Introduction
Mitochondrial Ca2+ homeostasis is crucial for balancing cell survival and death (Giacomello et al., 2007; Duchen et al., 2008). Mitochondrial Ca2+ uptake mechanisms across the inner mitochondrial membrane (IMM) are especially important for the regulation of ATP synthesis, the amplitude and spatiotemporal patterns of intracellular Ca2+ transients, the mitochondrial fission–fusion, dynamics, the opening of mitochondrial permeability transition pores (mPTPs), and the generation of reactive oxygen species (Gunter and Sheu, 2009; Csordás et al., 2011; Drago et al., 2011). Mitochondrial Ca2+ influx was dogmatically considered to result from a single transport mechanism mediated by the mitochondrial Ca2+ uniporter (MCU), principally a result of nearly complete inhibition by Ruthenium red and lanthanides (Gunter and Pfeiffer, 1990). However, subsequent studies have also identified additional Ca2+ uptake pathways, such as the rapid mode of uptake (RaM) (Sparagna et al., 1995; Buntinas et al., 2001; Bazil and Dash, 2011) and Coenzyme Q10 (Bogeski et al., 2011), which exhibit different Ca2+ affinity, uptake kinetics, and pharmacological characteristics from the original MCU theory.
Although the basic functional and pharmacological properties of various mitochondrial Ca2+ uptake mechanisms have been well studied, the molecular identities of the channels/transporters responsible for these mechanisms have not been well understood until recently. In this Perspective, we focus on the recent studies that attempted to uncover the molecular identities of mitochondrial Ca2+ influx mechanisms using genetic manipulations including small interfering RNA (siRNA) or knockout mice. In particular, we summarize here the recent discoveries of the molecular identity of MCU protein and also discuss the controversies of two other Ca2+ influx mechanisms, mitochondrial RyR type 1 (mRyR1) and leucine-zipper-EF-hand–containing transmembrane protein 1 (Letm1), together with the future directions in this research field.
Background
Mitochondria were originally found and studied simply as a cellular power-plant in the first half of the 20th century (Drago et al., 2011). Soon it was also recognized that Ca2+ stimulates the Krebs cycle and electron transport chain activity, which results in the stimulation of ATP synthesis (Balaban, 2009; Denton, 2009; Carafoli, 2010). Early studies in the 1960s to 1970s revealed that isolated mitochondria could take up a large quantity of Ca2+ (Deluca et al., 1962; Vasington and Murphy, 1962). Surprisingly, super-physiological high Ca2+ concentrations ([Ca2+]) (10–100 µM) were required to activate Ca2+ uptake into isolated mitochondria. However, in the intact cells, less than a 10-µM [Ca2+] increase in the cytosol by receptor stimulation indeed propagated into mitochondria matrix (Rizzuto et al., 1992; Jou et al., 1996). This discrepancy between isolated mitochondria and intact cells was partially resolved by the finding of high cytosolic [Ca2+] ([Ca2+]c) at microdomains between mitochondria and ER/SR, which possesses Ca2+-releasing channels, inositol 1,4,5-trisphosphate (IP3) receptor, and/or RyR because of their physical proximity (Rizzuto et al., 1998, 2009; Sharma et al., 2000; Csordás et al., 2010; Giacomello et al., 2010) (Figs. 1 and 3). The functional tight coupling between ER/SR and mitochondria is attributed to the inter-organelle tether proteins such as mitofusin 2 (de Brito and Scorrano, 2008; García-Pérez et al., 2011) (Fig. 1). These seminal discoveries have positioned mitochondria as one of the key players in the dynamic regulation of physiological Ca2+ signaling.
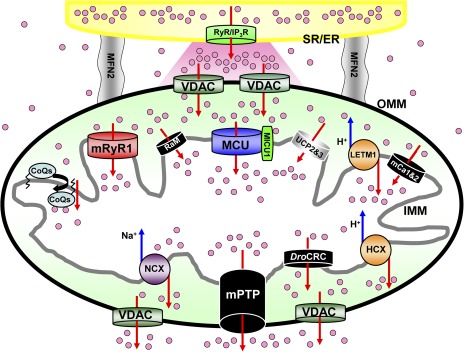
Figure 1.
Mitochondrial Ca2+ influx and efflux mechanisms. Schematic diagram of mitochondrial Ca2+ channels/transporters for influx and efflux mechanisms. The functional and morphological tight coupling of ER/SR (yellow) and mitochondria is attributed to the specific structure of inter-organelle tether proteins such as mito-fusion protein 2 (gray) (de Brito and Scorrano, 2008; García-Pérez et al., 2011). Ca2+-releasing sites of ER/SR, IP3 receptors (IP3R), or RyRs (RyR; green) are facing microdomains between mitochondria and ER/SR (shown as a pink region). Ca2+ release from ER/SR dramatically changes [Ca2+]c at this microdomain (Csordás et al., 2010; Giacomello et al., 2010). Then, mitochondria sense the high increases of [Ca2+]c at this microdomain, and [Ca2+]c propagates into the mitochondria matrix through a variety of Ca2+ channels/transporters (Rizzuto et al., 1992). Mitochondrial Ca2+ influx (upper part of this figure) is determined by the MCU (blue) (Baughman et al., 2011; De Stefani et al., 2011), RaM (black) (Sparagna et al., 1995; Buntinas et al., 2001; Bazil and Dash, 2011), HCX (Letm1; orange) (Jiang et al., 2009), mRyR1 (red) (Beutner et al., 2001, 2005), hydroxyl coenzyme Q10 (CoQs) (Bogeski et al., 2011), mCa1 and mCa2 (Michels et al., 2009), and UCP 2 and UPC 3 (Trenker et al., 2007) located at the IMM. MICU1 can bind to Ca2+ by its EF hand, but this protein does not make the channel pore because of its single-transmembrane structure (Perocchi et al., 2010). The mPTP (black) (Giacomello et al., 2007), NCX (purple) (Palty et al., 2010), and HCX (orange) (Jiang et al., 2009) contribute to Ca2+ efflux (lower part of this figure) in mammalian cells. Drosophila mitochondria possess another selective Ca2+ release channel (DroCRC; black) with unique featured characteristics intermediate between the permeability transition pore of yeast and mammals (von Stockum et al., 2011). Letm1 also works as a Ca2+ efflux pathway when [Ca2+]c becomes high (Jiang et al., 2009) (see also Figs. 2 and 3 E). Voltage-dependent anion-selective channels (VDAC; dark green) provide a pathway for Ca2+ and metabolite transport across the outer mitochondrial membrane (OMM). The channels/transporters for which molecular identities are still unknown are shown as black. Red arrows show Ca2+ movements, and blue arrows show other ion movements.
