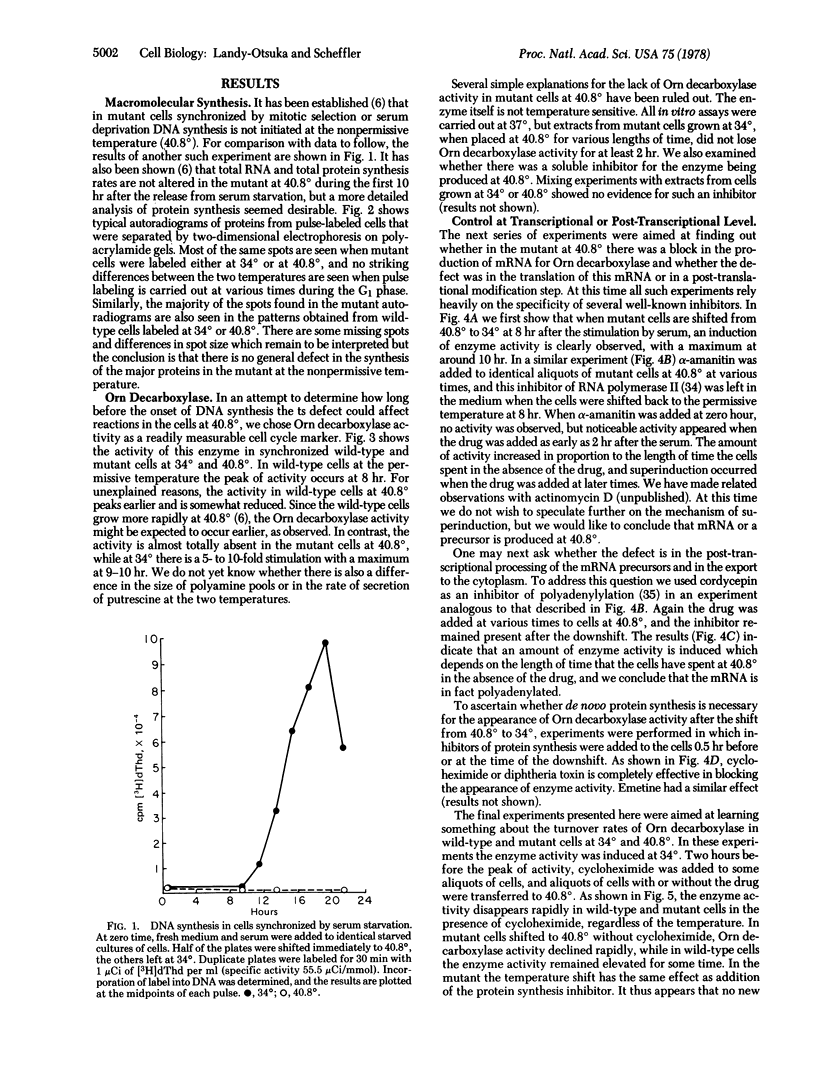
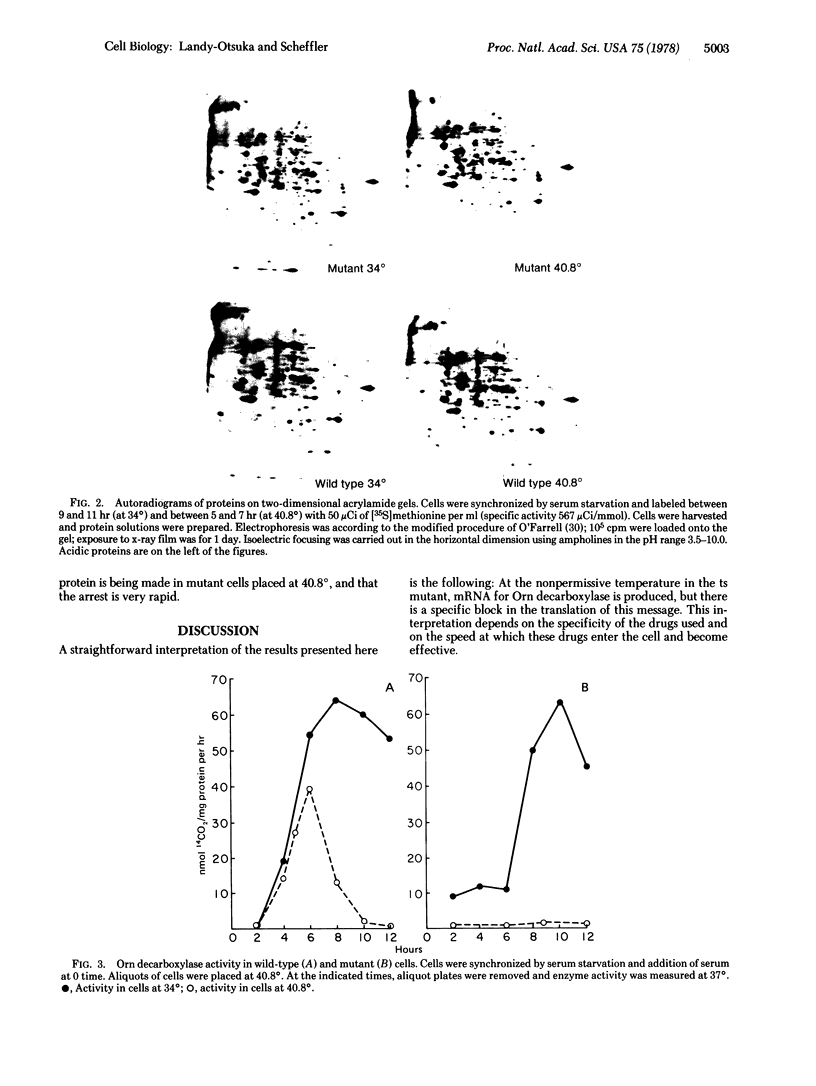
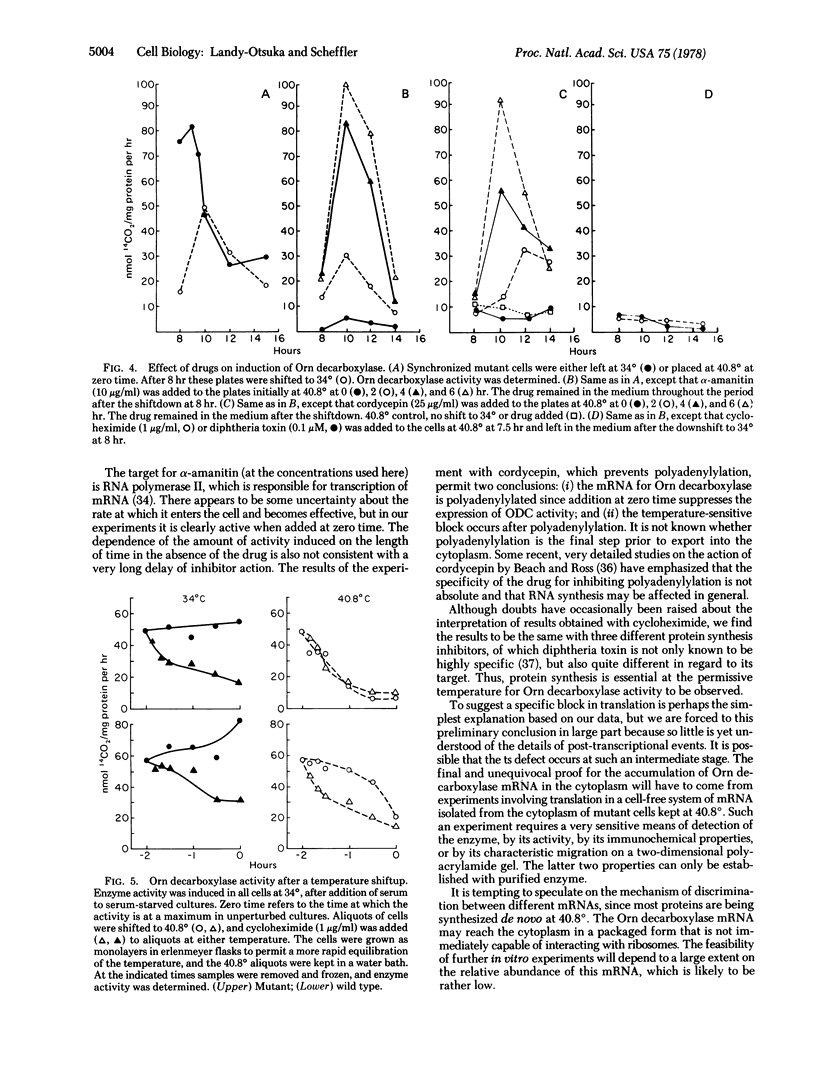
Abstract
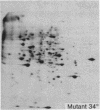
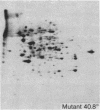
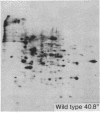
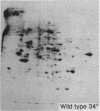
We have investigated the induction of ornithine decarboxylase (L-ornithine carboxy-lyase, EC 4.1.1.17) activity in a temperature-sensitive cell cycle mutant of Chinese hamster fibroblasts. This activity is not induced at the nonpermissive temperature, although the synthesis of the majority of proteins is normal. From a combination of studies with inhibitors of mRNA synthesis and maturation (alpha-amanitin, and cordycepin) and of proteins synthesis (cycloheximide, diphtheria toxin, and emetine), we conclude that the temperature-sensitive block is at the level of translation of one or more specific mRNAs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach L. R., Ross J. Cordycepin. An inhibitor of newly synthesized globin messenger RNA. J Biol Chem. 1978 Apr 25;253(8):2628–2632. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Meiss H. K., Basilico C. A temperature-sensitive cell cycle mutant of the BHK cell line. J Cell Physiol. 1974 Dec;84(3):397–408. doi: 10.1002/jcp.1040840308. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Pyridoxal 5'-phosphate and the regulation of ornithine decarboxylase activity and stability. Eur J Biochem. 1976 Aug 1;67(1):303–314. doi: 10.1111/j.1432-1033.1976.tb10662.x. [DOI] [PubMed] [Google Scholar]
