Abstract
The objective of this study was to evaluate the correlation between cyclooxygenase-2 (COX-2) and markers of cell proliferation and apoptosis, including, Bcl-2, Bax, Ki-67 and the type I insulin-like growth factor (IGF) receptor (IGF1-R) in ductal carcinoma in situ (DCIS) and infiltrating ductal carcinoma (IDC), present in the same surgical specimen. A total of 110 cases were evaluated using tissue microarrays. Cases were classified in scores from 0 to 3 according to pre-defined methods. The results showed that the positivity rates were COX-2 in 87% of cases in DCIS and IDC; Bcl-2 in 55% of cases in DCIS and IDC; Bax in 23% of cases in IDC and 19% in DCIS, IGF-1 in 24% of cases in DCIS and IDC; and Ki-67 in 81% of cases in DCIS and IDC. We also observed a positive correlation between the expression of COX-2 and IGF1-R (p=0.045). Our results demonstrate a positive correlation between the expression of COX-2 and IGF1-R in DCIS and IDC, demonstrating that they are involved in breast cancer carcinogenesis. Further studies are required to prove the effectiveness of COX-2 and IGF1-R inhibitors for the prevention and treatment of breast cancer, as well as to explain their mechanism of action.
Keywords: invasive ductal carcinomas, ductal carcinoma in situ, cyclooxygenase-2, type I insulin-like growth factor receptor, tissue microarrays
Introduction
Primary prevention of breast cancer is a necessity. Previous studies have demonstrated the effectiveness of drugs, such as selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) for the chemoprevention of breast cancer (1–4).
The utilization of SERMs is known to be effective in reducing the risk of breast cancer. Moreover, recent studies using AIs suggest that these drugs may be considerably more effective than SERMs in post-menopausal women. These agents are useful in cases of breast tumors that are hormone receptor (HR)-positive, since in negative cases no benefit is observed (5).
Li and Brown performed a study in transgenic mice that developed estrogen receptor (ER)-negative breast cancer, demonstrating that rexinoids (retinoid analogs), tyrosine kinase and cyclooxygenase-2 (COX-2) inhibitors have chemopreventive properties for these specific tumors (5).
Cyclooxygenase is an enzyme that catalyzes the conversion of arachidonic acid into prostaglandins (PGs) and is expressed as two isoenzymes: COX-1 and COX-2. The first is constitutive and mediates normal physiological functions, while the second is undetectable in most normal tissues but is induced by cytokines, growth factors, oncogenes and tumor promoters, thus contributing to the synthesis of PGs in inflammatory diseases and tumor tissues (6,7).
In breast cancer, COX-2 plays several roles, among which is the inhibition of apoptosis through the synthesis of PGE2, leading to the increased expression of anti-apoptotic protein B-cell lymphoma/leukemia-2 (Bcl-2) and decreased pro-apoptotic B-cell lymphoma 2-associated X protein (Bax), as well as the promotion of neoangiogenesis (8). Apoptosis is dependent on the balance between the anti-apoptotic protein Bcl-2 and the pro-apoptotic proteins Bax and Bak. In breast cancer, Bax plays a key role as a facilitator of apoptosis, a function that ensures the balance against the main anti-apoptotic member of this family, Bcl-2 (9).
Certain investigators have studied the correlation between the expression of COX-2 and the proteins Bcl-2 and Bax. Singh et al (10) observed that in MCF-7 breast cancer cell cultures overexpressing COX-2, high levels of Bcl-2 are also found and that these cells behave like more aggressive tumors. Zhu et al (11) analyzed the effects of the non-selective COX-2 inhibitor Aspisol® (a salicylic acid derivative) on breast cancer cells in vitro and in vivo, observing that the antitumor effect of Aspisol on breast cancer cells is possibly mediated by the induction of apoptosis, and may be correlated with the downregulation of COX-2 or Bcl-2 expression and the upregulation of caspase-3 or Bax.
COX-2 positively correlates with the cell proliferation marker Ki-67. Ristimäki et al (12) analyzed the expression of COX-2 in 1,576 infiltrating ductal carcinoma (IDC) cases and found that in instances of COX-2 overexpression (37.4% of cases) rates were high for Ki-67 (p<0.0001), indicating a more aggressive tumor. In another study, Boland et al (13) observed that in ductal carcinoma in situ (DCIS), which exhibited high levels of Ki-67, COX-2 expression was also positively correlated in 79% of cases.
