Abstract
We report experiments designed to test the hypothesis that the aqueous solubility of 11-cis-retinoids plays a significant role in the rate of visual pigment regeneration. Therefore, we have compared the aqueous solubility and the partition coefficients in photoreceptor membranes of native 11-cis-retinal and an analogue retinoid, 11-cis 4-OH retinal, which has a significantly higher solubility in aqueous medium. We have then correlated these parameters with the rates of pigment regeneration and sensitivity recovery that are observed when bleached intact salamander rod photoreceptors are treated with physiological solutions containing these retinoids. We report the following results: (a) 11-cis 4-OH retinal is more soluble in aqueous buffer than 11-cis-retinal. (b) Both 11-cis-retinal and 11-cis 4-OH retinal have extremely high partition coefficients in photoreceptor membranes, though the partition coefficient of 11-cis-retinal is roughly 50-fold greater than that of 11-cis 4-OH retinal. (c) Intact bleached isolated rods treated with solutions containing equimolar amounts of 11-cis-retinal or 11-cis 4-OH retinal form functional visual pigments that promote full recovery of dark current, sensitivity, and response kinetics. However, rods treated with 11-cis 4-OH retinal regenerated on average fivefold faster than rods treated with 11-cis-retinal. (d) Pigment regeneration from recombinant and wild-type opsin in solution is slower when treated with 11-cis 4-OH retinal than with 11-cis-retinal. Based on these observations, we propose a model in which aqueous solubility of cis-retinoids within the photoreceptor cytosol can place a limit on the rate of visual pigment regeneration in vertebrate photoreceptors. We conclude that the cytosolic gap between the plasma membrane and the disk membranes presents a bottleneck for retinoid flux that results in slowed pigment regeneration and dark adaptation in rod photoreceptors.
INTRODUCTION
Rhodopsin is one of the most prominent members of the G protein–coupled receptor super family. Members of this family comprise heptahelical transmembrane receptors that sense environmental changes, such as light, odors, hormones, neurotransmitters, and pheromones outside the cell to trigger appropriate cellular responses. Unlike most GPCR’s in which ligand binding triggers a cascade of reactions that result in an amplified cellular response, rhodopsin covalently binds its ligand, which operates as an inverse agonist in the receptor’s dark state. Light triggers an isomerization of the ligand from its 11-cis form to the all-trans form after light exposure, thus promoting activation of the receptor and its associated transduction cascade. The details of this cascade have been extensively reviewed (Shichida and Imai, 1998; Pugh and Lamb, 2000; Arshavsky et al., 2002). Additionally, in its ground state, rhodopsin is extremely stable, having a half-life of ∼420 yr (Baylor et al., 1980; Baylor, 1987). This high stability results in a low dark noise in the photoreceptor, allowing for photoreceptor responses to extremely low levels of light intensity, which are at or near the theoretical limit of detection.
Such extreme sensitivity has its drawbacks. Once activated, rhodopsin is said to be bleached, i.e., it is no longer functional for sensing visible light. As such, it must be regenerated to its ground state form containing 11-cis-retinal. This occurs normally through a chain of reactions collectively referred to as the visual cycle. In rods, the visual cycle is complex and requires the participation of both the photoreceptors and the retinal pigment epithelium (RPE). First, the all-trans-retinal is reduced to all-trans-retinol within the photoreceptor. This reaction is catalyzed by the enzyme retinol dehydrogenase, with NADPH as a cofactor (De Pont et al., 1970; Futterman et al., 1970; Palczewski et al., 1994). All-trans-retinol is then removed from the photoreceptors by a diffusive mechanism (Ala-Laurila et al., 2006; Wu et al., 2006) and, subsequently, taken up in the interphotoreceptor matrix. From here, it is translocated to the RPE (Okajima et al., 1990), where it is esterified, isomerized to 11-cis-retinol, and then oxidized to 11-cis-retinal. 11-cis-retinal then exits the RPE and is taken up by interphotoreceptor retinoid-binding protein (IRBP), which facilitates the translocation back to the photoreceptor outer segment.
Within the rod outer segment, opsin is located in membrane disks that are separated from the plasma membrane by a cytosolic gap (Mariani, 1986). In order for new visual pigment to form, 11-cis-retinal must be translocated across this gap to the membrane disks and bind to opsin in a multistep process that results in regeneration of rhodopsin and recovery of visual sensitivity. Retinal is a highly hydrophobic molecule (Szuts and Harosi, 1991), and the aqueous photoreceptor cytosol could present a barrier to its transport during dark adaptation.
In an extensive review article, Lamb and Pugh (2004) concluded that the regeneration of visual pigment and dark adaptation in the intact human retina is rate limited by a biochemical or biophysical mechanism involved in the translocation of retinal from the RPE to the rod outer segments. Here, we specifically investigate the processes within rod photoreceptors that regulate the translocation of retinoids that precede visual pigment regeneration.
We report experiments that were designed to test the hypothesis that the aqueous solubility of retinoids in the photoreceptor cytosol is an important determinant of pigment regeneration rate in vertebrate photoreceptors exposed to bright bleaching light. For our observations, we have chosen a retinoid analogue with increased aqueous solubility, which results from the addition of a hydroxyl group on the fourth position of the β-ionone ring of 11-cis-retinal, 11-cis 4-OH retinal. Our microspectrophotometric and electrophysiological recordings show a significantly more rapid rate of pigment regeneration and dark adaptation in salamander photoreceptors when their visual pigment is regenerated with 11-cis 4-OH retinal compared with 11-cis-retinal. Our data are consistent with a model in which passive diffusion governs the transport of cis-retinal across the cytosolic gap between intracellular disks and the plasma membrane. Previous studies of this work have appeared in abstract form (Frederiksen, R., R.K. Crouch, C.L. Makino, and M.C. Cornwall. 2010. The Association for Research in Vision and Ophthalmology Meeting. Abstr. 1112; Frederiksen, R., R.K. Crouch, B. Nickle, K.S. Chakrabarti, and M.C. Cornwall. 2011. The Association for Research in Vision and Ophthalmology Meeting. Abstr. 1179).
MATERIALS AND METHODS
Preparation of retinoids
11-cis-retinal was obtained as a gift from the National Eye Institute. 11-cis 4-OH retinal was synthesized and purified as previously described (Renk et al., 1981). Working solutions containing these retinoids were prepared for exogenous delivery to bleached photoreceptors in physiological saline solution. These retinoid-containing solutions were of four different types: dissolved in aqueous/ethanolic solution, loaded into lipid vesicles, bound to IRBP, or bound to BSA. In most cases, bleached cells were treated with ethanolic solutions. However, when another method was used, a specific note is provided in the text.
Retinoid in ethanolic solution was prepared in dim red light by dissolving 25 µg retinoid in 4 µl absolute ethanol in a conical vial. The saline solution was added, at first in small (9 × 5 µl) amounts and then in increasing amounts (50 µl and 2 × 450 µl) until the final volume was ∼1 ml. The peak absorbance (OD) of retinoid in this solution was measured using a conventional spectrophotometer (U-3010; Hitachi); its concentration was calculated as c = (OD380 l)/ε380, in which l is a 1-cm path length, and ε380 = 24,900 M−1 cm−1.
For solubility measurements, the concentration of retinoid was measured from its peak absorbance in ethanolic solutions prepared at the same dilution from a stock solution. For 11-cis-retinal and 11-cis 4-OH retinal, the wavelength of maximal absorbance in saline solution was 390 and 385 nm, respectively. The wavelength of maximal absorbance was the same in saline solution with 0.01% BSA and did not change with retinoid concentration.
The methods for the preparation of lipid vesicle solutions containing retinoid were similar to those described previously (Cornwall et al., 2000). This solution was stored for up to 24 h in a refrigerator before use. Solutions with retinoids bound to IRBP or BSA were prepared by exposing dried retinoid in vials to saline solutions to which these proteins had been added (10 µM IRBP or 0.2% BSA) and gently agitating these overnight at 4°C in darkness as described previously (Jones et al., 1989). Bovine IRBP was a gift from B. Wiggert (National Eye Institute, National Institutes of Health, Bethesda, MD). Procedures for the preparation and extraction of IRBP have been previously described (Redmond et al., 1985). We estimated the concentration spectrophotometrically using ε280 = 120,000 M−1 cm−1 for IRBP and an extinction coefficient for 11-cis-retinal as ε380 = 24,900 M−1 cm−1. Delipidated BSA was purchased from Sigma-Aldrich.
