Abstract
The present study aimed to retrospectively evaluate the efficacy and toxicities of adjuvant chemotherapy with paclitaxel and carboplatin (TC) following concurrent cisplatin-based chemoradiation (CCRT) in patients with cervical cancer with lymphadenopathy (N1). A total of 37 patients with FIGO stage IB2-IVA cervical carcinoma with N1 (median age 57 years, range 31–74 years) were enrolled. External beam radiation therapy was followed by high-dose-rate brachytherapy. In cases of suspected para-aortic lymphadenopathy or common iliac lymph node involvement, extended radiotherapy fields were applied. Positive lymph nodes were externally radiated. Cisplatin was administered weekly at a dose of 30 mg/m2 during external beam radiation therapy. Adjuvant therapy was administered to 17 patients and comprised carboplatin (6 mg/ml/min) and paclitaxel (175 mg/m2) administered monthly after CCRT, and repeated every 4 weeks for 3–6 cycles. Over a median 21.5-month follow-up, no significant differences were found in the recurrence rate, progression-free survival, overall survival, or median interval to recurrence with N1 cervical cancer patients between the two groups. Patients with para-aortic lymphadenopathy who received CCRT and adjuvant chemotherapy had a more favorable overall and disease-free survival than those treated with CCRT alone. However, 16/17 patients developed grade 3–4 leukopenia and 14/17 patients developed severe hematologic toxicity during adjuvant chemotherapy. In conclusion, adjuvant chemotherapy consisting of full dose TC therapy after CCRT was not well tolerated in general and exhibited no benefit to N1 cervical cancer patients. However, it may be of therapeutic advantage over CCRT alone in cervical cancer patients with para-aortic lymphadenopathy.
Keywords: cervical cancer, lymphadenopathy, chemoradiation, adjuvant chemotherapy
Introduction
It is estimated that in 2005 in Japan, 8,474 women were diagnosed with cervical carcinoma and approximately 2,500 women succumbed to the disease (1). A National Cancer Institute clinical announcement based on 5 trials concluded that concurrent cisplatin-based chemoradiation (CCRT) improved survival in patients with advanced cervical carcinoma (2–6). CCRT includes a combination of external beam radiotherapy and intracavity brachytherapy. In Japan, CCRT is generally performed in patients with locally advanced disease and/or lymphadenopathy, who are at high risk for recurrence.
Green et al showed that an absolute survival benefit of 12% was potentially attributable to the use of chemoradiotherapy (7). The Radiation Therapy Oncology Group trial (RTOG) 90-01 showed after long-term follow-up that CCRT did not decrease para-aortic recurrence, and that more than 50% of patients with para-aortic recurrence revealed distant metastases after CCRT (6). No standard treatment exists for cervical cancer with lymphadenopathy after CCRT. However, studies pertaining to adjuvant chemotherapy or consolidation chemotherapy after CCRT in cases of cervical cancer with lymphadenopathy are available. Kim et al (8) compared treatment with CCRT followed by adjuvant chemotherapy to treatment with CCRT alone in FIGO stage IB and IIB bulky cervical cancers. The chemotherapeutic agents they used comprised platinum followed by 5 consecutive daily infusions of 5-fluorouracil every 3 weeks, and resulted in no therapeutic advantage (8). However, a phase II study showed that consolidation chemotherapy consisting of cisplatin and 5-fluorouracil every 3 weeks after CCRT was tolerable and effective (9).
The present study retrospectively evaluated the efficacy and toxicities of adjuvant chemotherapy with paclitaxel and carboplatin followed by CCRT for patients with cervical cancer with lymphadenopathy.
Materials and methods
Patient eligibility
Patients with FIGO Stage IB2-IVA cervical carcinoma with lymphadenopathy who were treated with CCRT at Tokushima University Hospital, Japan, were enrolled. Patients who had received chemotherapy or radiotherapy prior to this study were excluded. Eligibility criteria included: age <75 years, Eastern Cooperation Oncology Group (ECOG) performance status score of 0–1, normal cardiovascular function, normal blood cell counts, and normal serum levels of blood urea nitrogen, creatinine, and bilirubin. Criteria for malignancy determined by positron emission tomography/computed tomography (PET/CT) or magnetic resonance imaging (MRI) were a pelvic or para-aortic lymph node with a short-axis dimension of ≥1 cm or a standard uptake value (SUV) of ≥3. Each patient provided written informed consent prior to enrollment.