The type I insulin-like growth factor (IGF) receptor (IGF1-R) is considered to be a crucial prognostic factor in breast cancer as it is expressed in most breast cancer epithelial cells and is essential for the malignant phenotype (14,15). Using cell culture assays, Levitt et al (16) found a positive correlation between COX-2 and IGF1-R, and that the use of celecoxib (a selective COX-2 inhibitor) determines a decreased expression of COX-2 and the subsequent downregulation of IGF1-R, resulting in decreased cell proliferation. Põld et al (17) studied lung cancer cell cultures and observed that cells that overexpress COX-2 exhibit an increase in anti-apoptotic and mitogenic signals induced by IGF-1.
Given the scarcity of studies evaluating the interaction between COX-2 and the proliferation markers IGF1-R and Ki-67, and the apoptosis markers Bcl-2 and Bax, the aim of this study was to evaluate the correlation between these proteins in DCIS and IDC in order to obtain information that may assist in the prevention and treatment of breast carcinoma.
Materials and methods
Patients
We selected 110 patients (age range, 26–90) surgically treated for breast cancer from the Mastology Clinics of the Department of Obstetrics and Gynecology of the Santa Casa Hospital, Brazil, between August 2002 and January 2008. Only the patients with IDC and DCIS in the same surgical specimen were selected for our study. The study was approved by the ethics committee of the hospital and informed consent was obtained from the patients who participated.
Histopathology and tissue microarray immunohistochemistry
Following the study of macroscopic specimens, selected fragments were dehydrated in ethanol, cleared by xylene and embedded in paraffin to create the blocks. The blocks were cut using a microtome calibrated for 4-mm cuts. The histological sections were stained with hematoxylin and eosin (H&E) and were read using a light microscope.
The DCIS cases were classified according to the presence or absence of comedonecrosis in comedocarcinomas and non-comedocarcinomas. The criteria proposed by Dabbs (18) and Elston and Ellis (19) were used for the classification of DCIS and IDC using the nuclear and histological grades, respectively.
The cases with IDC and adjacent DCIS were selected for the preparation of the tissue microarray. Two regions from each histological type were selected for composing the microarray; thus, each case was represented by four areas, two from DCIS and two from IDC from the same patient, comprising a total of 440 regions for analysis.
In the immunohistochemistry analysis we evaluated the expression of COX-2, Bcl-2, Bax, Ki-67 and IGF1-R using specific antibodies that were detected using the chromogenic substrate, diaminobenzidine. The sections were counterstained with Harris hematoxylin, followed by dehydration and mounting in Entellan with cover slips. The primary antibodies used in immunohistochemistry reactions were: COX-2 clone CX-294 (Dako; Glostrup, Denmark), Bcl-2 clone 124 (Dako), Bax clone 43-61 (Dako), Ki-67 clone MIB-1 (Dako) and IGF1-R clone 24-31 DBS® (Pleasanton, CA, USA) at dilutions of 1/80, 1/2000, 1/3000, 1/80 and 1/80, respectively.
Evaluation of the expression of COX-2, Bcl-2, Bax, Ki-67 and IGF1-R
The expression of COX-2, Bcl-2, Bax, Ki-67 and IGF1-R were evaluated by scores from two independent examiners.
We used the same criteria employed by Ristimäki et al (12) for the analysis of COX-2: score 0, no stained cells observed; score 1, cytoplasm and cell membrane stained diffusely and weakly (at least 10% of stained cells with strong intensity); score 2, cytoplasmic granular staining of the cell membrane and moderate to strong staining in 10–90% of cells; score 3, >90% of cells stained with strong intensity.
To classify the immunohistochemical expression of Bcl-2, Bax, IGF1-R and Ki-67 we used the same criteria for determining the score of the HER-2 according to the HercepTest® (Dako) (20): score 0, no staining observed or membrane staining observed in <10% of the tumor cells; score 1, a faint/barely perceptible membrane staining in >10% of tumor cells and the cells exhibit incomplete membrane staining; score 2, a weak to moderate complete staining observed in >10% of tumor cells; score 3, a strong complete membrane staining observed in >10% of tumor cells.
We used the same criteria as those used by Ristimäki et al (12), Boland et al (13), Shim et al (21) and Oliveira et al (22) to classify the immunohistochemical expression as positive or negative, 0 and 1 were regarded as negative and 2 or 3 as positive.
Statistical analysis
The correlation between COX-2, Bcl-2, Bax, IGF1-R and Ki-67 was assessed according to the Spearman's correlation. The Chi-square test was used to analyze nuclear grade, histological grade, age group, tumor size, lymph node status and hormonal status. We set 5% as the rejection level for the null hypothesis for all the parameters we evaluated (23). The data were evaluated using the statistical program SPSS® (Statistical Package for Social Sciences) version 14.0 for Microsoft Windows.