Retinoid solubility determinations
Stock solutions of 11-cis-retinal and 11-cis 4-OH retinal were prepared in ethanol at the following concentrations: 12.5 mM, 6.25 mM, 2.5 mM, 1.25 mM, 250 µM, and 125 µM. Before use, these were stored at −80°C. Amphibian saline solution and amphibian saline solution containing 0.01% BSA were prepared at the time of determination.
Retinoid solubility in aqueous solution was determined as follows: each stock was diluted 1:250 in saline solution and saline solution containing 0.01% BSA. The dilution was performed in steps in the following way: First, 4 µl of the retinoid stock solution was added to a vial. Second, a small amount of solvent was added to the vial, and the vial was gently shaken to ensure proper mixing. Addition of solvent and mixing were repeated until the final volume of 1 ml was reached. The exact volumes of solvent added to the sample were 5 µl four times, 26 µl once, 50 µl once, and finally, 450 µl twice. This gave approximate final concentrations of 50, 25, 10, 5, 1, and 0.5 µM retinoid. Absorbance spectra were then measured using a spectrophotometer (Cary 300 Bio UV-Visible; Varian, Inc.). The absorbance maximum in saline solution and BSA-containing saline solution was determined by fitting a polynomial function to the peak region of the spectrum. The concentration in saline solution and saline solution containing BSA was then calculated from the absorbance maxima using an extinction coefficient (ε = 20,800 M−1 cm−1) obtained by fitting a straight line to the linear range (three first data points) of the absorbance measurements of 11-cis 4-OH retinal (r = 0.999, P < 0.0001; Fig. 1 A, inset). This measured extinction coefficient was assumed to be the same for both species of retinal in both solutions. Experiments were performed in triplicate.
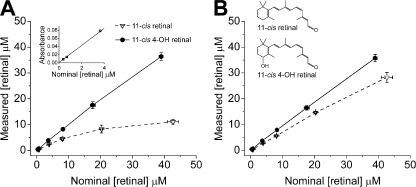
Figure 1.
11-cis 4-OH retinal is more soluble in aqueous buffer than 11-cis-retinal. (A and B) Plots of measured concentration of 11-cis 4-OH retinal (filled circles) and 11-cis-retinal (open triangles) against nominal retinoid concentration in Ringer’s solution. Values of measured concentration were estimated from spectral absorbance using an extinction coefficient (ε = 20,800 M−1 cm−1) obtained by fitting a straight line to the linear range (three first data points) of the absorbance measurements of 11-cis 4-OH retinal (inset in A). Data in A were measured on a solution that contained no BSA. The data in B were measured on a solution that contained 0.01% BSA. Continuous lines connect data points for 11-cis 4-OH retinal; dashed lines show 11-cis-retinal data points. The inset in B shows stick diagrams of the two species of retinal. Error bars represent means ± SD.
Determination of partition coefficient
Measurements of partition coefficients of retinoids in photoreceptor membrane were performed as described by Noy and Xu (1990). Stock solutions of 11-cis-retinal and 11-cis 4-OH retinal were prepared in ethanol at 10 mM. Bovine rod outer segment disk membrane was prepared from frozen retinae (Lawson Co.) as described previously (Papermaster and Dreyer, 1974). The rod outer segment disk membrane suspension was diluted in saline buffer (100 mM NaCl, 1 mM MgCl2, and 10 mM HEPES, pH 7.4). The concentration of phospholipids was estimated from absorbance spectra of rhodopsin in these suspensions (ε = 40,000 M−1 cm−1), assuming a 100:1 molar ratio of phospholipids to rhodopsin (Brown, 1994). In determinations of the partition coefficient for 11-cis-retinal, we mixed 8.56 × 10−9 mol phospholipid and 0.111 mol water. In the experiments with 11-cis 4-OH retinal, we used 8.56 × 10−8 mol phospholipid and 0.101 mol water. Ethanolic retinoid stock solutions were diluted stepwise in the saline buffer containing bovine disk membrane to a final volume of 2 ml in a similar way as described for the experiments to determine solubility. The dilution steps were 2 µl retinoid stock solution, four times 2 µl saline buffer, four times 10 µl saline buffer, three times 50 µl saline buffer, four times 200 µl saline buffer, and 1 ml saline buffer. Given the rapid rate of transfer of retinoids between lipid and aqueous phases (Noy and Xu, 1990), this stepwise dilution ensured enough time for the equilibration of the retinoid between the two phases. The sample was then centrifuged at 19,000 rpm for 30 min. The concentration of retinoid in the supernatant and the pellet was assessed spectrophotometrically in 0.1% Ammonyx LO (Sigma-Aldrich) using a spectrophotometer (Cary 300 Bio UV-Visible). Experiments were performed in triplicate. The partition coefficients for each retinoid, Keq, were calculated from their molar ratios (Noy and Xu, 1990):
| (1) |
in which n is the molar amount. The superscripts and subscripts denote solvent and solute, respectively.
Animals and preparation
Larval tiger salamanders, Ambystoma tigrinum, were obtained from a commercial supplier (Charles D. Sullivan Co.). All procedures were performed according to protocols approved by the Animal Care and Use Committee of Boston University School of Medicine and in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Salamanders were housed in a cold room maintained at 10°C in small water-filled tanks on a 12–12-h light–dark cycle. Animals were dark adapted for 12 h before all experiments. At the beginning of an experiment, an animal was decapitated and pithed caudally and rostrally under dim red light. All subsequent manipulations were performed under infrared light with the aid of infrared image converters (FJW Industries, Inc.). The retina was then isolated and placed in physiological saline solution, chopped into pieces, and triturated by repeated passage through the tip of a fire-polished Pasteur pipette. The physiological saline solution had the following composition: 111 mM NaCl, 2.5 mM KCl, 1.0 mM CaCl2, 1.6 mM MgCl2, 10 mM glucose, and 0.01% BSA. The pH was adjusted to 7.8 with 10 mM HEPES. The resulting cell suspension was then transferred to the recording chamber for single-cell suction pipette recordings or microspectrophotometry (MSP; see MSP section).
Electrophysiology
For electrophysiological recordings, a recording chamber containing retinal fragments and single isolated photoreceptors was placed on the stage of a modified inverted microscope (Invertoscope D; Carl Zeiss) in a light-tight Faraday cage. Isolated intact photoreceptors were identified from their images as viewed via a charge-coupled device camera (LCL-902HS; Watec) connected to a TV monitor. Cells were drawn, inner segment first, into a glass micropipette filled with physiological solution and connected to a patch clamp amplifier (EPC-7; List Associates). In this way, flash responses were measured as light-induced changes in the extracellular membrane current. Pipettes were fabricated from capillary glass tubing and fire polished to an inner diameter of 12 µm, which gave a tight fit to the inner segment of the photoreceptor. The resistance of the pipettes recorded in saline recording solution was ∼1 MΩ; this increased to ∼3 MΩ as the cell was drawn into the tip. In any given experiment, we recorded sensitivity, spectral sensitivity, dim flash responses, and dark noise in the dark-adapted state, after a bright bleach and after exogenous treatment with either 11-cis-retinal or 11-cis 4-OH retinal. The precise recording protocol and analysis used have been described previously (Corson et al., 1990; Jones et al., 1996; Ala-Laurila et al., 2007).
MSP
The spectral absorbance of native and 11-cis 4-OH–regenerated visual pigments was assessed using a single-beam microspectrophotometer. This instrument was modified from a similar device described previously (MacNichol, 1978; Cornwall et al., 1984; Jones et al., 1993). Measurements were made over the wavelength range of 300–800 nm at 2-nm intervals. A single spectral scan was completed in 1 s. The absorbance spectrum was calculated using Beer’s Law according to the relation OD = log10(Io/It), in which OD is optical density or absorbance, Io is the light transmitted through a cell-free space adjacent to the cell outer segment, and It is the light transmitted through the tissue. Absorption spectra could be measured with the polarization of the incident-measuring beam either parallel to the plane of the intracellular disks (T polarization) or parallel to the long axis of the outer segment (L polarization). Because of the dichroic nature of pigment within the outer segment, measurements were made in T polarization to optimize the measurement of dark-adapted visual pigment. All measurements reported here were made with light polarized in this orientation. The measuring beam was adjusted to fit within the center of the outer segment with the long axis of the slit parallel to the long axis of the outer segment, covering approximately one fourth of its width.
