Abstract
Previous studies reported that serum amyloid A (SAA) is elevated in patients with tumors, including breast cancer, compared to healthy controls. In addition, the levels of SAA increase gradually with tumor progression. In this study, we investigated the blood SAA level of breast cancer patients, and evaluated its potential as a serum biomarker for the early diagnosis of breast cancer and as a staging estimate. SAA protein was determined by enzyme-linked immunosorbent assay in serum samples from 30 healthy women, 21 women with benign diseases and 118 breast cancer patients who were subdivided into 4 groups based on their clinical characteristics. SAA levels were not statistically different in stage I breast cancer patients compared with the healthy controls and benign breast disease patients. SAA concentrations had medians of 0.63 µg/ml in normal healthy women, 0.76 µg/ml in patients with benign disease (p>0.05) and 0.82 µg/ml in stage I breast cancer patients (p>0.05). By contrast, SAA values in stage Ⅱ, Ⅲ and Ⅳ patients had a significantly higher median compared to those of the healthy, benign breast diseases and stage I groups (p<0.05). Breast cancer patients with lymph node (LN) metastasis or distant metastasis were found to have significantly higher SAA concentrations than those without metastases. SAA is not a suitable marker for early breast cancer diagnosis, but its level is correlated with the stage of breast cancer. Thus, it may be a good candidate marker for the staging and prognosis of breast cancer.
Keywords: serum amyloid A, breast cancer, staging, diagnosis
Introduction
In the last few decades, the incidence of breast cancer has increased; however, after 1990 the mortality rate of breast cancer decreased, due to widespread breast cancer screening and the general use of anticancer agents (1). The 5-year survival rates have improved to 98% for early stage and 39% for late stage breast cancer (2). Thus, early detection is vital to improve the prognosis of cancer patients. At present, mammography screening has made a substantial contribution to the reduction of breast cancer mortality (3,4). However, the limitation of mammography screening is that a breast tumor is required to be at least a few millimeters in size for detection (5). For solid tumors, the ideal detection threshold should be prior to the angiogenic switch; therefore, cancer imaging techniques are not enough. The tumor initiates malignant transformations, such as turning on the angiogenic switch, developing invasive properties, or even establishing distant micro-metastases, prior to detection (6). Early diagnosis of breast cancer requires non-invasive and specific biomarkers; among them, blood markers may offer a good surrogate selection.
High-throughput proteome analysis deciphering the global changes in protein expression holds promise for the discovery of novel molecular tumor markers. Using this technology, a number of proteins associated with breast cancer have been found. Among those identified candidate target proteins, serum amyloid A (SAA), as an acute-phase reactant, is up-regulated in a wide range of malignancies, such as lung, pancreatic, prostate, colon and gastric cancer (7-16). Being the precursor protein in inflammation-associated reactive amyloidosis, the SAA level in the blood is also increased in response to various insults including trauma, various inflammations and neoplasia (17). Therefore, it was formerly considered that the elevation of SAA in the serum of cancer patients is of liver origin rather than from tumor cells. However, recent data indicated that certain tumor cells (such as endometrial and colon cancer cells) also synthesize and secrete SAA (15,16,18,19). SAA is an inflammatory modulator that is involved in cholesterol metabolism and transport and is important in tumor progression (20-22). Clinically, SAA was increased gradually as tumors progressed and the SAA value showed a direct correlation with the stage of the tumor. In conventional renal cell carcinomas, expression of the SAA1 protein in tumor cells correlated with poor course-specific survival. SAA1 also supports tumor invasion and MMP expression (23). With regard to breast cancer, the proteomic mass spectrometry showed that the blood level of SAA is significantly higher in stage Ⅳ compared with stage I (24). High-fat diet in the F2 mouse, which was generated from a cross between the M16i polygenic obese and MMTV-PyMT mammary cancer models, is capable of significantly altering the expression of the SAA gene. These mice show a decrease in mammary cancer latency and an increase in pulmonary metastases (25). Moreover, elevated SAA and CRP were associated with reduced overall survival, regardless of adjustment for age, tumor stage, ethnicity and body mass index (26). In this study, we employed an enzyme immunoassay system to measure the SAA level in serum from breast cancer patients and healthy age-matched women, and our results revealed that SAA may be a good candidate marker for the staging and prognosis of breast cancer.
