Abstract
The purpose of this study was to investigate the effects of glyceollins on the suppression of tumorigenesis in triple-negative breast carcinoma cell lines. We further explored the effects of glyceollins on microRNA and protein expression in MDA-MB-231 cells. Triple-negative (ER-, PgR- and Her2/neu-) breast carcinoma cells were used to test the effects of glyceollins on tumorigenesis in vivo. Following this procedure, unbiased microarray analysis of microRNA expression was performed. Additionally, we examined the changes in the proteome induced by glyceollins in the MDA-MB-231 cells. Tumorigenesis studies revealed a modest suppression of MDA-MB-231 and MDA-MB-468 cell tumor growth in vivo. In response to glyceollins we observed a distinct change in microRNA expression profiles and proteomes of the triple-negative breast carcinoma cell line, MDA-MB-231. Our results demonstrated that the glyceollins, previously described as anti-estrogenic agents, also exert antitumor activity in triple-negative breast carcinoma cell systems. This activity correlates with the glyceollin alteration of microRNA and proteomic expression profiles.
Keywords: triple-negative breast cancer, microRNA, tumorigenesis, glyceollins
Introduction
Breast cancer afflicts approximately 1 in 8 women and is a leading cause of cancer-related mortality. Expression profiles of breast cancer exhibit a systematic variation and allow for the classification of breast cancer into five main groups: two estrogen receptor (ER)-positive (luminal A and B) and three ER-negative groups (normal breast-like, HER2-positive, and ‘basal-like’) (1). The term ‘triple-negative breast cancer’ (TNBC) represents a heterogeneous group of diseases and clearly does not comprise a ‘single entity’ (1). Although triple-negative cancer is not a synonym for basal-like cancer, basal-like cancers are preferentially negative for ER and progesterone receptor (PR) and lack HER2 expression (1). Basal-like breast carcinomas consistently express genes generally found in normal basal/myoepithelial cells of the breast, including high-molecular-weight ‘basal’ cytokeratins (CK; CK5/6, CK14 and CK17), vimentin, p-cadherin, αB crystallin, fascin and caveolins 1 and 2 (1). While it is clear that not all TNBC cases are characterized by the basal-like phenotype and vice versa, microarray-based expression analysis has demonstrated a great deal of overlap (1,2). Clinical similarities also exist between triple-negative tumors and basal-like tumors, including a higher prevalence in African-American women, more frequent incidence in younger patients, and greater aggressiveness than other molecular subgroups (1,3,4).
Of all breast cancers diagnosed approximately 75–80% are positive for ER and/or PR expression and 15–20% are positive for Her2/neu (5). Although these subtypes of disease are potentially susceptible to endocrine therapy and targeted therapy, such as trastuzumab, the remaining 10–15% of breast cancers diagnosed as triple-negative [ER(−), PR(−) and Her2/neu(−)] do not have defined therapeutic targets (6). TNBC has an aggressive clinical history as is evident by its rapid progression to a metastatic phenotype as well as a shorter time to death from distant recurrence as compared to ER(+) disease (1). It is therefore critical to identify novel targets in this disease entity.
The flavonoid family of phytochemicals, particularly those derived from soy, has received attention regarding their estrogenic activity as well as their effects on human health and disease (7–10). The observation that soy phytochemicals decrease the risk of breast cancer indicates a potential for the anti-tumorigenic activity of these compounds (11–13). Additionally, the ability of soy isoflavonoids to prevent carcinogen-induced mammary tumorigenesis further indicates the potential anti-tumorigenic effects of these compounds (14–16). Notably, the amount and type of isoflavonoid present in soy can be readily altered in response to external stimuli (17–20). We previously described an increased biosynthesis of the isoflavonoid phytoalexin compounds, glyceollins I, II and III, in soy plants grown under stressed conditions (elicited soy) (17,19,21).
We showed that glyceollins suppress the tumorigenesis of ER(+) and estrogen-dependent breast cancer systems, demonstrating a clear in vivo anti-estrogenic activity of glyceollins (22). Notably, during these studies we noted that in the absence of estrogen, glyceollin-treated tumors were significantly smaller than their negative control counterparts by day 14. This indicated that in addition to their anti-estrogenic activity, glyceollins may target ER-independent mechanisms regulating tumor cell proliferation and/or survival. In the present study, we evaluated from a biological approach the efficacy of glyceollins on TNBC tumorigenesis in immunocompromised Nu/Nu female mice. Additionally, we investigated the effects of glyceollins on microRNA (miR) expression in the triple-negative setting. In this study, we aimed to demonstrate that glyceollins act as a novel therapeutic agent in TNBC-suppressing tumorigenesis, regulating the expression of miR and altering the proteome of MDA-MB-231 cells.
Materials and methods
Cells and reagents
The MDA-MB-231 and MDA-MB-468 cell lines (human breast cancer negative for ER, PR and Her2/neu) were acquired from the American Type Culture Collection (Manassas, VA, USA) and cultured as previously described (22–24). Glyceollin mixture was isolated as previously described (22).
Xenograft model of tumorigenesis
Nu/Nu immunocompromised female mice (4–6 weeks old) were obtained from Charles River Laboratories (Wilmington, MA, USA). The animals were allowed a period of adaptation in a sterile and pathogen-free environment with food and water ad libitum. Mice were divided into treatment groups of 5 mice each: MDA-MB-231 + dimethyl sulfoxide (DMSO), MDA-MB-231 + glyceollins, MDA-MB-468 + DMSO, MDA-MB-468 + glyceollins. MDA-MB-231 and MDA-MB-468 cells were harvested in the exponential growth phase using a phosphate-buffered saline (PBS)/EDTA solution and washed. Viable cells (5×106) in 50 μl of sterile PBS suspension were mixed with 100 μl Reduced Growth Factor Matrigel (BD Biosciences, Bedford, MA, USA). Cells were injected bilaterally into the mammary fat pad using 27½ gauge sterile syringes. All procedures in animals were carried out under anesthesia using a mix of isofluorane and oxygen delivered by mask. Drug treatment (50 mg/kg/day glyceollins in DMSO/PBS) or vehicle (DMSO/PBS) injections were administered intraperitoneally daily for 14 days after palpable tumors had formed (MDA-MB-231, day 10; MDA-MB-468, day 25).