- Clark J. L. Specific induction of ornithine decarboxylase in 3T3 mouse fibroblasts by pituitary growth factors: cell density-dependent biphasic response and alteration of half-life. Biochemistry. 1974 Oct 22;13(22):4668–4674. doi: 10.1021/bi00719a031. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. Polyamine accumulation during lymphocyte transformation and its relation to the synthesis, processing, and accumulation of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4479–4487. doi: 10.1021/bi00746a028. [DOI] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Fuller D. J., Gerner E. W., Russell D. H. Polyamine biosynthesis and accumulation during the G1 to S phase transition. J Cell Physiol. 1977 Oct;93(1):81–88. doi: 10.1002/jcp.1040930111. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Wilson C. B., Martinez H. M. Polyamines: a high correlation with cell replication. FEBS Lett. 1975 Jan 15;50(1):1–4. doi: 10.1016/0014-5793(75)81026-x. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Kyriakidis D., Fong W. F., Canellakis E. S. Ornithine decarboxylase antizyme is a normal component of uninduced H-35 cells and rat liver. Eur J Biochem. 1977 Dec;81(3):545–550. doi: 10.1111/j.1432-1033.1977.tb11980.x. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Murden S. Effect of growth conditions on the activity of ornithine decarboxylase in cultured hepatoma cells. I. Effect of amino acid supply. J Cell Physiol. 1974 Jun;83(3):345–351. doi: 10.1002/jcp.1040830304. [DOI] [PubMed] [Google Scholar]
- Kane G., Basilico C., Basterga R. Transcriptional activity and chromatin structural changes in a temperature-sensitive mutant of BHK cells blocked in early G1. Exp Cell Res. 1976 Apr;99(1):165–173. doi: 10.1016/0014-4827(76)90691-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liskay R. M. A mammalian somatic "cell cycle" mutant defective in G1. J Cell Physiol. 1974 Aug;84(1):49–55. doi: 10.1002/jcp.1040840106. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Polyamine metabolism in enucleated mouse L-cells. J Cell Physiol. 1977 Nov;93(2):285–292. doi: 10.1002/jcp.1040930214. [DOI] [PubMed] [Google Scholar]
