Abstract
Previous studies demonstrated that preparation with recombinant human thyroid-stimulating hormone (rhTSH) for thyroid remnant ablation results in lower extrathyroidal radiation compared to hypothyroidism. The results of 50 radioiodine therapies (RITs) under rhTSH, regarding iodine half-life, were evaluated and compared with 50 RITs performed on patients with hypothyroidism following thyroxine withdrawal. The patients were treated with 3700 MBq (100 mCi) of 131I. Forty-eight hours after RIT, patients were measured with a radiation detector at a 1-meter (m) distance for evaluation of the effective dose (μSv/h). TSH and thyroglobulin (Tg) maximal values were also compared. rhTSH-stimulated patients had a significantly lower whole-body retention of 131I (8.5±7.3 μSv/h), extrapolated from the measurements of the effective dose at a 1-m distance, compared to endogenously stimulated patients (13.6±8.1 μSv/h; p=0.001). Furthermore, TSH mean and Tg median levels were significantly higher in the rhTSH-stimulated patients (89.9±15.3 mU/l and 7.7 ng/ml, respectively) compared to the hypothyroid group (59.2±25.1 mU/l and 3.3 ng/ml; p<0.001 and p=0.003, respectively). Compared to thyroid hormone withdrawal, the use of rhTSH prior to RIT was associated with significantly lower whole-body retention of 131I and with greater efficacy in reaching TSH levels greater than 30 mU/l, confirming data previously described.
Keywords: iodine, ablative therapy, recombinant human thyroid-stimulating hormone, differentiated thyroid carcinoma
Introduction
Differentiated thyroid cancer comprises the majority of thyroid malignancies (approximately 95%). Papillary thyroid cancer (PTC) accounts for 85% of differentiated thyroid cancers, and follicular thyroid cancer (FTC) for 10%. Total thyroidectomy is the initial treatment for the majority of patients with differentiated thyroid cancer. Postoperative remnant ablation with 131I is indicated for all patients with stage 3 and 4 disease and for some patients with stage 1 and 2 disease (1). It supplements surgery by destroying normal thyroid remnants, thus increasing the sensitivity of subsequent 131I whole-body scanning, and of serum thyroglobulin (Tg) measurements in detecting persistent or recurrent disease. It also destroys microscopic neoplastic tissue, decreasing the long-term recurrence rate (1–3).
Remnant ablation requires TSH stimulation. This may be accomplished by withdrawing thyroid hormone treatment or by using recombinant human thyroid-stimulating hormone (rhTSH). These approaches have been approved for ablative therapy and diagnostic purposes (1).
Certain studies have found that the two methods are equally effective in preparing patients for 131I remnant ablation, with a greater quality of life achieved when rhTSH is used (4–6). Other authors demonstrated that the use of rhTSH is associated with a significant decrease in whole-body irradiation (7–12), which may be relevant with regards to the radiation exposure of the general population. The amount of 131I retained depends on factors such as the presence of metastatic disease, hydration level, bowel functioning, pre-therapeutic diet and renal clearance (9). The preserved renal clearance in patients prepared with rhTSH should explain the lower whole-body retention in these individuals, since it is known that renal clearance of 131I is reduced to approximately 50% in hypothyroid patients (13).
We retrospectively evaluated 100 patients submitted to postoperative remnant ablation with 131I. Of those, 50 patients were prepared with thyroid hormone withdrawal and 50 patients were prepared with rhTSH, with particular emphasis on the whole-body retention of 131I, extrapolated from the measurements of the effective dose at a 1-meter (m) distance.
Materials and methods
The study was approved by the ethics committee of the hospital. A total of 100 randomly selected patients, submitted to postoperative remnant ablation with 131I at the Portuguese Oncology Institute of Lisbon (Portugal) in 2008, were evaluated retrospectively. The patients had undergone a low-iodine diet, and those with positive anti-Tg antibodies, <18 years of age or with impaired renal function (estimated by the creatinine blood level) were excluded.