Tumor size was measured every 2–3 days using digital calipers. The volume of the tumor was calculated using the formula: 4/3π LS2 (L = larger radius; S = shorter radius). At necroscopy animals were sacrificed by cervical dislocation after exposure to CO2. Tumors, uteri, livers, and lungs were removed and frozen in liquid nitrogen or fixed in 10% formalin for further analysis. All procedures involving these animals were conducted in compliance with State and Federal laws, standards of the US Department of Health and Human Services, and guidelines established by Tulane University Animal Care and Use Committee. The facilities and laboratory animals program of Tulane University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
miR microarray
MDA-MD-231 cells were plated at a density of 2 million cells in 25 cm2 flasks in normal culture media (Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin, 1% essential amino acids, 1% non-essential amino acids and 1% sodium pyruvate) and allowed to adhere overnight at 37°C, 5% CO2 and air. Cells were treated with glyceollins (10 μM) or DMSO for 18 h. Cells were harvested in PBS and collected by centrifugation, and total RNA was extracted using the miRNeasy kit (Qiagen) according to manufacturer's protocol. Enrichment for miRNA was not performed. Quantity and quality of RNA was determined by absorbance (260 and 280 nm). Microarray assay was performed by LC Sciences (Houston, TX, USA). The assay started from 5 μg total RNA sample, which was size fractionated using a YM-100 Microcon centrifugal filter (Millipore, Billerica, MA, USA) and the small RNAs (<300 nt) isolated were 3′-extended with a poly(A) tail using poly(A) polymerase. An oligonucleotide tag was then ligated to the poly(A) tail for later fluorescent dye staining; two different tags were used for the two RNA samples in dual-sample experiments. Hybridization was performed overnight on a μParaflo microfluidic chip using a micro-circulation pump (Atactic Technologies, Houston, TX, USA) (25,26). On the microfluidic chip, each detection probe consisted of a chemically modified nucleotide coding segment complementary to target miR (from miRBase, http://microrna.sanger.ac.uk/sequences/) or other RNA (control or customer defined sequences) and a spacer segment of polyethylene glycol to extend the coding segment away from the substrate. The detection probes were rendered by in situ synthesis using PGR (photogenerated reagent) chemistry. The hybridization melting temperatures were balanced by chemical modifications of the detection probes. Hybridization used 100 μl 6X SSPE buffer (0.90 M NaCl, 60 mM Na2HPO4, 6 mM EDTA, pH 6.8) containing 25% formamide at 34°C. After RNA hybridization, tag-conjugating Cy3 and Cy5 dyes were circulated through the microfluidic chip for dye staining. Fluorescence images were collected using a laser scanner (GenePix 4000B, Molecular Device, Sunnyvale, CA, USA) and digitized using Array-Pro image analysis software (Media Cybernetics, Bethesda, MD, USA). Data were analyzed by first subtracting the background and then normalizing the signals using a LOWESS (Locally-weighted Regression) filter (27). For two color experiments, the ratio of the two sets of detected signals (log2 transformed, balanced) and p-values of the t-test were calculated; differentially detected signals were those with p-values of <0.01. The array was performed using quadruplicate biological repeats. Full array data are available in Table I.
Table I.
microRNA microarray results for MDA-MB-231 cells treated with glyceollin (10 μM) for 18 h.
| No. | Reporter name | p-value | Group 1 | Group 2 | Log2 (G2/G1) |
|---|---|---|---|---|---|
| Control | Glyceollin | ||||
| Mean | Mean | ||||
| 108 | hsa-miR-1268 | 1.09E-05 | 1,732 | 918 | −0.92 |
| 156 | hsa-miR-130a | 3.61E-05 | 1,711 | 2,904 | 0.76 |
| 844 | hsa-miR-940 | 4.32E-05 | 204 | 730 | 1.84 |
| 287 | hsa-miR-197 | 4.59E-05 | 2,018 | 1,015 | −0.99 |
| 277 | hsa-miR-193a-5p | 5.45E-05 | 1,452 | 866 | −0.75 |
| 332 | hsa-miR-22 | 8.15E-05 | 3,807 | 5,788 | 0.60 |
| 372 | hsa-miR-29b | 1.53E-04 | 2,616 | 7,692 | 1.56 |
| 351 | hsa-miR-25 | 1.68E-04 | 6,161 | 4,963 | −0.31 |
| 808 | hsa-miR-877 | 1.75E-04 | 1,424 | 708 | −1.01 |
| 294 | hsa-miR-19b | 2.30E-04 | 3,160 | 4,253 | 0.43 |
| 345 | hsa-miR-23a* | 2.33E-04 | 1,205 | 669 | −0.85 |
| 828 | hsa-miR-923 | 2.80E-04 | 1,743 | 1,243 | −0.49 |
| 748 | hsa-miR-638 | 3.58E-04 | 2,729 | 3,946 | 0.53 |
| 621 | hsa-miR-542-5p | 3.66E-04 | 430 | 142 | −1.60 |
| 853 | hsa-miR-99a | 4.18E-04 | 13,257 | 16,348 | 0.30 |
| 246 | hsa-miR-185 | 4.43E-04 | 1,576 | 1,182 | −0.41 |
| 446 | hsa-miR-361-5p | 4.92E-04 | 4,553 | 3,574 | −0.35 |