Results
Patient characteristics
The aim of the present study was to evaluate the correlation between COX-2, IGF1-R, Ki-67, Bcl-2 and Bax levels in DCIS and IDC, present in the same surgical specimen. In addition, we observed whether there was any correlation between the expression of these biomarkers and age (younger or older than 50 years), tumor size (≤2 cm or >2 cm), histological grade, nuclear grade, axillary lymph node status and hormonal status. The ages of the patients at diagnosis ranged from 26 to 90 years old, with a mean age of 56.4 years, a standard deviation of 12.81 and median age of 55 years.
Evaluation of COX-2 expression
A total of 110 cases were evaluated by immunohistochemistry and the scores were defined from 0 to 3, according to the intensity and number of stained cells. The COX-2-positive expression was similar in DCIS and IDC in 87% of cases, demonstrating a high correlation (p<0.001). No statistically significant difference was found when we analyzed the expression of COX-2 with histological grade, nuclear grade, age, axillary lymph node status and hormonal status.
Immunoreactivity to Bcl-2, Bax, Ki-67 and IGF1-R
Bcl-2 was positive in 55% of the cases in IDC and DCIS; Bax in 23% of cases in IDC and 19% of cases in DCIS; IGF-1 in 24% of cases in IDC and DCIS and Ki-67 in 81% of cases in IDC and DCIS. Results of the statistical analyses of these data showed a statistically significant correlation between their expression in IDC and DCIS (p<0.001). As in the analysis of COX-2, no statistically significant difference was observed when we analyzed the expression of these biomarkers with histological grade, nuclear grade, age, axillary lymph node status and hormonal status (Table I).
Table I.
Expression of biomarkers according to scores.
| Scores | COX-2 (%) | Bcl-2 (%) | Bax (%) | Ki-67 (%) | IGF1-R (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IDC | DCIS | IDC | DCIS | IDC | DCIS | IDC | DCIS | IDC | DCIS | |
| 1 | 12.7 | 12.7 | 45.5 | 45.5 | 77.3 | 80.9 | 19.1 | 19.1 | 76.4 | 76.4 |
| 2 | 38.2 | 38.2 | 36.4 | 36.4 | 18.2 | 15.5 | 70 | 70 | 20 | 20 |
| 3 | 49.1 | 49.1 | 18.2 | 18.2 | 4.5 | 3.6 | 10.9 | 10.9 | 3.6 | 3.6 |
IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ.
Correlation between the expression of COX-2, IGF1-R, Ki-67, Bcl-2 and Bax
The expression of COX-2 in IDC and DCIS was positively correlated with the expression of IGF1-R (p=0.045; Fig. 1). We also found a negative correlation between the expression of Ki-67 and IGF1-R in IDC and DCIS (p=0.013). No statistically significant difference was observed when we analyzed the expression of COX-2, Bcl-2, Bax, Ki-67 and IGF1-R with histological grade, nuclear grade, age, axillary lymph node status and hormonal status (Tables II and III).
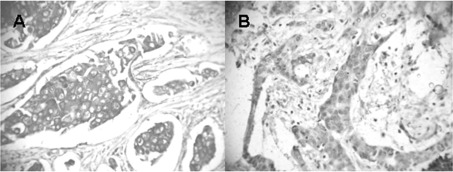
Figure 1.
IDC showing score 3 (A) COX-2 and (B) IGF1-R expression. IDC, invasive ductal carcinoma; IGF1-R, type I insulin-like growth factor receptor; COX-2, cyclooxygenase-2.
Table II.
Correlation between the expression of COX-2, Bcl-2, Bax, IGF-1 and Ki-67 in IDC in 110 cases.
| Variant | Statistic | COX-2 | Bcl-2 | Bax | IGF1-R | Ki-67 |
|---|---|---|---|---|---|---|
| COX-2 | Correlation | 1 | 0.138 | 0.113 | 0.191a | 0.029 |
| Significance (p) | - | 0.152 | 0.241 | 0.045 | 0.766 | |
| Bcl-2 | Correlation | 0.138 | 1 | -0.036 | 0.065 | 0.007 |
| Significance (p) | 0.152 | - | 0.712 | 0.497 | 0.940 | |
| Bax | Correlation | 0.113 | −0.036 | 1 | −0.055 | −0.019 |
| Significance (p) | 0.241 | 0.712 | - | 0.570 | 0.847 | |
| IGF1-R | Correlation | 0.191a | 0.065 | −0.055 | 1 | −0.236a |
| Significance (p) | 0.045 | 0.497 | 0.570 | - | 0.013 | |
| Ki-67 | Correlation | 0.029 | 0.007 | −0.019 | −0.236a | 1 |
| Significance (p) | 0.766 | 0.940 | 0.847 | 0.013 | - |
Spearman's correlation showing statistically significant correlations between COX-2 expressed in IDC, with IGF1-R expressed in IDC and DCIS.
