo with Sh mutations led to strikingly increased synaptic transmission, suggesting upregulation of presynaptic Sh current to limit excessive neurotransmitter release in the absence of Slo current. In addition, slo mutants displayed altered immunoreactivity intensity ratio between DGluRIIA and DGluRIIB receptor subunits. This modified receptor composition caused smaller mEJP amplitudes, further preventing excessive transmission in the absence of Slo current. Such compensatory regulation was prevented by rutabaga (rut) adenylyl cyclase mutations in rut slo double mutants, demonstrating a novel role of rut in homeostatic plasticity, in addition to its well-established function in learning behavior.
Keywords: Synaptic strength, dorsal-ventral differentials, rutabaga adenylyl cyclase, cAMP, Membrane excitability, Postsynaptic receptor composition
INTRODUCTION
Natural variations in synaptic strength, or differential responses of postsynaptic targets to presynaptic inputs, play crucial roles in matching the efficacy of individual synapses with different levels of activity demands. This mechanism is important for precise information transfer in neural circuits, in particular to support the functional organization of effectors in the various body plans. A well-studied example is the tonotopic processing of auditory information. There is a gradient of Ca2+-activated BK or Slowpoke (Slo) K+ channel-mediated IKCa in sensory hair cells controlling tuning frequency along the base-to-apex axis of the cochlea (Fettiplace and Fuchs, 1999). In addition, differential expression of Shaker (Sh) voltage-activated K+ (Kv1) channels contributes to tonotopic processing of auditory information corresponding to a gradient of membrane excitability and synaptic transmission in the chicken nucleus magnocellularis (Fukui and Ohmori, 2004) and in the rat lateral superior olive (Barnes-Davies et al., 2004). These examples highlight the importance of K+ channels in establishing natural gradients of neuronal activity and synaptic strength.
Another important aspect of regulation of synaptic strength is homeostatic maintenance of synaptic strength in individual neurons within a particular circuit. Neurons are able to restore proper synaptic functions when the neural circuits are destabilized following perturbations in membrane excitability, in neurotransmitter release machinery, in postsynaptic receptor functions, or in synaptic growth. For example, chronic blockade by TTX or enhancement by GABA receptor antagonists of mammalian neuronal activity in culture leads to a compensatory increase or decrease in postsynaptic response amplitude via regulation of AMPA receptor accumulation (O’Brien et al., 1998, Turrigiano et al., 1998). Similar homeostatic regulation occurs at Drosophila neuromuscular junctions. A change in postsynaptic activity by expressing a persistent outward K+ current, causing a hyperpolarized resting potential, results in a compensatory increase in presynaptic neurotransmitter release (Paradis et al., 2001). Mutations that decrease postsynaptic receptor density lead to an increase in presynaptic neurotransmitter release (Petersen et al., 1997). In addition, compensatory increases in synaptic areas and active zone numbers are induced by reduced number of synaptic varicosities to match the demand of target muscles (Stewart et al., 1996).
It has not been established how differential synaptic strength according to different body plans is related to homeostatic mechanisms following perturbations of synaptic strength. Here we document naturally occurring variations in synaptic strength along the dorsal-ventral (D-V) body axis in the body wall of Drosophila larvae that correlate with differential expression of Sh K+ currents affecting presynaptic neurotransmitter release and with regulation of postsynaptic receptor composition. Mutational and pharmacological analyses reveal that, in the absence of major repolarizing Slo K+ channels, synaptic strength and its D-V differentials are still maintained homeostatically through presynaptic upregulation of Sh currents and postsynaptic alterations in receptor composition. Mutations in rutabaga (rut) adenylate cyclase (AC) prevent these homeostatic adjustments in rut slo double mutants, indicating that cAMP is central to the homeostatic plasticity in addition to its well-known roles in behavioral plasticity.
EXPERIMENTAL PROCEDURES
Fly stocks
The fly stocks used in this study include: wild type (WT) Canton-S (CS); K+ channel mutants, including slowpoke (slo) (slo1, slo98, and slo4), Shaker (Sh) (ShM and Sh133), and Sh;;slo (Sh133;;slo1 and ShM;;slo98), and AC mutants rut (rut1 and rut1084) and rut slo (rut1;;slo1 and rut1;;slo1/slo4). Data collected from different alleles of slo and Sh indicate similar physiological phenotypes (see Table S1 in the Supplement Data). Thus, results from the different alleles are combined in analysis to increase statistical power. All these stocks were raised in the presence of conventional fly medium and maintained at room temperature.
Preparations and Electrophysiology
Preparation of wandering third instar larvae and intracellular recordings of excitatory junctional potentials (EJPs) were performed as described previously (Ueda and Wu, 2006). Evoked EJPs upon segmental nerve stimulation were recorded from abdominal segments 3 through 6, mostly from 4th and 5th segments, in HL3.1 saline (Feng et al., 2004) containing 0.2 mM of Ca2+, unless specified. For the analysis of D-V disparity, EJPs were sampled from multiple muscles in a single hemisegment (mostly from the 4th and 5th abdominal segments) per larva (Fig. 1B) to ensure stable muscle conditions throughout the experiment. The EJP amplitude from muscles 6/7 (V) was divided by that from muscle 1/9 (D) within the same hemisegment of body wall to calculate the EJP ratio (V/D). Miniature EJPs (mEJPs) with the resting membrane potential lower than −60 mV were analyzed by MiniAnalysis software (Synaptosoft Inc., Fort Lee, NJ, USA). Quantal content was calculated either by dividing the mean EJP amplitude by the mean mEJP amplitude at 0.2 mM Ca2+, or by calculating the log (1/failure rate) during 200 consecutive stimuli in the presence of 0.1 mM Ca2+. The correction procedure for nonlinear summation of synaptic potential (Martin, 1955) was applied for EJP ratio (V/D), quantal content, and drug-induced EJP change. All EJP data were collected using CLAMPEX (version 5.5, Molecular Devices Corporation, Sunnyvale, CA, USA) and analyzed using CLAMPFIT (version 6.0, Molecular Devices Corporation) and Origin software (version 6.0, OriginLab Corporation, Northampton, MA, USA). For selective blockade of Slo and Sh K+ channel function, ChTx (50 nM, Alomone Labs, Ltd., Jerusalem, Israel) and 4-AP (0.2 mM, otherwise indicated, Sigma-Aldrich Corp., St. Louis, MO, USA), respectively, were applied in the bath saline at least for 2 min before data collection.
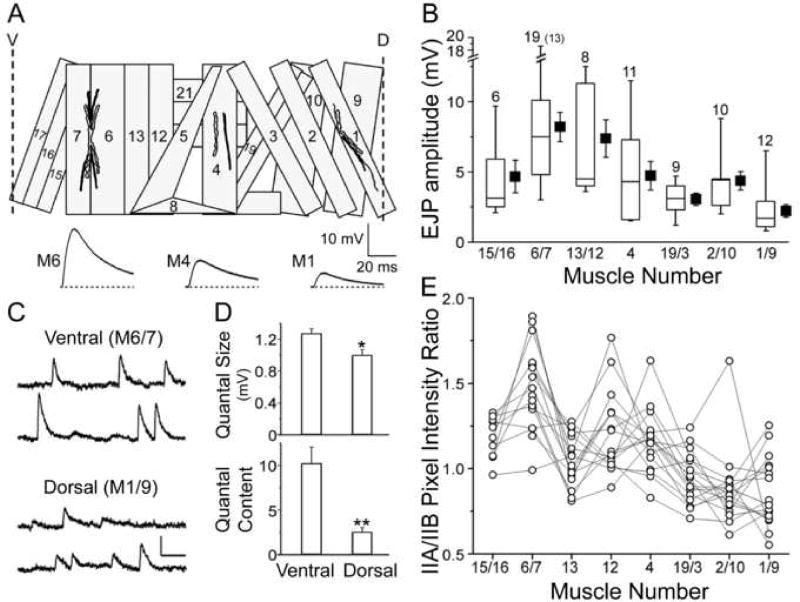
Fig. 1. Naturally occurring dorsal-ventral disparity in excitatory junctional potential (EJP) amplitudes correlated with differential regulation of presynaptic neurotransmitter release, postsynaptic receptor composition, and mEJP amplitudes.
(A) Larval body-wall musculature within a single hemisegment from the ventral (V) to dorsal (D) midline of a third instar larva, with neuromuscular junctions (NMJs, isomorphic) on muscles 6, 4, and 1 and corresponding representative EJPs. The numbering system for the identified muscles is shown. (B) Pooled EJP amplitude data from muscles in the abdominal hemisegments are shown in the box plots together with Mean ± SEM values with respect to their locations along the D-V axis. For each larva, 5 to 8 muscles in a single hemisegment are sampled. The total number of muscle fibers is indicated (equivalent to the number of larvae except for muscles 6/7, 19 muscle fibers from 13 larvae). The box plot in this and following figures represents 25 through 75% with median and 5 and 95% indicated by bars. (C) Two representative traces of spontaneous mEJPs in WT are shown for ventral (M6/7) and dorsal (M1/9) NMJs. Scale bar, 1 mV and 100 ms. (D) Average quantal size (mEJP amplitude) (top) and quantal content (bottom) at ventral (M6/7) and dorsal (M1/9) NMJs in WT. Note a significantly smaller quantal size and quantal content at dorsal NMJs. *, P<0.05 and *, P<0.01, t-test for ventral vs. dorsal NMJs. (E) Relative ratio of DGluRIIA over DGluRIIB (IIA/IIB) immunoreactivity visualized by double-labeling at the same type Ib NMJs reveals gradual changes along the D-V axis, resembling the D-V profile of EJP amplitude shown in (B) (see Experimental procedures). 17 hemisegments in 5 larvae.