Of the 100 patients, 50 were prepared with thyroid hormone withdrawal (hypothyroidism group): levothyroxine (LT4) was discontinued and switched to triiodothyronine (LT3) for 6 weeks, followed by LT3 withdrawal for 2 weeks prior to ablation. The remaining 50 patients were prepared with rhTSH (rhTSH group): rhTSH 0.9 mg was administered intramuscularly in the 2 days prior to ablative therapy.
Data on the maximal TSH and Tg levels were collected. Radioiodine therapy (RIT) regimen comprised 3700 MBq (100 mCi) of 131I and administered per os to all patients. None of the patients were submitted to diagnostic radioiodine scanning prior to ablative therapy.
The whole-body retention of 131I was extrapolated from the measurements of the effective dose at a standardized distance of 1 m between the probe and the patient, as recommended in the ATA guidelines (14). The probe was a whole-body counter, model TAM/S Single Area Monitor, from Tema Sinergie (Faenza, Italy).
Statistical analysis
Data are reported as the means ± SD. The differences between groups were tested for significance using the Student's t-test, Mann-Whitney test and χ2 test, where appropriate. P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic characteristics did not vary significantly between the two groups (Table I).
Table I.
Baseline characteristics of the patients.
| Hypothyroidism group | rhTSH group | p-value | |
|---|---|---|---|
| Number of patients | 50 | 50 | |
| Mean age | 50.4±13.2 | 48.3±14.6 | 0.456 |
| Gender (M:F) | 12:38 | 6:44 | 0.118 |
| Histology | |||
| Papillary | 32 | 33 | |
| Follicular | 6 | 2 | |
| Papillary, follicular variant | 10 | 13 | 0.442 |
| Papillary, tall cell variant | 0 | 1 | |
| Papillary, oncocytic variant | 2 | 1 | |
rhTSH, recombinant human thyroid-stimulating hormone.
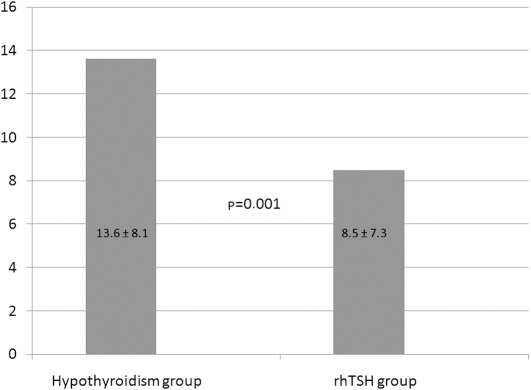
The whole-body retention of 131I, extrapolated from the measurements of the effective dose at 1 m, was significantly lower in the rhTSH group at 8.5±7.3 μS/h versus 13.6±8.1 μS/h in the hypothyroid group (p=0.001; Fig. 1).
Figure 1.
Effective dose at 1 meter (μS/h) in the two groups. rhTSH, recombinant human thyroid-stimulating hormone.
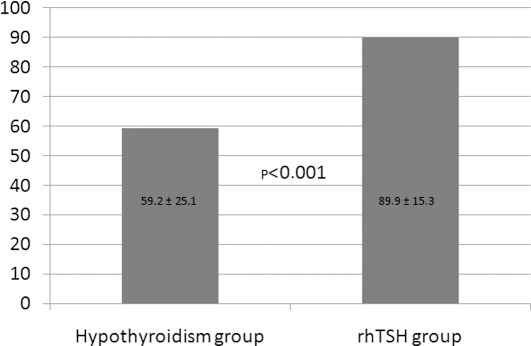
All rhTSH-stimulated patients reached TSH >30 mU/l, while 7 patients (14%) in the hypothyroid group had maximal TSH <30 mU/l (Fig. 2). Mean maximal TSH values were significantly higher in the rhTSH group at 89.9±15.3 mU/l versus 59.2±25.1 mU/l in the hypothyroid group (p<0.001).
Figure 2.
Mean TSH values (mU/l) in the two groups are shown. TSH, thyroid-stimulating hormone; rhTSH, recombinant human TSH.