P<0.05 denotes statistically significant differences.
IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; IGF1-R, type I insulin-like growth factor receptor.
Table III.
Correlation between the expression of COX-2, Bcl-2, Bax, IGF-1 and Ki-67 in DCIS in 110 cases.
| Variant | Statistic | COX-2 | Bcl-2 | Bax | IGF1-R | Ki-67 |
|---|---|---|---|---|---|---|
| COX-2 | Correlation | 1 | 0.138 | 0.113 | 0.191a | 0.029 |
| Significance (p) | - | 0.152 | 0.172 | 0.045 | 0.766 | |
| Bcl-2 | Correlation | 0.138 | 1 | 0.039 | 0.065 | 0.007 |
| Significance (p) | 0.152 | - | 0.688 | 0.497 | 0.940 | |
| Bax | Correlation | 0.113 | 0.039 | 1 | −0.018 | 0.124 |
| Significance (p) | 0.172 | 0.688 | - | 0.221 | 0.198 | |
| IGF1-R | Correlation | 0.191a | 0.065 | −0.018 | 1 | −0.236a |
| Significance (p) | 0.045 | 0.497 | 0.221 | - | 0.013 | |
| Ki-67 | Correlation | 0.029 | 0.007 | 0.124 | −0.236a | 1 |
| Significance (p) | 0.766 | 0.940 | 0.198 | 0.013 | - |
Spearman's correlation showing statistically significant correlations between COX-2 expressed in DCIS, with IGF1-R expressed in IDC and DCIS.
P<0.05 denotes statistically significant differences.
IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; IGF1-R, type I insulin-like growth factor receptor.
Discussion
The overexpression of the COX-2 enzyme in breast tumors is a significant biological marker. Its expression has been reported in numerous studies aimed at demonstrating its role in the carcinogenesis and natural history of breast cancer through mechanisms involving cytokines, growth factors, apoptotic agents and tumor promoters (21,24,25). Epidemiological studies have suggested that non-steroidal anti-inflammatory drugs confer a moderate degree of benefit against breast cancer (26).
In the present study, we found COX-2 (as detected by immunohistochemistry) in 87% of the cases of IDC and DCIS evaluated. Similar results were found by Davies et al (27), who observed COX-2 expression in 79% of the 80 cases of IDC, and Shim et al (21), who found COX-2 in IDC and DCIS in 81.6% of the 38 cases they studied. Conversely, Half et al (28) found the presence of COX-2 in 43% of 42 cases of pure IDC evaluated. Similar numbers were observed by Ristimäki et al (12) who analyzed 1,576 cases of IDC, and found COX-2 expression in 39.9% of cases.
Oliveira et al (22) analyzed the expression of COX-2 in IDC, DCIS and normal epithelium and correlated these levels with nuclear grade, histological grade and the presence of comedonecrosis. These results are similar to those found in the present study, demonstrating positive results for COX-2 in 87% of cases of IDC, 85% of cases of DCIS and 74.5% of normal control patients. These data demonstrated a high correlation between the expression of COX-2 in IDC, DCIS and normal epithelium (p<0.001).
In the analysis of COX-2 expression in DCIS we found a positivity rate of 87%. This rate is similar to that found by other authors. Shim et al (21) analyzed 46 cases of pure DCIS, and observed a positivity rate of of 85%. Shim et al (21), Half et al (28), Tan et al (29), Boland et al (13) and Perrone et al (30), found positivity rates of 63, 67, 80, 76 and 88%, respectively. This finding demonstrates homogeneity in the expression of COX-2 in DCIS, which is not observed in invasive disease.
The mechanisms by which COX-2 is upregulated in breast cancer are unknown, but one possibility is that tumor cells express a more active enzyme owing to intrinsic mechanisms. The inactivation of tumor suppressor genes, such as p53, and the activation of proto-oncogenes, including Ras and HER-2/neu are involved in these mechanisms (12,31,32).
Recent studies indicate that anti-inflammatory drugs may inhibit cell proliferation and induce apoptosis in tumor cells. In an experimental study, Wang et al (33) examined how celecoxib, a COX-2 inhibitor, is associated with the NF-κB pathway and apoptosis in breast cancer cell cultures treated with celecoxib. As a result, they deduced that cell proliferation was significantly decreased (p<0.05) in the celecoxib-treated group when compared with the control group, who received no medication, leading to the conclusion that celecoxib may inhibit cell proliferation and induce apoptosis through downregulation of COX-2 and NF-κB.
We showed that in the presence of two tumor components in the same surgical specimen (IDC and DCIS), the rate of positivity in IDC and DCIS was similar, probably as a result of the paracrine action of one component over the other. Our findings denote a positive correlation between the expression of COX-2 in DCIS and IDC. It appears that the expression of COX-2 in the in situ component upregulates the enzyme in the infiltrating carcinoma, since its levels are generally higher in DCIS.