Immunohistochemistry
Dissected larvae were fixed with either 100% ice-cold methanol for 5 min for immunostaining of DGluRIIA, otherwise with 3.7% formaldehyde in phosphate buffer saline for 20 min. The primary antibodies used include: monoclonal FasII (1D4, 1:4, Developmental Studies Hybridoma Bank (DSHB), Univ. of Iowa) and DGluRIIA antibody (8B4D2, 1:10, DSHB, Univ. of Iowa), and rabbit polyclonal Dlg (1:20,000, Dr. V. Budnik at Univ. of Massachusetts, Worchester), DGluRIIB (1:1000) and DGluRIIC (1:2000) antibody (Dr. A. DiAntonio at Washington Univ., St. Louis). The fluorescent secondary antibodies at 1:50 or 1:200 were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). For direct comparison of DGluRIIA, DGluRIIB, and DGluRIIC density among different genotypes, all larvae were treated within single tubes and washout chambers throughout the entire procedures.
Imaging and Analysis
Images of NMJs on muscles 6/7 (ventral) and 1(dorsal) from abdominal segments 3 through 6 were collected using a M1024 confocal microscope (BioRad, Hercules, CA, USA) with an oil-immersed 60X objective. The average pixel intensity of fluorescent signals was measured from the collapsed Z-stacked images using Image J software (NIH image, USA) by outlining the boundary of the entire type Ib boutons (Atwood et al., 1993). The boundary was determined by enclosing the area of pixel intensity at least 30% beyond the background level. The D/V ratio of pixel intensity was then calculated from the pixel intensity measured from dorsal (M1) and ventral (M6/7) NMJs within the same hemisegment.
For direct comparison across genotypes of DGluRII subunit density, settings for exposure and reading were fixed for all larvae in the same batch processed and stained simultaneously. The intensity readings were normalized to the average value from the ventral NMJs of WT (see Figs. 4 and S1 in the Supplement Data). The DGluRIIA/DGluRIIB (IIA/IIB) ratio was compared between double-labeled ventral and dorsal NMJs and across genotypes.
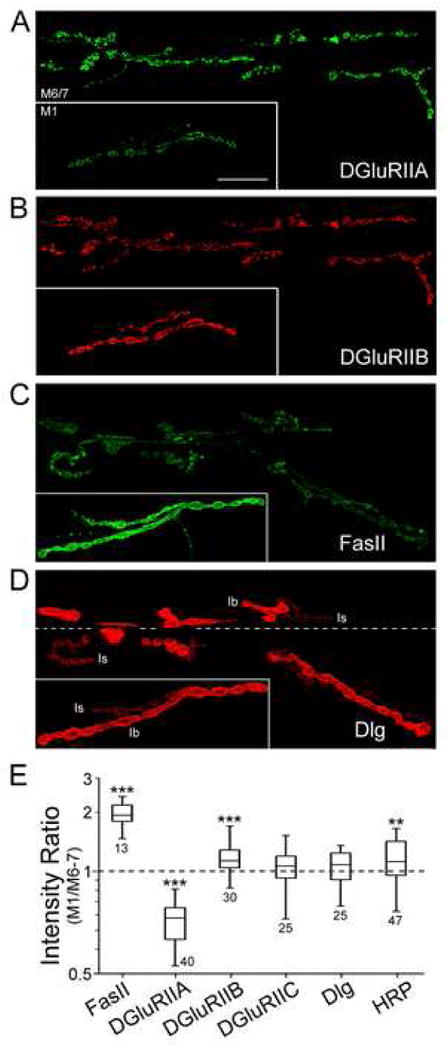
Fig. 4. Altered postsynaptic receptor composition and synaptic protein density in slo mutants.

Immunoreactivity against FasII (A1 and A2), DGluRIIA (B1 and B2), and Dlg (C1 and C2) in slo and Sh mutants is shown for both ventral (M6/7) and dorsal (M1) NMJs. (D) Ratio of pixel intensity (M1/M6–7) among WT, slo, and Sh. Note a significant difference in the FasII, DGluRIIA, and Dlg ratios in slo compared with WT and/or Sh. The number of NMJs examined in WT, slo, and Sh: 13, 18, and 8 for FasII; 40, 28, and 21 for DGluRIIA; 30, 18, and 17 for DGluRIIB; 25, 12, and 4 for DGluRIIC; 25, 18, and 6 for Dlg; 47, 27, and 35 for HRP. WT data from Fig. 3 are shown as reference. (E) Decrease in the DGluRIIA/IIB (IIA/IIB) ratio in slo NMJs. The IIA/IIB ratio from DGluRIIA and DGluRIIB double staining experiments is normalized to that in WT ventral NMJs (dashed lines). The number of ventral and dorsal NMJs examined: 15 and 15 for WT, 20 and 19 for slo, and 20 and 20 for Sh. Mean ± SD in D and E. ***, P<0.001, *, P<0.05, one-way ANOVA.
Statistical Analyses
For comparison between ventral and dorsal NMJs within a genotype, or between two genotypes, two-tailed t-test was routinely carried out against the null hypothesis of equal values between NMJs or genotypes. When each value was compared among more than two genotypes, one-way ANOVA was carried out with the tests for equal variance and multiple comparisons. In both cases, P-value less than 0.05 was considered to be significantly different. All statistical analyses were performed using Origin software (version 6.0, OriginLab Corporation).
RESULTS
Naturally occurring, systematic variations in synaptic strength along the dorsal-ventral body axis and the potential pre- and post-synaptic mechanisms
Neuromuscular junctions (NMJs) formed in the body-wall musculature of the third instar larvae of Drosophila have been one of the best-studied systems for synaptic transmission. The arrangement of body-wall muscles in each abdominal hemisegment is well characterized, consisting of 30 muscles in each segment (Fig. 1A). Each muscle is poly-innervated by multiple axons that form synaptic boutons along terminal branches, which differ in their shape and size (Johansen et al., 1989). Among these muscles, ventral longitudinal muscles including muscles 7, 6, 13, and 12 have been most frequently utilized for the effects of mutations on synaptic responses or excitatory junctional potentials (EJPs). In contrast, synaptic transmission in other muscles in the hemisegment, including the dorsal musculature, has not been described in detail.
In this study, we found a systematic variation in evoked synaptic responses among NMJs of the identified muscles within a single hemisegment of WT larvae. Synaptic strength indicated by EJP amplitude at 0.2 mM [Ca2+]o in HL3.1 saline was consistently greater in ventral muscles, represented by muscle 6, than in dorsal muscles, represented by muscle 1 (Fig. 1A). The pooled data from abdominal longitudinal muscles (Fig. 1B, 6 to 13 larvae) show a trend of increasing EJP amplitudes from dorsal to ventral midlines. Notably, the diagonal muscles closest to the ventral midline, muscles 15 and 16, actually displayed EJPs of smaller amplitudes.
Fig. 6. Contribution of Sh current in the natural D-V disparity in synaptic strength and its homeostatic upregulation in slo mutants.
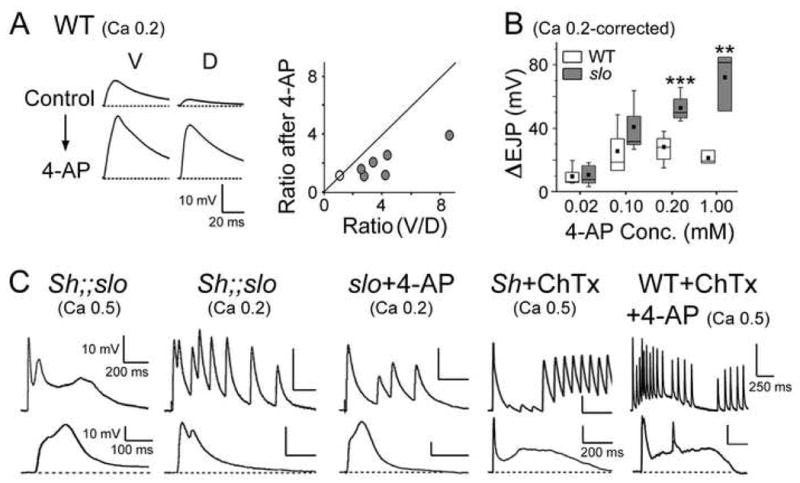
(A) Proportionally greater increase in EJP amplitude induced by 4-AP treatment at dorsal than ventral NMJs in WT. As shown in the ensemble plot (right), the EJP V/D ratios are reduced after 4-AP treatment in nearly all samples, as indicated by gray circles below the 45-degree diagonal line. (B) Greater EJP increase (ΔEJP) in slo after sequential application of 4-AP with increasing concentrations at ventral NMJs. The number of larvae examined, WT vs. slo at [4-AP]: 8 vs. 6 at both 0.02 and 0.1; 6 vs. 5 at 0.2; 2 vs. 3 at 1.0 mM. ***, P<0.001, **, P<0.01, t-test between WT and slo. (C) Two representative examples of EJP responses demonstrating presynaptic hyperexcitability caused by suppression of both Slo and Sh currents upon mutational or pharmacological (4-AP and ChTx) manipulations. Scale bar, 10 mV and 200 ms for the top row, and 10 mV and 100 ms for the bottom row, unless otherwise indicated.