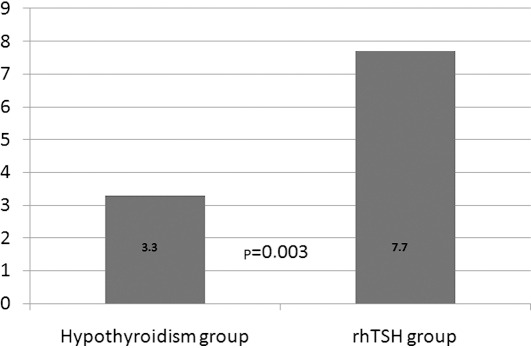
Maximal Tg values were also significantly different between the two groups (p=0.003) with a median of 7.7 ng/ml in the rhTSH group versus 3.3 ng/ml in the hypothyroid group (Fig. 3). Undetectable Tg levels were present in 20 cases (40%) in the hypothyroid group and in 11 cases (22%) in the rhTSH group (p=0.05).
Figure 3.
Median Tg values (ng/ml) in the two groups are shown. rhTSH, recombinant human thyroid-stimulating hormone; Tg, thyroglobulin.
Discussion
Preparation for RIT following hormone withdrawal is usually well tolerated but may be harmful in a subset of patients (6,7,15), such as the elderly, patients with cardiac dysfunction or patients with psychiatric conditions. Other individuals, including those with hypopituitarism, may be unable to endogenously raise TSH. In these circumstances the use of rhTSH is advisable.
There is general agreement that rhTSH administration provides a better quality of life. One of the major drawbacks for its more generalized use is its cost (16). Although it is believed that it can be counterbalanced by improved productivity and reduced work absenteeism when hypothyroidism is avoided (17,18), its exact economic impact is difficult to ascertain (19).
Our primary goal was to evaluate the whole-body retention time of 131I, extrapolated from the measurements of the effective dose at a 1-m distance, associated with each strategy of preparing patients for postoperative remnant ablation with 131I. We found lower whole-body retention of 131I in the rhTSH group. Our results, regarding whole-body retention of 131I and maximal TSH values reached, support previously reported data (5,10–13,20).
Although it was not our primary objective, the comparison of peak Tg levels between groups demonstrated that the levels were significantly higher in the rhTSH group. There are several possible explanations for this finding. One is that TSH levels in the rhTSH group are higher than in the hypothyroid group, which clearly stimulates the release of Tg by the remaining thyroid tissue. Another reason is that the acute stimulation obtained with rhTSH releases Tg stored in thyroid cells, whereas a more chronic TSH stimulation, as obtained with thyroid hormone withdrawal, gradually depletes the pool of stored Tg. A previous study has also documented higher Tg levels with rhTSH preparation compared to those of hypothyroidism (12), although it was interpreted as a consequence of the more aggressive tumor subtypes in the group of patients prepared with rhTSH. This is not the case in our study, since no differences were observed in TNM staging between the two groups (data not shown).
The safety of rhTSH use for diagnostic and ablative purposes has been well documented (16,21). The question of whether the sudden increase in TSH resulting from rhTSH administration may lead to tumor expansion (10) in patients with occult metastatic disease, or whether the more prolonged TSH stimulation related to hypothyroidism is more life-threatening, remains to be resolved. Although studies have been carried out to address the efficacy and safety of rhTSH in metastatic disease (22,23), its use has not yet been approved for this purpose.
In conclusion, in our series, the use of rhTSH in the preparation of patients for postoperative remnant ablation with 131I was associated with lower radioiodine toxicity and with greater efficacy in achieving the desirable TSH level (>30 mU/l). However, the use of rhTSH was also associated with greater Tg levels and an undetectable Tg level was more frequently found in the hypothyroid group (40%) as compared to the rhTSH group (22%; p=0.05). Since an undetectable Tg level at the time of postoperative 131I remnant ablation generally reflects complete remission (24–26), patients treated with rhTSH who do not have undetectable Tg levels when treated with 131I may not be regarded as disease-free. Therefore, we believe that clinicians should not disregard the possibility of complete tumor eradication in patients presenting with low, but not undetectable, Tg levels at the time of ablation with rhTSH.