At the beginning of an MSP experiment, a dark-adapted retina was chopped and triturated, and the solution containing retinal fragments as well as isolated intact cells was placed on a quartz coverslip window coated with concanavalin A (Sigma-Aldrich) located in the bottom of a 2-mm-deep Plexiglas recording chamber. The tissue was allowed to settle, and cells were allowed to adhere to the quartz window for ≤5 min to minimize their movement. After this period, the cells were superfused at a rate of 4 ml/min with saline solution identical to that used in electrophysiological experiments. An isolated intact cell was identified from its projected image on the screen of the infrared video system. A baseline absorbance spectrum was measured from an area adjacent to the selected cell. Then, the outer segment was oriented in the beam path with the long axis of the slit oriented parallel to the long axis of the outer segment, and an absorbance measurement in this area was made. The absorbance spectrum was then calculated. Generally, 10 complete sample scans and 10 baseline scans were averaged to increase the signal-to-noise ratio of a measured spectrum. A single spectral scan was measured to have bleached <0.1% of the visual pigment per scan.
Dark-adapted spectra of the visual pigment measured in this way were then compared with spectra measured immediately after and at different times after exposure to a 505-nm light from a light-emitting diode light source calibrated to bleach >95% of the visual pigment. Such recordings of postbleach absorption spectra were continued for ≤60 min after bleaching. From measurements such as these, it was possible to measure directly the fraction of the pigment that was photoactivated by the bleaching light. After pigment bleaching had taken place, the tissue was exposed exogenously to a solution containing either 11-cis-retinal or 11-cis 4-OH retinal. Measurements of the kinetics and extent of visual pigment regeneration that occurred during this subsequent dark period were made.
Stopped-flow experiments of visual pigment regeneration
Stopped-flow experiments were used to measure regeneration of visual pigment in detergent micelles and nanodiscs. These used salamander rod opsin, wild-type (WT) bovine rod opsin, and 2/282 bovine rod opsin mutant containing an engineered disulfide bond between two introduced cysteine residues, N2C and D282C (Xie et al., 2003). The engineered disulfide confers enhanced thermal stability to the opsin, such that binding experiments can be undertaken in detergent solution (Gross et al., 2003; Xie et al., 2003). Crystal structures of the 2/282 mutant, in both the dark state (Standfuss et al., 2007) and active form (Standfuss et al., 2011), show the protein to be identical to that of native rhodopsin, except for the missing oligosaccharyl chain at position 2 and the presence of electron density corresponding to a disulfide bond connecting the two side chain sulfur atoms at positions 2 and 282. In addition, functional studies performed to date show that this mutant form behaves as does WT in all ways except with respect to stability of the opsin form in detergent solution (Gross et al., 2003; Xie et al., 2003).
All opsins were cloned into the pMT3 expression vector (Franke et al., 1988). The coding sequence for salamander rod opsin was modified to encode the last eight amino acids of bovine rod opsin (1D4 epitope) at its C terminus to allow for purification using the 1D4 antibody (Molday and MacKenzie, 1983). All opsins were transfected into HEK293 GnTI− cells (Reeves et al., 2002) using standard calcium phosphate–mediated procedures. Transfected cells were cared for and harvested as previously described (Reeves et al., 2002). Purification of expressed rod opsin in its apoprotein form was performed as previously reported (Gross et al., 2003), except the final wash steps and elution steps were performed in 10 mM HEPES, pH 7.4, 200 µM MgCl2, 3 mM NaN3, and 0.02% (wt/vol) n-dodecyl-β-d-maltoside (DDM; stop-flow [sf] reaction buffer). A dual-beam spectrophotometer (U-3210; Hitachi) was used to determine opsin concentration using ε280 = 65,000 M−1 cm−1 (Liu et al., 1996).
For stopped-flow assays performed in detergent micelles, 2× stock solutions were prepared containing 1 µM opsin in sf reaction buffer. 2× retinal stock solutions were prepared containing 6 µM retinal in sf reaction buffer. Concentrations of retinal were determined using ε380 = 24,935 M−1 cm−1 for 11-cis-retinal and 11-cis 4-OH retinal.
For nanodisc preparations, MSP1D1 was expressed in Escherichia coli and purified as previously described (Bayburt et al., 2002). Nanodiscs were formed by mixing POPC (Avanti Polar Lipids, Inc.), which was dried and resolubilized in 10% (wt/vol) DDM, MSP1D1, and purified opsin in a 70:1:0.06 molar ratio followed by removal of detergents using Bio-Beads SM-2 (Bio-Rad Laboratories). After detergent removal, opsin-containing nanodiscs were purified by affinity chromatography as previously described (Bayburt et al., 2007) using wash and elution buffers consisting of 20 mM HEPES, pH 7.5, 0.1 mM MgCl2, 120 mM NaCl, and 3 mM NaN3 (nanodisc reaction buffer). The concentration of opsin-containing nanodiscs was calculated using ε280 = 107,000 M−1 cm−1, corresponding to ε280 = 65,000 M−1 cm−1 for opsin (Liu et al., 1996) and ε280 = 42,000 M−1 cm−1 for the nanodisc (each nanodisc contains two MSP1D1 chains, ε280= 21,000 M−1 cm−1; Bayburt et al., 2002). Naked nanodiscs were formed by mixing POPC and MSP1D1 in a 70:1 molar ratio followed by detergent removal. Naked nanodiscs were purified by gel filtration on a column (Superdex 200 10/300 GL; GE Healthcare) using nanodisc reaction buffer as the running buffer. The concentration of naked nanodiscs was calculated using ε280 = 42,000 M−1 cm−1.
For stopped-flow assays performed in nanodiscs, 2× opsin stock solutions were prepared containing 1 µM opsin-embedded nanodiscs in nanodisc reaction buffer. 2× retinal stock solutions were prepared containing 6 µM retinal and 1 µM of naked nanodiscs in nanodisc reaction buffer. Retinal concentrations were determined spectrophotometrically. Because of the high value of the retinoid partition coefficients, at the lipid and retinoid concentrations used, the concentrations of 11-cis-retinal and 11-cis 4-OH retinal in the lipid phase in these experiments were very similar (the calculated concentration of 11-cis-retinal in the lipid phase was ∼20% higher than that of 11-cis 4-OH retinal). Thus, any difference in regeneration rates is not caused by any difference in their concentrations in the lipid phase.
Reaction kinetics was measured using a stopped-flow (SX20; Applied Photophysics) instrument at 25°C with an optical path length of 1 cm. The slit width of the light source was 0.5 mm, corresponding to a wavelength bandwidth of 2.3 nm. Reactions were initiated by combining equal volumes of opsin and retinal stock solutions to achieve a final concentration of 0.5 µM opsin and 3 µM retinal. Data were recorded at 0.05-s intervals for 50 s (detergent micelle assays) or 0.1-s intervals for 500 s (nanodisc assays) by continuous monitoring at 500 nm for pigment formation. Data were signal averaged over three measurements to reduce background noise. The data were normalized by the relation ΔA = (A − Amin)/(Amax − Amin), in which A is the absorbance at any time, Amax is the maximum absorbance value, and Amin is the value of minimum absorbance.