To explore the potential mechanisms of this profile of synaptic variation, we carried out quantal analysis that reveals differences in quantal size (mEJP amplitude or the postsynaptic response to a single vesicle release) and quantal content (the number of presynaptic vesicle released). Significantly, mEJP amplitude was larger in ventral (muscle 6 or 7, or M6/7) than dorsal (muscle 1 or 9, or M1/9) muscles (see Fig. 1C for two sample traces and 1D for the pooled data, P<0.05), parallel to the observed EJP amplitude (Fig. 1B). Quantal content was subsequently determined for ventral and dorsal NMJs. EJP amplitudes were corrected for non-linear summation to estimate the quantal content accurately (see Experimental procedures). Our results demonstrate that the EJP quantal content was higher at ventral than dorsal NMJs (Fig. 1D, M6/7 vs. 1/9, P<0.01).
Recent studies have proposed a link between quantal size and glutamate receptor subunit composition at Drosophila larval NMJs. The relative abundance of DGluRIIA and DGluRIIB, two of the five identified glutamate receptor subunits in Drosophila (Schuster et al., 1991, Petersen et al., 1997, Featherstone et al., 2005, Qin et al., 2005), influences the quantal size (Petersen et al., 1997, DiAntonio et al., 1999, Marrus et al., 2004). Indeed, our results support a close relationship between receptor composition and quantal size. A clear dorsal-ventral (D-V) difference in receptor composition was observed when the pixel intensity ratio between DGluRIIA and DGluRIIB immunoreactivity (IIA/IIB) was calculated from the more prominent type Ib boutons in multiple NMJs within a single hemisegment (see Experimental procedures) (Fig. 1E). The ratios in type Is boutons co-varied with those in type Ib boutons at the same NMJs (data not shown). The IIA/IIB ratio difference along the D-V axis was mostly due to the varying density of DGluRIIA without significant variations in DGluRIIB (see Fig. S1 in the Supplement Data). It is noteworthy that a greater ratio was obtained from M6/7 than M1/9, consistent with their quantal size difference. Overall, the pooled data from 17 hemisegments in 5 simultaneously processed larvae displayed a gradual change in the IIA/IIB ratio along the D-V axis despite apparent deviations in muscles 15/16 and 13 (Fig. 1E). This profile of D-V receptor composition paralleled that of EJP amplitude (Fig. 1B).
Therefore, our analysis for quantal release and immunostaining suggests distinct pre-and post-synaptic mechanisms that are coupled with the D-V differences in synaptic strength, i.e. regulation of presynaptic neurotransmitter release, or quantal content, and postsynaptic receptor composition influencing mEJP amplitude, or quantal size.
D-V differences in synaptic protein expression
Further immunohistochemical analysis indicated that the D-V difference in the receptor density is restricted to DGluRIIA (Schuster et al., 1991) and, to a lesser extent, DGluRIIB (Petersen et al., 1997), but not DGluRIIC, one of the shared subunits among glutamate receptor subtypes (Featherstone et al., 2005, Qin et al., 2005). The D-V difference in receptor density was represented by relative pixel intensity of M1 over M6/7 for each subunit within the same hemisegment (see Experimental procedures). Our results indicate a D/V ratio consistently smaller than 1.0 for DGluRIIA (Figs. 2A and 2E), but slightly higher than 1.0 for DGluRIIB subunits (Figs. 2B and 2E). In contrast, DGluRIIC density did not differ between ventral and dorsal NMJs (Fig. 2E), suggesting similar density of total glutamate receptor subtypes at both NMJs.
Fig. 2. Differential distribution of synaptic proteins between ventral and dorsal NMJs.
Immunoreactivity against DGluRIIA (A), DGluRIIB (B), FasII (C), and Dlg (D) is shown for ventral (M6/7) and dorsal (M1) NMJs in WT. The approximate boundary between muscles 6 (bottom) and 7 (top) is indicated by a dashed line in (D). Scale bar, 20 μm. (E) D-V differences in the density of receptor and other synaptic proteins. The D/V (M1/M6–7) ratio of pixel intensity is shown with the number of NMJs examined (see Experimental procedures for the measurement). Note opposite trends in the ratios of DGluRIIA vs. FasII, and, to a lesser extent, DGluRIIB. ***, P<0.001, **, P<0.01, *, P<0.05, t-test against the null hypothesis of equal D-V intensity (or the ratio of 1.0).
Comparisons of D/V density ratios for other synaptic proteins also revealed a differential distribution of an adhesion molecule, Fasciclin II (FasII) (Bastiani et al., 1987) (Fig. 2C), of which expression is affected by varying activity levels at Drosophila larval NMJs upon environmental or genetic manipulations (Schuster et al., 1996b, Sigrist et al., 2003). The D/V ratio was consistently greater than 1.0 for FasII in all preparations examined, indicating higher density at dorsal NMJs (Fig. 2E, P<0.001). In contrast, Discs-Large (Dlg) (Lahey et al., 1994, Budnik et al., 1996), an invertebrate scaffold protein homologous to PSD-95 required for clustering of FasII and Sh K+ channels (Tejedor et al., 1997, Thomas et al., 1997, Zito et al., 1997), did not show any D-V density differences (Figs. 2D and 2E). Furthermore, the immunoreactivity to anti-HRP, which recognizes pan-neuronal markers in insects (Jan and Jan, 1982), showed a slightly higher intensity at dorsal NMJs (Fig. 2E, P<0.01). However, HRP density did not co-vary with FasII density in double-labeled preparations (data not shown).
Such a D-V difference in FasII density led us to examine the possibility that it contributes to the functional synaptic variation (Fig. 1). However, fasII mutants displayed D-V differences in both EJP amplitudes and receptor density similar to WT (data not shown). Therefore, differential distribution of FasII is not required for the D-V disparity in synaptic strength described above, supporting the idea that FasII is involved in plasticity of NMJ morphology, but not synaptic efficacy (Schuster et al., 1996a, Schuster et al., 1996b).
Effects of elimination of Ca2+-activated Slo and voltage-activated Sh K+ channels on synaptic strength
We then examined how the pre- and post-synaptic mechanisms described above are affected by changes in activity level using K+ channel mutations. Among K+ channels, the Ca2+-activated Slo and voltage-activated Sh K+ channels have been first characterized molecularly in Drosophila (Kamb et al., 1987, Papazian et al., 1987, Atkinson et al., 1991) and their mutational effects on larval neuromuscular transmission have been well described (Jan et al., 1977, Wu et al., 1983, Gho and Ganetzky, 1992, Warbington et al., 1996). Sh IA is important for axonal repolarization and nerve terminal excitability control (Tanouye et al., 1981, Ganetzky and Wu, 1983, Tanouye and Ferrus, 1985, Ueda and Wu, 2006). Slo (BK) channels are co-localized at presynaptic terminals with voltage-gated Ca2+ channels, a major source of Ca2+ influx for transmitter release (Robitaille et al., 1993, Yazejian et al., 1997, Marrion and Tavalin, 1998), and thus provide an effective feedback mechanism to limit Ca2+ influx-induced transmitter release. We first examined how these two important repolarizing channels affect natural variations in synaptic strength along the D-V axis.
Surprisingly, at a physiological [Ca2+]o level (0.5 mM or higher), EJP amplitude and D-V differences in slo and Sh mutants were not severely affected as compared to WT (Fig. 3A, top traces; see Fig. S2 in the Supplement Data for the pooled data), contrary to the expected effects from a loss of major repolarizing force to stabilize membrane excitability. It should be noted that under this condition (0.5 mM Ca2+ in HL3.1 saline) EJP amplitudes are closer to the reported response amplitudes obtained from HL3 saline containing 1.5 mM of Ca2+ (Stewart et al., 1994, Feng et al., 2004). EJPs described here did not reach saturation, as indicated by further increase in the amplitude at even higher Ca2+ levels (1.0 or 1.5 mM), and displayed considerable variations in the amplitudes (Fig. S2 in the Supplement Data). Therefore, these results indicate that these slo and Sh mutant NMJs can indeed perform normal synaptic function under a physiological condition, suggesting the presence of homeostatic mechanisms to compensate the loss of these important K+ channel functions.
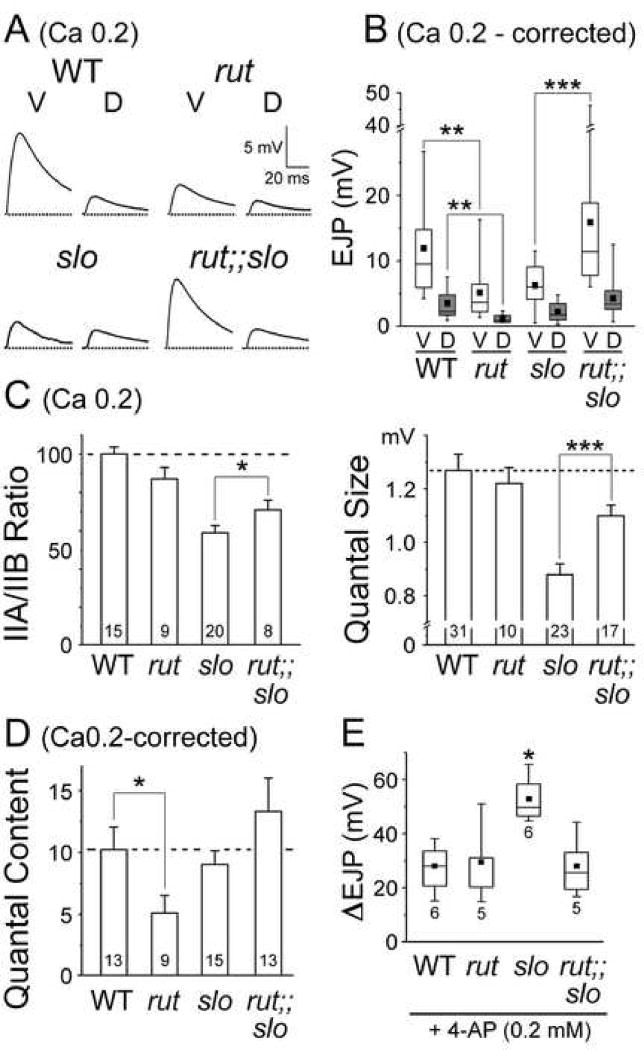
Fig. 3. Homeostatic maintenance of synaptic strength in slo and Sh mutants and their Ca2+-dependent mutant phenotypes in EJP amplitudes.