A comparison of the expression of COX-2 according to tumor size did not yield statistically significant differences between the two groups, data consistent with the findings obtained by Half et al (28). However, these data are not equivalent to those obtained by Ristimäki et al (12) and Shim et al (21), who found a greater expression directly proportional to tumor size. These results may be explained by the specific analysis of invasive tumors by these authors, as in our study and that carried out by Half et al (28), where samples containing DCIS and IDC in the same specimen were evaluated.
Evaluation of COX-2 expression according to the patients' age showed no difference between the groups. These data were similar to those obtained by Ristimäki et al (12).
An analysis of the correlation between the expression of COX-2 and axillary lymph node status did not yield any difference between the lymph node groups of patients with COX-2 expression, these data being consistent with the results obtained by Shim et al (21). Li et al (34) analyzed the correlation between the expression of COX-2 and lymph node metastases and found a higher rate of axillary lymph nodes in the group of patients with overexpressed COX-2 (p=0.012). This difference in results may be explained by the small number of COX-2-negative patients in our study.
When we compared the expression of COX-2 with HR, no significant difference between the two groups was observed, which was similar to the findings by Half et al (28) and Shim et al (21).
An analysis of the expression of Bcl-2 and Bax revealed that Bcl-2 was positive in 55% of cases for IDC and DCIS, while Bax was present in 23% of IDC and 19% of DCIS cases. Our rate of positivity in DCI for Bcl-2 was similar to that found by Yang et al (35) who observed 48% of cases overexpressing Bcl-2, but differs from the Bax analysis where the results showed 54% of cases with positivity. We observed a statistically significant correlation between the expression of Bcl-2 in DCIS and IDC, and the same was noted for Bax (p<0.001). These data differ from those found by Mintz et al (36), who observed a higher expression of Bcl-2 in DCIS than in IDC.
The balance between the expression of the anti-apoptotic Bcl-2 and pro-apoptotic Bax gene is considered a good indicator of apoptotic activity in tumor cells. Martínez-Arribas et al (37) studied the apoptosis associated with the two proteins in 86 specimens of breast tumors, where 14 patients had received neoadjuvant chemotherapy. Their results revealed that Bcl-2 overexpression correlated with the presence of estrogen and progesterone receptors in cases without previous treatment, and high rates of apoptosis were significantly correlated with the expression of the progesterone receptor (p=0.037). Our results found no correlation between the expression of Bcl-2 and Bax and the hormonal receptors.
There was also no correlation between the expression of COX-2 and Bcl-2 and Bax, which is similar to findings described by Arun et al (38). In their study on the apoptosis induction in human breast cancer cells, Michael et al (39) found an association between a high expression of COX-2 and a low expression of Bcl-2.
In the analysis of Bax we found 23% positivity for IDC and 19% for DCIS, which is a significant correlation (p<0.001). We found no correlation between the expression of Bcl-2 and the expression of Bax, which differs from results reported by Martínez-Arribas et al (37), who found a statistically significant association between the two proteins (p=0.0063).
We observed a high expression of Ki-67 (81%) for IDC and DCIS (p=0.001). This positivity correlation is consistent with the results reported by Hoque et al (40), who concluded that there is no increased expression of Ki-67 in IDC associated with DCIS. However, Mylonas et al (41) reported a higher expression of Ki-67 in IDC (p=0.05) than in DCIS and attributed this finding to the evidence that DCIS has a lower potential for malignancy than IDC.
We found no correlation between the expression of Ki-67 and COX-2, which is not consistent with the study by Boland et al, who found a positive association between the group with high positivity for Ki-67 and COX-2 (p<0.001) for IDC and DCIS, respectively (13).
We observed a positive correlation between the expression of Ki-67 and hormonal status (p<0.01) in DCIS and IDC. These data are consistent with the findings of Faratian et al (42); however, Boland et al (13) observed the opposite, showing that cases with a high Ki-67 expression positively correlated with negative HR (p=0.003).
Our analysis of the expression of IGF1-R yielded 24% positivity for IDC and DCIS (p<0.001). When analyzing the expression of IGF1-R in positive HR cases we observed a decrease in IGF1-R expression to 20% in ER-positive and 19% in PR-positive cases. As in our study, Shimizu et al (43) found no correlation between the overexpression of IGF1-R and the variables of age, axillary lymph node status, histological grade and hormonal status, concluding that its prognostic value is limited.
IGF1-R is a transmembrane tyrosine kinase involved in breast cancer proliferation, survival and metastasis (44). The activities of IGF-1 and 2 are tightly regulated by a network of binding proteins and targeted degradation mechanisms. This complex regulatory system is disrupted in breast cancer, leading to excess IGF1-R signaling (45).
