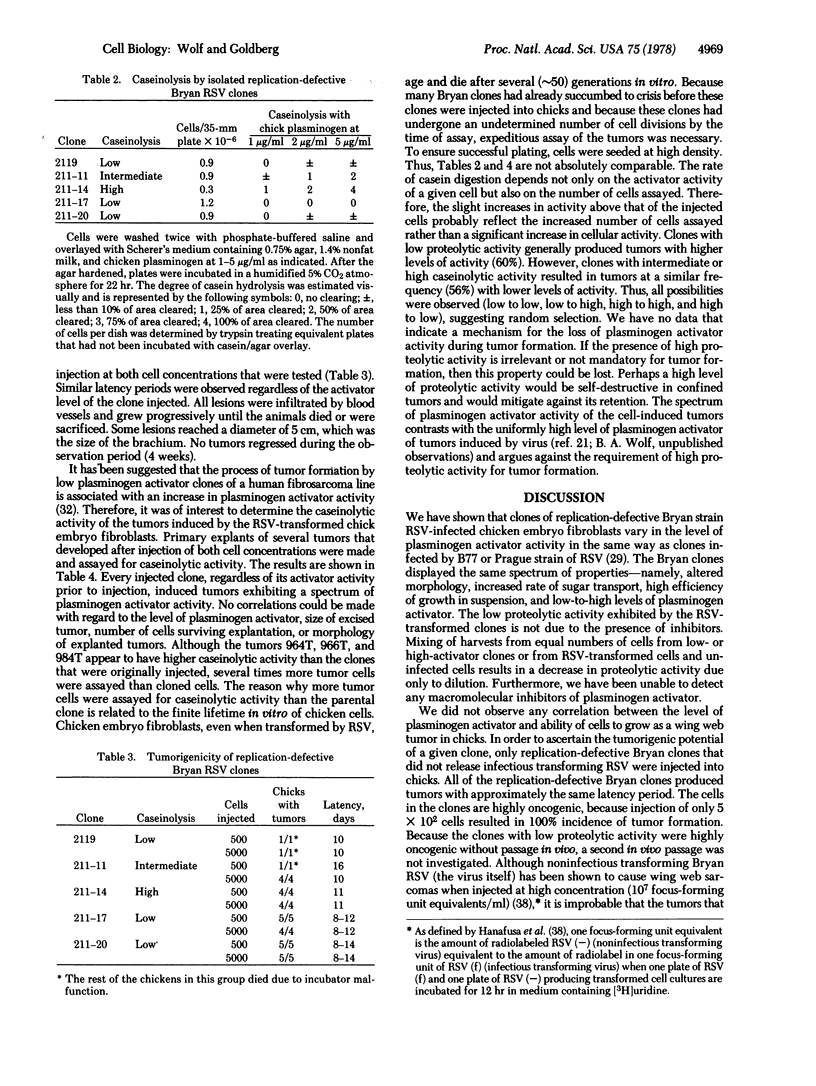
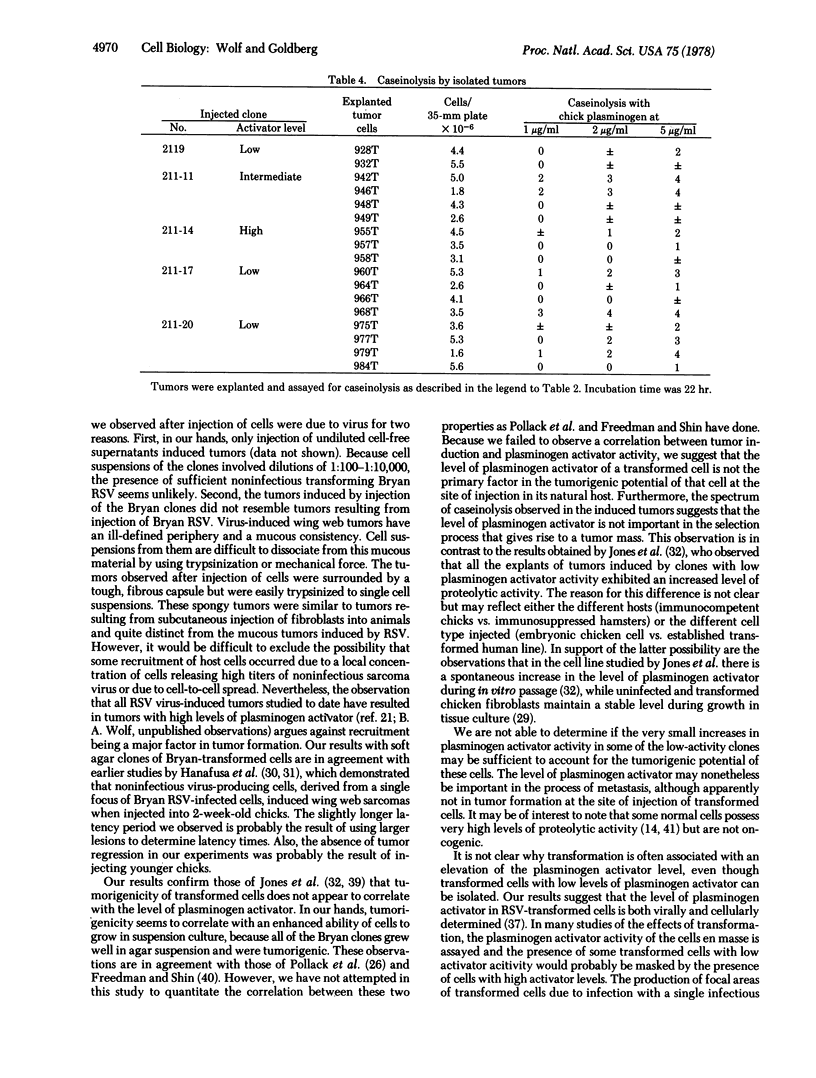
Abstract
We have previously isolated, from agar suspension culture, clones of chicken embryo fibroblasts transformed by B77 and Prague strains of Rous sarcoma virus (RSV) that varied in the expression of plasminogen activator activity [Wolf, B. A. & Goldberg, A. (1976) Proc. Natl. Acad. Sci. USA 73, 3613-3617]. All of the clones exhibited an altered cellular morphology, an increased rate of sugar transport, and a high efficiency of colony formation in agar suspension regardless of the level of plasminogen activator. Because B77 and Prague strains of RSV replicate as well as cause sarcomas in chickens, the tumorigenicity of the transformed cells could not be evaluated with clones of these cells. In order to determine the oncogenicity of clones with various levels of plasminogen activator, it was necessary to isolate cells transformed by the replication-defective Bryan strain of RSV, which release noninfectious virus. All of the agar suspension clones of transformed cells, derived by infection of chicken embryo cells with replication-defective Bryan RSV, fell within the continuum observed for B77- and Prague-transformed clones with respect to altered morphology, increased rate of sugar transport, efficiency of colony formation in agar suspension, and variations in plasminogen activator activity. All of the clones, regardless of the level of plasminogen activator, produced tumors when as few as 5 × 102 cells were injected into the wing web of 1-day-old chicks. The latency period for tumor formation after injection of cells was similar regardless of the level of plasminogen activator of the injected cell. Primary explants of tumors resulting from inoculation of clones having low, intermediate, or high activator activity displayed a spectrum of activator activity.
Keywords: protease, caseinolysis, fibrinolysis, soft agar colony formation, altered morphology
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Balduzzi P. C., Murphy H. Plaque assay of avian sarcoma viruses using casein. J Virol. 1975 Sep;16(3):707–711. doi: 10.1128/jvi.16.3.707-711.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Isolement et étude d'un mutant conditionnel du virus de Rous à capacité transformante thermosensible. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2430–2433. [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Chou I. N., Black P. H., Roblin R. O. Non-selective inhibition of transformed cell growth by a protease inhibitor. Proc Natl Acad Sci U S A. 1974 May;71(5):1748–1752. doi: 10.1073/pnas.71.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Friis R. R., Toyoshima K., Vogt P. K. Conditional lethal mutants of avian sarcoma viruses. I. Physiology of ts 75 and ts 149. Virology. 1971 Feb;43(2):375–389. doi: 10.1016/0042-6822(71)90310-2. [DOI] [PubMed] [Google Scholar]