| 378 | hsa-miR-301a | 5.19E-04 | 1,176 | 1,436 | 0.29 |
| 95 | hsa-miR-125b | 8.65E-04 | 26,192 | 23,016 | −0.19 |
| 375 | hsa-miR-29c | 1.03E-03 | 2,157 | 4,445 | 1.04 |
| 830 | hsa-miR-92a | 1.04E-03 | 10,508 | 9,547 | −0.14 |
| 789 | hsa-miR-720 | 1.04E-03 | 2,194 | 1,622 | −0.44 |
| 238 | hsa-miR-182 | 1.17E-03 | 2,216 | 1,493 | −0.57 |
| 492 | hsa-miR-423-5p | 1.22E-03 | 5,631 | 3,810 | −0.56 |
| 78 | hsa-miR-1246 | 1.28E-03 | 561 | 368 | −0.61 |
| 774 | hsa-miR-663 | 1.49E-03 | 1,023 | 2,102 | 1.04 |
| 282 | hsa-miR-195 | 1.62E-03 | 217 | 543 | 1.33 |
| 32 | hsa-miR-10a | 2.23E-03 | 1,375 | 762 | −0.85 |
| 513 | hsa-miR-454 | 2.58E-03 | 1,855 | 1,070 | −0.79 |
| 235 | hsa-miR-181c | 2.64E-03 | 892 | 1,806 | 1.02 |
| 212 | hsa-miR-151-5p | 2.96E-03 | 8,728 | 7,418 | −0.23 |
| 272 | hsa-miR-1915 | 3.17E-03 | 1,135 | 1,470 | 0.37 |
| 407 | hsa-miR-320c | 3.31E-03 | 9,712 | 8,731 | −0.15 |
| 397 | hsa-miR-30d | 3.51E-03 | 5,669 | 6,753 | 0.25 |
| 176 | hsa-miR-138 | 3.83E-03 | 3,588 | 4,796 | 0.42 |
| 315 | hsa-miR-21 | 3.96E-03 | 21,133 | 18,983 | −0.15 |
| 211 | hsa-miR-151-3p | 4.09E-03 | 3,811 | 2,996 | −0.35 |
| 515 | hsa-miR-455-3p | 4.21E-03 | 1,666 | 1,068 | −0.64 |
| 523 | hsa-miR-486-5p | 4.50E-03 | 777 | 389 | −1.00 |
| 237 | hsa-miR-181d | 4.51E-03 | 540 | 2,691 | 2.32 |
| 363 | hsa-miR-28-5p | 5.90E-03 | 983 | 1,137 | 0.21 |
| 391 | hsa-miR-30a* | 5.98E-03 | 1,452 | 1,015 | −0.52 |
| 439 | hsa-miR-34a | 6.31E-03 | 1,307 | 1,644 | 0.33 |
| 31 | hsa-miR-107 | 6.42E-03 | 7,287 | 8,200 | 0.17 |
| 356 | hsa-miR-26b | 6.45E-03 | 3,013 | 3,902 | 0.37 |
| 405 | hsa-miR-320a | 6.58E-03 | 10,409 | 9,155 | −0.19 |
| 124 | hsa-miR-1280 | 7.49E-03 | 4,155 | 5,245 | 0.34 |
| 8 | hsa-let-7d* | 7.73E-03 | 754 | 497 | −0.60 |
| 38 | hsa-miR-1180 | 8.08E-03 | 984 | 501 | −0.97 |
| 292 | hsa-miR-19a | 8.08E-03 | 208 | 602 | 1.54 |
| 343 | hsa-miR-224 | 8.83E-03 | 2,601 | 1,362 | −0.93 |
| 686 | hsa-miR-584 | 9.53E-03 | 2,478 | 1,818 | −0.45 |
| The following transcripts are statistically significant but have low signals (signal <500) | |||||
| 365 | hsa-miR-296-5p | 9.44E-04 | 139 | 304 | 1.13 |
| 125 | hsa-miR-1281 | 3.24E-03 | 205 | 440 | 1.10 |
| 352 | hsa-miR-25* | 3.32E-03 | 152 | 74 | −1.04 |
| 528 | hsa-miR-489 | 4.29E-03 | 460 | 183 | −1.33 |
| 269 | hsa-miR-1913 | 4.37E-03 | 126 | 288 | 1.19 |
| 553 | hsa-miR-505* | 5.53E-03 | 340 | 130 | −1.39 |
| 464 | hsa-miR-374b | 6.50E-03 | 201 | 282 | 0.49 |
| 778 | hsa-miR-665 | 8.19E-03 | 55 | 160 | 1.54 |
| 245 | hsa-miR-184 | 9.20E-03 | 274 | 36 | −2.93 |
Proteomics analysis
MDA-MB-231 cells were treated with DMSO or glyceollin-mix (10 μM) for 18 h. Cells were harvested and run using 2D-electrophoresis. The first dimensional electrophoresis was performed using a Protean IEF cell unit (BioRad, Hercules, CA, USA). Precast 11-cm IPG strips with a pH range of 5–8 were used to separate the proteins based on their isoelectric pH. The second dimensional electrophoresis was carried out in a BioRad Criterion electrophoresis cell system. Stained gels were scanned with a Gel Doc-XR image system (BioRad) and analyzed with the PDQuest software (version 8.01). The proteins of interest were marked for excision and excised from gels using a Quest Spot cutter (BioRad), digested and analyzed by an LC-Nanospray-MS system. The tandem MS spectra were analyzed against the ipi.human.v3.27 database using SEQUEST software and tabulated.
Statistical analysis
Studies were analyzed by the unpaired Student's t-test (Graph Pad Prism V.4) and p-values of <0.05 were considered statistically significant.
Results and Discussion
Glyceollins partially suppress growth of triple-negative breast tumor growth in vivo
To determine the therapeutic relevance of glyceollins in the triple-negative setting, MDA-MB-231 and MDA-MB-468 cells were used in an in vivo xenograft model of tumorigenesis. Immunocompromised female nude mice were injected in the mammary fat pad (MFP) with either MDA-MB-231 (Fig. 1A) or MDA-MB-468 (Fig. 1B) cells mixed with reduced growth factor matrigel. After palpable tumor formation (MDA-MB-231, day 10; MDA-MB-468, day 25), mice were randomized into treatment groups (n=5) and treated with vehicle or glyceollins (50 mg/kg/day). Tumor volume of MDA-MB-231 and MDA-MB-468 cells treated with glyceollins showed decreased tumor growth compared to vehicle-treated control tumors at endpoint analysis (Fig. 1, 64.36±21.29 mm3 and 58.16±11.28 mm3, respectively). These results demonstrate the tumor-suppressive effects of glyceollins on triple-negative breast carcinoma cell lines and indicate the clinical significance and therapeutic potential of glyceollins in the TNBC.