Patient characteristics
Between September 2005 and December 2009, 37 patients with FIGO stage IB2-IVA cervical cancer who had pelvic and/or para-aortic lymphadenopathy were treated with CCRT with or without adjuvant chemotherapy. Patient characteristics are shown in Table I. The median age was 57 years (range 31–74). Distribution of patients by stage was: IB2, n=3; II, n=17; III, n=13; and IVA, n=4. Histology revealed squamous cell carcinoma in 33 patients (89%) and other cell types in 4 patients (11%). Characteristics of patients in the CCRT-alone and CCRT plus adjuvant chemotherapy (CCRT-CTX) groups are shown in Table I. The CCRT-alone group comprised 20 patients, whereas the CCRT-CTX group comprised 17 patients. A total of 15 patients had para-aortic lymph node enlargement >10 mm on the minimum diameter as assessed by PET-CT or MRI. In the CCRT alone group, 4 patients had para-aortic lymphadenopathy and 11 patients had involvement of ≥3 pelvic lymph nodes. In the CCRT-CTX group, 11 patients had para-aortic lymphadenopathy and 12 patients had involvement of ≥3 pelvic lymph nodes.
Table I.
Patient characteristics (n=37).
| Characteristics | CCRT-alone group | CCRT-CTX group | p-value |
|---|---|---|---|
| Number of patients | 20 | 17 | |
| Median age (years) (range) | 57 (31–72) | 53 (32–72) | |
| FIGO stage | |||
| IB | 2 | 1 | NS |
| IIA | 1 | 0 | |
| IIB | 9 | 7 | |
| IIIA | 1 | 1 | |
| IIIB | 5 | 6 | |
| IVA | 2 | 2 | |
| Histology | |||
| Squamous cell carcinoma | 20 | 13 | 0.036 |
| Non-squamous cell carcinoma | 0 | 4 | |
| No. of pelvic lymph node involvement | |||
| 1 | 4 | 2 | NS |
| 2 | 5 | 3 | |
| 3 | 2 | 5 | |
| ≥4 | 9 | 7 | |
| Para-aortic lymph node involvement | |||
| Positive | 4 | 11 | |
| Negative | 16 | 6 | 0.007 |
| CCRT: Number of CDDP cycles | |||
| 1 | 0 | 1 | NS |
| 2 | 2 | 1 | |
| 3 | 3 | 3 | |
| 4 | 6 | 3 | |
| 5 | 8 | 10 |
CCRT, concurrent chemoradiotherapy; CTX, chemotherapy; NS, not significant; CDDP, cisplatin.
Radiotherapy
All 37 patients underwent radiotherapy (RT) with a combination of external beam RT (EBRT) and intracavitary brachytherapy according to the general rules for clinical and pathological management of uterine cervical cancer (10). EBRT consisting of whole pelvic RT (WPRT) and center-shielding WPRT was delivered using a 10-MV photon by a linear accelerator (PRIMUS High-Energy; Toshiba Medical Systems Co., Tochigi, Japan) with each patient in the supine position. Gross tumor volume (GTV) was defined as the primary tumor and involved lymph nodes. Clinical target volume (CTV) encompassed the GTV with 0.5-cm isotropic margin and uterus, presacral, common iliac, internal iliac, upper external iliac, and obturator lymph node. Planning target volume (PTV) was defined as the CTV with an isotropic margin of 1 cm. A total of 5 weekly fractions of 2.0 Gy per fraction, at a total dose of 20–40 Gy in 10–20 fractions, were delivered to the isocenter with WPRT using a four-field box technique. The dose distributions were computed using the Convolution Algorithm implemented in the Xio planning system (CMS Inc., St. Louis, MO, USA). These fields realized dose delivery to the PTV of not <95% of the prescription dose. An additional 10–30 Gy in 5–15 fractions was delivered with center-shielding WPRT using anteroposterior and posteroanterior parallel-opposing fields after WPRT at a total dose of 50 Gy in 25 fractions. High-dose-rate (HDR) intracavitary brachytherapy using a high-activity Ir-192 source was started 7–21 days after the initiation of EBRT. HDR intracavitary brachytherapy and EBRT were not administered on the same day. A total dose of 15 Gy in 3 fractions or 24 Gy in 4 fractions at Point A was delivered by weekly HDR brachytherapy. When para-aortic lymph node or common iliac lymph node involvement was suspected, patients were treated with extended EBRT fields including para-aortic lymph nodes using anteroposterior and postroanterior parallel-opposing portals. The superior border of the para-aortic field was a transverse line through the T12-L1 intervertebral space. The total dose delivered to the whole pelvis and para-aortic lymph node was 45 Gy adminsitered at a dose of 1.8 Gy per fraction. Concomitant boost of 6–10 Gy in 3–5 fractions was added to the metastatic lymph nodes in all clinically node-positive patients.