Materials and methods
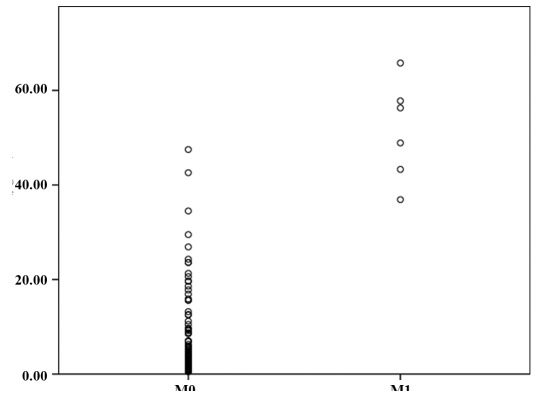
Patients and samples. A total of 118 breast carcinoma patients and 51 healthy age-matched female individuals were recruited from the Beijing Tiantan Hospital Affiliated to Capital Medical University, China. The ages of these patients ranged between 26 and 83 (mean, 54.5). Fasting morning serum samples were obtained from the patients with breast carcinoma and healthy age-matched women. Serum samples were frozen and maintained at -80˚C until the assay was conducted. All 169 breast cancer patients and volunteers signed informed consent forms for sample collection. The main characteristics of the patients with respect to tumor size, stage, nodal status and distant metastasis are shown in Table I. The study was approved by the ethics committee of the hospital.
Table I.
Clinical characteristics of breast cancer patients analyzed for SAA concentration in blood by ELISA.
Enzyme-linked immunosorbent assay. Serum concentrations of SAA were determined using a commercially available Human SAA ELISA Kit (YES Biotech Laboratories Ltd., Canada) according to the manufacturer's instructions. Samples that fell outside the linear detection range of the standard curves were diluted and re-measured. Briefly, 100 µl standard reagent or sample duplicate was added to the appropriate well with antibody pre-coated in the microtiter plate. The plate was then covered and incubated for 1 h at room temperature; plates were then washed 5 times with washing buffer (1X). Conjugate buffer (100 µl) was added to each well, the plates were incubated for 1 h at room temperature and then washed 5 times. Substrate solution (100 µl) was added to each well, followed by incubation for 15 min at room temperature. Subsequently, 100 µl stop solution was added to each well and mixed thoroughly. The optical density (OD) at 450 nm was read using a spectrophotometer (SLT Labinstruments, Salzburg, Austria) within 30 min. The concentration of SAA in the sera was interpolated from a standard curve, which was generated using the respective recombinant protein.
Statistical analysis. SPSS 17.0 statistical software was used for statistical analysis. SAA serum concentrations among the various groups (i.e., healthy controls, benign breast disease, and breast cancer groups with different degree of differentiation) were summarized as medians and ranges. The Wilcoxon rank-sum test or the Kruskal-Wallis test was used to evaluate the differences in SAA concentrations between the study groups. Spearman's rank correlation was also used to check the correlation between SAA concentrations and stages of breast cancer. For all tests, p<0.05 was considered to indicate a statistically significant difference.
Results
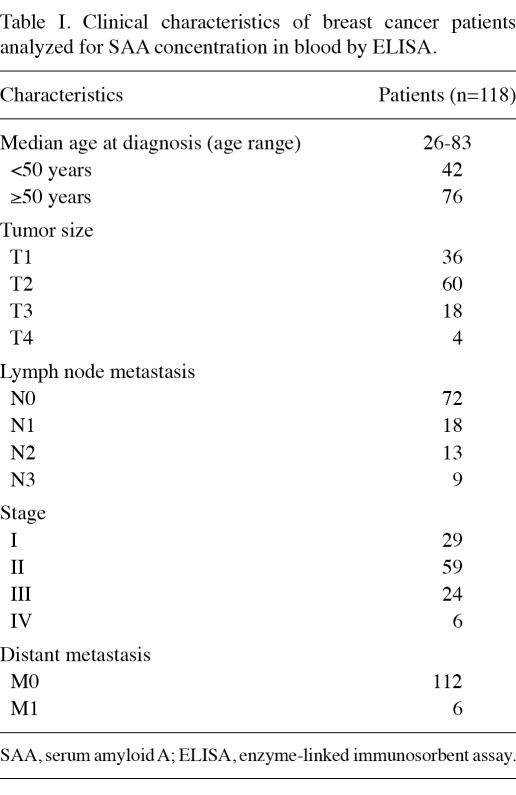