Figure 1.
Glyceollin decreases tumorigenesis of triple-negative breast carcinoma in vivo. Nu/Nu female mice (4–6 weeks old) were injected in the MFP with 5×106 (A) MDA-MB-231 or (B) MDA-MB-468 cells. After tumor formation (10 and 25 days, respectively) mice were administered daily intraperitoneal injections of vehicle or glyceollins (50 mg/kg) for (A) 9 or (B) 7 days. Tumors were measured via digital caliper. Bars are the mean tumor volume at endpoint (normalized to vehicle) ± SEM.
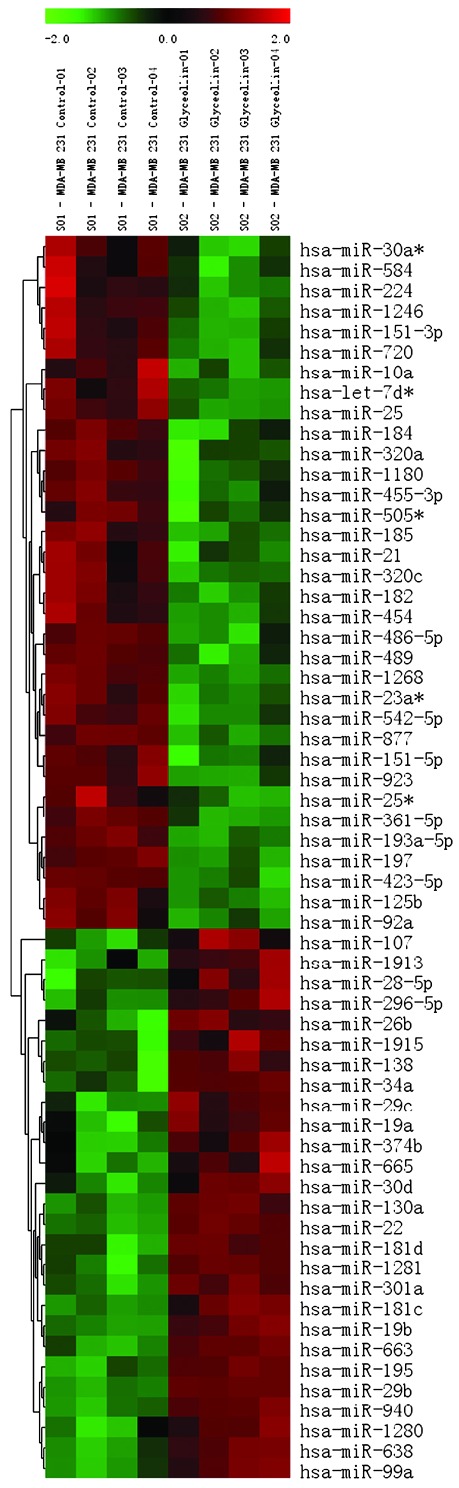
Glyceollins alter the miRnome of triple-negative breast carcinoma cells consistent with tumor-suppressive effects
Altered miR expression is common among a number of types of cancer and this dysregulation is known to promote tumorigenesis, hormone independence and drug resistance, epithelial-mesenchymal transition (EMT) and metastasis (28–39). miR microarray analysis of MDA-MB-231 cells treated with glyceollins for 18 h revealed a number of changes in the miRNA expression profile compared to vehicle treated cells. Fig. 2 shows a heat map of miR expression changes for 4 independent samples. Tables II and III show the miRs found to have a significantly altered expression (increased or decreased, respectively) in response to treatment with glyceollins (p<0.01). A number of the miRs demonstrating a significantly increased expression following treatment with glyceollins have been characterized as tumor suppressers inhibiting cell cycle and proliferation (miR-181c/d), EMT and metastasis (miR-22, 29b/c, 30d, 34a, 195), or directly targeting known oncogenes (miR-26b). Those miRs with a significantly decreased expression induced by glyceollins have been identified as oncomiRs with roles in promoting tumorigenesis (miR-21, 193-5p) and metastasis (miR-185, 224).
Figure 2.
Glyceollins regulation of microRNA expression in MDA-MB-231 cells. Heatmap of microRNA changes induced by treatment with glyceollins (10 μM) after 18 h in MDA-MB-231 cells. microRNAs demonstrating statistically significant changes in expression are shown (p<0.01). Green indicates down-regulated expression and red indicates up-regulated expression of microRNAs. Individual samples are shown in columns while specific microRNAs are indicated by rows as labeled.
Table II.
microRNA with increased expression following glyceollin treatment.
| miRNA | Mean fold-change | p-value | miRNA | Mean fold-change | p-value |
|---|---|---|---|---|---|
| 19a | 2.91 | <0.01 | 130a | 1.69 | <0.001 |
| 19b | 1.35 | <0.001 | 301a | 1.22 | <0.001 |
| 22 | 1.52 | <0.001 | 138 | 1.34 | <0.01 |
| 26b | 1.29 | <0.01 | 181c | 2.03 | <0.01 |
| 181d | 4.99 | <0.01 | |||
| 28-5p | 1.16 | <0.01 | 195 | 2.51 | <0.01 |
| 29b | 2.95 | <0.001 | 638 | 1.44 | <0.001 |
| 29c | 2.06 | <0.01 | |||
| 30d | 1.19 | <0.01 | 663 | 2.06 | <0.01 |
| 34a | 1.26 | <0.01 | 940 | 3.58 | <0.001 |
| 99a | 1.23 | <0.001 | 1280 | 1.27 | <0.01 |
| 107 | 1.13 | <0.01 | 1915 | 1.29 | <0.01 |
Table III.
microRNA with decreased expression following glyceollin treatment.