RESULTS
Aqueous solubility and partition coefficients
We performed experiments to compare the solubility of 11-cis-retinal and 11-cis 4-OH retinal chromophores in aqueous solution. Fig. 1 plots retinal concentrations as determined from the absorption at the wavelength of maximal absorbance of 11-cis-retinal and 11-cis 4-OH retinal (390 and 385 nm, respectively) in saline solution containing no BSA (Fig. 1 A) and in saline solution containing 0.01% BSA (Fig. 1 B). In both cases, it can be clearly seen that the concentration of dissolved 11-cis 4-OH retinal is higher than that of 11-cis-retinal at each nominal concentration. It is also clearly evident that even when present at the very low concentrations used in our experiments, BSA significantly increases the measured concentration of retinal in solution. The linearity of the plots for 11-cis 4-OH retinal suggests that it is mostly soluble in both saline solution buffer and saline solution with BSA up to a concentration of ≥40 µM. It can also be seen in Fig. 1 that the concentration of dissolved 11-cis-retinal in saline solution levels off, which also suggests that it is not as soluble as 11-cis 4-OH retinal. In Table 1, we present values of fractional solubility at a 10-µM measured concentration (the concentration used in the physiology experiments). We define fractional solubility as the ratio between measured (spectrophotometrically) and nominal (calculated from mass) concentration. The values in Table 1 show that at a 10-µM measured concentration, 11-cis-retinal has a solubility that is only about a third of the solubility of 11-cis 4-OH retinal in aqueous buffer without BSA and about three quarters of that measured in the presence of 0.01% BSA.
Table 1.
Biochemical properties of 11-cis-retinal and 11-cis 4-OH retinal
| Biochemical property | 11-cis-retinal | 11-cis 4-OH retinal |
| Fractional solubilitya | ||
| Ringer’s solution | 0.34 | 1 |
| Ringer’s solution, 0.01% BSA | 0.74 | 1 |
| Partition coefficientb | ||
| Keq | (1.6 ± 0.5) × 108 | (2.9 ± 0.2) × 106 |
| Pigment formation rate constant, Rc | ||
| Bovine 2/282 opsin, micelles | 0.74 s−1 | 0.15 s−1 |
| Bovine 2/282 opsin, nanodiscs | 0.019 s−1 | 0.010 s−1 |
| Bovine WT opsin, nanodiscs | 0.015 s−1 | 0.007 s−1 |
| Salamander opsin, nanodiscs | 0.053 s−1 | 0.027 s−1 |
We also measured the partition coefficient, Keq, of 11-cis-retinal and 11-cis 4-OH retinal in photoreceptor cell membranes (Table 1). 11-cis-retinal has a partition coefficient of (1.6 ± 0.5) × 108 (n = 3). The corresponding value for 11-cis 4-OH retinal is (2.9 ± 0.2) × 106 (n = 3). Thus, 11-cis-retinal partitions into the cell membrane roughly two orders of magnitude better than 11-cis 4-OH retinal. However, we must point out that the partition coefficients of both species of retinal are extremely high. Thus, when an intact rod is exposed to an external concentration of either 11-cis-retinal or 11-cis 4-OH retinal, it is expected that the concentration of either chromophore in the cytosol next to the plasma membrane will rapidly equilibrate to a concentration set by its aqueous solubility.
Rate of pigment regeneration and recovery of sensitivity
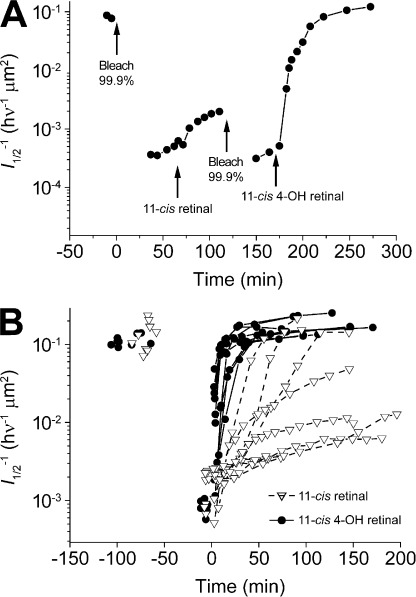
The experiments illustrated in Fig. 2 demonstrate the consequence of increased solubility of 11-cis 4-OH retinal compared with 11-cis-retinal as well as the rapid partition of these two retinoids into bleached salamander rods to promote recovery of flash sensitivity. Fig. 2 A plots the time course of sensitivity recovery that occurs first when bleached rods are treated with 11-cis-retinal and thereafter with its 4-OH analogue. After bleaching and exogenous treatment with 11-cis-retinal, we observed a slow and partial recovery of sensitivity. However, when bleached again and treated with the same concentration of 11-cis 4-OH retinal, the rod recovered sensitivity and dark current fully over the subsequent 80 min to the prebleach dark-adapted level. In Fig. 2 B, we present results from multiple sensitivity recovery rate experiments. On average, this recovery after 11-cis 4-OH treatment occurred as a single exponential function with a time constant of 26.0 ± 2.0 min (±SEM). In some experiments, recovery occurred much faster, in as little as 8 min. In contrast to the results with the 4-OH analogue, recovery after such exhaustive bleaching and 11-cis-retinal treatment was significantly slower, often requiring >2 h to complete. Because of the very large variability in the recovery rates after treatment with 11-cis-retinal, we were unable to obtain a reliable estimate of the mean recovery time under these same conditions (Fig. 2 B). Sometimes no recovery of sensitivity was observed. Recovery was most often slow and only partial. In no case was recovery observed to be faster than when 11-cis 4-OH retinal was used.
Figure 2.
Isolated bleached salamander rods recover sensitivity faster after substantial bleaching of the visual pigment when exogenously treated with 11-cis 4-OH retinal compared with when treated with 11-cis-retinal. (A) The graph plots the time course of sensitivity changes (l1/2) in a rod during darkness before and after a bleach, after subsequent treatment with 10 µM 11-cis-retinal in 0.1% ethanolic solution. At 120 min, the rod was then exposed to a second bleach and finally, 80 min later, treated with 10 µM 11-cis 4-OH retinal in 0.1% ethanolic solution. (B) Recovery of sensitivity measured in single isolated rods treated as in A. The mean fitted time constant for recovery of sensitivity after treatment with 11-cis 4-OH retinal was 26.0 min; n = 9. Data points are plotted ± SEM. Because of the large variability, we were unable to obtain a reliable fit for the recovery of sensitivity after treatment with 11-cis-retinal. However, the mean time constant for recovery was considerably longer that the 26 min found for the 4-OH retinal.
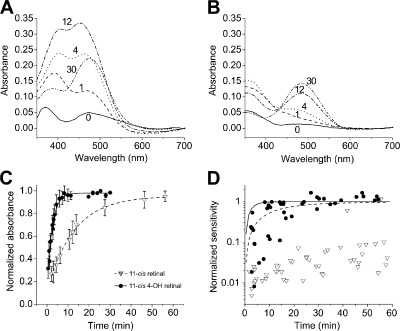
The spectral data plotted in Fig. 3 provide insight into the much more rapid recovery of sensitivity after 11-cis 4-OH retinal treatment compared with 11-cis-retinal treatment. Here, absorption spectra were measured transversely in intact outer segments during the time course of pigment regeneration after treatment with these two chromophores, 11-cis 4-OH retinal in Fig. 3 A and 11-cis-retinal in Fig. 3 B. The results plotted in Fig. 3 C show that there are substantial differences in the rates with which native and 4-OH visual pigments can be regenerated when intact bleached rods are treated with solutions containing the same exogenous concentration of 11-cis-retinal or 11-cis 4-OH retinal. The mean rate constant for regeneration of 4-OH rhodopsin is R4-OH = 0.0064 s−1, whereas that for 11-cis-rhodopsin is R11-cis = 0.0013 s−1, a difference of more than fivefold. From these data, we can calculate the initial rate in moles per second at which the pigment of an individual rod outer segment is being regenerated: K = Rπr2lCrh. Here, we view the rod outer segment as a cylinder with the radius (r) = 5.7 µm, length (l) = 29.8 µm (estimated from bright-field images of the cells; unpublished data), and a pigment concentration of Crh = 3.5 mM (Hárosi, 1975). From these values, we calculate that the initial pigment regeneration rates for individual rod outer segments are K11-cis = 1.4 × 10−17 mol s−1 and K4-OH = 6.8 × 10−17 mol s−1 for 11-cis-retinal and 11-cis 4-OH retinal, respectively. Fig. 3 D plots normalized sensitivity recovery (Fig. 3 D, symbols) and normalized pigment regeneration (Fig. 3 D, lines) as a function of time. It is apparent that sensitivity recovery lags pigment regeneration, both when treated with 11-cis-retinal (Fig. 3 D, open triangles and dashed line) and when treated with 11-cis 4-OH retinal (Fig. 3 D, closed circles and solid line). The reason for this difference is presently unknown.