Chemotherapy
The protocol for concurrent chemotherapy was based on a previous report, and consisted of cisplatin administered weekly at a dose of 30 mg/m2 during EBRT (11). Granisetron (3 mg) was routinely administered as an anti-emetic treatment, and 8 mg of dexamethasone was received by patients who complained of severe nausea. Cisplatin was withheld if grade ≥3 gastrointestinal toxicities appeared, or when either the leukocyte count was <3,000/mm3 or the platelet count was <100,000/3.
Adjuvant chemotherapy
TC therapy [carboplatin (AUC = 6) and paclitaxel (175 mg/m2)] was administered monthly following CCRT. Paclitaxel was administered as a 3-h intravenous infusion and carboplatin as a 2-h intravenous infusion. Chemotherapy was repeated every 4 weeks for 3–6 cycles. Toxicity of the regimen was determined according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. In cases of severe toxicity (grade ≥3), chemotherapy was postponed until toxic symptoms disappeared. Chemotherapy was interrupted when the leukocyte count was <3,000/mm3, platelet count was <100,000/mm3, hemoglobin was <80 g/l, or grade ≥3 non-hematologic toxicity developed.
Study endpoint
The primary endpoint was acute toxicity. Acute toxicity was assessed according to the National Cancer Institute-Common Toxicity Criteria (NCI-CTC). Secondary endpoints were treatment failures, survival rates, disease-free survival rate, and late toxicity. Late toxicity for RT was assessed according to the RTOG criteria. Overall survival (OS) was calculated from the time of initiation of therapy to the time of death or last visit. Progression-free survival (PFS) was measured from the initiation of therapy to the date of last visit or the radiographic evidence of progressive disease.
Statistical analysis
Treatment outcomes were compared between the CCRT-alone and CCRT-CTX groups retrospectively. Survival curves were estimated by the Kaplan-Meier method and compared using the log-rank test. The differences between the two groups according to treatment assignment and patient characteristics were assessed using the Chi-square analysis.
Results
Toxicity
A total of 151 cycles of CCRT and 78 cycles of adjuvant chemotherapy were administered. Table II shows the patients who developed acute toxicity. A total of 27 patients (73%) received ≥4 cycles of cisplatin chemotherapy (CDDP) during RT. In the remaining 4 patients, CDDP was interrupted by gastrointestinal toxicity in 3 patients, and by failure of renal function in 1 patient. None of the patients succumbed to their disease during treatment. During CCRT, grade 3 leukopenia accounted for 48.6% (18 patients) of toxicity cases. Grade 3–4 anemia occurred in 4 patients (11%). No patients suffered from thrombocytopenia during CCRT. A total of 19 patients were treated with extended field radiotherapy. Table IIA shows the toxicity with or without para-aortic irradiation. A total of 12 patients (63%) who were treated with extended field irradiation developed grade 3–4 hematologic toxicity. No significant difference was observed in grade 3–4 hematologic toxicity with or without para-aortic irradiation during CCRT (p=0.121). Grade 3 gastrointestinal toxicity during CCRT occurred in 5 patients (13%).
Table II.