| miRNA | Mean fold-change | p-value | miRNA | Mean fold-change | p-value |
|---|---|---|---|---|---|
| 10a | −1.80 | <0.01 | 361-5p | −1.27 | <0.001 |
| 21 | −1.11 | <0.01 | 423-5p | −1.47 | <0.01 |
| 23a* | −1.80 | <0.001 | 454 | −1.73 | <0.01 |
| 25 | −1.24 | <0.001 | 455-3p | −1.56 | <0.01 |
| 30a* | −1.43 | <0.01 | 486-5p | −2.00 | <0.01 |
| 92a | −1.10 | <0.01 | 542-5p | −3.03 | <0.001 |
| 125b | −1.14 | <0.001 | 584 | −1.37 | <0.01 |
| 151-3p | −1.27 | <0.01 | 720 | −1.36 | <0.01 |
| 151-5p | −1.17 | <0.01 | |||
| 182 | −1.48 | <0.01 | 877 | −2.01 | <0.001 |
| 185 | −1.33 | <0.001 | 923 | −1.40 | <0.001 |
| 193a-5p | −1.68 | <0.001 | 1180 | −1.96 | <0.01 |
| 197 | −1.99 | <0.001 | 1246 | −1.53 | <0.01 |
| 224 | −1.91 | <0.01 | 1268 | −1.89 | <0.001 |
| 320a | −1.14 | <0.01 | let-7da | −1.52 | <0.01 |
| 320c | −1.11 | <0.01 |
Among the most highly expressed miRs following treatment with glyceollins were miR-19a/b, 22, 29b/c, 181c/d, 195, 663 and 940. Notably, a number of the miRs that demonstrated glyceollin-induced expression have previously been documented as having tumor-suppressive effects. For example, miR-22 has been classified as a tumor-suppressive miR in metastatic breast cancers, as it has been shown to target oncogenes EVI-1, ERBB3 and CDC25C (40), as well the pro-metastatic gene EZR in ovarian cancer (41,42). miR-26b inhibits glioma tumor cell proliferation, survival, and migration by directly targeting EPHA2 (43). Further evidence of the tumor-suppressive nature of miR-26b include its ability to induce apoptosis via repression of SLC7A11 and the decreased expression of miR-26b in breast carcinoma patient samples (44).
miR-29b/c have been shown to directly inhibit the cell cycle transcription factor MYBL2 and in turn induce tumor cell senescence (45). miR-29 also plays a role in maintaining adequate cell adhesion by regulating extracellular matrix proteins (46) including collagens (47,48) and elastin (49), and regulates cell survival by targeting the anti-apoptotic MCL1 (50). Furthermore, miR-29 has been shown to induce expression of the tumor suppressor p53 by inhibiting the Rho-GTPase CDC42 (51).
Decreased expression of miR-181c due to hypermethylation has been observed in gastric carcinoma and its targets include the oncogenes NOTCH4 and KRAS (52). Additionally, miR-195 expression is significantly decreased in breast carcinoma patient samples (53), and a decreased expression of miR-195 has been correlated with decreased survival and increased metastasis in colorectal cancers (54). Direct targets of miR-195 include the oncogene RAF1, cell cycle regulators CCND1 (53) and CCNE1 (55), as well as the anti-apoptotic BCL2 (56).
The remaining two miRs with fold changes >2 have also been found to play anti-tumorigenic roles in cancer. miR-663 inhibits AP-1 activity by directly targeting JunB and JunD (57) and has been described as a tumor suppressor in gastric cancer (58). In silico predicted targets (TargetScan and miRANDA) of miR-940 include RhoA, a prominent mediator of invasion and metastasis.
Notably, although miR-19 is often referred to as an oncomiR due to its inclusion in the miR-17–92 oncogenic cluster, Zhang et al have recently demonstrated the ability of miR-19 to directly target tissue factor (TF), a known promoter of cancer cell survival, angiogenesis, and metastasis (59). Therefore, miR-19 may also play a tumor-suppressive role in breast cancer.
Among the most downregulated miRs following treatment with glyceollins were 193a-5p, 197, 224, 486-5p, and 542-5p, all of which have been associated with cancer progression. For instance, miR-193a-5p has been shown to target pro-apoptotic p73 and limit the effects of chemotherapy (60), while the oncomiR miR-197 has been shown to directly target the tumor suppressor, FUS1 (61). miR-224 has been associated with cancer progression (62) and enhanced cell migration and invasion by increasing the expression of the pro-invasive PAK4 and MMP-9 (63). Additionally, miR-224 and miR-486-5p promote cell migration and invasion by targeting the tumor suppressor CD40 (64,65).
miR-542-5p expression has also been associated with maintenance of the mesenchymal phenotype (66), a key characteristic of the TNBC phenotype and driver of cell motility and invasiveness. The reversal of the mesenchymal phenotype to a more epithelial morphology through the process of mesenchymal-to-epithelial transition (MET) represents an area of high-impact research for the development of novel therapeutics.
Although not a marked change, treatment with glyceollins decreases the expression of miR-21. miR-21 is one of the most established and highly researched miRs for its oncogenic role in cancer (67), and has been shown to be highly overexpressed in TNBC (68). The expression of miR-21 in breast tumors has been associated with poor prognosis (31), development of drug resistance (69,70), and increased rate of recurrence (39). Targets of miR-21 include prominent tumor suppressors PTEN (71,72) and PDCD4 (73), as well as inhibitors of metastasis, such as TIMP3 (74) and TPM1 (75,76).
The function of the remaining two miRs downregulated more than 1.95-fold by glyceollins, miR-877 and 1180, has yet to be determined at the time of this publication. Although the function of these miRs is not currently known, putative targets predicted by TargetScan include p53 inducible nuclear protein 2 (TP53INP2) and the cell cycle regulator, CDC40 (putative targets for miR-877); a regulator of cell adhesion, PUNC, the pro-apoptotic gene, BAD and BAMBI, a negative regulator of TGFβ known to mediate cell transformation (putative targets of miR-1180).