Figure 3.
Regeneration of visual pigment in isolated salamander rods after extensive bleaching is faster when treated exogenously with 11-cis 4-OH retinal than with 11-cis-retinal. (A) Absorbance spectra measured microspectrophotometrically for comparison of the time course of retinoid partition and pigment regeneration in a bleached salamander rod photoreceptor outer segment after treatment with 11-cis 4-OH retinal. Two peaks appear: one at 385 nm, the retinoid loading into the outer segment, and the other at 478 nm reports the regeneration of the visual pigment. Note that rapid loading of 11-cis 4-OH retinal in the outer segment precedes pigment formation. The numbers in the plot indicate time in minutes after retinal treatment. (B) Absorbance spectra taken after exogenous application of 11-cis-retinal as in A. Note the smaller retinoid peak, compared with A, in the near UV region of the spectrum. The numbers in the panel indicate time in minutes after retinal treatment. (C) Time course of regeneration of visual pigment measured microspectrophotometrically in intact rods treated with 11-cis 4-OH retinal and 11-cis-retinal. Transverse OD in outer segments of intact rods was measured at 520 nm and normalized to avoid bias from retinoid absorbance at shorter wavelength (see A and B). Bleached rods treated with 11-cis-retinal regenerate pigment with a time constant of 12.8 min, n = 6; those treated with 11-cis 4-OH retinal regenerate pigment with a time constant of 2.6 min, n = 5. In both cases, regeneration data are fitted as simple exponential functions. The retinal concentration was 10 µM in 0.1% ethanolic solution. Error bars show means ± SEM. (D) Normalized recovery of sensitivity of bleached rods treated with 11-cis-retinal (open triangles) or 11-cis 4-OH retinal (closed circles). The lines are single exponential functions that were fitted to pigment regeneration data in C. The dashed line represent pigment regeneration with 11-cis-retinal, and the solid line represents 11-cis 4-OH retinal. Note that in both cases, pigment regeneration precedes the recovery of sensitivity.
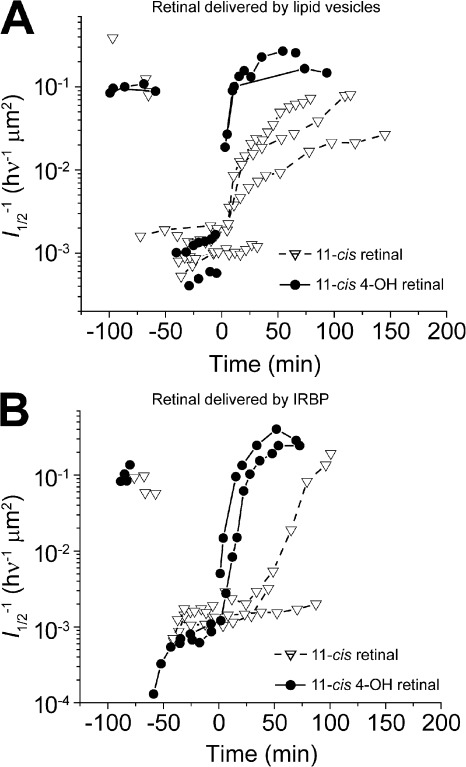
The data shown in Figs. 2 and 3 make it clear that exogenous application of 11-cis 4-OH retinal to intact photoreceptors results in faster rhodopsin regeneration compared with 11-cis-retinal. Although this is consistent with the higher solubility of 11-cis 4-OH retinal, there are several steps intervening between the extracellular application of the retinoid and rhodopsin formation, and we have examined them in turn. First, we have looked at facilitation of the transfer of retinoid to the photoreceptor outer segment membrane. To address this question, we designed experiments to test different ways by which this may take place. Four delivery mechanisms were tested. These were (1) a 0.1% ethanolic saline solution, (2) a 0.2% BSA-containing solution in which retinoid was complexed to nonspecific lipid binding sites on the protein, (3) a lipid vesicle solution in which retinoid is partitioned into lipid vesicles suspended in the bulk solution, and (4) a 10 µM bovine IRBP solution in which retinoid was bound to specific retinoid binding sites on the protein. Of these vehicles, only the IRBP solution approximates the environment that exists in the extracellular matrix surrounding the rods. Retinoid-containing solutions were applied to the bath to a final effective concentration that we estimate to be 10 µM. Figs. 2 and 4 (sensitivity recovery rates with other vehicles) illustrate that when allowance was made for small differences in retinoid concentration, we were unable to detect any significant differences in sensitivity recovery kinetics, regardless of vehicle. In all four cases, kinetics observed after 11-cis 4-OH retinal treatment were accelerated compared with those after treatment with 11-cis-retinal (BSA; unpublished data).
Figure 4.
Effect of different retinoid delivery vehicles on the rate of sensitivity recovery after bleaching. (A and B) Both graphs plot the time course of sensitivity (l1/2) changes in individual rods during darkness before and after a bleach and then after treatment with 10 µM 11-cis-retinal or with 10 µM 11-cis 4-OH retinal. (A) Time course of sensitivity recovery when lipid vesicles are used as the delivery vehicle. (B) Time course of sensitivity recovery when IRBP is used as the delivery vehicle.
Pigment formation in vitro
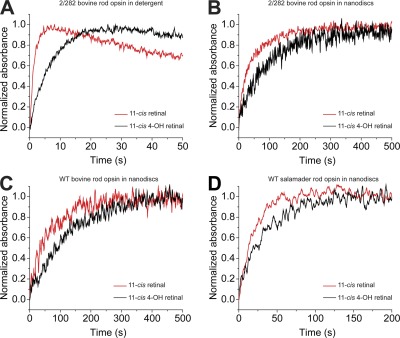
We next addressed whether the faster pigment regeneration and recovery of sensitivity that we observed can be attributed to the interaction of opsin with retinal. To answer this question, we measured pigment regeneration rates in vitro in detergent solution as well as lipid membranes. These results are summarized in Fig. 5. For analysis in detergent, we determined rate constants for pigment regeneration using bovine opsin containing the stabilizing mutations N2C and D282C (2/282; Xie et al., 2003). The stabilizing mutations were necessary for detergent-solubilized experiments to avoid denaturation of the opsin. In detergent, regeneration with 11-cis-retinal occurred fivefold faster than regeneration with 11-cis 4-OH retinal (Fig. 5 A). These results are in contrast to the results with intact cells showing faster regeneration with 11-cis 4-OH retinal (Fig. 3 C). To test whether a membrane environment affects the rate constant, we made further measurements using discoidal lipid bilayer membranes (nanodiscs; Bayburt and Sligar, 2010). Regeneration rate constants were measured for bovine WT, bovine 2/282, and salamander rod opsins reconstituted with retinal. By using nanodiscs (Fig. 5 B), measurements using WT rod opsins were possible because of the increased stability provided by support in a lipid bilayer. Analysis of regeneration rates in nanodiscs reveals that the time course for regeneration with both species of retinal is comparable for bovine WT and 2/282 rod opsins (Fig. 5, B and C). In contrast, regeneration occurs faster for salamander rod opsin than bovine rod opsin (Fig. 5 D). For all opsins analyzed, pigment formation with 11-cis-retinal was at least two times faster than with 11-cis 4-OH retinal. Rate constants for all of these measurements are presented in Table 1.
Figure 5.