SAA protein in controls and at different breast cancer stages. The concentrations of SAA protein in normal controls and in patients at various stages of breast cancer were determined (Fig. 1). Non-Gaussian distributions of values were identified (Kolmogorov-Smirnov test), and evaluation of the data was performed using the non-parametric Wilcoxon rank-sum test or the Kruskal-Wallis test. The results of the statistical assessment showed the median serum SAA concentration to be 0.82 µg/ml (range, 0.21-4.53) in stage Ⅰ breast cancer patients (n=29) and 0.63 µg/ml (range, 0.21-2.60) and 0.76 µg/ml (range, 0.15-1.60) in the healthy controls (n=30) and benign breast diseases (n=21). No statistically significant difference was found in SAA concentrations among the healthy controls, benign breast disease and stage I breast cancer patients (p>0.05), but the level of SAA in increasing stages of breast cancer was gradually elevated. The median serum SAA concentration of stage Ⅱ (n=59), stage Ⅲ (n=24) and stage Ⅳ (n=6) was 3.90 µg/ml (range, 1.02-10.48), 19.645 µg/ml (range, 6.98-41.2) and 52.6 µg/ml (range, 36.9-65.8), respectively. The level of SAA in these stages of breast cancer was significantly different, and significantly higher than the healthy controls, benign breast disease and stage I breast cancer patients (p<0.05). Moreover, SAA concentrations correlated positively with tumor stages (rs=0.837, p<0.05). These results indicate that SAA concentrations are correlated with the clinical stage of breast cancer.
Figure 1.
Concentrations of SAA in controls, benign breast disease and breast cancer patients at various stages of disease are shown. The Kruskal-Wallis test shows significant differences between the groups (p<0.05). Differences between certain groups are indicated by p-values calculated by the Mann-Whitney U-test. Spearman's rank correlation was used to check the correlation between SAA concentrations and the stage of breast cancer. SAA, serum amyloid A.
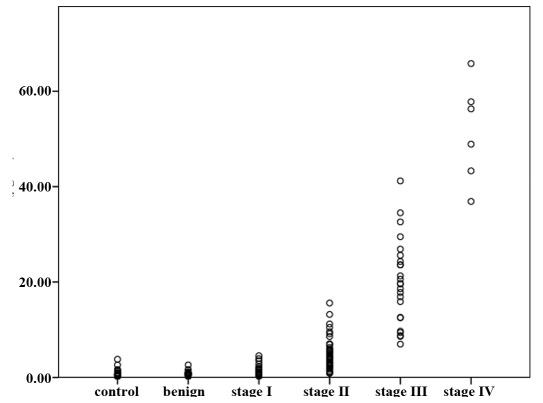
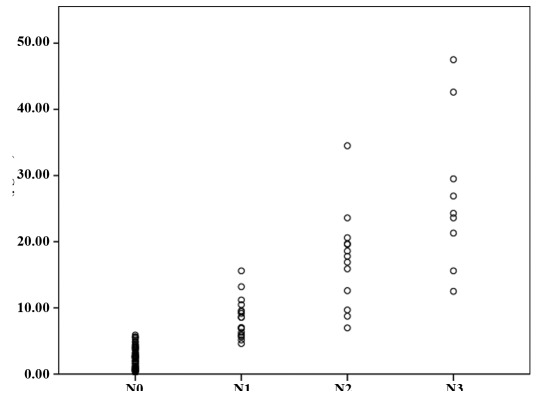
Correlation of SAA protein with established clinicopathological data. We further analyzed the correlation between SAA blood concentration and clinical features in patients with breast cancer. Age was not significantly correlated with concentration (p>0.05), whereas a significant correlation was observed with tumor size. We performed an exploratory analysis and compared SAA levels between different lymph node metastasis grades of breast cancer. The median serum SAA concentrations of N0 (n=72), N1 (n=18), N2 (n=13) and N3 (n=6) were 2.42 µg/ml (range, 0.21-5.46), 7.81 (range, 4.6-10.48), 17.80 µg/ml (range, 6.98-34.5) and 24.3 µg/ml (range, 12.5-47.5), respectively. A significant difference (p<0.05) among these groups was noted (Fig. 2). Additional data were available concerning the character of distant metastases. In this respect, there was a significant difference in median serum SAA concentrations between patients with and without distant metastases (3.80 vs. 52.6 µg/ml, p<0.05) (Fig. 3).
Figure 2.