Glyceollins alter the proteome of MDA-MB-231 breast carcinoma cells in a manner indicative of tumor suppressive effects
The treatment of MDA-MB-231 cells with glyceollins for 18 h generated distinct protein spot patterns as analyzed by 2D-gel electrophoresis. Sequence analysis of selected spots revealed a number of proteins up- and downregulated by glyceollins (Table IV). While each of these spots represents a target for validation and mechanistic analysis, two proteins identified are known to play key roles in breast cancer tumorigenesis and progression. We observed an almost 25-fold upregulation of NME1 (NM23-H1) by glyceollin treatment. NME1 is a known metastasis suppressor gene (77) and is a putative target of two miRs with a significantly decreased expression following treatment with glyceollins, miR-486-5p and miR-542-5p. Notably, as mentioned above, miR-542-5p expression has been linked to the maintenance of the mensenchymal phenotype. Decreased expression of miR-542-5p, as well as an increased expression of NME1, indicates a reversal of EMT and a suppression of metastasis by glyceollins. A second target identified via our proteomics approach is vimentin, whose expression was downregulated more than 13-fold by glyceollins. Vimentin is a marker for epithelial-to-mesenchymal transition and is highly expressed in numerous TNBCs and cell lines including MDA-MB-231 (78). Vimentin is a proven target of miR-30d (79) and a predicted target of miR-138, both found to be significantly increased by glyceollins. These data suggest that the effects of glyceollins on TNBC cell lines are achieved via regulation of miRs, which in turn regulate known oncogenes and tumor suppressors. Taken together, these data indicate that treatment of TNBC cells with glyceollins inhibit tumorigenesis and induce a miR expression profile correlative to a less aggressive phenotype.
Table IV.
Effects of glyceollins on the MDA-MB-231 cell proteome.
| Spot | Gene symbol | Gene name | Mean intensity ratio | p-value |
|---|---|---|---|---|
| 203 | EEF1D | Elongation factor 1-δ | 36.50 | <0.01 |
| 1004 | ARHGDIA | Rho GDP-dissociation inhibitor 1 | 115.85 | <0.001 |
| 2102 | CLIC1 | Chloride intracellular channel protein 1 | 129.94 | <0.001 |
| 2103 | TPD52L2 | Isoform 2 of tumor protein D54 | 43.90 | <0.01 |
| 2204 | EIF2S1 | Eukaryotic translation initiation factor 2 subunit 1 | 55.62 | <0.01 |
| 3103 | CLIC4 | Chloride intracellular channel protein 4 | 44.37 | <0.01 |
| 5004 | NME1 | Non-metastatic cells 1 (NM23-H1) | 24.89 | <0.01 |
| 5304 | GIPC1 | PDZ domain-containing protein GIPC1 | 21.41 | <0.01 |
| 5405 | MAP2K2 | Mitogen-activated protein kinase kinase 2 | 29.96 | <0.01 |
| 5406 | TARDBP | TAR DNA-binding protein 43 | 29.96 | <0.01 |
| 7706 | VIM | Vimentin | −13.84 | <0.01 |
| 9903 | DDX1 | ATP-dependent RNA helicase DDX1 | −34.75 | <0.001 |
| 9904 | KHSRP | Far upstream element-binding protein 2 | −31.31 | <0.01 |
| 2304 | SEC13 | SEC13-related protein | −10.07 | <0.01 |
| 5506 | HNRPH1 | Heterogeneous nuclear ribonucleoprotein H1 | −28.63 | <0.001 |
| 7202 | HNRPH3 | Heterogeneous nuclear ribonucleoprotein H3 | −66.35 | <0.01 |
| 8404 | HNRPD | Heterogeneous nuclear ribonucleoprotein D0 | −15.28 | <0.001 |
| 3605 | FKBP4 | FK506-binding protein | −17.83 | <0.01 |
In conclusion, the results from our study demonstrate the ability of glyceollins to inhibit tumor growth of the triple-negative breast carcinoma cell lines, MDA-MB-231 and MDA-MB-468. Furthermore, it is known that the dysregulation of miR expression is a characteristic of numerous cancer cancer types including breast carcinoma. miR microarray analysis of MDA-MB-231 cells treated with glyceollins revealed significant alterations of the miR expression profile consistent with a less aggressive phenotype.
Acknowledgements
This study was supported by: The US Department of Agriculture 58-6435-7-019 (S.M.B. and M.E.B.), the National Institutes of Health/National Center for Research Resources P20RR020152 (B.M.C.-B.), National Center for Research Resources RCMI program through Grant 5G12RR026260-02 (G.W.), and the Office of Naval Research Grant 09-10 N00014-10-1-0270 (M.E.B.).