In the present study, when we compared the expression of IGF1-R with hormonal status, the correlation was negative, although data from the literature does not show the association between IGF1-R and HR. Shimizu et al (43) and Law et al (46) found no association between the overexpression of IGF1-R and ER/PR.
When we compared COX-2 and IGF1-R expression we found a statistically significant correlation (p<0.05). This finding is consistent with that of Henriksen et al (47), who, by using the semi-quantitative method as recommended by the HercepTest (quantifying their results in scores 0, 1, 2 and 3), found a positive correlation between COX-2 and IGF1-R (p<0.01).
Põld et al (17) reported that high systemic levels of IGF-1 and the correlation with the insulin-like growth factor-binding protein 3 (IGFBP-3) has emerged as a potential risk marker for tumors with COX-2 overexpression.
There is a hypothesis that COX-2 increases the viability and proliferation of tumor cells that express IGF being accompanied by the processes of facilitating auto-phosphorylation of IGF1-R and a decreased expression of IGFBP-3. All these actions may be interpreted as amplifiers of the mitogenic process and as crucial for maintaining the activity of cellular immortality determined by IGF-1 and IGF-2, being consistent, thus explaining the link between COX-2 and IGF1-R (17).
Due to the interrelation between COX-2 and IGF1-R by the mechanisms described previously, the use of a combination therapy with COX-2 and tyrosine kinase inhibitors correlated with IGF1-R may be promising (17).
Several experimental and epidemiological studies have demonstrated the role of COX-2 inhibitors in the prevention of breast carcinoma. In a study that examined the growth inhibition of breast cancer epithelial cells treated with the COX-2 inhibitor, celecoxib, Levitt and Pollak (48) observed that following application of this drug, the induction of apoptosis occurred in these cells as well as a decrease in IGF-1, concluding that these two biomarkers are closely related and may assist in the prevention and treatment of breast cancer.
The development of effective medications that modulate the carcinogenesis of breast tumors is crucial for the prevention and treatment of breast cancer. Recent data suggest that the use of tyrosine kinase, HER-2 and IGF1-R inhibitors is a good strategy for the treatment of breast cancer (49). New studies suggest that the use of metformin inhibits tumor growth in cells in vitro. In an experimental study, Zakikhani et al (50) found that metformin is capable of inducing apoptosis and inhibiting cellular proliferation in breast cancer cells. There are few population studies concerning this drug. Jiralerspong et al (51) evaluated patients who underwent neoadjuvant chemotherapy and who used metformin, and observed a better response when compared with the group that did not use metformin, demonstrating the antitumor effect of this drug.
There is currently a lack of studies involving COX-2 inhibitors and drugs that inhibit activation of IGF1-R. Our results have demonstrated the interrelation between COX-2 and IGF1-R. However, further studies are required to confirm the effectiveness and elucidation of the mechanisms and pathways involved in the use of COX-2 inhibitors, in combination with metformin and IGF1-R inhibitors, in order that they may be used in the prevention and treatment of breast cancer.
References
- 1.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Powles T, Eeles R, Ashley SE, Easton D, Chang J, Dowsett L. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Maisonneuve P, Sacchini V, Rotmensz N, Boyle P. Tamoxifen for breast cancer among women hysterectomised. Lancet. 2002;359:1122–1124. doi: 10.1016/S0140-6736(02)08159-X. [DOI] [PubMed] [Google Scholar]
- 4.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Arimidex, tamoxifen, alone or in combination (ATAC) trialists' group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Brown PH. Prevention of ER-negative breast cancer. Recent Results Cancer Res. 2009;181:121–134. doi: 10.1007/978-3-540-69297-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 7.Dempke W, Rie C, Grothey A, Schmoll H. Cyclooxygenase-2: a novel target for cancer chemotherapy. J Cancer Res Clin Oncol. 2001;127:411–417. doi: 10.1007/s004320000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krcova Z, Ehrmann J, Krejci V, Eliopoulos A, Kolar Z. TPL-2/COT and COX-2 in breast cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:21–25. doi: 10.5507/bp.2008.003. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Arribas F, Martín-Garabato E, Zapardiel I, Sánchez J, Lucas AR, Tejerina A, Schneider J. Bax expression in untreated breast cancer: an immunocytometric study of 255 cases. Anticancer Res. 2008;28:2595–2598. [PubMed] [Google Scholar]