References
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al. Revised management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Eustatia-Rutten CF, Smit JW, Romijn JA, Van der Kleij- Corssmit EP, Pereira AM, Stokkel MP, Kievit J. Diagnostic value of serum thyroglobulin measurements in the follow-up of differentiated thyroid carcinoma, a structured meta-analysis. Clin Endocrinol (Oxf) 2004;61:61–74. doi: 10.1111/j.1365-2265.2004.02060.x. [DOI] [PubMed] [Google Scholar]
- 3.Toubeau M, Touzery C, Arveux P, Chaplain G, Vaillant G, Berriolo A, Riedinger J, Boichot C, Cochet A, Brunotte F. Predictive value for disease progression of serum thyroglobulin levels measured in the postoperative period and after 131I ablation therapy in patients with differentiated thyroid cancer. J Nucl Med. 2004;45:988–994. [PubMed] [Google Scholar]
- 4.Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. 1977;50:799–807. doi: 10.1259/0007-1285-50-599-799. [DOI] [PubMed] [Google Scholar]
- 5.Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, Sherman S, Haugen B, Corone C, Molinaro E, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 6.Ladenson PW, Braverman LE, Ebner SA, Brucker-Davis F, Cooper D, Garber J, Wondisford F, Davies T, DeGroot L, Daniels G, et al. Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. New Eng J Med. 1997;337:888–896. doi: 10.1056/NEJM199709253371304. [DOI] [PubMed] [Google Scholar]
- 7.Haugen B, Pacini F, Reiners C, Schlumberger M, Ladenson P, Sherman S, Cooper D, Graham K, Braverman L, Skarulis M, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 8.Pilli T, Brianzoni E, Capoccetti F, Castagna MG, Fattori S, Poggiu A, Rossi G, Ferretti F, Guarino E, Burroni L, et al. A comparison of 1850 (50 mCi) and 3700MBq (100 mCi) 131-iodine administered doses for recombinant thyrotropin-stimulated postoperative thyroid remnant ablation in differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3542–3546. doi: 10.1210/jc.2007-0225. [DOI] [PubMed] [Google Scholar]
- 9.Sisson JC, Shulkin BL, Lawson S. Increasing efficacy and safety of treatments of patients with well-differentiated thyroid carcinoma by measuring body retentions of 131I. J Nucl Med. 2003;44:898–903. [PubMed] [Google Scholar]
- 10.Papadimitriou D, Kottou S, Oros L, Ilias I, Molfetas M, Tsapaki V, Perris A, Christakopoulou I. Differentiated thyroid cancer: comparison of therapeutic iodine 131 biological elimination after discontinuation of levothyroxine versus administration of recombinant human thyrotropin. Annals Nucl Med. 2006;20:63–67. doi: 10.1007/BF02985593. [DOI] [PubMed] [Google Scholar]
- 11.Remy H, Borget I, Leboulleux S, Guilabert N, Lavielle F, Garsi J, Bournaud C, Gupta S, Schlumberger M, Ricard M. 131I effective half-life and dosimetry in thyroid cancer patients. J Nucl Med. 2008;49:1445–1450. doi: 10.2967/jnumed.108.052464. [DOI] [PubMed] [Google Scholar]
- 12.Menzel C, Kranert W, Döbert N, Diehl M, Fietz T, Nadja H, Berner U, Grünwald F. rhTSH stimulation before radiodine therapy in thyroid cancer reduces the effective half-life of 131I. J Nucl Med. 2003;44:1065–1068. [PubMed] [Google Scholar]
- 13.Hänscheid H, Lassmann M, Luster M, Thomas S, Pacini F, Ceccarelli C, Ladenson P, Wahl R, Schlumberger M, Ricard M, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006;47:648–654. [PubMed] [Google Scholar]
- 14.Sisson JC, Freitas J, McDougall IR, Dauer LT, Hurley JR, Brierley JD, Edinboro CH, Rosenthal D, Thomas MJ, Wexler JA, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid. 2011;21:335–346. doi: 10.1089/thy.2010.0403. [DOI] [PubMed] [Google Scholar]