Characteristics of patients in the CCRT-alone and CCRT plus adjuvant chemotherapy groups.
| A, Acute systemic toxicity during CCRT. | |||
|---|---|---|---|
| Non-extended field n=18 (%) |
Extended field n=19 (%) |
p-value | |
| ≥4 cycles of CDDP | 14 (78) | 13 (68) | 0.713 |
| Number of patients with grade 3–4 acute toxicity | |||
| Hematologic toxicity | 8 (44) | 12 (63) | 0.121 |
| Leukopenia | 7 (38) | 11 (58) | |
| Thrombocytopenia | 0 (0) | 0 (0) | |
| Anemia | 1 (5.3) | 3 (16) | |
| Gastrointestinal toxicity | 1 (5.3) | 4 (22) | 0.153 |
| B, Acute systemic toxicity during adjuvant chemotherapy. | |||
|---|---|---|---|
| Non-extended field n=4 (%) |
Extended field n=13 (%) |
p-value | |
| Number of patients with grade 3–4 acute hematologic toxicity | 4 (100) | 13 (100) | NS |
| Leukopenia | 4 (100) | 12 (92) | |
| Thrombocytopenia | 0 (0) | 3 (23) | |
| Anemia | 0 (0) | 7 (54) | |
| C, Late treatment-related toxicities. | ||||
|---|---|---|---|---|
| CCRT-alone (n=20) | CCRT-CTX (n=17) | |||
| Grade 1–2 | Grade 3–5 | Grade 1–2 | Grade 3–5 | |
| Site of effect | ||||
| Gastrointestinal | 3 | 1 | 2 | 1 |
| Genitourinary | 0 | 0 | 1 | 0 |
| Insufficiency fractures | 1 | 0 | 2 | 0 |
Adjuvant chemotherapy following CCRT and complications of treatment
Adjuvant CT consisted of 3–6 cycles of TC therapy after CCRT. Only 1 patient was treated with weekly TC therapy due to severe hematologic toxicity during CCRT, and 2 patients were treated with docetaxel and carboplatin (DC) therapy due to numbness. The median interval of CCRT and adjuvant chemotherapy was 44 days. The median inter-cycle intervals of chemotherapy were 28±8.7, 28±7.1, 35±14.5, 28±6.7 and 28±9.3 days. Table IIB shows that during adjuvant chemotherapy grade 3–4 leukopenia occurred in almost all of the patients (16/17 patients). Grade 3 or higher anemia and thrombocytopenia occurred in 7 and 3 patients, respectively. Severe hematologic toxicity in 14 patients (82%) resulted in a dose reduction of the agents by 20%. Due to prolonged hematologic toxicities, 2 patients discontinued chemotherapy. Chemotherapy schedules were delayed and dose-reduced in 14 patients (82%) and 2 patients (5%) discontinued adjuvant chemotherapy due to hematologic toxicity. Adjuvant chemotherapy comprising full-dose TC therapy following CCRT was not well tolerated in general.
Late complications of treatment are shown in Table IIC. A total of 9 patients (24%) had grade 2 late complications. There were 2 incidences of grade 3 or 4 gastrointestinal toxicity, but no late treatment-related fatalities.
Treatment outcome
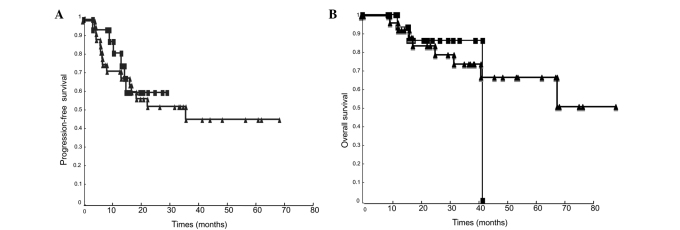
The median follow-up was 21.5 months (range 14.8–36). A total of 13 patients (35%) had recurrence (8 in the CCRT-alone group, and 5 in the CCRT-CTX group). No significant differences were found in the recurrence rate, PFS (p=0.751) or OS (p=0.813) (Fig. 1). The median interval to recurrence was 6.7 months (range 4.3–22.) without adjuvant chemotherapy and 10.4 months (range 3.2–14.8) with adjuvant chemotherapy. In the CCRT-alone group, 8 patients had recurrence, and the recurrence sites were inside the radiation field in 6 patients and outside the radiation field in 3 patients (Table III). In the CCRT-CTX group, 5 patients had recurrence, and the recurrence sites were inside the radiation field in 4 patients and outside the radiation field in 2 patients. The cumulative incidence of lymph node metastases was 20 and 23%. The incidence of distant metastases, including para-aortic lymph node metastases, was not reduced by adjuvant chemotherapy. A total of 7 patients had locoregional recurrences: 5 pelvic recurrences without adjuvant chemotherapy and only 2 recurrences with adjuvant chemotherapy. No significant differences were observed in the patterns of failure between the two groups.