- 10.Singh B, Cook KR, Vincent L, Hall CS, Berry JA, Multani AS, Lucci A. Cyclooxygenase-2 induces genomic instability, BCL2 expression, doxorubicin resistance, and altered cancer-initiating cell phenotype in MCF7 breast cancer cells. J Surg Res. 2008;147:240–246. doi: 10.1016/j.jss.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhu XG, Tao L, Mei ZR, Wu HP, Jiang ZW. Aspisol inhibits tumor growth and induces apoptosis in breast cancer. Exp Oncol. 2008;30:289–294. [PubMed] [Google Scholar]
- 12.Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 13.Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423–429. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheen-Chen SM, Chou FF, Hsu W, Huang CC, Eng HL, Tang RP. Lack of prognostic value of insulin-like growth factor-1 in patients with breast cancer: analysis with tissue microarray. Anticancer Res. 2007;27:3541–3544. [PubMed] [Google Scholar]
- 15.Werner H, Bruchim I. The insulin-like growth factor-1 receptor on the oncogene. Arch Physiol Biochem. 2009;115:58–71. doi: 10.1080/13813450902783106. [DOI] [PubMed] [Google Scholar]
- 16.Levitt RJ, Buckley J, Blouin MJ, Schaub B, Triche TJ, Pollak M. Growth inhibition of breast epithelial cells by celecoxib is associated with up-regulation of insulin-like growth factor binding protein-3 expression. Biochem Biophys Res Commun. 2004;316:421–428. doi: 10.1016/j.bbrc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- 17.Põld M, Krysan K, Põld A, Dohadwala M, Heuze-Vourc'h N, Mao JT, Riedl KL, Sharma S, Dubinett SM. Cyclooxygenase-2 modulates the insulin-like growth factor axis in non-small-cell lung cancer. Cancer Res. 2004;64:6549–6555. doi: 10.1158/0008-5472.CAN-04-1225. [DOI] [PubMed] [Google Scholar]
- 18.Dabbs DJ. Ductal carcinoma of the breast: nuclear grade as a predictor of S-phase fraction. Human Pathol. 1993;24:652–656. doi: 10.1016/0046-8177(93)90246-d. [DOI] [PubMed] [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2006;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 21.Shim JY, Jung An H, Lee YH, Kim SK, Lee KP, Lee KS. Over-expression of Cyclooxygenase-2 is associated with breast carcinoma and its poor prognostic factors. Mod Pathol. 2003;16:1199–1204. doi: 10.1097/01.MP.0000097372.73582.CB. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira VM, Piato S, Silva MA. Correlation of cyclooxygenase-2 and aromatase immunohistochemical expression in invasive ductal carcinoma, ductal carcinoma in situ, and adjacent normal epithelium. Breast Cancer Res Treat. 2006;95:235–241. doi: 10.1007/s10549-005-9010-1. [DOI] [PubMed] [Google Scholar]
- 23.Bernard R, editor. Fundamentals of Biostatistics. 2nd edition. Buxbury Press; Boston, MA: 1986. [Google Scholar]
- 24.Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 25.Buskens CJ, Sivula A, van Rees BP, Haglund C, Offerhaus GJ, van Lanschot JJ, Ristimäki A. Comparison of cyclooxygenase 2 expression in adenocarcinomas of the gastric cardia and distal esophagus. Gut. 2003;52:1678–1683. doi: 10.1136/gut.52.12.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh-Ranger G, Salhab M, Mokbel K. The role of cyclooxygenase-2 in breast cancer: review. Breast Cancer Res Treat. 2008;109:189–198. doi: 10.1007/s10549-007-9641-5. [DOI] [PubMed] [Google Scholar]
- 27.Davies G, Salter J, Hills F, Martin L, Sacks N, Dowset M. Correlation of cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin Cancer Res. 2003;9:2652–2656. [PubMed] [Google Scholar]
- 28.Half E, Tang XT, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- 29.Tan KB, Yong WP, Putti TC. Cyclooxygenase-2 expression: a potential prognostic and predictive marker for high-grade ductal carcinoma in situ of the breast. Histopathology. 2004;44:24–28. doi: 10.1111/j.1365-2559.2004.01774.x. [DOI] [PubMed] [Google Scholar]
- 30.Perrone G, Santini D, Vincenzi B, Zagami M, La Cesa A, Bianchi A, Altomare V, Primavera A, Battista C, Vetrani A, Tonini G, Rabitti C. COX-2 expression in DCIS: correlation with VEGF, HER-2/neu, prognostic molecular markers and clinicopathological features. Histopathology. 2005;46:561–568. doi: 10.1111/j.1365-2559.2005.02132.x. [DOI] [PubMed] [Google Scholar]
- 31.Howe LR, Subbaramaiah K, Brown AM, Dannenberg AJ. Cyclooxygenase-2: a target for prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]