- 15.Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997;7:613–619. doi: 10.1089/thy.1997.7.613. [DOI] [PubMed] [Google Scholar]
- 16.Haugen B, Cooper D, Emerson C, Luster M, Maciel R, Biscolla R, Mazzaferri E, Medeiros-Neto G, Reiners C, Robbins R, et al. Expanding indications for recombinant human TSH in thyroid cancer. Thyroid. 2008;18:687–694. doi: 10.1089/thy.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mernagh P, Campbell S, Dietlein M, Luster M, Mazzaferri E, Weston AR. Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: the German perspective. Eur J Endocrinol. 2006;155:405–414. doi: 10.1530/eje.1.02223. [DOI] [PubMed] [Google Scholar]
- 18.Borget I, Corone C, Nocaudie M, Allyn M, Iacobelli S, Schlumberger M, De Pouvourville G. Sick leave for follow-up control in thyroid cancer patients: comparison between stimulation with Thyrogen and thyroid hormone withdrawal. Eur J Endocrinol. 2007;156:531–538. doi: 10.1530/EJE-06-0724. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Cheung K, Mehta P, Roman S, Walker H, Sosa J. To stimulate or withdraw? A cost-utility analysis of recombinant human thyrotropin versus thyroxine withdrawal for radioiodine ablation in patients with low-risk differentiated thyroid cancer in the United States. J Clin Endocrin Metab. 2010;96:1672–1680. doi: 10.1210/jc.2009-1803. [DOI] [PubMed] [Google Scholar]
- 20.Rosário P, Borges M, Purisch S. Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicity. J Nucl Med. 2008;49:1776–1782. doi: 10.2967/jnumed.108.050591. [DOI] [PubMed] [Google Scholar]
- 21.David A, Blotta A, Bondanelli M, Rossi R, Roti E, Braverman L, Busutti L, Uberti E. Serum thyroglobulin concentrations and 131I whole-body scan results in patients with differentiated thyroid carcinoma after administration of recombinant human thyroid-stimulating hormone. J Nucl Med. 2001;42:1470–1475. [PubMed] [Google Scholar]
- 22.Lippi F, Capezzone M, Angelini F, Taddei D, Molinaro E, Pinchera A, Pacini F. Radioiodine treatment of metastatic differentiated thyroid cancer in patients on L-thyroxine, using recombinant human TSH. Eur J Endoc. 2001;144:5–11. doi: 10.1530/eje.0.1440005. [DOI] [PubMed] [Google Scholar]
- 23.Jarzab B, Handkiewicz-Junak D, Roskosz J, Puch Z, Wygoda Z, Kukulska A, Jurecka-Lubieniecka B, Hasse-Lazar K, Turska M, Zajusz A. Recombinant human TSH-aided radioiodine treatment of advanced differentiated thyroid carcinoma: a single-centre study of 54 patients. Eur J Nucl Med Molec Imaging. 2003;30:1077–1086. doi: 10.1007/s00259-003-1190-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS, Kim SC, Hong SJ, Shong YK. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrin Metab. 2005;90:1440–1445. doi: 10.1210/jc.2004-1771. [DOI] [PubMed] [Google Scholar]
- 25.Piccardo A, Arecco F, Morbelli S, Bianchi P, Barbera F, Finessi M, Corvisieri S, Pestarino E, Foppiani L, Villavecchia G, Cabria M, Orlandi F. Low thyroglobulin concentrations after thyroidectomy increase the prognostic value of undetectable thyroglobulin levels on levo-thyroxine suppressive treatment in low-risk differentiated thyroid cancer. J Enddocrinol Invest. 2010;33:83–87. doi: 10.1007/BF03346558. [DOI] [PubMed] [Google Scholar]
- 26.Karam M, Feustel PJ, Postal ES, Cheema A, Goldfarb CR. Successful thyroid tissue ablation as defined by a negative whole-body scan or an undetectable thyroglobulin: a comparative study. Nucl Med Commun. 2005;26:331–336. doi: 10.1097/00006231-200504000-00005. [DOI] [PubMed] [Google Scholar]