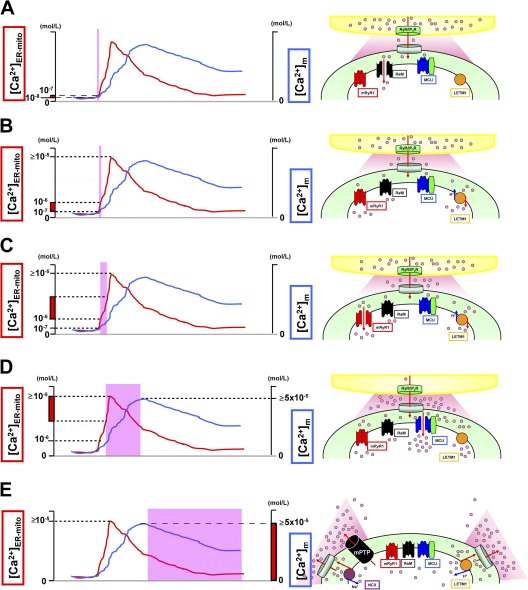
Figure 3.
Activation/inactivation patterns of Ca2+ influx/efflux mechanisms. (A) At first, RaM (black) is activated at a very initial phase of [Ca2+]ER-mito transient (<200 nM), with faster Ca2+ uptake kinetics (ms time scale). (B) Letm1 (orange) starts to uptake Ca2+ at ≥200 nM [Ca2+]ER-mito. (C) mRyR1 (red) starts to open at ≅1 µM [Ca2+]ER-mito, with a fivefold faster Ca2+ transport compared with the MCU, and inactivates before [Ca2+]ER-mito reaches the peak. (D) Finally, MCU (blue) starts to activate at >1 µM [Ca2+]ER-mito, and the activity increases in a [Ca2+]ER-mito–dependent manner. At this point, Letm1 (orange) shifts from Ca2+-uptake mode to Ca2+-efflux mode. (E) mPTP (black) and NCX (purple) contribute to Ca2+ efflux in mammalian cells and form the decay phase of [Ca2+]m transient. Letm1 also works as a Ca2+ efflux pathway at this phase. The channels/transporters of which the molecular identities are still unknown are shown as black. Red arrows show Ca2+ movements, and blue arrows show other ion movements.
The driving forces for mitochondrial Ca2+ uptake are the membrane potential (ΔΨm) and [Ca2+] gradient across IMM. For MCU, Ca2+ is taken into the mitochondrial matrix down its electrochemical gradient without transport of another ion (Kirichok et al., 2004). Basically, for each Ca2+ transported through MCU, there is a net transfer of two positive charges into matrix resulting in a drop of ΔΨm, which is energetically unfavorable. However, the Ca2+-stimulated respiration will not only compensate the loss of ΔΨm by the efflux of H+ through electron transport chain, but it will also produce a net gain of ATP. In addition, multiple Ca2+ efflux mechanisms work in concert aiming to expedite a transient and an oscillatory nature rather than a tonic and a steady-state change of matrix [Ca2+] ([Ca2+]m).
Mitochondrial Ca2+ efflux mechanism is also important for cellular Ca2+ homeostasis, as is mitochondrial Ca2+ influx mechanism. The proposed mitochondrial Ca2+ efflux mechanisms are Na2+ dependent (Palty et al., 2010) and/or H+ dependent (Jiang et al., 2009). Na2+-dependent mitochondrial Ca2+ efflux was first documented more than 30 years ago using cardiac mitochondria (Carafoli et al., 1974), and it has been shown that Na+–Ca2+ exchanger (NCX) is the primary Ca2+ efflux mechanism in cardiac mitochondria (Maack et al., 2006; see also Denton and McCormack, 1985; Gunter and Pfeiffer, 1990). Moreover, a strong candidate for the molecular identity of the mitochondrial NCX has been recently reported (Na+- or Li+-dependent Ca2+ transport) (Palty et al., 2010) (Figs. 1 and 3 E). On the other hand, Letm1 originally identified as a H+–K+ exchanger, has been recently reported to function as a critical component of a mitochondrial H+–Ca2+ exchanger (HCX), but its role in Ca2+ extrusion is still controversial (Figs. 1 and 3 E) (see also next section). Interestingly, the protein expression of NCX is particularly robust in excitable cells including heart and brain (Palty et al., 2010), whereas the activity of the HCX is primarily found in nonexcitable cells, suggesting that there exist tissue-specific mitochondrial Ca2+ efflux mechanisms. In addition, the idea that mPTP can also serve as a rapid Ca2+ efflux mechanism has gained appreciation as stated in several recent reviews (Gunter and Sheu, 2009; Bernardi and von Stockum, 2012). It has been shown that mPTP can open and close transiently (“flicker”) at its low conductance state (Zoratti and Szabò, 1995); thus, it serves as one of the physiological Ca2+ efflux mechanisms (Figs. 1 and 3 E). However, under certain cellular stresses that lead to Ca2+ overload and/or overproduction of reaction oxygen species, mPTP can open constantly, causing the release of cytochrome c and subsequently leading to cell death (Giacomello et al., 2007). Finally, a recent report shows that Drosophila melanogaster mitochondria possess another selective Ca2+ release channel (shown as “DroCRC” in Fig. 1) with unique featured characteristics between the mPTP of yeast and mammals, such as inhibition by Pi but not by ADP and cyclosporine A (as in the mPTP of yeast mitochondria), and the existence of voltage- and redox-sensitive regulatory sites (as in the mPTP of mammalian cells) (von Stockum et al., 2011).