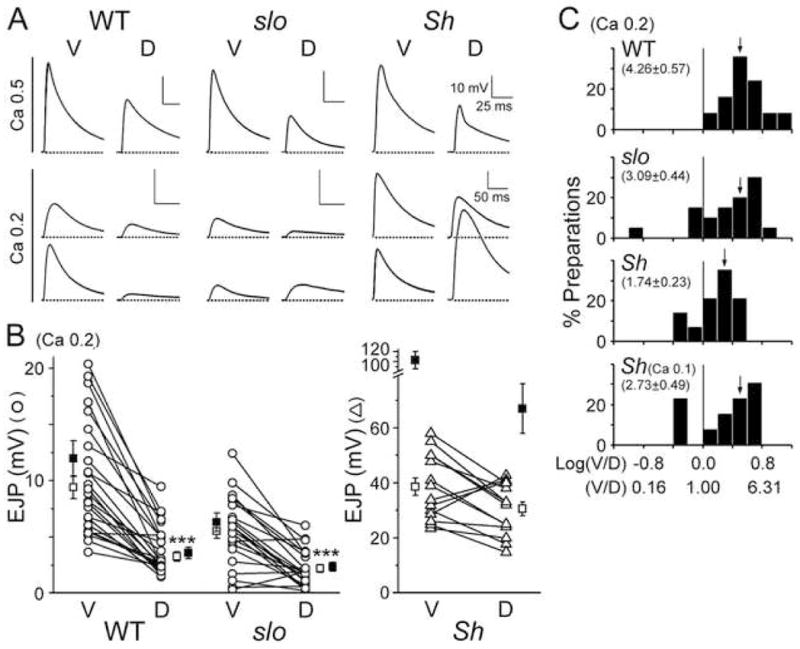
(A) D-V differences in EJP amplitude from WT, slo, and Sh in the presence of 0.5 (top) and 0.2 (bottom) mM [Ca2+]o in HL3.1 saline. Top traces in the Ca 0.2 panel represent the general trends while those at the bottom depict extreme cases, including the reversed D-V differentials, with larger EJPs at dorsal NMJs in mutants. Scale bar, 10 mV and 25 ms, unless otherwise indicated. (B) Pooled data for D-V differences at a low Ca2+ level (0.2 mM). Ventral (V) and dorsal (D) EJP data of the same larva are linked. Note larger EJPs at dorsal NMJs in subsets of slo and Sh mutants (lines with positive slopes). Number of larvae: 25 for WT, 22 for slo, and 14 for Sh. Open and closed symbols, Mean ± SEM before and after the correction procedure for non-linear summation, respectively (see Experimental procedures). ***, P<0.001, t-test for ventral vs. dorsal NMJs. (C) Histogram for the EJP ratio (V/D) in linear and logarithmic scales (see Experimental procedures). Vertical line in each plot distinguishes the normal (right) and reversed (left) D-V differences. Note cases with reversed differences in slo and Sh. Mean (indicated by arrows) and SEM in parentheses.
In contrast to a lack of clear mutant phenotypes at physiological Ca2+ levels, striking mutant phenotypes were revealed at low [Ca2+]o (0.2 mM) in Sh and slo larvae (Fig. 3A, bottom two traces). Consistent with previous reports (Jan et al., 1977, Wu et al., 1983, Ueda and Wu, 2006), Sh mutations induced a significant increase in EJP amplitude at both ventral and dorsal NMJs, as expected from enhanced nerve excitability (Fig. 3A). At this Ca2+ level, Sh EJPs were more than 4-fold greater than WT EJPs, or greater than 10-fold in transmitter release when non-linear correction of EJPs was considered (Fig. 3B). However, in slo mutants, we found a significant reduction, but not enhancement, in EJPs at ventral NMJs (Figs. 3A and 3B). This paradoxical result, also described in a previous report (Warbington et al., 1996), is contrary to the expected increase in membrane excitability due to a loss of Slo channels. In addition to altered EJP amplitudes, comparing the D-V muscle pairs (M6/7 for ventral (V) and M1/9 for dorsal (D) NMJs) for individual larvae revealed disruptions in the D-V difference in synaptic strength in Sh and slo mutants (EJP amplitude in Fig. 3B and the V/D ratio in Fig. 3C). Despite variable amplitudes among preparations, D-V pairs from the same hemisegment connected by lines nevertheless showed a clear difference in WT (Fig. 3B, P<0.001, V vs. D at 0.2 mM [Ca2+]o). Fig. 3B also demonstrates a preferential effect of Sh mutations on dorsal EJPs in addition to the average increase in synaptic strength (P<0.0001, WT vs. Sh). On average, EJP amplitude was increased to a greater extent at dorsal NMJs with some extreme cases of dorsal EJPs becoming greater than ventral EJPs (see Fig. 3A, bottom traces for an example; see also lines with positive slopes in Fig. 3B and columns left to the vertical line in Fig. 3C), resulting in no clear overall D-V difference in mean EJP amplitude. On the other hand, slo EJP amplitude was reduced, mostly at ventral NMJs (Fig. 3B, P<0.005, WT vs. slo ventral NMJs). Again, in some cases of slo mutants, dorsal, but not ventral, NMJs displayed greater EJPs (bottom traces in Fig. 3A; see also Figs. 3B and 3C). These extreme cases of reversed D-V differentials were also found in mutants at different levels of low [Ca2+]o (see Fig. 3C for Sh, 0.1 and 0.2 mM), but have not been observed in WT.
Modification of synaptic protein distribution and postsynaptic receptor composition in slo mutants
Disrupted D-V differentials at slo and Sh NMJs revealed at low [Ca2+]o levels (Fig. 3) suggest that these two major repolarizing K+ channels may play important roles in the D-V profile of synaptic strength. We first examined whether there are changes in distribution of synaptic proteins in the mutants as compared WT. Fig. 4D depicts mean of D/V ratios of synaptic protein density as well as SD to indicate the range of the variability. In Sh mutants, we did not detect significant changes in D/V ratios of the proteins from those shown in Fig. 2 for WT, except for Dlg (Figs. 4A–D).
In contrast, slo mutations induced clear changes in the distribution of FasII and Dlg compared to WT. We found in slo mutants a significant reduction in D-V differences for FasII and differential D-V intensities for Dlg that was evenly distributed in WT (Figs. 4A1, 4C1, and 4D). The significance of these alterations remains unclear. As mentioned above, synaptic strength as well as glutamate receptor density in fasII and dlg mutants were not different from WT (data not shown). Thus, it is unlikely that altered distributions of FasII and Dlg in slo mutants correlate with the profile of synaptic strength. Whether modified distributions of these molecules in slo mutants can lead to alterations in synaptic features other than transmission efficacy needs to be further investigated in future studies.
Significantly, slo mutations induced a change in DGluRIIA density along the D-V axis. While there was no change in the D/V ratio of DGluRIIB and DGluRIIC densities (Fig. 4D), it was significantly reduced for DGluRIIA density in slo mutants (Figs. 4B1 and 4D, P<0.05 for WT vs. slo). Calculation of DGluRIIA/IIB (IIA/IIB) ratio at ventral (M6/7) and dorsal (M1) NMJs within the same hemisegment again indicated a preferential effect of slo mutations on ventral NMJs, where the IIA/IIB ratio was more drastically decreased than at dorsal NMJs compared to WT and Sh (Fig. 4E, see also Figs. S1 and S3 in the Supplement Data for the relative density of DGluRIIA and DGluRIIB at double-stained NMJs and IIA/IIB ratio throughout a single hemisegment, respectively).
Modification of quantal size and quantal content by slo and Sh mutations
The preferential effect of slo mutations on receptor composition (IIA/IIB ratio) at ventral NMJs was reflected by a clear reduction in quantal size at ventral, but not dorsal, NMJs (Fig. 5, P<0.01 for WT vs. slo, P<0.05 for Sh vs. slo). While mEJP amplitudes were larger at ventral than dorsal NMJs in both WT and Sh larvae (P<0.05), such difference disappeared in slo mutants (Figs. 5A and 5B, top panel). Together with a more drastically reduced IIA/IIB ratio at ventral NMJs (Fig. 4E), these results demonstrate a postsynaptic modification that leads to smaller EJP amplitudes in slo mutants at lower Ca2+ levels (Fig. 3).
Fig. 5. Modified quantal size and quantal content in slo and Sh mutants.