References
- 1.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 2.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progeste- rone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a popu- lation-based study from the California cancer registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst. 2007;99:1657–1659. doi: 10.1093/jnci/djm228. [DOI] [PubMed] [Google Scholar]
- 6.Reis-Filho JS, Westbury C, Pierga JY. The impact of expression profiling on prognostic and predictive testing in breast cancer. J Clin Pathol. 2006;59:225–231. doi: 10.1136/jcp.2005.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr. 2010;140:1350S–1354S. doi: 10.3945/jn.109.118315. [DOI] [PubMed] [Google Scholar]
- 8.Murkies AL, Wilcox G, Davis SR. Clinical review 92: Phytoestrogens. J Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 9.Tham DM, Gardner CD, Haskell WL. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998;83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 10.Humfrey CD. Phytoestrogens and human health effects: weighing up the current evidence. Nat Toxins. 1998;6:51–59. doi: 10.1002/(sici)1522-7189(199804)6:2<51::aid-nt11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Collins-Burow BM, Burow ME, Duong BN, McLachlan JA. Estrogenic and antiestrogenic activities of flavonoid phytochemicals through estrogen receptor binding-dependent and -independent mechanisms. Nutr Cancer. 2000;38:229–244. doi: 10.1207/S15327914NC382_13. [DOI] [PubMed] [Google Scholar]
- 12.Collins BM, McLachlan JA, Arnold SF. The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids. 1997;62:365–372. doi: 10.1016/s0039-128x(96)00246-2. [DOI] [PubMed] [Google Scholar]
- 13.Fournier DB, Erdman JW, Jr, Gordon GB. Soy, its components, and cancer prevention: a review of the in vitro, animal, and human data. Cancer Epidemiol Biomarkers Prev. 1998;7:1055–1065. [PubMed] [Google Scholar]
- 14.Barnes S. The chemopreventive properties of soy isoflavonoids in animal models of breast cancer. Breast Cancer Res Treat. 1997;46:169–179. doi: 10.1023/a:1005956326155. [DOI] [PubMed] [Google Scholar]
- 15.Lamartiniere CA, Zhang JX, Cotroneo MS. Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity. Am J Clin Nutr. 1998;68:1400S–1405S. doi: 10.1093/ajcn/68.6.1400S. [DOI] [PubMed] [Google Scholar]
- 16.Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H, Vollmer G. Phytoestrogens and carcinogenesis-differential effects of genistein in experimental models of normal and malignant rat endometrium. Hum Reprod. 2001;16:997–1006. doi: 10.1093/humrep/16.5.997. [DOI] [PubMed] [Google Scholar]
- 17.Boue SM, Carter CH, Ehrlich KC, Cleveland TE. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J Agric Food Chem. 2000;48:2167–2172. doi: 10.1021/jf9912809. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya M, Ward EWB. Resistance, susceptibility and accumulation of glyceollins I-III in soybean organs inoculated with Phytophthora megasperma f. sp. Glycinea. Physiol and Mol Plant Pathol. 1986;29:227–237. [Google Scholar]
- 19.Boue SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agric Food Chem. 2003;51:2193–2199. doi: 10.1021/jf021114s. [DOI] [PubMed] [Google Scholar]
- 20.Graham TL, Graham MY. Glyceollin elicitors induce major but distinctly different shifts in isoflavonoid metabolism in proximal and distal soybean cell populations. Mol Plant Microbe Interact. 1991;4:60–68. [Google Scholar]
- 21.Burow ME, Boue SM, Collins-Burow BM, et al. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J Clin Endocrinol Metab. 2001;86:1750–1758. doi: 10.1210/jcem.86.4.7430. [DOI] [PubMed] [Google Scholar]
- 22.Salvo VA, Boue SM, Fonseca JP, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12:7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes LV, Muir SE, Elliott S, et al. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat. 2010;121:293–300. doi: 10.1007/s10549-009-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burow ME, Tang Y, Collins-Burow BM, et al. Effects of environmental estrogens on tumor necrosis factor alpha-mediated apoptosis in MCF-7 cells. Carcinogenesis. 1999;20:2057–2061. doi: 10.1093/carcin/20.11.2057. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Gulari E, Zhou X. In situ synthesis of oligonucleotide microarrays. Biopolymers. 2004;73:579–596. doi: 10.1002/bip.20005. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Q, Hong A, Sheng N, et al. microParaflo biochip for nucleic acid and protein analysis. Methods Mol Biol. 2007;382:287–312. doi: 10.1007/978-1-59745-304-2_19. [DOI] [PubMed] [Google Scholar]
- 27.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winter J, Diederichs S. MicroRNA biogenesis and cancer. Methods Mol Biol. 2011;676:3–22. doi: 10.1007/978-1-60761-863-8_1. [DOI] [PubMed] [Google Scholar]
- 30.Allen KE, Weiss GJ. Resistance may not be futile: microRNA biomarkers for chemoresistance and potential therapeutics. Mol Cancer Ther. 2011;9:3126–3136. doi: 10.1158/1535-7163.MCT-10-0397. [DOI] [PubMed] [Google Scholar]
- 31.Gandellini P, Profumo V, Folini M, Zaffaroni N. MicroRNAs as new therapeutic targets and tools in cancer. Expert Opin Ther Targets. 2011;15:265–279. doi: 10.1517/14728222.2011.550878. [DOI] [PubMed] [Google Scholar]
- 32.Nana-Sinkam SP, Croce CM. MicroRNA dysregulation in cancer: opportunities for the development of microRNA-based drugs. IDrugs. 2011;13:843–846. [PubMed] [Google Scholar]
- 33.Ma L, Weinberg RA. MicroRNAs in malignant progression. Cell Cycle. 2008;7:570–572. doi: 10.4161/cc.7.5.5547. [DOI] [PubMed] [Google Scholar]
- 34.O'Day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2011;12:201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 36.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Wang L, Matyunina LV, Hill CG, McDonald JF. Overexpression of miR-429 induces mesenchymal-to-epithelial transition (MET) in metastatic ovarian cancer cells. Gynecol Oncol. 2011;121:200–205. doi: 10.1016/j.ygyno.2010.12.339. [DOI] [PubMed] [Google Scholar]
- 38.Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2011;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 39.Ota D, Mimori K, Yokobori T, et al. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int J Oncol. 2011;38:955–962. doi: 10.3892/ijo.2011.926. [DOI] [PubMed] [Google Scholar]
- 40.Patel JB, Appaiah HN, Burnett RM, et al. Control of EVI-1 oncogene expression in metastatic breast cancer cells through microRNA miR-22. Oncogene. 2011;30:1290–1301. doi: 10.1038/onc.2010.510. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Liang S, Yu H, Zhang J, Ma D, Lu X. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol. 2011;119:543–548. doi: 10.1016/j.ygyno.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Liang SH, Lu X. Potential role of ezrin and its related microRNA in ovarian cancer invasion and metastasis. Zhonghua Fu Chan Ke Za Zhi. 2011;45:787–792. [PubMed] [Google Scholar]