Through the multiple experimental approaches, several different types of mitochondrial Ca2+ uptake mechanisms, in addition to classical MCU, were functionally isolated, including: (a) RaM (Sparagna et al., 1995; Buntinas et al., 2001; Bazil and Dash, 2011), (b) RyR type1 (RyR1) (Beutner et al., 2001, 2005; Altschafl et al., 2007; Ryu et al., 2011), (c) Ca2+-selective conductance (mCa) 1 and 2 (Michels et al., 2009), (d) IMiCa (Kirichok et al., 2004), (e) Coenzyme Q10 (Bogeski et al., 2011), (f) uncoupling proteins (UCPs) 2 and 3 (Trenker et al., 2007), and (g) Letm1 (Jiang et al., 2009) (summarized in Fig. 1). Among these studies, RyR1 was found as the first mitochondrial Ca2+ uptake mechanism with a known molecular identity, but molecular identities of RaM (Sparagna et al., 1995; Buntinas et al., 2001; Bazil and Dash, 2011) and mCa1 and mCa2 (Michels et al., 2009) have not yet been clarified (see Fig. 1; the channels/transporters for which molecular identities are still unknown are shown in black). IMiCa was recently recorded from mitoplasts (Kirichok et al., 2004), providing direct electrophysiological demonstration for the existence of a Ca2+-selective ion channel, which would possibly fit to the originally predicted channel nature of MCU (Gunter and Pfeiffer, 1990). Through RNA interference studies, several groups have recently proposed novel candidate proteins that involve the mitochondrial Ca2+ uptake mechanism, such as Letm1 (Jiang et al., 2009) and MICU1 (Perocchi et al., 2010). Finally, two papers have come out very recently at the same time from two different groups, reporting that the coiled-coil domain–containing protein 109A (CCDC109A) is the molecular identity of MCU (Baughman et al., 2011; De Stefani et al., 2011). Because of the length limitation, only MCU, mRyR1, and Letm1 are discussed in detail in this Perspective.
MCU
UCPs 2 and 3 were proposed as the molecular identity of MCU by an siRNA study (Trenker et al., 2007). This view was soon challenged by Clapham’s group, who showed that dsRNAs against Drosophila mitochondrial UCPs did not affect mitochondrial Ca2+ and H+ concentration (Jiang et al., 2009). Interestingly, a recent report from Demaurex’s group also showed that UCP3 is not an MCU, but it alters ER/SR Ca2+ ATPase activity by decreasing mitochondrial ATP production (De Marchi et al., 2011). Next, Mootha’s group identified a protein that is an important regulator of mitochondrial Ca2+ uptake mechanism, using bioinformatics and siRNA screening, termed MICU1 (Perocchi et al., 2010). MICU1 has two Ca2+-binding EF hands but only one putative transmembrane domain, which seems unlikely to form a Ca2+ channel pore and be an MCU itself (Fig. 1). After the discovery of MICU1, Mootha’s group moved to whole genome phylogenetic profiling, genome-wide RNA coexpression analysis, and organelle-wide protein coexpression analysis to predict proteins being functionally related to MICU1, which is thought to be an ancillary subunit of MCU (Baughman et al., 2011). The analysis predicted that a transmembrane protein previously identified as CCDC109A is MCU. Using slightly different approaches, De Stefani et al. (2011) also identified the same protein as MCU at the same time. The characteristics of MCU found by these two groups are as follows: (a) CCDC109A (MCU) has two transmembrane domains, which seems likely to make a Ca2+ channel pore; (b) using RNA interference studies, knockdown of MCU dramatically reduces mitochondrial Ca2+ uptake in isolated mitochondria or in living cells, and this effect was rescued by overexpression of MCU; (c) MCU down-regulation itself does not affect mitochondrial O2 consumption, ATP synthesis, ΔΨm, and mitochondrial morphology; and (d) site-specific mutations at the pore region in MCU show loss of function or a dominant-negative effect. Moreover, Rizzuto’s group reconstituted MCU in lipid bilayers and recorded Ruthenium red–sensitive Ca2+ current with 6–7-pS single-channel activity (De Stefani et al., 2011). The most obvious discrepancy between these two groups’ data is the topology of MCU (Drago et al., 2011). Both groups had a consensus proposal that MCU consists of two transmembrane domains and forms oligomer to be a Ca2+ channel. However, Rizzuto’s group proposed that C and N terminals face intermembrane space (De Stefani et al., 2011), and Mootha’s group proposed the opposite direction: C and N terminals face matrix (Baughman et al., 2011). The C and N terminals of the channels are generally important regions for receiving various kinds of posttranslational modifications including phosphorylation by second messengers or kinases, which would modulate the channel function (Dai et al., 2009). Therefore, the discrepancy in the topology of MCU will need to be resolved for understanding the modulation of MCU functions by signaling molecules from cytosol or matrix.
mRyR1
One of the candidates for the mitochondrial Ca2+ uptake mechanism with a known molecular identity is the mitochondrial RyR in cardiac cells reported from our group (Beutner et al., 2001, 2005). Three different RyR isoforms (RyR1, RyR2, and RyR3) have been cloned, and different physiological and pharmacological properties between these isoforms have been identified (Lanner et al., 2010). In cardiac cells, intracellular Ca2+ release and muscle contraction were mainly controlled by isoform RyR2 located in the SR (Lanner et al., 2010). Although RyR1 is also detectable both at mRNA and at protein levels in cardiac tissue (Münch et al., 2000; Jeyakumar et al., 2002), its functional and physiological roles in the heart had not been fully understood for a long time. We first showed that a low level of functional RyR is also expressed at heart IMM (shown by high affinity binding of [3H]ryanodine, immunogold staining, RT-PCR, and Western blot) and has a role of fast Ca2+ uptake pathway (Beutner et al., 2001, 2005) (Figs. 2 and 3 C). Furthermore, RyR in cardiac mitochondria exhibits remarkably similar biochemical, pharmacological, and functional properties to those of RyR1 in skeletal muscle SR, but not to those of RyR2 found in cardiac SR. Therefore, we termed it as mRyR1 (mitochondrial RyR1) (Fig. 1). The molecular identity of mRyR1 was carefully analyzed and confirmed by a variety of functional and biochemical experiments using not only native heart but also transgenic heart (RyR1 knockout mice) (Beutner et al., 2005).