- Melero J. A., Smith A. E. Possible transcriptional control of three polypeptides which accumulate in a temperature-sensitive mammalian cell line. Nature. 1978 Apr 20;272(5655):725–727. doi: 10.1038/272725a0. [DOI] [PubMed] [Google Scholar]
- Milman G., Lee E., Ghangas G. S., McLaughlin J. R., George M., Jr Analysis of HeLa cell hypoxanthine phosphoribosyltransferase mutants and revertants by two-dimensional polyacrylamide gel electrophoresis: evidence for silent gene activation. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4589–4593. doi: 10.1073/pnas.73.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieber M., Bacalao J. Alterations in nuclear phosphoproteins of a temperature-sensitive Chinese hamster cell line exposed to non-permissive conditions. Exp Cell Res. 1974 Apr;85(2):334–338. doi: 10.1016/0014-4827(74)90134-7. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H., Robinson H., Carbonell A. W. DNA synthesis and mitosis in a temperature sensitive Chinese hamster cell line. J Cell Physiol. 1973 Dec;82(3):333–338. doi: 10.1002/jcp.1040820303. [DOI] [PubMed] [Google Scholar]
- Rossini M., Baserga R. RNA synthesis in a cell cycle-specific temperature sensitive mutant from a hamster cell line. Biochemistry. 1978 Mar 7;17(5):858–863. doi: 10.1021/bi00598a017. [DOI] [PubMed] [Google Scholar]