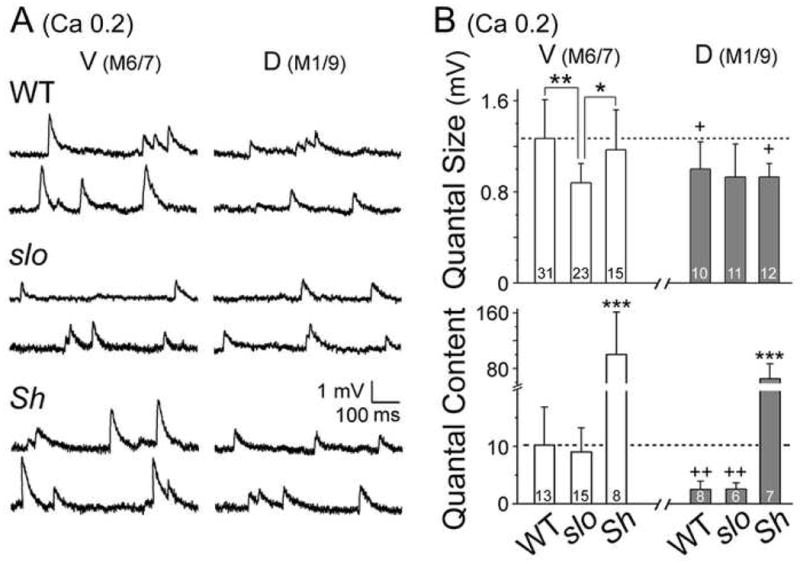
(A) Two sample traces of mEJPs recorded from ventral (M6/7) and dorsal (M1/9) NMJs of each genotype. (B) Pooled data of quantal size (top) and quantal content (bottom) with the number of larvae examined indicated. Note a significant reduction in quantal size at slo ventral NMJs and drastic increase in Sh quantal content. WT data in Fig. 1D are shown as reference. Mean ± SD; ***, P<0.001, **, P<0.01, *, P<0.05, one-way ANOVA among genotypes. +, P<0.05, ++, P<0.01, t-test for ventral vs. dorsal NMJs within a genotype.
One important feature of Sh mutations is the enhanced EJPs, more extreme at dorsal NMJs, at 0.2 mM [Ca2+]o (Fig. 3). Significantly, unaltered receptor composition and quantal size in Sh mutants (Figs. 4E, 5A, and 5B, top panel) gave rise to extremely large quantal content when quantal analysis was performed (see Experimental procedures). Notably, the resultant quantal contents for ventral and dorsal EJPs were equally large, whereas in WT dorsal quantal content was about 3-fold smaller (Fig. 5B, bottom panel, V vs. D, P<0.01). Unexpectedly, quantal content in slo mutants was not significantly different from WT in the absolute scale (Fig. 5B, bottom panel). Similar quantal contents between WT and slo were also observed upon failure analysis (data not shown, see Experimental procedures). This unexpected result, plus Sh-induced increases in quantal content and EJP amplitude at dorsal NMJs, led us to examine a potential presynaptic mechanism for the homeostatic regulation of synaptic strength in slo and for the natural D-V disparity in synaptic strength, i.e. differential Sh current expression (Fig. 3A, see below).
An important clue to a higher abundance of presynaptic Sh currents at dorsal NMJs was discovered when WT preparations were treated with 4-aminopyridine (4-AP), a selective blocker for Sh IA. Upon removal of Sh IA, EJPs at dorsal NMJs increased proportionally greater than those at ventral NMJs (Fig. 6A, left). Furthermore, pooled data from seven larvae indicated a decrease in the EJP ratios (V/D) following 4-AP treatment (Fig. 6A, right). Therefore, the synaptic transmitter release capability of dorsal NMJs is at least comparable to that of ventral NMJs once Sh IA is removed. The results collectively support the hypothesis that higher expression of Sh current at dorsal NMJs more effectively restricts transmitter release and hence causes lower quantal content. Together with smaller quantal size, suppressed quantal content by upregulated Sh IA may thus underlie smaller EJPs at dorsal NMJs in WT.
Homeostatic upregulation of presynaptic Sh IA in slo mutants
In vertebrate species, it is established that Slo (BK) channels, co-localized at presynaptic terminals with voltage-gated Ca2+ channels, act as a major transmitter release regulator (Robitaille et al., 1993, Yazejian et al., 1997, Marrion and Tavalin, 1998). It is therefore surprising that lack of BK currents in slo mutants did not cause greatly enhanced synaptic transmission (Fig. 3) with similar amount of transmitter release, as indicated by comparable quantal contents between WT and slo at a low Ca2+ level (0.2 mM, Fig. 5B, bottom). These observations raise a possibility of a compensatory mechanism that minimizes the effect of a loss of Slo channels to retain synaptic function at physiological Ca2+ levels.
We found evidence of upregulated Sh IA in slo mutants when we examined changes in EJPs following 4-AP treatment. Serial application of 4-AP with increasing concentrations revealed a far greater effect in slo than WT. Enhancement of EJPs in WT was detectable at 0.02 mM of 4-AP, but reached a saturated level beyond 0.1 mM (Fig. 6B, ΔEJP). In contrast, EJPs in slo mutants continued to increase without a clear saturation effect up to 1.0 mM, and therefore the EJP increment became progressively greater than that in WT (Fig. 6B, at 0.2 mM or higher [4-AP], P<0.001 or P<0.01). Remarkably, at higher 4-AP concentrations, EJP amplitude of slo mutants became larger than that of WT (data not shown). It is important to note that 4-AP treatment in WT did not enhance EJP amplitude beyond the extent displayed by Sh mutants at different Ca2+ concentrations and that 4-AP treatment did not further increase EJPs in Sh mutants (data not shown). These imply that a major portion of IA in presynaptic terminals is mediated by Sh rather than Shal (Covarrubias et al., 1991) and that the observed 4-AP effects reflect manipulation of mainly Sh IA.
The functional significance of increased Sh IA currents in slo mutants was demonstrated in Sh;;slo double mutants and 4-AP-treated slo single mutants. Upon elimination of Sh IA in slo mutants, EJPs were significantly prolonged along with the occurrence of supernumerary EJPs following a single stimulus at both low and physiological [Ca2+]o (Fig. 6C). These phenotypes, characteristic of presynaptic hyperexcitability (Ganetzky and Wu, 1983), were also reproduced by combination of Charybdotoxin (ChTx), a selective blocker of Slo current, with 4-AP in WT, or by ChTx treatment in Sh mutants (Fig. 6C), confirming both Slo and Sh as major K+ currents controlling presynaptic excitability. It is therefore necessary to compensate the loss of BK currents in slo mutants by a major repolarization force, in this case, Sh IA, which prevents excessive vesicle release.
cAMP-dependent homeostatic regulation of synaptic strength in slo mutants
Functions of ion channels, including Slo and Sh, are often regulated by second messenger pathways such as the cAMP-dependent cascade (Zhong and Wu, 1993, Esguerra et al., 1994, Jonas and Kaczmarek, 1996). On the other hand, activities of these pathways are regulated in Ca2+- and activity-dependent manners, e.g. during induction of Ca2+-dependent LTP, involving delicate control of Ca2+ influx by voltage- and Ca2+-activated ion channels (Ferguson and Storm, 2004). We found that both pre- and post-synaptic homeostatic mechanisms activated in slo mutants (Figs. 4, 5, and 6) are affected by mutations of rut encoding a Ca2+/CaM-dependent AC that produces cAMP in a Ca2+-dependent manner (Dudai et al., 1983, Livingstone et al., 1984). We observed in rut1 mutants greatly reduced EJP amplitude (to a lesser extent in rut1084, data not shown) at both ventral and dorsal NMJs compared to WT (Figs. 7A and 7B, P<0.01), consistent with reduced Ca2+ influx in rut presynaptic terminals (Ueda and Wu, 2003). Nevertheless, the rut mutants displayed a clear D-V difference in EJP amplitude (Fig. 7B, V vs. D, P<0.01).
Fig. 7. Actions of rut AC in homeostatic regulation of synaptic strength in slo mutants through modifications of postsynaptic receptor composition, quantal size, and 4-AP-sensitive Sh IA.
(A) Representative traces of EJPs at 0.2 mM [Ca2+]o for WT, slo, rut, and rut;;slo. Note similar EJP sizes between WT and rut;;slo, in contrast to smaller sizes in both slo and rut mutants. (B) Pooled EJP amplitudes summarized in box plots. The number of larvae examined in WT, rut, slo, and rut;;slo: 25, 15, 22, and 19 for ventral; 25, 10, 22, and 11 for dorsal NMJs. (C) Increases in IIA/IIB ratio and quantal size at ventral NMJs in rut;;slo that are partially restored to WT values. rut;;slo double mutants significantly differ from slo in postsynaptic receptor composition (IIA/IIB) (left) and correlated quantal size (right). (D) Quantal content decrease in rut and enhancement in rut;;slo double mutants at ventral NMJs. (E) EJP increase at ventral NMJs upon 4-AP treatment (0.2 mM) in rut, slo, and rut;;slo. Mean ± SEM with numbers of NMJs (IIA/IIB ratio) and larvae (quantal size, quantal content, and ΔEJP). EJP amplitudes for (B), (D), and (E) are corrected for non-linear summation (see Experimental procedures). Data shown in (C) and (D) are normalized to WT values indicated by dashed lines. ***, P<0.001, **, P<0.01, *, P<0.05, t-test (B–D) and one-way ANOVA (ΔEJP in (E)).
We first examined the effect of rut on homeostatic mechanisms in rut;;slo double mutants at low [Ca2+]o, at which slo phenotypes were most striking (Fig. 3). Surprisingly, the average EJP amplitude of rut;;slo double mutants was greater than either single mutants (Fig. 7A) and with WT-like D-V differences (Fig. 7B, V vs. D, P<0.01). The enhancement of EJP amplitude was more clearly observed at ventral NMJs (Fig. 7B, slo vs. rut;;slo, P<0.001), where slo mutant phenotypes were more striking in the homeostatic modifications of quantal size and receptor composition, i.e. the IIA/IIB ratio (Figs. 4 and 5).