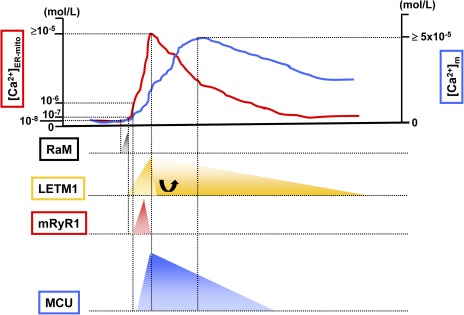
Figure 2.
Mitochondrial Ca2+ influx mechanisms during cytosolic/mitochondrial Ca2+ transient. The top of the figure is an example of Ca2+ transient at microdomains between mitochondria and ER/SR ([Ca2+]ER-mito; red line) and Ca2+ transient at the mitochondrial matrix ([Ca2+]m; blue line). RaM (black) shows 50-fold faster Ca2+ transport compared with the MCU, and the activation peak is at 50 nM of extra-mitochondrial Ca2+. Letm1 (orange) can be activated at ≥200 nM of extra-mitochondrial Ca2+, but at high [Ca2+]ER-mito condition, a role of Letm1 shifts to Ca2+ efflux rather than Ca2+ uptake into the mitochondrial matrix. mRyR1 (red) can start to be activated at 1 µM of extra-mitochondrial Ca2+, with a fivefold faster Ca2+ transport compared with the MCU. 2 µM is the half-maximal concentration for Ca2+-dependent activation of mRyR1, and 20 µM is the half-maximal concentration for Ca2+-dependent inhibition. Thus, mRyR1 inactivates before [Ca2+]ER-mito reaches the peak. A lower concentration of extra-mitochondrial Ca2+ (such as 200–300 nM) does not activate MCU, and at least >1 µM Ca2+ is needed for the initial activation. The estimated half-maximal concentration for the activation of MCU is ≅20 mM.
Recently, we also performed electrophysiological experiments to directly demonstrate the existence of mRyR1, which clearly showed the predicted channel nature of skeletal RyR1 (Altschafl et al., 2007; Ryu et al., 2011). At first, we performed electrophysiological experiments using a conventional lipid bilayer system (Altschafl et al., 2007). The activity of RyR1, but not RyR2, was observed in lipid bilayers of mRyRs purified from heart IMM fraction. Neither SR nor outer mitochondrial membrane markers were detected in these mRyR1 preparations. Next, we characterized the biophysical and pharmacological properties of native single mRyR1 channels in heart mitoplast using the patch-clamp technique (Ryu et al., 2011). We observed a novel 225-pS cation–selective channel in heart mitoplasts that exhibited multiple subconductance states, which was blocked by high concentrations of ryanodine and Ruthenium red, the known inhibitors of RyRs. Ryanodine exhibited a concentration-dependent modulation of this channel, with low concentrations stabilizing a subconductance state and with high concentrations abolishing activity (Ryu et al., 2011). The channel prosperities of Ca2+-dependent [3H] ryanodine binding and the channel modulation by caffeine (Beutner et al., 2001, 2005) implicate that the topology of mRyR1 is the same as RyR1 at the SR (Du et al., 2002) because these agonist-binding sites are facing cytosol. Therefore, we hypothesize that C and N terminals face intermembrane space (cytosolic side), and S1–S2, S3–S4, and S5–S6 linkers face the mitochondrial matrix side. However, further experiments will be needed to confirm the topology of mRyR1 by using other modulators that either act from the cytosolic side (ATP, FK-binding proteins, calmodulin, phosphorylation by protein kinase A, or Ca2+/calmodulin-binding protein II) or from the matrix side (Ca2+) (Lanner et al., 2010).
Collectively, our studies show the molecular and functional existence of mRyR1 in heart mitochondria and clearly distinguish it from previously identified mitochondrial ion channels. It is worthwhile to mention that unlike MCU (which is a highly Ca2+-selective and low conductance ion channel as such would not strongly affect ΔΨm; Drago et al., 2011), RyR is a poorly Ca2+-selective large cation channel (Ryu et al., 2011), and thus opening of this channel might collapse ΔΨm, which is energetically unfavorable. This dichotomy would be explained as follows: (a) the expression number of RyR1 in a single mitochondrion is very small (Beutner et al., 2001; Ryu et al., 2011), and thus the depolarization of ΔΨm might be localized only near the site of mRyR1, and the rest of the cristae membrane preserves its voltage; (b) the bell-shape Ca2+ dependency with a rapid Ca2+ activation and inactivation profile of this channel (see also Figs. 2 and 3, C and D) would minimize the ΔΨm change; and (c) any small decrease in ΔΨm then can be readily compensated by the Ca2+-dependent activation of dehydrogenases in tricarboxylic acid cycle and F0F1-ATP synthase. Collectively, the mRyR may be uniquely poised to sequester Ca2+ during a transient and rapid excitation–contraction coupling process in cardiac muscle cells.