- 32.Benoit V, Relic B, Leval X, Chariot A, Merville MP, Bours V. Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene. 2004;23:1631–1635. doi: 10.1038/sj.onc.1207295. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Liu LH, Shan BE, Zhang C, Sang MS, Li J. Celecoxib promotes apoptosis of breast cancer cell line MDA-MB-231 through down-regulation of the NK-κB pathway. Chin J Cancer. 2009;28:569–574. [PubMed] [Google Scholar]
- 34.Li RX, Shi F, Wu YY, Wu Y, Guo JJ, Dong DF. The relationship between lymphatic metastasis and serum vascular endothelial growth factor C and cyclooxygenase 2 expression in breast cancer. Zhonghua Yi Xue Za Zhi. 2008;88:88–91. [PubMed] [Google Scholar]
- 35.Yang L, Zhu X, Ran L. Correlations of HER-2, PCNA, Bcl-2, and Bax expression to prognosis of breast cancer. Ai Zheng. 2007;26:756–761. (In Chinese) [PubMed] [Google Scholar]
- 36.Mintz PJ, Habib NA, Jones LJ, Giamas G, Lewis JS, Bowen RL, Coombes RC, Stebbing J. The phosphorylated membrane estrogen receptor and cytoplasmic signaling and apoptosis proteins in human breast cancer. Cancer. 2008;113:1489–1495. doi: 10.1002/cncr.23699. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Arribas F, Nuñez-Villar MJ, Lucas AR, Sanchez J, Tejerina A, Schneider J. Immunofluorometric study of Bcl-2 and BAX expression in clinical fresh tumor samples from breast cancer patients. Anticancer Res. 2003;23:565–568. [PubMed] [Google Scholar]
- 38.Arun B, Kilic G, Yen C, et al. Loss of FHIT expression in breast cancer is correlated with poor prognostic markers. Cancer Epidemiol Biomarkers Prev. 2005;14:1681–1685. doi: 10.1158/1055-9965.EPI-04-0278. [DOI] [PubMed] [Google Scholar]
- 39.Michael MS, Badr MZ, Badawi AF. Inhibition of cyclooxygenase-2 and activation of peroxisome proliferator-activated receptor-γ synergistically induces apoptosis and inhibits growth of human breast cancer cells. Int J Mol Med. 2003;11:733–736. [PubMed] [Google Scholar]
- 40.Hoque A, Menter DG, Sahin AA, Sneige N, Lippman SM. No increased Ki67 expression in ductal carcinoma in situ associated with invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:153–154. [PubMed] [Google Scholar]
- 41.Mylonas I, Makovitzky J, Jeschke U, Briese U, Friese K, Gerber B. Expression of Her2/neu, steroid receptors (ER and PR), Ki67 and p53 in invasive mammary ductal carcinoma associated with ductal carcinoma in situ (DCIS) versus invasive breast cancer alone. Anticancer Res. 2005;25:1719–1723. [PubMed] [Google Scholar]
- 42.Faratian D, Munro A, Twelves C, Bartlett JM. Membranous and cytoplasmic staining of Ki67 is associated with HER2 and ER status in invasive breast carcinoma. Histopathology. 2009;54:254–257. doi: 10.1111/j.1365-2559.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu C, Hasegawa T, Tani Y, Takahashi F, Takeuchi M, Watanabe T, Ando M, Katsumata N, Fujiwara Y. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol. 2004;35:1537–1542. doi: 10.1016/j.humpath.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Sachdev D, Wang C, Hubel A, Gaillard-Kelly M, Yee D. Detection and downregulation of type I IGF receptor expression by antibody-conjudated quantum dots in breast cancer cells. Breast Cancer Res Treat. 2009;114:277–285. doi: 10.1007/s10549-008-0014-5. [DOI] [PubMed] [Google Scholar]
- 45.Ellis MJ, Jenkins S, Hanfelt J, Redington ME, Taylor M, Leek R, Siddle K, Harris A. Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat. 1998;52:175–184. doi: 10.1023/a:1006127621512. [DOI] [PubMed] [Google Scholar]
- 46.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, et al. Phosphorylated insulin-like growth factor-1/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–103246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 47.Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: a comparison between whole sections and tissue microarrays. J Clin Pathol. 2007;60:397–404. doi: 10.1136/jcp.2005.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levitt R, Pollak M. Insulin-like growth factor-I antagonizes the antiproliferative effects of cyclooxygenase-2 inhibitors on BxPC-3 pancreatic cancer cells. Cancer Res. 2002;62:7372–7376. [PubMed] [Google Scholar]
- 49.Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:485–498. doi: 10.1007/s10911-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 50.Zakikhani M, Blouin MJ, Piura E, Pollak MN. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat. 2010;123:271–279. doi: 10.1007/s10549-010-0763-9. [DOI] [PubMed] [Google Scholar]
- 51.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]