We found that rut mutants retained a similar IIA/IIB ratio and quantal size (Fig. 7C) comparable to WT. However, in rut;;slo double mutants, both the IIA/IIB ratio and quantal size were increased compared to slo (Fig. 7C, slo vs. rut;;slo, P<0.05 for IIA/IIB ratio, P<0.001 for quantal size). There was a clear correlation between these two parameters as we observed the same descending order of rut, rut;;slo, and slo in their values. The action of rut AC in this case is likely postsynaptic since IIA/IIB ratios can vary even among NMJs with a common presynaptic motoneuron identity (i.e. innervation of type Is branches in M6/7, 13, and 12), as mentioned above.
In addition to such postsynaptic modification, a presynaptic role for rut was also suggested by comparisons in quantal content and 4-AP response between slo single and rut;;slo double mutants (Figs. 7D and 7E). Quantal content was significantly lower in rut1 mutants (Fig. 7D, P<0.05, rut vs. WT), as deduced from smaller EJP amplitude with unaltered quantal size (Figs. 7B and 7C, right panel). However, quantal content of rut;;slo double mutants appeared to be enhanced compared with the single mutants (Fig. 7D, P<0.05 for rut vs. rut;;slo, P<0.07 for slo vs. rut;;slo). Importantly, such enhancement may involve Sh IA adjustment since application of 4-AP (0.2 mM) in rut;;slo mutants no longer yielded an extreme EJP increment as observed in slo single mutants (Fig. 7E, P<0.05 for slo vs. other genotypes; see also Fig. 6B). Therefore, our results indicate that, together with postsynaptic modifications in receptor composition and quantal size, presynaptic upregulation of Sh current triggered by slo mutations may also involve cAMP-dependent pathways.
DISCUSSION
Synaptic strength profile along the D-V axis of larval body-wall musculature and the roles of Slo and Sh K+ currents in its homeostatic maintenance
Systematic variations in synaptic strength according to the body plans provide mechanisms for ordered information transfer in neural circuits. In this study, we, for the first time, analyzed a novel D-V disparity in synaptic strength at Drosophila larval body-wall NMJs. In Drosophila larvae, a variation along the anterior-posterior (A-P) axis has been reported for the number of synaptic boutons (Budnik et al., 1990). While the A-P variation in synaptic morphology is derived from motoneurons of the same identities among body segments, the D-V differential in synaptic strength involves motoneurons of different identities within the same segment (Johansen et al., 1989, Landgraf et al., 1997). It is worth pointing out that the D-V profile of EJP amplitude does not correlate with the number of boutons in different muscles (data not shown). In addition, we did not find receptor composition differences for identified NMJs along the A-P axis (see Fig. S4 in the Supplement data), in contrast to gradual changes along the D-V axis (Fig. 1E), highlighting two types of morphological and systematic functional variations. Equipped with microchetae on the ventral cuticle, ventral muscles likely face greater mechanical demands upon crawling than dorsal muscles, which may require different levels of synaptic strength.
Fig. 8A summarizes the findings and integrates the plausible pre- and post-synaptic mechanisms that may underlie the natural variations in synaptic strength. Analysis of the D-V profile of synaptic strength in vivo indicated a potential physiological significance of postsynaptic glutamate receptor composition (DGluRIIA/IIB) (Fig. 8A). With shared subunits, e.g. DGluRIIC, competition between DGluRIIA and DGluRIIB for receptor assembly is thought to determine single channel conductance and kinetics, and thus quantal size (Petersen et al., 1997, DiAntonio et al., 1999). Indeed, we found D-V difference in quantal size correlated with differential IIA/IIB ratios, but not DGluRIIC density, at both type Ib (Figs. 1, 2, and 5) and Is NMJs (data not shown), providing a strong support for the proposed functional consequences of subunit competition. In addition to such postsynaptic mechanism, regulation of presynaptic excitability by differential expression of Sh current also contributes to the D-V differentials in synaptic strength (Fig. 8A). This is supported by the stronger effect of 4-AP as well as Sh mutations at dorsal than ventral NMJs (Figs. 3, 5B, and 6A).
Fig. 8. Roles of Slo and Sh K+ channels in the natural variation in synaptic strength along the D-V axis and cAMP-dependent synaptic homeostasis.
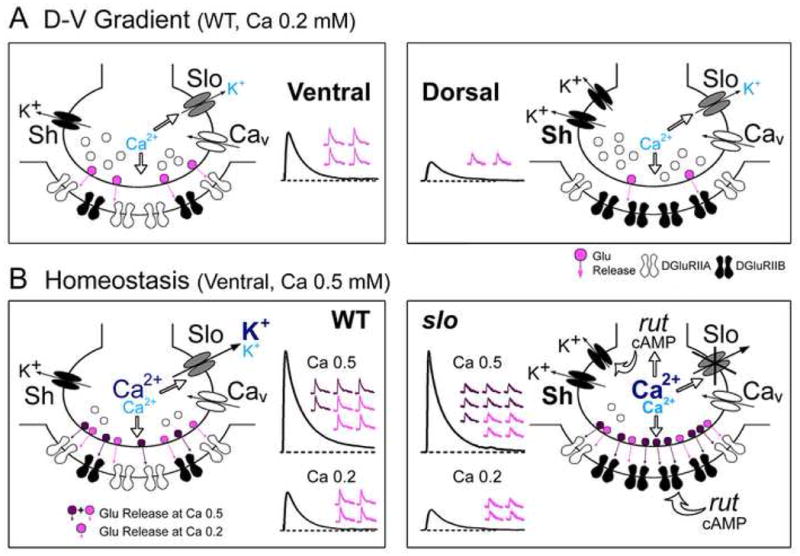
(A) Plausible pre- and post-synaptic mechanisms underlying the D-V disparity in synaptic strength. Note the increased number of Sh channels correlated with reduced number of vesicle release and more abundant DGluRIIB subunits associated with smaller mEJP amplitude at dorsal (right) compared to ventral (left) NMJs. (B) As Ca2+ influx increases at higher [Ca2+]o (0.5 mM, dark blue), full-blown Slo current (dark blue) plays a major role in repolarizing membrane and controlling vesicle release (left). In slo mutants (right), the loss of a negative feedback onto Ca2+ influx provided by Slo current may lead to an increase in [Ca2+]i (bold), which, in turn, may initiate rut AC/cAMP-dependent presynaptic upregulation of Sh current, compensating in part for the loss of Slo current (note enhanced releases in slo). Concomitant postsynaptic decrease in IIA/IIB ratio and mEJP amplitude via rut regulation leads to further restoration of synaptic strength (note similar EJPs between WT and slo as the ensemble quantal events in top traces). However, with less Slo current activation at lower [Ca2+]o (0.2 mM, light blue), Sh IA plays a dominant role in regulating membrane repolarization and vesicle release. A combination of upregulated presynaptic Sh current and reduced IIA/IIB ratio and mEJP amplitude would then lead to a smaller EJP in slo mutants (bottom traces).
These pre- and post-synaptic mechanisms are homeostatically modified in slo mutants to retain synaptic function at physiological Ca2+ levels (Fig. 8B, 0.5 mM). Sh IA acts as a major repolarizing force at low [Ca2+]o (Fig. 5B), where repolarization provided by Ca2+-activated Slo current is minimal. Enhanced neurotransmitter release in Sh mutants is most striking at low [Ca2+]o, but progressively less obvious as [Ca2+]o rises to physiological levels (Fig. 3A), where Slo current becomes full-blown. This suggests a contrasting role for Slo current at higher [Ca2+]o in controlling presynaptic excitability (Fig. 8B, left, dark blue), as indicated by striking presynaptic hyperexcitability induced by removal of Slo in Sh mutants (Fig. 6C). The fact that slo mutants do not display obvious abnormalities in EJPs at physiological [Ca2+]o (Fig. 8B, right) indicates the existence of homeostatic restoration mechanisms. At lower [Ca2+]o (0.2 mM, light blue), the paradoxical reduction in slo EJP amplitude is resolved by a presynaptic upregulation of compensatory repolarizing forces, Sh IA (Fig. 8B, right), supported by greater effect of 4-AP on EJP amplitude in slo compared to WT (Fig. 6B). Additional modifications in postsynaptic receptor composition were also found in slo, contributing to reduced quantal size (Figs. 4E, 5 and 8B, right). The combined effects of decreased quantal size and nearly normal quantal content at lower [Ca2+]o lead to a smaller EJPs, but result in almost normal EJP amplitude at physiological [Ca2+]o (Fig. 8B, right).
Activation of cAMP-dependent homeostatic mechanisms
Our finding of upregulated Sh IA in slo presynaptic terminals is an interesting parallel to the recent report in cultured Drosophila embryonic neurons that reduced Slo current following the loss of a major Ca2+ current is compensated by upregulated Sh IA (Peng and Wu, 2007). These observations in Drosophila corroborate previous reports that neuronal excitability is homeostatically regulated through modulation of ionic currents in response to manipulations of a specific membrane conductance (Marder and Goaillard, 2006). For example, overexpression of IA in crustacean stomatogastric ganglion neurons induces concomitant increase in the hyperpolarization-activated current, with an end result of maintained neuronal firing patterns (MacLean et al., 2003). In mutant mice defective in Cav2.1 channels, total Ca2+ current density in forebrain neurons remains unaltered due to compensatory increase in Cav1 activity (Etheredge et al., 2007).