Figure 1.
Survival curve among patients with lymphadenopathy in the CCRT-only and CCRT-CTX groups. (A) Progression free-survival (p=0.751). (B) Overall survival (p=0.813). ■, CCRT-CTX group; ▲, CCRT-only group.
Table III.
Pattern of recurrence.
| A, Pattern of recurrence. | |||
|---|---|---|---|
| CCRT-alone (n=20) | CCRT-CTX (n=17) | p-value | |
| Recurrence | 8 (40%) | 5 (29%) | |
| Locoregional failure | 5 (25%) | 2 (12%) | NS |
| Lymph node failure | 4 (20%) | 4 (23%) | NS |
| Distant metastasis (excluding para-aortic failure) | 2 (10%) | 1 (6%) | NS |
| Inside radiation field | 6 (30%) | 4 (23%) | NS |
| Outside radiation field | 3 (15%) | 2 (12%) | NS |
| B, Pattern of recurrence of patients who underwent para-aortic lymphadenopathy (n=15). | |||
|---|---|---|---|
| CCRT-alone (n=4) | CCRT-CTX (n=11) | p-value | |
| Recurrence | 3 (75%) | 4 (36%) | NS |
| Locoregional failure | 2 | 1 | NS |
| Lymph node failure | 2 | 4 | NS |
| Distant metastasis (excluding para-aortic failure) | 1 | 3 | NS |
NS, not significant.
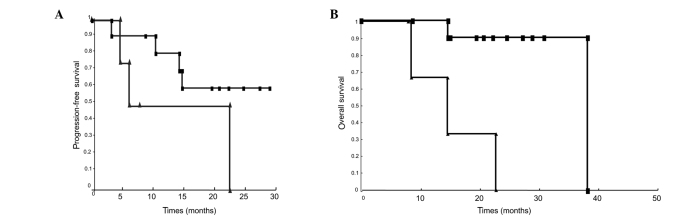
Para-aortic lymphadenopathy was demonstrated by imaging in 15 patients: 4 in the CCRT-alone group and 11 in the CCRT-CTX group. A total of 6 patients (40%) with para-aortic lymphadenopathy had lymph node failure: 2 patients without adjuvant chemotherapy and 4 patients with adjuvant chemotherapy. In the CCRT-CTX group, the estimated 2-year PFS was 60.1% and OS was 90% (Fig. 2).
Figure 2.
Survival curve among patients who had para-aortic lymphadenopathy in the CCRT-only and CCRT-CTX groups. (A) Progression-free survival (p=0.120). (B) Overall survival (p=0.001). ■, CCRT-CTX group; ▲, CCRT-only group.
Discussion
Lymph node metastasis is one of the most significant prognostic factors of cervical cancer. According to the literature, the incidence of lymph node involvement increases with the FIGO stage: it occurs in 12–22% of stage IB, 10–27% of stage IIA, and 34–43% of stage IIB (12). A GOG (Gynecology Oncology Group) study reported that the para-aortic lymph node metastasis in cervical cancer was 6% for stage I, 16% for stage II, and 25% for stage III (13). In the GOG study, the patients underwent surgical staging, and the 3-year survival for those with stage IIB and IIIB cancers was 25%. Para-aortic lymph involvement was also a significant prognostic factor (14). Para-aortic node metastases occurred in 76% of patients with positive common iliac lymph nodes, and in 19% of patients without common iliac lymph node involvement. Common iliac and para-aortic lymph node metastases are associated with markedly poor survival.