- Rupniak H. T., Paul D. Inhibition of spermidine and spermine synthesis leads to growth arrest of rat embryo fibroblasts in G1. J Cell Physiol. 1978 Feb;94(2):161–170. doi: 10.1002/jcp.1040940205. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Stambrook P. J. Cell cycle specific fluctuations in adenosine 3':5'-cyclic monophosphate and polyamines of Chinese hamster cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1482–1486. doi: 10.1073/pnas.72.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler I. E. Conditional lethal mutants of Chinese hamster cells: mutants requiring exogenous carbondioxide for growth. J Cell Physiol. 1974 Apr;83(2):219–230. doi: 10.1002/jcp.1040830208. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Wigglesworth N. M. A temperature-sensitive function in a Chinese hamster line affecting DNA synthesis. J Cell Physiol. 1973 Dec;82(3):339–347. doi: 10.1002/jcp.1040820304. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Wigglesworth N. M. Studies on a Chinese hamster line that is temperature sensitive for the commitment to DNA synthesis. J Cell Physiol. 1974 Aug;84(1):127–133. doi: 10.1002/jcp.1040840114. [DOI] [PubMed] [Google Scholar]
- Talavera A., Basilico C. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J Cell Physiol. 1977 Sep;92(3):425–436. doi: 10.1002/jcp.1040920310. [DOI] [PubMed] [Google Scholar]
- Tenner A., Zieg J., Scheffler I. E. Glycoprotein synthesis in a temperature-sensitive Chinese hamster cell cycle mutant. J Cell Physiol. 1977 Feb;90(2):145–160. doi: 10.1002/jcp.1040900202. [DOI] [PubMed] [Google Scholar]
- Wieland T. Poisonous principles of mushrooms of the genus Amanita. Four-carbon amines acting on the central nervous system and cell-destroying cyclic peptides are produced. Science. 1968 Mar 1;159(3818):946–952. doi: 10.1126/science.159.3818.946. [DOI] [PubMed] [Google Scholar]