These homeostatic mechanisms may require long-term modifications of pre- or post-synaptic machinery. Acute application of ChTx to block Slo channels in WT at low [Ca2+]o failed to reproduce the characteristic slo mutant phenotypes of reduced EJP amplitudes (data not shown). Similarly, compensatory upregulation of Sh current in cultured neurons also requires chronic reduction of Ca2+ currents (Peng and Wu, 2007). Thus, these observations suggest that chronic, but not acute, dysfunction of ion channels is required to induce homeostatic compensation.
Our results demonstrate the involvement of a prevalent signal transduction pathway, the cAMP cascade, in synaptic homeostasis. It is well known that the cAMP cascade is critical for behavioral plasticity such as learning and memory (Keene and Waddell, 2007), for activity-dependent synaptic plasticity (Zhong and Wu, 1991, Davis et al., 1996, Schuster et al., 1996b, Renger et al., 2000), and for regulation of Ca2+ and K+ currents in neurons and muscles (Zhong and Wu, 1993, Zhao and Wu, 1997, Bhattacharya et al., 1999, Ueda and Wu, 2003). In addition, hyperexcitability-induced synaptic overgrowth by K+ channel mutations (Budnik et al., 1990, Zhong et al., 1992) is modified by mutations in cAMP metabolism (Zhong et al., 1992, Zhong and Wu, 2004), suppressed by rut, but enhanced by increased cAMP levels by dunce mutations affecting cAMP-specific phosphodiesterase (Dudai et al., 1976, Byers et al., 1981).
The functional link between cAMP-dependent pathways and K+ channel-related synaptic plasticity rests on the activity-dependent accumulation of Ca2+, an important regulator of rut AC activity (Dudai and Zvi, 1984, Livingstone et al., 1984). Slo channels are colocalized with voltage-activated Ca2+ channels and provide a negative feedback to regulate Ca2+-activated events (Salkoff et al., 2006). Thus, enhanced Ca2+ influx during development due to the lack of Slo-related negative feedback may trigger a cascade of cAMP-dependent events, leading to pre- and post-synaptic homeostatic modifications observed in slo mutants (Fig. 8B).
Such rut AC-dependent homeostatic mechanisms may involve downstream effectors, including cAMP-dependent protein kinase (PKA) for protein phosphorylation and cAMP response element-binding protein (CREB) for gene expression (Nguyen and Woo, 2003). PKA-dependent regulation of Sh and Slo currents has been demonstrated in Drosophila neurons and muscles (Zhong and Wu, 1993, Esguerra et al., 1994, Yao and Wu, 2001). In addition, elevated PKA activity at larval NMJs reduces quantal size, presumably through modulation of glutamate receptor channel (DGluRIIA) (Davis et al., 1998). However, since acute blockade of Slo current by ChTx treatment does not initiate homeostatic processes (see above), long-term homeostatic modifications observed in slo mutants likely involve CREB-dependent gene expression.
Future studies in synaptic homeostasis
It should be noted that slo-induced modifications are not fully prevented in rut;;slo double mutants. Their postsynaptic receptor composition and associated quantal size are still not identical to WT, albeit significantly different from those in slo (Fig. 7C), suggesting potential involvement of other Ca2+-dependent second-messenger cascades in homeostasis. It will be interesting to examine whether Ca2+/CaM-dependent kinase II, known to modulate K+ currents (Yao and Wu, 2001), activity-dependent synaptic plasticity (Wang et al., 1994, Park et al., 2002), and behavioral plasticity (Griffith et al., 1993, Jin et al., 1998) in Drosophila, also participates in synaptic homeostasis.
Our results highlight the significance of Ca2+ influx regulated by Slo K+ channels in homeostasis. It will be important to determine the source of Ca2+ influx that triggers compensatory pre- and post-synaptic modifications. Two Ca2+ channel genes, cacophony (cac) (Smith et al., 1996, Kawasaki et al., 2000, Rieckhof et al., 2003, Peng and Wu, 2007) and Dmca1D for the major neuronal and muscular Ca2+ channels (Gielow et al., 1995, Zheng et al., 1995), respectively, have been identified in Drosophila. Reduced Slo current associated with compensatory increase in Sh IA in cultured cac neurons (Peng and Wu, 2007) suggests a similar contribution of cac channels in presynaptic homeostasis. It will be interesting to ask whether Dmca1D-dependent Ca2+ influx contributes to postsynaptic homeostatic mechanisms through functional coupling with Slo channels.
The diversity of K+ channels, characterized by distinct gating, activation and inactivation properties (Armstrong, 2003), may lead to different profiles of Ca2+ influx in subcellular regions upon their dysfunction (Berke et al., 2006). Therefore, it will be interesting to see whether mutations in other K+ channels can trigger homeostatic adjustments different from those observed in slo.
Supplementary Material
Fig. S1. Effects of slo and Sh mutations on DGluRIIA and DGluRIIB density. Absolute pixel intensity for DGluRIIA and DGluRIIB immunoreactivity in double labeling experiments is compared among simultaneously processed WT, slo, and Sh larvae and measurements are normalized to that of WT ventral NMJs in the same batch, indicated by dashed lines. The number of ventral (V) and dorsal (D) NMJs examined: 15 and 15 for WT, 20 and 19 for slo, and 20 and 20 for Sh. Mean ± SD indicated. ***, P<0.001, **, P<0.01, one-way ANOVA among genotypes: +, P<0.05, +++, P<0.001, t-test for ventral (V) vs. dorsal (D) NMJs within a genotype.
Fig. S2. Similarities in EJP amplitudes and D-V differentials among WT, slo, and Sh larvae in HL3.1 saline containing 0.5 mM Ca2+.
EJP amplitudes in the scatter plots are corrected for non-linear summation (see Experimental procedures). Mean ± SEM are indicated. Number of larvae examined, ventral (V, M6/7) vs. dorsal (D, M1/9) NMJs: WT, 10 vs. 7; slo, 12 vs. 5; Sh, 11 vs. 5. *, P<0.05 and ***, P<0.001, t-test for ventral vs. dorsal NMJs.
Fig. S3. Altered receptor composition at slo NMJs throughout a single hemisegment. Reduced DGluRIIA/IIB (IIA/IIB) ratio for type Ib NMJs is found in slo mutants (slo98) compared to WT preparations stained and processed simultaneously (see Experimental procedures). See also Fig. 4 for the ratios between WT and slo in M6/7 and M1. WT data in Fig. 1E are shown as reference. The number of segments (larvae), 17 (5) for WT and 9 (3) for slo.
Fig. S4. Similar postsynaptic receptor immunoreactivity and IIA/IIB intensity ratio along the anterior-to-posterior (A-P) axis of larval body-wall musculature.
Signals from DGluRIIA and DGluRIIB immunoreactivity and their ratio between ventral (V, M6/7) and dorsal (D, M1) NMJs are color-coded for individual segments from the 3rd (AR3) to 6th (AR6) segments. There are no clear trends indicating a variation along the A-P axis. Readings from dorsal NMJs are normalized with respect to ventral NMJ values.
Acknowledgments
We thank Drs. Vivian Budnik and Aaron DiAntonio for the Dlg and DGluRIIB/IIC antibody, respectively. This work was supported by NIH grants NS26528 and HD18577 to CFW.