Pigment regeneration in solutions of four different recombinant opsin models is slower when treated with 11-cis 4-OH retinal than when treated with 11-cis-retinal. Pigment formation from opsin and retinal was monitored as an increase in absorbance at 500 nm. (A) Regeneration was analyzed in DDM detergent micelles using recombinant bovine rod opsin containing the stabilizing mutations N2C and D282C (2/282). (B–D) Regeneration in POPC lipid bilayers was analyzed using nanodiscs containing 2/282 bovine rod opsin (B), WT bovine rod opsin (C), and WT salamander rod opsin (D). Pigment regeneration is shown to proceed at a slower rate in nanodiscs than in detergent micelles; however, under all tested conditions, the rate of regeneration with 11-cis 4-OH retinal is slower than with 11-cis-retinal. Comparison of bovine 2/282 with WT rod opsins shows the stabilizing mutations do not influence the regeneration rate. Additionally, a much faster regeneration rate is shown for salamander rod opsin compared with bovine rod opsin. Data for all traces were signal averaged from three experiments. The concentrations used were 0.5 µM opsin and 3 µM retinal. Rate constants for the regenerations are presented in Table 1.
Properties of 11-cis 4-OH visual pigment
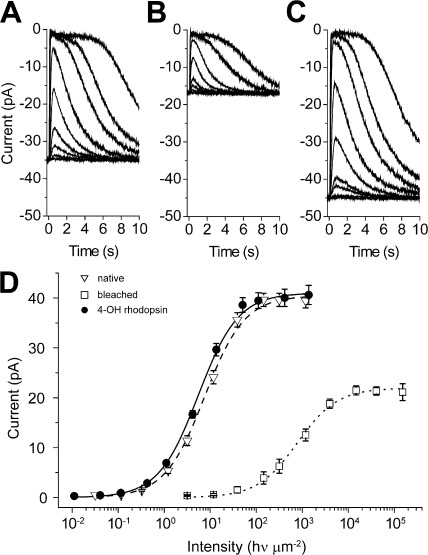
Given the differences in the rates of pigment formation and recovery of sensitivity that are illustrated in Figs. 2, 3, and 4, we sought to understand whether there were additional differences in the kinetics of transduction and adaptation. Data in Figs. 6, 7, and 8 show that the physiological properties of rods that have been bleached and regenerated with 11-cis 4-OH retinal are very similar to those expected in the native state. Flash response families that are plotted in Fig. 6 (A–C) were elicited while the rod was dark adapted (Fig. 6 A), 60 min after exposure to a bright light that bleached >99% of the visual pigment (Fig. 6 B), and then 120 min after exposure to a solution containing 10 µM 11-cis 4-OH retinal (Fig. 6 C). As measured by the saturated response amplitude, the maximum level of the dark current in this cell recovered in the steady state to ∼40% of the dark-adapted value within 60 min. Subsequent exposure to the retinoid resulted in greater than full recovery of dark current over the subsequent 80–100 min. The intensity/response relations in Fig. 6 D plot the mean behavior of cells treated as in Fig. 6 A. These results demonstrate that sensitivity in the steady state after the period of bleaching (Fig. 6 D, open squares) recovered to a level that was approximately two orders of magnitude less than in the dark-adapted state (Fig. 6 D, open triangles). Previous work has demonstrated that this steady-state level of desensitization is caused by a combination of quantum catch loss that results from a reduced ability of the outer segment to catch photons together with cellular desensitization that results from the presence within the cell of a large amount of naked opsin (Cornwall and Fain, 1994; Jones et al., 1996). Response amplitude and sensitivity were observed to recover fully after subsequent exogenous treatment with 11-cis 4-OH retinal.
Figure 6.
Intact isolated rods completely recover dark current and sensitivity after bleaching of the visual pigment and exogenous treatment with 11-cis 4-OH retinal. (A–C) Flash response families recorded in response to 20-ms flashes in dark-adapted conditions (A), after 99% of the pigment had been photoactivated (bleached; B), and after exogenous application of 11-cis 4-OH retinal (C). (D) Mean response/intensity relations measured under conditions in A–C in darkness before bleach, 60 min after the bleach, and after retinoid treatment (Mean ± SEM, n = 19).The retinal concentration was 10 µM in 0.1% ethanolic solution.
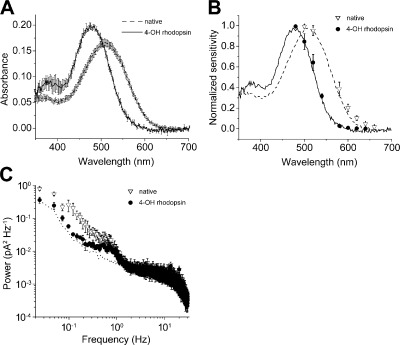
Figure 7.
Absorption and spectral sensitivity of isolated rod outer segments regenerated with 11-cis 4-OH retinal is blue shifted in respect to rods containing native visual pigment. (A) Absorption spectra of native dark-adapted rod photoreceptor visual pigment (λmax = 509 nm, n = 7) and of pigment regenerated with 11-cis 4-OH retinal (λmax = 478 nm, n = 7) measured microspectrophotometrically in intact outer segments. (B) Normalized spectral sensitivity recorded from rods in the native dark-adapted state (n = 5) and after bleach and regeneration with 11-cis 4-OH retinal (n = 7). The lines represent the normalized absorbance of native dark-adapted rod photoreceptor visual pigment (dashed line) and of 11-cis 4-OH retinal (continuous line). (C) The blue-shifted 4-OH rhodopsin (n = 4) has a lower level of dark noise power than native rhodopsin (n = 4) as can be seen in the plot of power spectral density. This is caused by the higher activation energy that follows from shorter wavelength absorption. Instrumentation noise that was recorded with the cell in the electrode and in the presence of bright saturating light is indicated by the dotted line. The retinal concentration in the experiment was 10 µM in 0.1% ethanolic solution. Data points are plotted ± SEM in all graphs.
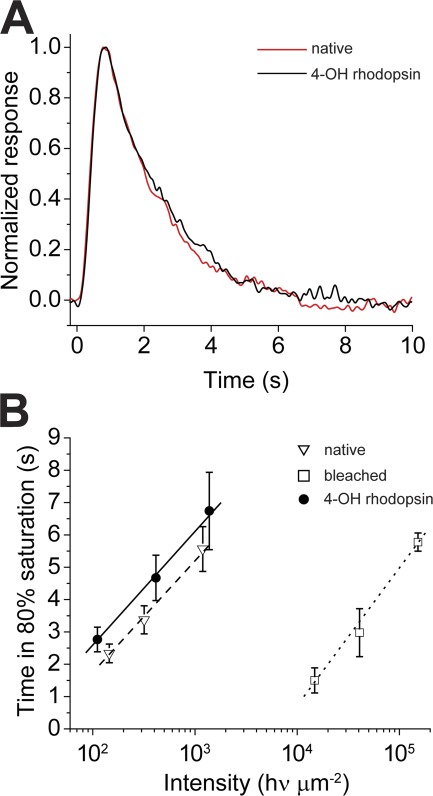
Figure 8.
Flash response kinetics to dim and bright flashes is similar in rods containing 4-OH rhodopsin compared with rods containing native visual pigment. (A) Normalized dim flash responses in dark-adapted native state (n = 8) and when bleached and regenerated with 11-cis 4-OH retinal (n = 8). There is no significant difference in the time course of the responses. The responses have been smoothed by adjacent averaging. (B) Plots of the time necessary for responses to bright saturating flash responses to recover to 80% of saturation amplitude. Measurements made in a background of darkness when outer segments contained native visual pigment (dashed line; n = 9), after 99.9% bleached (dotted line; n = 9), and after regeneration with 11-cis 4-OH retinal (solid line; n = 9). The retinal concentration was 10 µM in 0.1% ethanolic solution. Data points in B are plotted ± SEM.
Results illustrated in Fig. 6 are consistent with the notion that accelerated recovery of flash response amplitude and sensitivity of bleached rods after 11-cis 4-OH retinal treatment is caused by the regeneration of functional visual pigment as normally occurs during dark adaptation. For instance, the kinetics of the response family (Fig. 6 A) and the response intensity relation (Fig. 6 D, filled circles), both measured after treatment with 11-cis 4-OH retinal, are not significantly different from those observed before bleach. The recovery of dark-adapted sensitivity and flash response amplitude caused by the formation of a rhodopsin-like visual pigment is demonstrated in Fig. 7. Here, intact rod outer segments containing the newly formed pigment, 11-cis 4-OH rhodopsin, have a mean peak transverse OD of 0.20 ± 0.004, whereas rods containing native rhodopsin have a mean OD that is 0.17 ± 0.004. The peak absorbance of 11-cis 4-OH rhodopsin is at ∼478 nm, whereas the native visual pigment absorbs maximally at 509 nm (Fig. 7 A). These values are roughly consistent with those published previously (Corson et al., 1990). Fig. 7 B also shows that native spectral sensitivity and that observed after 11-cis 4-OH retinal regeneration are consistent, within experimental error, to their respective spectral absorbance curves, as measured transversely in intact rods. As shown in Fig. 7 C, rods containing 4-OH rhodopsin exhibit a lower level of dark noise, indicating less spontaneous activations of the transduction cascade compared with cells containing native visual pigment. This finding is consistent with those of previous studies, which have reported that Purkinje shifted pigments are less noisy as a result of an increased activation energy (Barlow, 1957; Ala-Laurila et al., 2004, 2007).