Concentrations of SAA in various lymph node metastasis grades of breast cancer are shown. The Kruskal-Wallis test shows significant differences between the groups (p<0.05). Differences between certain groups are indicated by p-values calculated by the Mann-Whitney U-test. SAA, serum amyloid A.
Figure 3.
Concentrations of SAA in breast cancer patients with or without distant metastasis. Differences between certain groups are indicated by p-values calculated by the Mann-Whitney U-test (p<0.05). SAA, serum amyloid A.
Discussion
The current clinical diagnostic approach to breast cancer is fundamentally based on non-invasive radiology studies, which suggests the diagnosis, but still requires tissue sampling and pathological examination to confirm malignancy. Although there have been significant advances in imaging technology, many breast abnormalities remain indeterminate following conventional evaluation. Application of genomic and proteomic high-throughput approaches holds the promise of identifying new blood biomarkers, which provide a convenient, relatively noninvasive approach to diagnosing and monitoring patients.
Using proteomic techniques, we found that the level of SAA in the blood of breast cancer stage IV patients is significantly higher than in stage I patients. SAA has received considerable attention as a potential marker for neoplastic activity, as it was found that its concentration in blood was higher in many tumor patients than in healthy controls, and that it had an inverse correlation with patient survival. In addition, Biran et al (27) reported an overall elevation of median SAA concentrations in metastatic vs. early stages of breast, colorectal and lung tumors (15.0 vs. 4.3 mg/l). However, as an acute-phase protein, SAA levels in blood are transiently increased as much as 1000-fold in response to inflammation (17,28). Therefore, we speculate that SAA is a non-specific biomarker of breast cancer, and that the level of SAA in the blood of patients may be correlated with the stage. In the present study, we found that there is no difference in the SAA level in stage Ⅰ compared with healthy controls or breast benign tumor patients. Similarly, Ramankulov et al (29) reported that the SAA concentrations in controls and in the N0M0 group of renal cell carcinoma (RCC) were not different. By contrast, Chan et al (30) found that the SAA concentration was higher in gastric cancer patients than in healthy subjects and gastric ulcer patients, but of the 96 gastric cancer patients, only 65 of the patients exhibited TNM stages III and IV, which may have affected the results. Although an increasing amount of clinical proteomic data demonstrates that SAA concentrations increase in tumors and that certain tumor cells secrete SAA, SAA is not a suitable marker for the early diagnosis of breast cancer.
Our findings also correspond to those of other reports whereby increased SAA concentrations are observed in metastatic patients suffering from breast, colorectal, gastric, lung, ovarian and pancreatic tumors (12,27,30-33). Results of recent studies have demonstrated that higher concentrations of SAA were associated with increasing concentrations of CRP, and that elevated SAA and CRP were associated with reduced disease-free survival and reduced overall survival in breast cancer patients, regardless of adjustment for age, tumor stage, race and body mass index (26,34,35). The results indicated that inflammation is closely linked to tumor progression and that inflammatory status may be an important prognostic factor for breast cancer. Additionally, reduction of the inflammatory reaction by, for example, improving diet quality may benefit breast cancer survivors (25,35,36).
The origin of SAA is different in various tumors. For example, in certain tumors, such as renal cancer, SAA mainly originates from the inflammatory cells. It is plausible that there is no difference in blood SAA between the early stage of tumors and healthy controls, and that the level of SAA rises gradually as tumor progression stimulates the inflammatory reaction. SAA may decrease following surgery or chemotherapy, which eliminates inflammatory stimulating factors. Therefore, it may be a suitable biomarker for detecting and monitoring the progressive growth of the tumor, and evaluating prognosis and therapy effects. In colorectal, endometrial, lung and unknown types of cancer, however, SAA in the blood not only derives from inflammatory cells, but also from corresponding tumor cells (15,16,18,37). Notably, SAA1 and SAA4 are upregulated in colon carcinomas, and are barely detectable in normal colon tissues. SAA1 and SAA2 corresponding proteins are the predominant circulating SAA proteins during the acute phase response, but SAA4 is constitutively expressed and its protein product is a constituent of normal, non-acute phase, high-density lipoprotein (18). Thus, SAA combined with other markers or the level of different subtypes of SAA may improve specificity and sensitivity for diagnostic screening.
In conclusion, our results suggest that an elevated SAA level in breast cancer patients is indicative of metastatic lesions and correlated with the stage of disease. SAA is not a suitable marker for early diagnosis. However, it is a significant prognostic factor that may be useful in determining the staging and prognosis of breast cancer.