- Goetz I. E., Weinstein C., Roberts E. Effects of protease inhibitors on growth of hamster tumor cells in culture. Cancer Res. 1972 Nov;32(11):2469–2474. [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Involvement of cell and serum proteases in exposing tumor-specific agglutinin sites. Ann N Y Acad Sci. 1974;234(0):348–354. doi: 10.1111/j.1749-6632.1974.tb53047.x. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS, II. SPECIFICATION OF RSV ANTIGENICITY BY HELPER VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:41–48. doi: 10.1073/pnas.51.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Aoki T., Kawai S., Miyamoto T., Wilsnack R. E. Presence of antigen common to avian tumor viral envelope antigen in normal chick embryo cells. Virology. 1973 Nov;56(1):22–32. doi: 10.1016/0042-6822(73)90284-5. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Benedict W. F. Fibrinolytic activity in a human fibrosarcoma cell line and evidence for the induction of plasminogen activator secretion during tumor formation. Cell. 1975 Oct;6(2):245–252. doi: 10.1016/0092-8674(75)90015-x. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Rhim J. S., Isaacs H., Jr, McAllister R. M. The relationship between tumorigenicity, growth in agar and fibrinolytic activity in a line of human osteosarcoma cells. Int J Cancer. 1975 Oct 15;16(4):616–621. doi: 10.1002/ijc.2910160411. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Mott D. M., Fabisch P. H., Sani B. P., Sorof S. Lack of correlation between fibrinolysis and the transformed state of cultured mammalian cells. Biochem Biophys Res Commun. 1974 Nov 27;61(2):621–627. doi: 10.1016/0006-291x(74)91002-x. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Ossowski L., Reich E. Plasminogen, the serum proenzyme activated by factors from cells transformed by oncogenic viruses. J Biol Chem. 1974 Jul 10;249(13):4306–4311. [PubMed] [Google Scholar]
- Schnebli H. P., Burger M. M. Selective inhibition of growth of transformed cells by protease inhibitors. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3825–3827. doi: 10.1073/pnas.69.12.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- Weber M. J. Inhibition of protease activity in cultures of rous sarcoma virus-transformed cells: effect on the transformed phenotype. Cell. 1975 Jul;5(3):253–261. doi: 10.1016/0092-8674(75)90100-2. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Goldberg A. R. Rous-sarcoma-virus-transformed fibroblasts having low levels of plasminogen activator. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3613–3617. doi: 10.1073/pnas.73.10.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]