To determine whether differences exist between photoactivation of the visual transduction cascade by rhodopsin and 4-OH rhodopsin, we recorded the mean dim flash response of the native dark-adapted state (before bleaching) and that after complete pigment regeneration with 11-cis 4-OH retinal (Fig. 8 A). This comparison is based on observations made previously demonstrating that dim flash responses that have an amplitude <20–30% of those produced by bright saturating flashes display kinetics that reflect those of underlying reactions in the visual transduction cascade (Lamb and Pugh, 1992; Jones, 1995; Fain et al., 2001). For the mean responses (n = 9) shown in Fig. 8 A, the light intensity was adjusted so that a response could be detected approximately every two to three flashes. The responses to a total of 90 flashes were recorded and averaged in the native dark-adapted state (Fig. 8 A, red) or when regenerated with 11-cis 4-OH retinal (Fig. 8 A, black). We observed no detectable difference between them. The time to peak was measured to be τpeak4-OH = 0.82 s and τpeaknative = 0.79 s; the exponential recovery time was measured to be τrec4-OH = 1.90 s and τrecnative = 1.79 s. We also evaluated the time dependence of response recovery after exposure to very bright supersaturating light flashes by measuring the time required for the response to recover to a level that is 80% of the maximum (Fig. 8 B). A large body of previous work has demonstrated that the slope of this relation provides an evaluation of the enzymatic step in the transduction cascade that rate limits response recovery (Pepperberg et al., 1992, 1994). By this test, we observed no significant difference in response recovery kinetics between rods containing native visual pigment and rods bleached and regenerated with 11-cis 4-OH retinal.
DISCUSSION
The experiments presented here test the hypothesis that the passive transport of retinal through the cytoplasm between the plasma and disk membranes could constitute an important determinant of the rate of pigment regeneration. To do this, we have compared the rates of pigment regeneration and sensitivity recovery in salamander rod photoreceptors that have been bleached and subsequently exposed to exogenous solutions containing native 11-cis-retinal and 11-cis 4-OH retinal. 11-cis 4-OH retinal differs from 11-cis-retinal in that it has an additional hydroxyl group attached to position 4 on the β-ionone ring, thus increasing its aqueous solubility. The data presented here demonstrate the following: (a) The aqueous solubility of 11-cis 4-OH retinal is substantially greater than for 11-cis-retinal. (b) 11-cis-retinal has a partition coefficient that is roughly 100 times higher than that of 11-cis 4-OH retinal, although both species of retinal partition extremely well into lipid membranes. (c) When exogenously supplied to an extensively bleached vertebrate rod photoreceptor, 11-cis 4-OH retinal combines with opsin to form a fully functional blue-shifted visual pigment, whose physiological properties can be predicted from its spectral absorbance. (d) The rate of pigment regeneration and sensitivity recovery as well as the return of response kinetics to prebleach levels that occur when 11-cis 4-OH retinal is applied exogenously to bleached rods is over fivefold faster than occurs when similarly bleached rods are treated with 11-cis-retinal. These more rapid rates of pigment regeneration and sensitivity recovery occur even though the rate of pigment regeneration measured in solutions containing opsin and 11-cis 4-OH retinal is slower than in solutions containing 11-cis-retinal. (e) The sensitivity and response kinetics that are fully restored to dark-adapted values in rods containing 4-OH rhodopsin demonstrate that the photosensitivity of 4-OH rhodopsin is close to that of rhodopsin.
These results demonstrate that there exists a close correlation between the aqueous solubility of retinoids and the rate at which pigment regeneration and sensitivity recovery occur in bleached isolated rods. This is in spite of the fact that the intrinsic rate at which 4-OH rhodopsin forms is slower than the rate of rhodopsin formation, as we demonstrate in in vitro measurements of pigment regeneration, both with bovine opsin expressed in solution or salamander rod opsin expressed in nanodiscs (Fig. 5).
In order for the visual pigment to regenerate in a rod photoreceptor, 11-cis-retinal must be transported from the extracellular space to the internal membrane disks and bind to opsin. This process can be broken down into the following steps: (1) transfer of 11-cis-retinal from the extracellular space into the plasma membrane, (2) transfer of 11-cis-retinal from plasma membrane to the cytosolic aqueous phase, (3) diffusion of 11-cis-retinal within the cytosol, (4) transfer of 11-cis-retinal from the cytosolic aqueous phase into disk membrane, and (5) reaction of 11-cis-retinal with opsin resulting in regeneration of visual pigment.
The data summarized in Table 1 show that both 11-cis-retinal and 4-OH retinal partition extremely well into lipid membranes. In effect, this means that the external plasma membrane is unlikely to act as a barrier for retinal movement, as the retinoid concentration in the cytosol next to the plasma membrane will quickly equalize to a concentration set by the aqueous solubility of retinal. This is supported by the fact that we observed no substantial differences in the rate of recovery of sensitivity when we used different delivery methods of retinal (Fig. 4). Thus, steps 1, 2, and 4 are expected to be very fast, consistent with the rapid rate for the transfer of retinol between membranes and aqueous solution of 0.64 s−1 as reported previously (Noy and Xu, 1990). The high partition coefficient values together with a rapid equilibrium rate constant for retinoids eliminates the plasma membrane as a potential bottleneck for the rate of pigment regeneration and dark adaptation. It follows that the regeneration is limited either by step 3, the diffusion across the cytosol, or by step 5, the reaction rate of 11-cis-retinal with opsin.
We believe that the intrinsic rates of regeneration that we have measured in in vitro experiments (step 5) cannot explain the pigment regeneration rates that we observe in intact rod photoreceptors. Our argument is as follows: The in vitro regeneration rates we observed (R11-cis = 0.053 s−1 and R4-OH = 0.027 s−1) are much faster than those observed in intact cells (R11-cis = 0.0013 s−1 and R4-OH = 0.0064 s−1). These rates of pigment regeneration are given by
| (2) |
in which k is the second order rate constant, and Cretinal and Copsin are the concentrations of retinal and opsin in the membrane, respectively. The in vitro regeneration experiments with nanodiscs were made using very low concentrations of opsin (0.5 µM) and retinal (3 µM). A naked nanodisc contains ∼250 lipid molecules (Bayburt et al., 2007). When containing opsin, a nanodisc contains ∼200 lipid molecules (Bayburt et al., 2007). Equal concentrations of naked nanodiscs and opsin-containing nanodiscs were mixed. Thus, the concentrations of retinal and opsin in the nanodiscs are Copsin = 1 opsin/450 lipids and Cretinal = 6 retinals/450 lipids. The corresponding opsin concentration in a rod outer segment is ∼1 opsin per 100 molecules of lipid. At early times, in the linear range, the pseudo–first order rate constants for regeneration are proportional to the rates of pigment regeneration. Thus, using Eq. 2, together with the experimentally obtained rates, R, and the concentrations given in this paragraph, we can calculate the retinal concentrations, Cretinal, in the rod outer segment disks from Cretinal-cell = (Rcell/Rnanodisc) × (Cretinal-nanodisc Copsin-nanodisk/Copsin-cell) and obtain C11-cis = 7.4 × 10−5 (mole retinal/mole lipid) and C4-OH = 7.0 × 10−4 (mole retinal/mole lipid). In the cytosolic aqueous phase next to the disk membrane, the retinal concentrations can be estimated from the known partition coefficients (Eq. 1). For a water concentration of 55 M, we get C11-cis = 2.5 × 10−11 M and C4-OH = 1.3 × 10−8 M. These concentrations are several orders of magnitudes lower than the concentrations of retinal delivered at the plasma membrane, indicating that the rate of pigment regeneration would be higher if higher retinoid concentrations were achieved at the disk membrane. Thus, pigment regeneration (step 5) is not limiting.