References
- 1.Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. Lancet. 2005;365:1727–1741. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- 2.Redondo M, Rivas-Ruiz F, Guzman-Soler MC, Labajos C. Monitoring indicators of health care quality by means of a hospital register of tumours. J Eval Clin Pract. 2008;14:1026–1030. doi: 10.1111/j.1365-2753.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 3.Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405–1410. doi: 10.1016/S0140-6736(03)13143-1. [DOI] [PubMed] [Google Scholar]
- 4.Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005;293:1245–1256. doi: 10.1001/jama.293.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding J, Warren R, Warsi I. et al. Evaluating the effectiveness of using standard mammogram form to predict breast cancer risk: case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:1074–1081. doi: 10.1158/1055-9965.EPI-07-2634. [DOI] [PubMed] [Google Scholar]
- 6.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–4021. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le L, Chi K, Tyldesley S. et al. Identification of serum amyloid A as a biomarker to distinguish prostate cancer patients with bone lesions. Clin Chem. 2005;51:695–707. doi: 10.1373/clinchem.2004.041087. [DOI] [PubMed] [Google Scholar]
- 8.Diamandis EP. Identification of serum amyloid a protein as a potentially useful biomarker for nasopharyngeal carcinoma. Clin Cancer Res. 2004;10:5293. doi: 10.1158/1078-0432.CCR-04-0377. author reply 5293-5294. [DOI] [PubMed] [Google Scholar]
- 9.Koomen JM, Shih LN, Coombes KR. et al. Plasma protein profiling for diagnosis of pancreatic cancer reveals the presence of host response proteins. Clin Cancer Res. 2005;11:1110–1118. [PubMed] [Google Scholar]
- 10.Howard BA, Wang MZ, Campa MJ, Corro C, Fitzgerald MC, Patz EF Jr. Identification and validation of a potential lung cancer serum biomarker detected by matrix-assisted laser desorption/ionization-time of flight spectra analysis. Proteomics. 2003;3:1720–1724. doi: 10.1002/pmic.200300514. [DOI] [PubMed] [Google Scholar]
- 11.Gao WM, Kuick R, Orchekowski RP. et al. Distinctive serum protein profiles involving abundant proteins in lung cancer patients based upon antibody microarray analysis. BMC Cancer. 2005;5:110. doi: 10.1186/1471-2407-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoi K, Shih LC, Kobayashi R. et al. Serum amyloid A as a tumor marker in sera of nude mice with orthotopic human pancreatic cancer and in plasma of patients with pancreatic cancer. Int J Oncol. 2005;27:1361–1369. [PubMed] [Google Scholar]
- 13.Dai S, Wang X, Liu L. et al. Discovery and identification of Serum Amyloid A protein elevated in lung cancer serum. Sci China C Life Sci. 2007;50:305–311. doi: 10.1007/s11427-007-0053-x. [DOI] [PubMed] [Google Scholar]
- 14.Dowling P, O'Driscoll L, Meleady P. et al. 2-D difference gel electrophoresis of the lung squamous cell carcinoma versus normal sera demonstrates consistent alterations in the levels of ten specific proteins. Electrophoresis. 2007;28:4302–4310. doi: 10.1002/elps.200700246. [DOI] [PubMed] [Google Scholar]
- 15.Cocco E, Bellone S, El-Sahwi K. et al. Serum amyloid A (SAA): a novel biomarker for uterine serous papillary cancer. Br J Cancer. 2009;101:335–341. doi: 10.1038/sj.bjc.6605129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocco E, Bellone S, El-Sahwi K. et al. Serum amyloid A: a novel biomarker for endometrial cancer. Cancer. 2010;116:843–851. doi: 10.1002/cncr.24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000;7:64–69. doi: 10.1097/00062752-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Gutfeld O, Prus D, Ackerman Z. et al. Expression of serum amyloid A, in normal, dysplastic, and neoplastic human colonic mucosa: implication for a role in colonic tumorigenesis. J Histochem Cytochem. 2006;54:63–73. doi: 10.1369/jhc.5A6645.2005. [DOI] [PubMed] [Google Scholar]
- 19.Liang L, Qu L, Ding Y. Protein and mRNA characterization in human colorectal carcinoma cell lines with different metastatic potentials. Cancer Invest. 2007;25:427–434. doi: 10.1080/07357900701512258. [DOI] [PubMed] [Google Scholar]