Evidence for the use of CCRT has been established for patients without para-aortic node metastases through the pathological evaluation of lymph node status by surgical staging. In the present study, lymphadenectomy was not performed prior to CCRT, as is common in Japan. Treatment was planned by analysis of MRI or PET-CT, and not by surgical staging. Evaluation of lymph node involvement with non-invasive techniques such as MRI provides a sensitivity and specificity near 80% when nodes are >1 cm. Such percentages may decrease to 24–48% in cases of lymph nodes with small metastases (14,15). Compared to the results observed with regards to CT alone, PET-CT may detect smaller para-aortic disease (16).
At least 7 trials have demonstrated an apparent improvement in local pelvic failures [19.3% (223/1155) in a CCRT group versus 31.7% (313/986) in an RT-alone group]. CDDP in CCRT may have a synergistic effect on micrometastases (17). In addition, Green et al demonstrated a significant benefit of CCRT on local and distant recurrences (7). In the present study, 73% (33/37) of patients had ≥3 cycles of CDDP, and 81% (30/37) of patients had no local pelvic failures.
For the purpose of modulating micrometastases and lymph node metastases, patients with pelvic or para-aortic lymph node involvement were treated with extended field radiotherapy. CCRT with extended field pelvic para-aortic irradiation has been reported (18,19). A GOG study showed that radiotherapy with extended field was completely treated in 37% of patients at 8 weeks and 68% of patients at 9 weeks (19). TP therapy [paclitaxel (40 mg/m2) and cisplatin (40 mg/m2)] with extended pelvic and para-aortic beam RT was acceptable. In the present study, grade ≥3 acute hematologic toxicity was observed in 63% (12/19) of patients who received extended field radiation and CDDP (30 mg/m2) chemotherapy as CCRT. The regimen with pelvic and para-aortic lymph irradiation was tolerable.
In a prospective study, Choi et al reported that consolidation chemotherapy with cisplatin and 5-fluorouracil following CCRT was well tolerated and effective in patients with locally advanced cervical carcinoma (9). Zhang et al reported CCRT with paclitaxel (135 mg/m2) and nedaplatin (60 mg/m2) followed by consolidation chemotherapy with FIGO stage IIB–IIIB cervical cancer (20). These authors found that consolidation chemotherapy every 3 weeks for 4 cycles was effective and well tolerated: 31 patients (91%) received more than 3 cycles of consolidation chemotherapy.
TP therapy has been reported to be highly effective in advanced and recurrent cervical cancer (21). TC therapy has been reported to be as effective as TP therapy (22). The chemotherapy comprising paclitaxel and a platinum agent has been evaluated, and in the present study, TC therapy after CCRT was administered as adjuvant chemotherapy and comprised paclitaxel (175 mg/m2) and carboplatin (AUC = 6). Adjuvant chemotherapy following CCRT increased the severity of the hematologic toxicities, whereas the frequency of other toxicities was not different between the 2 groups. In the two groups, a total of 5% (2/37) of patients had grade ≥3 late toxicities. In the RTOG 90-01 study, grade ≥3 late gastrointestinal toxicities occurred 3–9% of the time over 5 years (6). In the present study, 3 more cycles of chemotherapy consisting of paclitaxel and carboplatin were administered following CCRT. Adjuvant chemotherapy schedules were either delayed or dose-reduced in 14 patients (82%), and 2 of 37 patients (5%) were unable to receive adjuvant chemotherapy due to toxicity. Therefore, due to the small number of patients, it is unclear as to whether the chemotherapy dose in this study was tolerable.
In conclusion, patients with para-aortic lymph node metastasis in stage IB2-IVA cervical cancer who received CCRT and adjuvant chemotherapy had a more favorable survival than those treated with CCRT alone. However, no advantage of adjuvant chemotherapy was observed in patients with pelvic and para-aortic lymphadenopathy. Future clinical trials are required for confirmation of the clinical efficacy of adjuvant chemotherapy.
References
- 1.Center for Cancer Control and Information Services, National Cancer Center, Japan, 2010 (ganjoho.jp).