Our first report on the identification of RyR1 in cardiac mitochondria over 10 years ago (Beutner et al., 2001) has not yet drawn high research activity on this topic, both in the cardiac and mitochondrial field, because of the following reasons: (a) the difficulty in functional separation of the very low level of (∼5%) mRyR1 from a high abundance of (∼95%) SR-located RyR2; (b) the lack of genetic approaches to dissect mRyR1 function from MCU until recently (see also above section); and (c) the sparse information about RyR1 links to human diseases, with the exception of skeletal muscle–related diseases. However, this landscape has been gradually changing by these recent exciting reports: (a) the expression of RyRs in mitochondria has been confirmed in a variety of cell types including osteoblasts (Sun et al., 2002), endothelial cells (Uehara et al., 2004), and neuronal cells (Norman et al., 2008); (b) non-skeletal muscle and non–SR-RyR phenotypes related to human pathologies are being progressively reported, such as in neurological diseases, which include HIV induction of cortical neuron injury via activation of both ER-RyR and mRyR (Norman et al., 2008; Perry et al., 2010) and positive outcome on treating patients in neurointensive care units with dantrolene (Muehlschlegel and Sims, 2009), a more selective inhibitor of RyR1 that is frequently used for treating malignant hyperthermia; (c) an intriguing clinical report, using a genome-wide association study, shows that RyR1 (not RyR2) single-nucleotide polymorphisms are associated with the risk for the development of electrocardiographic left ventricular hypertrophy (Hong et al., 2012); and (d) in a knock-in mouse model with heterozygous RyR1 (I4898T), related to a human central core disease, the ventricular chamber formation develops abnormally (Zvaritch et al., 2007).
Important future directions of cardiac mRyR research will uncover fundamental questions including: (a) What is the relative contribution of mRyR1 and other Ca2+ transporters in cellular Ca2+ homeostasis and ATP synthesis in beating heart in vivo? (b) What are the implications of mRyR in RyR1-linked diseases such as malignant hyperthermia (MacLennan, 1992), central core disease (Zhang et al., 1993), and cardiac hypertrophy (Hong et al., 2012)? (c) Does mRyR1 also exist in other excitable cells such as neuron, vascular smooth muscle cells, and skeletal muscle cells? If it does, what are the physiological and pathological implications?
Letm1 (Ca2+–H+ antiporter)
Using siRNA genome-wide screening in Drosophila, Jiang et al. (2009) reported that mitochondrial protein Letm1, originally known as K+–H+ exchanger, can play a role as Ca2+–H+ antiporter. They proposed that Letm1 is localized at the inner membrane (Fig. 1) and transports one Ca2+ and extracts one H+ (Figs. 2 and 3 B). Knockdown of Letm1 abolished only the initial fast mitochondrial Ca2+ uptake, but it still showed sustained Ca2+ increase, suggesting that Letm1 works at low [Ca2+]c for Ca2+ uptake (Figs. 2 and 3 B). Letm1 activity was inhibited by both Ruthenium red, an inhibitor of MCU, and CGP37157, an inhibitor of mitochondrial NCX. Similar data were also recently reported by Waldeck-Weiermair et al. (2011). This scenario seems like the revival story of Moyle and Mitchell (1977), which raises several points of discussion (see the Perspective by Nowikovsky et al. in this issue): (a) one Ca2+ for one H+ antiporter does not favor Ca2+ influx physiologically, according to the electrochemical gradients of Ca2+ and pH; (b) Ca2+ influx by Letm1 might be in part mediated by the changes in ΔΨm through K+ fluxes because of Letm1 being itself a K+–H+ exchanger; and (c) CGP37157 had not been shown to inhibit IP3-mediated [Ca2+]m increase. It can be anticipated that new experimental results will appear in future publications to resolve this controversy.
Mitochondrial Ca2+ influx mechanism and human diseases
Disruption of cellular Ca2+ homeostasis is associated with human diseases (Berridge et al., 2003) such as cardiovascular (Bers, 2008; Lanner et al., 2010), skeletal muscle (Lyfenko et al., 2004; Lanner et al., 2010) and neurological diseases (Vicencio et al., 2010). However, the relative contributions of individual mitochondrial Ca2+ influx mechanisms to the disease pathogenesis are still not well understood.
As of today, around 300 mutations have been identified in RyR, and some of these mutations are directly associated with human diseases (Lanner et al., 2010). For instance, RyR1 gene mutations are involved in several debilitating and/or life-threatening muscle diseases including malignant hyperthermia (MacLennan, 1992), central core disease (Zhang et al., 1993), heat/exercise-induced exertional rhabdomyolysis (Capacchione et al., 2010), multiminicore disease (Ferreiro et al., 2002), and atypical periodic paralyses (Zhou et al., 2010). More importantly, RyR1 mutations found in human malignant hyperthermia and central core disease exhibit abnormal Ca2+ regulation in cardiac mitochondria (Gross, P., N. Sokolova, S. Provazza, G. Beutner, and S.S. Sheu. 2011. 65th Annual Meeting of The Society of General Physiologists. Abstr. 30) and basal bioenergetic abnormalities in skeletal muscle mitochondria (Giulivi et al., 2011). Given the facts that mitochondria communicate closely with ER/SR, disruption of the mRyR1 Ca2+ influx mechanism may contribute to the initiation and development of these diseases. Similarly, Letm1 is involved in respiratory chain biogenesis and in the pathogenesis of seizures in the Wolf–Hirschhorn syndrome (McQuibban et al., 2010; Zotova et al., 2010). With the most recent discovery on the molecular identity of MCU, continued research in this field will certainly help our understating on the contribution of mitochondrial Ca2+ in the pathogenesis of human diseases.
Future perspective and conclusion
Historically, the mitochondrial Ca2+ influx mechanism has been an important topic in cell biology, despite the relatively slow progress in revealing the molecular identities of Ca2+-transporting proteins. Using multiple research tools, such as gene-screening analysis, genetic manipulation, and updated biochemical, pharmacological, cell biological, and electrophysiological techniques, has led to the recent groundbreaking discoveries in MICU1, MCU, and Letm1 molecular identities. This advance in the cloning of mitochondrial Ca2+ channels/transporters will provide essential information for studying (a) the regulatory mechanism underlying mitochondrial Ca2+ uptake, such as posttranslational modifications of these channel/transporter functions; (b) the design or discovery of more specific inhibitors/activators to each channel/transporter for the potential development of therapeutic drugs; and, furthermore, (c) the molecular mechanisms underlying mitochondrial Ca2+-mediated human diseases.