Abbreviations
- NMJs
neuromuscular junctions
- Sh (Sh)
Shaker (Shaker)
- slo (Slo)
slowpoke (Slowpoke)
- rut
rutabaga
- AC
Adenylyl cyclase
- IIA
DGluRIIA
- IIB
DGluRIIB
- V
ventral
- D
dorsal
- M
muscle(s)
- EJP
excitatory junctional potential
- mEJP
miniature excitatory junctional potential
- IA
transient A-type K+ current
- IK(Ca)
large conductance, Ca2+-activated K+ current
- 4-AP
4-aminopyridine
- ChTx
Charybdotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong CM. Voltage-gated K channels. Sci STKE. 2003;2003:re10. doi: 10.1126/stke.2003.188.re10. [DOI] [PubMed] [Google Scholar]
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Barker MC, Osmani F, Forsythe ID. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur J Neurosci. 2004;19:325–333. doi: 10.1111/j.0953-816x.2003.03133.x. [DOI] [PubMed] [Google Scholar]
- Bastiani MJ, Harrelson AL, Snow PM, Goodman CS. Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell. 1987;48:745–755. doi: 10.1016/0092-8674(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Berke BA, Lee J, Peng IF, Wu CF. Sub-cellular ca(2+) dynamics affected by voltage- and ca(2+)-gated k(+) channels: regulation of the soma-growth cone disparity and the quiescent state in drosophila neurons. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.06.051. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Gu GG, Singh S. Modulation of dihydropyridine-sensitive calcium channels in Drosophila by a cAMP-mediated pathway. J Neurobiol. 1999;39:491–500. doi: 10.1002/(sici)1097-4695(19990615)39:4<491::aid-neu3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981;289:79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Covarrubias M, Wei AA, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/s0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Uzzan A, Zvi S. Abnormal activity of adenylate cyclase in the Drosophila memory mutant rutabaga. Neurosci Lett. 1983;42:207–212. doi: 10.1016/0304-3940(83)90408-1. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Zvi S. Adenylate cyclase in the Drosophila memory mutant rutabaga displays an altered Ca2+ sensitivity. Neurosci Lett. 1984;47:119–124. doi: 10.1016/0304-3940(84)90416-6. [DOI] [PubMed] [Google Scholar]
- Esguerra M, Wang J, Foster CD, Adelman JP, North RA, Levitan IB. Cloned Ca(2+)-dependent K+ channel modulated by a functionally associated protein kinase. Nature. 1994;369:563–565. doi: 10.1038/369563a0. [DOI] [PubMed] [Google Scholar]
- Etheredge JA, Murchison D, Abbott LC, Griffith WH. Functional compensation by other voltage-gated Ca2+ channels in mouse basal forebrain neurons with Ca(V)2.1 mutations. Brain Res. 2007;1140:105–119. doi: 10.1016/j.brainres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Rohrbough J, Liebl F, Karr J, Sheng Q, Rodesch CK, Broadie K. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci. 2005;25:3199–3208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Ueda A, Wu CF. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology (Bethesda) 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Fukui I, Ohmori H. Tonotopic gradients of membrane and synaptic properties for neurons of the chicken nucleus magnocellularis. J Neurosci. 2004;24:7514–7523. doi: 10.1523/JNEUROSCI.0566-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Gho M, Ganetzky B. Analysis of repolarization of presynaptic motor terminals in Drosophila larvae using potassium-channel-blocking drugs and mutations. J Exp Biol. 1992;170:93–111. doi: 10.1242/jeb.170.1.93. [DOI] [PubMed] [Google Scholar]
- Gielow ML, Gu GG, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci. 1995;15:6085–6093. doi: 10.1523/JNEUROSCI.15-09-06085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, Greenspan RJ. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci U S A. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY, Dennis MJ. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977;198:87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Jin P, Griffith LC, Murphey RK. Presynaptic calcium/calmodulin-dependent protein kinase II regulates habituation of a simple reflex in adult Drosophila. J Neurosci. 1998;18:8955–8964. doi: 10.1523/JNEUROSCI.18-21-08955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci. 1989;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Kaczmarek LK. Regulation of potassium channels by protein kinases. Curr Opin Neurobiol. 1996;6:318–323. doi: 10.1016/s0959-4388(96)80114-0. [DOI] [PubMed] [Google Scholar]
- Kamb A, Iverson LE, Tanouye MA. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987;50:405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Bossing T, Technau GM, Bate M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J Neurosci. 1997;17:9642–9655. doi: 10.1523/JNEUROSCI.17-24-09642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron. 2003;37:109–120. doi: 10.1016/s0896-6273(02)01104-2. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR. A further study of the statistical composition on the end-plate potential. Journal of Physiology. 1955;130:114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Park D, Coleman MJ, Hodge JJ, Budnik V, Griffith LC. Regulation of neuronal excitability in Drosophila by constitutively active CaMKII. J Neurobiol. 2002;52:24–42. doi: 10.1002/neu.10066. [DOI] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation. J Neurosci. 2007;27:1072–1081. doi: 10.1523/JNEUROSCI.4746-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Qin G, Schwarz T, Kittel RJ, Schmid A, Rasse TM, Kappei D, Ponimaskin E, Heckmann M, Sigrist SJ. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J Neurosci. 2005;25:3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger JJ, Ueda A, Atwood HL, Govind CK, Wu CF. Role of cAMP cascade in synaptic stability and plasticity: ultrastructural and physiological analyses of individual synaptic boutons in Drosophila memory mutants. J Neurosci. 2000;20:3980–3992. doi: 10.1523/JNEUROSCI.20-11-03980.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996a;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996b;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Ultsch A, Schloss P, Cox JA, Schmitt B, Betz H. Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science. 1991;254:112–114. doi: 10.1126/science.1681587. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, Schuster CM. Experience-dependent strengthening of Drosophila neuromuscular junctions. J Neurosci. 2003;23:6546–6556. doi: 10.1523/JNEUROSCI.23-16-06546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel alpha1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Schuster CM, Goodman CS, Atwood HL. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J Neurosci. 1996;16:3877–3886. doi: 10.1523/JNEUROSCI.16-12-03877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye MA, Ferrus A. Action potentials in normal and Shaker mutant Drosophila. J Neurogenet. 1985;2:253–271. doi: 10.3109/01677068509102322. [DOI] [PubMed] [Google Scholar]
- Tanouye MA, Ferrus A, Fujita SC. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981;78:6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor FJ, Bokhari A, Rogero O, Gorczyca M, Zhang J, Kim E, Sheng M, Budnik V. Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U, Kim E, Kuhlendahl S, Koh YH, Gundelfinger ED, Sheng M, Garner CC, Budnik V. Synaptic clustering of the cell adhesion molecule fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Ueda A, Wu CF. REDUCED SYNAPTIC STRENGTH AND INCREASED VARIABILITY OF AXONAL TERMINAL BRANCHES IN DROSOPHILA MEMORY MUTANTS: PRESYNAPTIC IONIC MECHANISMS. Program No 45712 2003 Abstract Viewer/Itinerary Planner; 2003; Washington, DC: Society for Neuroscience; 2003. Online. [Google Scholar]
- Ueda A, Wu CF. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab mutations. J Neurosci. 2006;26:6238–6248. doi: 10.1523/JNEUROSCI.0862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Renger JJ, Griffith LC, Greenspan RJ, Wu CF. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM kinase II-inhibited synapses. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Warbington L, Hillman T, Adams C, Stern M. Reduced transmitter release conferred by mutations in the slowpoke-encoded Ca2(+)-activated K+ channel gene of Drosophila. Invert Neurosci. 1996;2:51–60. doi: 10.1007/BF02336660. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Haugland FN, Liu AX. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983;220:1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- Yao WD, Wu CF. Distinct roles of CaMKII and PKA in regulation of firing patterns and K(+) currents in Drosophila neurons. J Neurophysiol. 2001;85:1384–1394. doi: 10.1152/jn.2001.85.4.1384. [DOI] [PubMed] [Google Scholar]
- Yazejian B, DiGregorio DA, Vergara JL, Poage RE, Meriney SD, Grinnell AD. Direct measurements of presynaptic calcium and calcium-activated potassium currents regulating neurotransmitter release at cultured Xenopus nerve-muscle synapses. J Neurosci. 1997;17:2990–3001. doi: 10.1523/JNEUROSCI.17-09-02990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ML, Wu CF. Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J Neurosci. 1997;17:2187–2199. doi: 10.1523/JNEUROSCI.17-06-02187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Feng G, Ren D, Eberl DF, Hannan F, Dubald M, Hall LM. Cloning and characterization of a calcium channel alpha 1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J Neurosci. 1995;15:1132–1143. doi: 10.1523/JNEUROSCI.15-02-01132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Budnik V, Wu CF. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci. 1992;12:644–651. doi: 10.1523/JNEUROSCI.12-02-00644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutabaga mutants of Drosophila. J Neurogenet. 1993;9:15–27. doi: 10.3109/01677069309167273. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Neuronal activity and adenylyl cyclase in environment-dependent plasticity of axonal outgrowth in Drosophila. J Neurosci. 2004;24:1439–1445. doi: 10.1523/JNEUROSCI.0740-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K, Fetter RD, Goodman CS, Isacoff EY. Synaptic clustering of Fascilin II and Shaker: essential targeting sequences and role of Dlg. Neuron. 1997;19:1007–1016. doi: 10.1016/s0896-6273(00)80393-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effects of slo and Sh mutations on DGluRIIA and DGluRIIB density. Absolute pixel intensity for DGluRIIA and DGluRIIB immunoreactivity in double labeling experiments is compared among simultaneously processed WT, slo, and Sh larvae and measurements are normalized to that of WT ventral NMJs in the same batch, indicated by dashed lines. The number of ventral (V) and dorsal (D) NMJs examined: 15 and 15 for WT, 20 and 19 for slo, and 20 and 20 for Sh. Mean ± SD indicated. ***, P<0.001, **, P<0.01, one-way ANOVA among genotypes: +, P<0.05, +++, P<0.001, t-test for ventral (V) vs. dorsal (D) NMJs within a genotype.
Fig. S2. Similarities in EJP amplitudes and D-V differentials among WT, slo, and Sh larvae in HL3.1 saline containing 0.5 mM Ca2+.
EJP amplitudes in the scatter plots are corrected for non-linear summation (see Experimental procedures). Mean ± SEM are indicated. Number of larvae examined, ventral (V, M6/7) vs. dorsal (D, M1/9) NMJs: WT, 10 vs. 7; slo, 12 vs. 5; Sh, 11 vs. 5. *, P<0.05 and ***, P<0.001, t-test for ventral vs. dorsal NMJs.
Fig. S3. Altered receptor composition at slo NMJs throughout a single hemisegment. Reduced DGluRIIA/IIB (IIA/IIB) ratio for type Ib NMJs is found in slo mutants (slo98) compared to WT preparations stained and processed simultaneously (see Experimental procedures). See also Fig. 4 for the ratios between WT and slo in M6/7 and M1. WT data in Fig. 1E are shown as reference. The number of segments (larvae), 17 (5) for WT and 9 (3) for slo.
Fig. S4. Similar postsynaptic receptor immunoreactivity and IIA/IIB intensity ratio along the anterior-to-posterior (A-P) axis of larval body-wall musculature.
Signals from DGluRIIA and DGluRIIB immunoreactivity and their ratio between ventral (V, M6/7) and dorsal (D, M1) NMJs are color-coded for individual segments from the 3rd (AR3) to 6th (AR6) segments. There are no clear trends indicating a variation along the A-P axis. Readings from dorsal NMJs are normalized with respect to ventral NMJ values.