- 2.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 3.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 4.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, Clarke-Pearson DL, Liao SY. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para–aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 5.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, III, Walker JL, Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 6.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 7.Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Colingwood M, Williams CJ. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim YB, Cho JH, Keum KC, Lee CG, Seong J, Suh CO, Kim GE. Concurrent chemoradiotherapy followed by adjuvant chemotherapy in uterine cervical cancer patients with high-risk factors. Gynecol Oncol. 2007;104:58–63. doi: 10.1016/j.ygyno.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Choi CH, Lee JW, Kim WY, Nam HR, Kim BG, Hum SJ, Lee JH, Bae DS. Phase II study of consolidation chemotherapy after concurrent chemoradiation in cervical cancer: preliminary results. Int J Rad Oncol Biol Phys. 2007;68:817–822. doi: 10.1016/j.ijrobp.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 10.Japan Society of Gynecologic Oncology. Cervical cancer treatment guidelines. 2007. [Google Scholar]
- 11.Ikushima H, Osaki K, Furutani S, Yamashita K, Kawanaka T, Kishida Y, Wamoto S, Takegawa Y, Kudoh T, Nishitani H. Chemoradiation therapy for cervical cancer: toxicity of concurrent weekly cisplatin. Rad Med. 2006;24:115–121. doi: 10.1007/BF02493277. [DOI] [PubMed] [Google Scholar]
- 12.Sakuragi N. Up-to-date management of lymph node metastasis and the role of tailred lymphadenenctomy in cervical cancer. Int J Clin Oncol. 2007;12:165–175. doi: 10.1007/s10147-007-0661-2. [DOI] [PubMed] [Google Scholar]
- 13.Berman ML, Keys H, Creasman W, Reasman W, DiSaia P, Bundy B, Blessing J. Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes. Gynecol Oncol. 1984;19:8–16. doi: 10.1016/0090-8258(84)90151-3. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti PP, Basile S, Angioli R. Pelvic and aortic lymphadenectomy in cervical cancer: the standardization of surgical procedure and its clinical impact. Gynecol Oncol. 2009;113:284–290. doi: 10.1016/j.ygyno.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Vidaurreta J, Bermudez A, Di Paola G, Sardi J. Laparoscopic staging in locally advanced cervical carcinoma: a new possible philosophy? Gynecol Oncol. 1999;75:366–371. doi: 10.1006/gyno.1999.5597. [DOI] [PubMed] [Google Scholar]
- 16.Grigsby PW, Dehdashi F, Siegel BA. FDG-PET evaluation of carcinoma of the cervix. Clin Pos Image. 1999;2:105–109. doi: 10.1016/s1095-0397(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 17.Rose PG, Bundy BN. Chemoradiation for locally advanced cervical cancer: does it help? J Clin Oncol. 2002;20:891–893. doi: 10.1200/JCO.2002.20.4.891. [DOI] [PubMed] [Google Scholar]
- 18.Grigsby PW, Perez CA, Chao KS, Herog T, Mutch DG, Rader J. Radiation therapy for carcinoma of the cervix with biopsy-proven positive para-aortic lymph nodes. Int J Rad Oncol Biol Phys. 2001;49:733–738. doi: 10.1016/s0360-3016(00)00806-3. [DOI] [PubMed] [Google Scholar]
- 19.Walker JL, Morrison A, DiSilvestro P, von Gruenige VE. A phase I/II study of extended field radiation therapy with concomitant paclitaxel and cisplatin chemotherapy in patients with cervical carcinoma metastatic to the para-aortic lymph nodes. Gynecol Oncol. 2009;112:78–84. doi: 10.1016/j.ygyno.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MQ, Liu SP, Wang XE. Concurrent chemotherapy with paclitaxel and nedaplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: preliminary results of phase II study. Int Rad Oncol Biol Phys. 2010;78:821–827. doi: 10.1016/j.ijrobp.2009.08.069. [DOI] [PubMed] [Google Scholar]
- 21.Rose PG, Lessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 1999;17:2676–2680. doi: 10.1200/JCO.1999.17.9.2676. [DOI] [PubMed] [Google Scholar]
- 22.Sit AS, Kelley JL, Gallion HH, Kunschner AJ, Edwards RP. Paclitaxel and carboplatin for recurrence or persistent cancer of the cervix. Cancer Invest. 2004;22:368–373. doi: 10.1081/cnv-200029062. [DOI] [PubMed] [Google Scholar]