- 20.Michaeli A, Finci-Yeheskel Z, Dishon S, Linke RP, Levin M, Urieli-Shoval S. Serum amyloid A enhances plasminogen activation: implication for a role in colon cancer. Biochem Biophys Res Commun. 2008;368:368–373. doi: 10.1016/j.bbrc.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 21.Malle E, Sodin-Semrl S, Kovacevic A. Serum amyloid A: an acute-phase protein involved in tumour pathogenesis. Cell Mol Life Sci. 2009;66:9–26. doi: 10.1007/s00018-008-8321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Cai L, Wang H. et al. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 2011;36:3887–3899. doi: 10.1038/onc.2011.112. [DOI] [PubMed] [Google Scholar]
- 23.Paret C, Schon Z, Szponar A, Kovacs G. Inflammatory protein serum amyloid A1 marks a subset of conventional renal cell carcinomas with fatal outcome. Eur Urol. 2010;57:859–866. doi: 10.1016/j.eururo.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Schaub NP, Jones KJ, Nyalwidhe JO. et al. Serum proteomic biomarker discovery reflective of stage and obesity in breast cancer patients. J Am Coll Surg. 2009;208:970–978. doi: 10.1016/j.jamcollsurg.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 25.La Merrill M, Gordon RR, Hunter KW, Threadgill DW, Pomp D. Dietary fat alters pulmonary metastasis of mammary cancers through cancer autonomous and non-autonomous changes in gene expression. Clin Exp Metastasis. 2010;27:107–116. doi: 10.1007/s10585-009-9302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce BL, Ballard-Barbash R, Bernstein L. et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biran H, Friedman N, Neumann L, Pras M, Shainkin-Kestenbaum R. Serum amyloid A (SAA) variations in patients with cancer: correlation with disease activity, stage, primary site, and prognosis. J Clin Pathol. 1986;39:794–797. doi: 10.1136/jcp.39.7.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein PS, Skinner M, Sipe JD, Lokich JJ, Zamcheck N, Cohen AS. Acute-phase proteins or tumour markers: the role of SAA, SAP, CRP and CEA as indicators of metastasis in a broad spectrum of neoplastic diseases. Scand J Immunol. 1984;19:193–198. doi: 10.1111/j.1365-3083.1984.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramankulov A, Lein M, Johannsen M. et al. Serum amyloid A as indicator of distant metastases but not as early tumor marker in patients with renal cell carcinoma. Cancer Lett. 2008;269:85–92. doi: 10.1016/j.canlet.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Chan DC, Chen CJ, Chu HC. et al. Evaluation of serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol. 2007;14:84–93. doi: 10.1245/s10434-006-9091-z. [DOI] [PubMed] [Google Scholar]
- 31.Khan N, Cromer CJ, Campa M, Patz EF Jr. Clinical utility of serum amyloid A and macrophage migration inhibitory factor as serum biomarkers for the detection of nonsmall cell lung carcinoma. Cancer. 2004;101:379–384. doi: 10.1002/cncr.20377. [DOI] [PubMed] [Google Scholar]
- 32.Glojnaric I, Casl MT, Simic D, Lukac J. Serum amyloid A protein (SAA) in colorectal carcinoma. Clin Chem Lab Med. 2001;39:129–133. doi: 10.1515/CCLM.2001.022. [DOI] [PubMed] [Google Scholar]
- 33.Moshkovskii SA, Serebryakova MV, Kuteykin-Teplyakov KB. et al. Ovarian cancer marker of 11.7 kDa detected by proteomics is a serum amyloid A1. Proteomics. 2005;5:3790–3797. doi: 10.1002/pmic.200401205. [DOI] [PubMed] [Google Scholar]
- 34.Pierce BL, Neuhouser ML, Wener MH. et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114:155–167. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George SM, Neuhouser ML, Mayne ST. et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19:2220–2228. doi: 10.1158/1055-9965.EPI-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villasenor A, Ambs A, Ballard-Barbash R. et al. Dietary fiber is associated with circulating concentrations of C-reactive protein in breast cancer survivors: the HEAL study. Breast Cancer Res Treat. 2011;129:485–494. doi: 10.1007/s10549-011-1474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung HJ, Ahn JM, Yoon YH. et al. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J Proteome Res. 2011;10:1383–1395. doi: 10.1021/pr101154j. [DOI] [PubMed] [Google Scholar]