The long-sought mystery of the molecular identity of MCU has just been uncovered, but other studies have also identified additional Ca2+ uptake pathways that exhibit different function and pharmacology from MCU. The idea that more than one Ca2+ influx mechanism exists in mitochondria has gradually gained wider recognition because each cell type (especially excitable vs. nonexcitable cells) possesses different size/frequency of Ca2+ oscillations (or transients). It is reasonable to predict that different tissues coordinate mitochondria Ca2+ influx in different fashion by using a different combination and expression ratio of these channels/transporters. For example, mRyR1, which has a high velocity of Ca2+ uptake and Ca2+ sensitivity, is a perfect candidate to mainly regulate effective Ca2+-induced ATP productions in cardiac cells in a beat to beat manner. IMiCa density has begun to be recorded from different tissues using mitoplast patch clamp, and interestingly, IMiCa density in the heart seems to be much smaller than in other tissues (Fieni, F., and Y. Kirichok. 2011. 65th Annual Meeting of The Society of General Physiologists. Abstr. 56A). Future studies are destined to provide new evidence regarding the diversity of Ca2+ influx mechanisms in different cell types/tissues, which will allow us to understand the relative contribution and/or cross talk between each mitochondrial Ca2+ transporter in each organ.
In conclusion, mitochondrial Ca2+ is crucial in governing energy production, Ca2+ homeostasis, and cell fate. Revealing the molecular identities of mitochondrial Ca2+ influx mechanisms provides us with the passwords to access a new field of study by establishing animal models to address the relationship between the mitochondrial Ca2+ uptake mechanism and human physiology and diseases.
This Perspectives series includes articles by Sheu et al., Zhang et al., Balaban, Santo-Domingo and Demaurex, Wei and Dirksen, Nowikovsky et al., and Galloway and Yoon.
Acknowledgments
We thank Mr. Stephen Hurst and Dr. Bong Sook Jhun for their reading and comments on our manuscript.
This research is supported by National Institutes of Health grants (RO1HL-033333, RO1HL-093671, and R21HL-110371 to S.-S. Sheu).
Shey-Shing Sheu served as guest editor.
Footnotes
Abbreviations used in this paper:
- [Ca2+]c
- cytosolic [Ca2+]
- [Ca2+]m
- matrix [Ca2+]
- CCDC109A
- coiled-coil domain–containing protein 109A
- ΔΨm
- membrane potential
- HCX
- H+–Ca2+ exchanger
- IMM
- inner mitochondrial membrane
- IP3
- inositol 1,4,5-trisphosphate
- Letm1
- leucine-zipper-EF-hand–containing transmembrane protein 1
- mCa
- Ca2+-selective conductance
- MCU
- mitochondrial Ca2+ uniporter
- mPTP
- mitochondrial permeability transition pore
- mRyR1
- mitochondrial RyR type 1
- NCX
- Na+–Ca2+ exchanger
- RaM
- rapid mode of uptake
- siRNA
- small interfering RNA
- UCP
- uncoupling protein
References
- Altschafl B.A., Beutner G., Sharma V.K., Sheu S.S., Valdivia H.H. 2007. The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim. Biophys. Acta. 1768:1784–1795 10.1016/j.bbamem.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Balaban R.S. 2009. The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta. 1787:1334–1341 10.1016/j.bbabio.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 476:341–345 10.1038/nature10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazil J.N., Dash R.K. 2011. A minimal model for the mitochondrial rapid mode of Ca²+ uptake mechanism. PLoS ONE. 6:e21324 10.1371/journal.pone.0021324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P., von Stockum S. 2012. The permeability transition pore as a Ca(2+) release channel: New answers to an old question. Cell Calcium. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Bootman M.D., Roderick H.L. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Bers D.M. 2008. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 70:23–49 10.1146/annurev.physiol.70.113006.100455 [DOI] [PubMed] [Google Scholar]
- Beutner G., Sharma V.K., Giovannucci D.R., Yule D.I., Sheu S.S. 2001. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 276:21482–21488 10.1074/jbc.M101486200 [DOI] [PubMed] [Google Scholar]
- Beutner G., Sharma V.K., Lin L., Ryu S.Y., Dirksen R.T., Sheu S.S. 2005. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim. Biophys. Acta. 1717:1–10 10.1016/j.bbamem.2005.09.016 [DOI] [PubMed] [Google Scholar]
- Bogeski I., Gulaboski R., Kappl R., Mirceski V., Stefova M., Petreska J., Hoth M. 2011. Calcium binding and transport by coenzyme Q. J. Am. Chem. Soc. 133:9293–9303 10.1021/ja110190t [DOI] [PubMed] [Google Scholar]
- Buntinas L., Gunter K.K., Sparagna G.C., Gunter T.E. 2001. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim. Biophys. Acta. 1504:248–261 10.1016/S0005-2728(00)00254-1 [DOI] [PubMed] [Google Scholar]
- Capacchione J.F., Sambuughin N., Bina S., Mulligan L.P., Lawson T.D., Muldoon S.M. 2010. Exertional rhabdomyolysis and malignant hyperthermia in a patient with ryanodine receptor type 1 gene, L-type calcium channel alpha-1 subunit gene, and calsequestrin-1 gene polymorphisms. Anesthesiology. 112:239–244 10.1097/ALN.0b013e3181c29504 [DOI] [PubMed] [Google Scholar]
- Carafoli E. 2010. The fateful encounter of mitochondria with calcium: how did it happen? Biochim. Biophys. Acta. 1797:595–606 10.1016/j.bbabio.2010.03.024 [DOI] [PubMed] [Google Scholar]
- Carafoli E., Tiozzo R., Lugli G., Crovetti F., Kratzing C. 1974. The release of calcium from heart mitochondria by sodium. J. Mol. Cell. Cardiol. 6:361–371 10.1016/0022-2828(74)90077-7 [DOI] [PubMed] [Google Scholar]