- 43.Wu N, Zhao X, Liu M, et al. Role of microRNA-26b in glioma development and its mediated regulation on EphA2. PLoS One. 2011;6:e16264. doi: 10.1371/journal.pone.0016264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XX, Li XJ, Zhang B, et al. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett. 2011;585:1363–1367. doi: 10.1016/j.febslet.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Martinez I, Cazalla D, Almstead LL, Steitz JA, DiMaio D. miR-29 and miR-30 regulate B-Myb expression during cellular senescence. Proc Natl Acad Sci USA. 2011;108:522–527. doi: 10.1073/pnas.1017346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villarreal G, Jr, Oh DJ, Kang MH, Rhee DJ. Coordinated Regulation of Extracellular Matrix Synthesis by the MicroRNA-29 Family in the Trabecular Meshwork. Invest Ophthalmol Vis Sci. 2011;52:3391–3397. doi: 10.1167/iovs.10-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maurer B, Stanczyk J, Jungel A, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2011;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott CE, Grunhagen J, Jager M, et al. MicroRNAs differentially expressed in postnatal aortic development downregulate elastin via 3′ UTR and coding-sequence binding sites. PLoS One. 2011;6:e16250. doi: 10.1371/journal.pone.0016250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto Y, Akiyama Y, Otsubo T, Shimada S, Yuasa Y. Involvement of epigenetically silenced microRNA-181c in gastric carcinogenesis. Carcinogenesis. 2011;31:777–784. doi: 10.1093/carcin/bgq013. [DOI] [PubMed] [Google Scholar]
- 53.Li D, Zhao Y, Liu C, et al. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clin Cancer Res. 2011;7:1722–1730. doi: 10.1158/1078-0432.CCR-10-1800. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Wang J, Ma H, Zhang J, Zhou X. Downregulation of miR-195 correlates with lymph node metastasis and poor prognosis in colorectal cancer. Med Oncol. 2011 Mar 10; doi: 10.1007/s12032-011-9880-5. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 55.Sekiya Y, Ogawa T, Iizuka M, Yoshizato K, Ikeda K, Kawada N. Down-regulation of cyclin E1 expression by microRNA-195 accounts for interferon-beta-induced inhibition of hepatic stellate cell proliferation. J Cell Physiol. 2011;226:2535–2542. doi: 10.1002/jcp.22598. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun. 2011;400:236–240. doi: 10.1016/j.bbrc.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 57.Tili E, Michaille JJ, Adair B, et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis. 2011;31:1561–1566. doi: 10.1093/carcin/bgq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan J, Hu H, Zhou Z, et al. Tumor-suppressive mir-663 gene induces mitotic catastrophe growth arrest in human gastric cancer cells. Oncol Rep. 2011;24:105–112. doi: 10.3892/or_00000834. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Yu H, Lou JR, et al. MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells. J Biol Chem. 2011;286:1429–1435. doi: 10.1074/jbc.M110.146530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ory B, Ramsey MR, Wilson C, et al. A microRNA-dependent program controls p53-independent survival and chemosensitivity in human and murine squamous cell carcinoma. J Clin Invest. 2011;121:809–820. doi: 10.1172/JCI43897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du L, Schageman JJ, Subauste MC, et al. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol Cancer Res. 2009;7:1234–1243. doi: 10.1158/1541-7786.MCR-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arndt GM, Dossey L, Cullen LM, et al. Characterization of global microRNA expression reveals oncogenic potential of miR-145 in metastatic colorectal cancer. BMC Cancer. 2009;9:374. doi: 10.1186/1471-2407-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q, Wang G, Shan JL, et al. MicroRNA-224 is upregulated in HepG2 cells and involved in cellular migration and invasion. J Gastroenterol Hepatol. 2011;25:164–171. doi: 10.1111/j.1440-1746.2009.05971.x. [DOI] [PubMed] [Google Scholar]
- 64.Mees ST, Mardin WA, Sielker S, et al. Involvement of CD40 targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas. Ann Surg Oncol. 2009;16:2339–2350. doi: 10.1245/s10434-009-0531-4. [DOI] [PubMed] [Google Scholar]
- 65.Wingett DG, Vestal RE, Forcier K, Hadjokas N, Nielson CP. CD40 is functionally expressed on human breast carcinomas: variable inducibility by cytokines and enhancement of Fas-mediated apoptosis. Breast Cancer Res Treat. 1998;50:27–36. doi: 10.1023/a:1006012607452. [DOI] [PubMed] [Google Scholar]
- 66.Castilla MA, Moreno-Bueno G, Romero-Perez L, et al. Micro- RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol. 2011;223:72–80. doi: 10.1002/path.2802. [DOI] [PubMed] [Google Scholar]
- 67.Bonci D. MicroRNA-21 as therapeutic target in cancer and cardiovascular disease. Recent Pat Cardiovasc Drug Discov. 2011;5:156–161. doi: 10.2174/157489010793351962. [DOI] [PubMed] [Google Scholar]
- 68.Radojicic J, Zaravinos A, Vrekoussis T, Kafousi M, Spandidos DA, Stathopoulos EN. MicroRNA expression analysis in triple-negative (ER, PR and Her2/neu) breast cancer. Cell Cycle. 2011;10:507–517. doi: 10.4161/cc.10.3.14754. [DOI] [PubMed] [Google Scholar]
- 69.Mei M, Ren Y, Zhou X, et al. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol Cancer Res Treat. 2011;9:77–86. doi: 10.1177/153303461000900109. [DOI] [PubMed] [Google Scholar]
- 70.Gong C, Yao Y, Wang Y, et al. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem. 2011;286:19127–19137. doi: 10.1074/jbc.M110.216887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi L, Bart J, Tan LP, et al. Expression of miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia of the breast in relation to ductal carcinoma in situ and invasive carcinoma. BMC Cancer. 2009;9:163. doi: 10.1186/1471-2407-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 74.Song B, Wang C, Liu J, et al. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res. 2010;29:29. doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 76.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 77.Russell RL, Pedersen AN, Kantor J, et al. Relationship of nm23 to proteolytic factors, proliferation and motility in breast cancer tissues and cell lines. Br J Cancer. 1998;78:710–717. doi: 10.1038/bjc.1998.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer – observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 79.Joglekar MV, Patil D, Joglekar VM, et al. The miR-30 family microRNAs confer epithelial phenotype to human pancreatic cells. Islets. 2009;1:137–147. doi: 10.4161/isl.1.2.9578. [DOI] [PubMed] [Google Scholar]