- Csordás G., Várnai P., Golenár T., Roy S., Purkins G., Schneider T.G., Balla T., Hajnóczky G. 2010. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell. 39:121–132 10.1016/j.molcel.2010.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G., Varnai P., Golenar T., Sheu S.S., Hajnoczky G. 2011. Calcium transport across the inner mitochondrial membrane: Molecular mechanisms and pharmacology. Mol. Cell. Endocrinol. 353:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Hall D.D., Hell J.W. 2009. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 89:411–452 10.1152/physrev.00029.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito O.M., Scorrano L. 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 456:605–610 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- De Marchi U., Castelbou C., Demaurex N. 2011. Uncoupling protein 3 (UCP3) modulates the activity of Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by decreasing mitochondrial ATP production. J. Biol. Chem. 286:32533–32541 10.1074/jbc.M110.216044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 476:336–340 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca H.F., Engstrom G.W., Rasmussen H. 1962. The action of vitamin D and parathyroid hormone in vitro on calcium uptake and release by kidney mitochondria. Proc. Natl. Acad. Sci. USA. 48:1604–1609 10.1073/pnas.48.9.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R.M. 2009. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 1787:1309–1316 10.1016/j.bbabio.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Denton R.M., McCormack J.G. 1985. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am. J. Physiol. 249:E543–E554 [DOI] [PubMed] [Google Scholar]
- Drago I., Pizzo P., Pozzan T. 2011. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 30:4119–4125 10.1038/emboj.2011.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G.G., Sandhu B., Khanna V.K., Guo X.H., MacLennan D.H. 2002. Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1). Proc. Natl. Acad. Sci. USA. 99:16725–16730 10.1073/pnas.012688999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M.R., Verkhratsky A., Muallem S. 2008. Mitochondria and calcium in health and disease. Cell Calcium. 44:1–5 10.1016/j.ceca.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Ferreiro A., Monnier N., Romero N.B., Leroy J.P., Bönnemann C., Haenggeli C.A., Straub V., Voss W.D., Nivoche Y., Jungbluth H., et al. 2002. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann. Neurol. 51:750–759 10.1002/ana.10231 [DOI] [PubMed] [Google Scholar]
- García-Pérez C., Schneider T.G., Hajnóczky G., Csordás G. 2011. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am. J. Physiol. Heart Circ. Physiol. 301:H1907–H1915 10.1152/ajpheart.00397.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M., Drago I., Pizzo P., Pozzan T. 2007. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 14:1267–1274 10.1038/sj.cdd.4402147 [DOI] [PubMed] [Google Scholar]
- Giacomello M., Drago I., Bortolozzi M., Scorzeto M., Gianelle A., Pizzo P., Pozzan T. 2010. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell. 38:280–290 10.1016/j.molcel.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Giulivi C., Ross-Inta C., Omanska-Klusek A., Napoli E., Sakaguchi D., Barrientos G., Allen P.D., Pessah I.N. 2011. Basal bioenergetic abnormalities in skeletal muscle from ryanodine receptor malignant hyperthermia-susceptible R163C knock-in mice. J. Biol. Chem. 286:99–113 10.1074/jbc.M110.153247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter T.E., Pfeiffer D.R. 1990. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258:C755–C786 [DOI] [PubMed] [Google Scholar]
- Gunter T.E., Sheu S.S. 2009. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim. Biophys. Acta. 1787:1291–1308 10.1016/j.bbabio.2008.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.W., Shin D.J., Lee S.H., Son N.H., Go M.J., Lim J.E., Shin C., Jang Y., Oh B. 2012. Common variants in RYR1 are associated with left ventricular hypertrophy assessed by electrocardiogram. Eur. Heart J. 33:1250–1256 [DOI] [PubMed] [Google Scholar]
- Jeyakumar L.H., Gleaves L.A., Ridley B.D., Chang P., Atkinson J., Barnett J.V., Fleischer S. 2002. The skeletal muscle ryanodine receptor isoform 1 is found at the intercalated discs in human and mouse hearts. J. Muscle Res. Cell Motil. 23:285–292 10.1023/A:1022091931677 [DOI] [PubMed] [Google Scholar]
- Jiang D., Zhao L., Clapham D.E. 2009. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 326:144–147 10.1126/science.1175145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou M.J., Peng T.I., Sheu S.S. 1996. Histamine induces oscillations of mitochondrial free Ca2+ concentration in single cultured rat brain astrocytes. J. Physiol. 497:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y., Krapivinsky G., Clapham D.E. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 427:360–364 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Lanner J.T., Georgiou D.K., Joshi A.D., Hamilton S.L. 2010. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2:a003996 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyfenko A.D., Goonasekera S.A., Dirksen R.T. 2004. Dynamic alterations in myoplasmic Ca2+ in malignant hyperthermia and central core disease. Biochem. Biophys. Res. Commun. 322:1256–1266 10.1016/j.bbrc.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Maack C., Cortassa S., Aon M.A., Ganesan A.N., Liu T., O’Rourke B. 2006. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ. Res. 99:172–182 10.1161/01.RES.0000232546.92777.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D.H. 1992. The genetic basis of malignant hyperthermia. Trends Pharmacol. Sci. 13:330–334 10.1016/0165-6147(92)90101-B [DOI] [PubMed] [Google Scholar]
- McQuibban A.G., Joza N., Megighian A., Scorzeto M., Zanini D., Reipert S., Richter C., Schweyen R.J., Nowikovsky K. 2010. A Drosophila mutant of LETM1, a candidate gene for seizures in Wolf-Hirschhorn syndrome. Hum. Mol. Genet. 19:987–1000 10.1093/hmg/ddp563 [DOI] [PubMed] [Google Scholar]
- Michels G., Khan I.F., Endres-Becker J., Rottlaender D., Herzig S., Ruhparwar A., Wahlers T., Hoppe U.C. 2009. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 119:2435–2443 10.1161/CIRCULATIONAHA.108.835389 [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. 1977. Electric charge stoicheiometry of calcium translocation in rat liver mitochondria. FEBS Lett. 73:131–136 10.1016/0014-5793(77)80964-2 [DOI] [PubMed] [Google Scholar]
- Muehlschlegel S., Sims J.R. 2009. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit. Care. 10:103–115 10.1007/s12028-008-9133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch G., Bölck B., Sugaru A., Schwinger R.H. 2000. Isoform expression of the sarcoplasmic reticulum Ca2+ release channel (ryanodine channel) in human myocardium. J. Mol. Med. 78:352–360 10.1007/s001090000122 [DOI] [PubMed] [Google Scholar]
- Norman J.P., Perry S.W., Reynolds H.M., Kiebala M., De Mesy Bentley K.L., Trejo M., Volsky D.J., Maggirwar S.B., Dewhurst S., Masliah E., Gelbard H.A. 2008. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS ONE. 3:e3731 10.1371/journal.pone.0003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., et al. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 107:436–441 10.1073/pnas.0908099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. 2010. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 467:291–296 10.1038/nature09358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S.W., Barbieri J., Tong N., Polesskaya O., Pudasaini S., Stout A., Lu R., Kiebala M., Maggirwar S.B., Gelbard H.A. 2010. Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax. J. Neurosci. 30:14153–14164 10.1523/JNEUROSCI.1042-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Simpson A.W., Brini M., Pozzan T. 1992. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 358:325–327 10.1038/358325a0 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 280:1763–1766 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R., et al. 2009. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta. 1787:1342–1351 10.1016/j.bbabio.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S.Y., Beutner G., Kinnally K.W., Dirksen R.T., Sheu S.S. 2011. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J. Biol. Chem. 286:21324–21329 10.1074/jbc.C111.245597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.K., Ramesh V., Franzini-Armstrong C., Sheu S.S. 2000. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 32:97–104 10.1023/A:1005520714221 [DOI] [PubMed] [Google Scholar]
- Sparagna G.C., Gunter K.K., Sheu S.S., Gunter T.E. 1995. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J. Biol. Chem. 270:27510–27515 10.1074/jbc.270.46.27510 [DOI] [PubMed] [Google Scholar]
- Sun L., Adebanjo O.A., Koval A., Anandatheerthavarada H.K., Iqbal J., Wu X.Y., Moonga B.S., Wu X.B., Biswas G., Bevis P.J., et al. 2002. A novel mechanism for coupling cellular intermediary metabolism to cytosolic Ca2+ signaling via CD38/ADP-ribosyl cyclase, a putative intracellular NAD+ sensor. FASEB J. 16:302–314 10.1096/fj.01-0705com [DOI] [PubMed] [Google Scholar]
- Trenker M., Malli R., Fertschai I., Levak-Frank S., Graier W.F. 2007. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 9:445–452 10.1038/ncb1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara K., Onoue H., Jeyakumar L.H., Fleischer S., Uehara A. 2004. Localization of ryanodine receptor 3 in the sinus endothelial cells of the rat spleen. Cell Tissue Res. 317:137–145 10.1007/s00441-004-0904-8 [DOI] [PubMed] [Google Scholar]
- Vasington F.D., Murphy J.V. 1962. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J. Biol. Chem. 237:2670–2677 [PubMed] [Google Scholar]
- Vicencio J.M., Lavandero S., Szabadkai G. 2010. Ca2+, autophagy and protein degradation: thrown off balance in neurodegenerative disease. Cell Calcium. 47:112–121 10.1016/j.ceca.2009.12.013 [DOI] [PubMed] [Google Scholar]
- von Stockum S., Basso E., Petronilli V., Sabatelli P., Forte M.A., Bernardi P. 2011. Properties of Ca(2+) transport in mitochondria of Drosophila melanogaster. J. Biol. Chem. 286:41163–41170 10.1074/jbc.M111.268375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck-Weiermair M., Jean-Quartier C., Rost R., Khan M.J., Vishnu N., Bondarenko A.I., Imamura H., Malli R., Graier W.F. 2011. Leucine zipper EF hand-containing transmembrane protein 1 (Letm1) and uncoupling proteins 2 and 3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. J. Biol. Chem. 286:28444–28455 10.1074/jbc.M111.244517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen H.S., Khanna V.K., De Leon S., Phillips M.S., Schappert K., Britt B.A., Browell A.K., MacLennan D.H. 1993. A mutation in the human ryanodine receptor gene associated with central core disease. Nat. Genet. 5:46–50 10.1038/ng0993-46 [DOI] [PubMed] [Google Scholar]
- Zhou H., Lillis S., Loy R.E., Ghassemi F., Rose M.R., Norwood F., Mills K., Al-Sarraj S., Lane R.J., Feng L., et al. 2010. Multi-minicore disease and atypical periodic paralysis associated with novel mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul. Disord. 20:166–173 10.1016/j.nmd.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti M., Szabò I. 1995. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1241:139–176 [DOI] [PubMed] [Google Scholar]
- Zotova L., Aleschko M., Sponder G., Baumgartner R., Reipert S., Prinz M., Schweyen R.J., Nowikovsky K. 2010. Novel components of an active mitochondrial K(+)/H(+) exchange. J. Biol. Chem. 285:14399–14414 10.1074/jbc.M109.059956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaritch E., Depreux F., Kraeva N., Loy R.E., Goonasekera S.A., Boncompagni S., Kraev A., Gramolini A.O., Dirksen R.T., Franzini-Armstrong C., et al. 2007. An Ryr1I4895T mutation abolishes Ca2+ release channel function and delays development in homozygous offspring of a mutant mouse line. Proc. Natl. Acad. Sci. USA. 104:18537–18542 10.1073/pnas.0709312104 [DOI] [PMC free article] [PubMed] [Google Scholar]