Instead, the most likely bottleneck is at the cytosolic gap (step 3) between the plasma membrane and the intracellular disk membranes in which the bulk of the bleached opsin is located. This notion is consistent with a simple diffusional model of retinal translocation across the cytosolic gap based on Fick’s second law of diffusion (Carslaw and Jaeger, 1959; Crank, 1975) J = −D dC/dx, in which J is the retinal flux per unit area, and D is the diffusion coefficient for retinal taken to be 5 × 10−6 cm2 s−1 (Szuts and Harosi, 1991). Given the high partition coefficient for retinoid in the membrane and its rapid equilibration between aqueous and lipid phases (Noy and Xu, 1990), we set the boundary condition (concentration) in the cytosol next to the plasma membrane, Cpm, to be equal to the aqueous solubility. In this case, we predict this to be the amount of retinoid that can be dissolved in aqueous solution (i.e., at 10 µM nominal concentration; Fig. 1). The cytosolic concentration of retinoid at the disk membrane boundary, Cdm, will be much smaller than that at the plasma membrane because of the presence of a large sink in the form of opsin on the disk membrane. This is indeed borne out by the calculations of the concentration for Cdm from the pigment regeneration rates. If we view the rod outer segment as a cylinder with the same size as used in the calculation of the regeneration rate (radius [r] = 5.7 µm and length [l] = 29.8 µm; see Results), the flux, ψ, becomes ψ = D 2πrl (Cpm − Cdm)/δ, in which δ represents the cytosolic gap between the plasma and disk membrane of 0.05 µm (Mariani, 1986). Because Cpm >> Cdm initially, we can approximate the previous equation as ψ = D 2πrl Cpm/δ.
It is apparent from this equation that the flux of retinoid across the cytosolic gap is, at least at initial times, directly proportional to the concentration in the cytosol just inside the plasma membrane, Cpm, which equals the solubility in the cytosol. Using values from Fig. 1 (Cpm = 3.5 µM for 11-cis-retinal and Cpm = 10 µM for 11-cis 4-OH retinal), we determine the fluxes to be ψ11-cis = 3.6 × 10−17 mol s−1 and ψ4-OH = 1.10−16 mol s−1 for 11-cis-retinal and 11-cis 4-OH retinal, respectively. These values are reasonably close to the regeneration rates in intact cells, which we have measured: K11-cis = 1.4 × 10−17 mol s−1 for 11-cis-retinal and K4-OH = 6.8 × 10−17 mol s−1 for 11-cis 4-OH retinal, calculated from our MSP data (see Results). Thus, the model, at least partly, explains the five times faster regeneration rate of 4-OH rhodopsin compared with rhodopsin that we have observed in intact cells.
With the cytosolic gap acting as a barrier, the aqueous solubility of retinal seems to be an important factor setting the rate of regeneration of the visual pigment after a substantial bleach. The model suggests that the higher aqueous solubility of 11-cis 4-OH retinal is sufficient to drive a greater retinoid flux across the cytoplasmic gap in spite of its lower partition into the membrane and, thus, driving visual pigment regeneration at a higher rate. We believe that this is the first observation that solubility of cis-retinoids may play an important role in the rate of pigment regeneration and sensitivity recovery. This may have important pharmacological implications for the design of retinoid drugs that may be used in the treatment of disorders of visual pigment regeneration in human patients.
Acknowledgments
This study was supported by National Institutes of Health/National Eye Institute grants EY04939 (to R.K. Crouch), TG NS007292 (to B. Nickle), EY009514 (to D. Oprian), EY14850 (to Y. Koutalos), and EY01157 (to M.C. Cornwall). R.K. Crouch is a Research to Prevent Blindness Senior Scientific Investigator.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- DDM
- n-dodecyl-β-d-maltoside
- IRBP
- interphotoreceptor retinoid-binding protein
- MSP
- microspectrophotometry
- RPE
- retinal pigment epithelium
- sf
- stop flow
- WT
- wild type
References
- Ala-Laurila P., Donner K., Koskelainen A. 2004. Thermal activation and photoactivation of visual pigments. Biophys. J. 86:3653–3662 10.1529/biophysj.103.035626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P., Kolesnikov A.V., Crouch R.K., Tsina E., Shukolyukov S.A., Govardovskii V.I., Koutalos Y., Wiggert B., Estevez M.E., Cornwall M.C. 2006. Visual cycle: Dependence of retinol production and removal on photoproduct decay and cell morphology. J. Gen. Physiol. 128:153–169 10.1085/jgp.200609557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P., Donner K., Crouch R.K., Cornwall M.C. 2007. Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods. J. Physiol. 585:57–74 10.1113/jphysiol.2007.142935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky V.Y., Lamb T.D., Pugh E.N., Jr 2002. G proteins and phototransduction. Annu. Rev. Physiol. 64:153–187 10.1146/annurev.physiol.64.082701.102229 [DOI] [PubMed] [Google Scholar]
- Barlow H.B. 1957. Purkinje shift and retinal noise. Nature. 179:255–256 10.1038/179255b0 [DOI] [PubMed] [Google Scholar]
- Bayburt T.H., Sligar S.G. 2010. Membrane protein assembly into Nanodiscs. FEBS Lett. 584:1721–1727 10.1016/j.febslet.2009.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayburt T.H., Grinkova Y.V., Sligar S.G. 2002. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2:853–856 10.1021/nl025623k [DOI] [Google Scholar]
- Bayburt T.H., Leitz A.J., Xie G., Oprian D.D., Sligar S.G. 2007. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 282:14875–14881 10.1074/jbc.M701433200 [DOI] [PubMed] [Google Scholar]
- Baylor D.A. 1987. Photoreceptor signals and vision. Proctor lecture. Invest. Ophthalmol. Vis. Sci. 28:34–49 [PubMed] [Google Scholar]
- Baylor D.A., Matthews G., Yau K.W. 1980. Two components of electrical dark noise in toad retinal rod outer segments. J. Physiol. 309:591–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.F. 1994. Modulation of rhodopsin function by properties of the membrane bilayer. Chem. Phys. Lipids. 73:159–180 10.1016/0009-3084(94)90180-5 [DOI] [PubMed] [Google Scholar]
- Carslaw H.S., Jaeger J.C. 1959. Conduction of Heat in Solids. Second edition. Oxford University Press, Oxford, England, UK. 510 pp [Google Scholar]
- Cornwall M.C., Fain G.L. 1994. Bleached pigment activates transduction in isolated rods of the salamander retina. J. Physiol. 480:261–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., MacNichol E.F., Jr, Fein A. 1984. Absorptance and spectral sensitivity measurements of rod photoreceptors of the tiger salamander, Ambystoma tigrinum. Vision Res. 24:1651–1659 10.1016/0042-6989(84)90323-7 [DOI] [PubMed] [Google Scholar]
- Cornwall M.C., Jones G.J., Kefalov V.J., Fain G.L., Matthews H.R. 2000. Electrophysiological methods for measurement of activation of phototransduction by bleached visual pigment in salamander photoreceptors. Methods Enzymol. 316:224–252 10.1016/S0076-6879(00)16726-6 [DOI] [PubMed] [Google Scholar]
- Corson D.W., Cornwall M.C., MacNichol E.F., Mani V., Crouch R.K. 1990. Transduction noise induced by 4-hydroxy retinals in rod photoreceptors. Biophys. J. 57:109–115 10.1016/S0006-3495(90)82511-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crank J. 1975. The Mathematics of Diffusion. Second edition. Oxford University Press, Oxford, England, UK. 414 pp [Google Scholar]
- De Pont J.J., Daemen F.J., Bonting S.L. 1970. Biochemical aspects of the visual process. VII. Equilibrium conditions in the formation of retinylidene imines. Arch. Biochem. Biophys. 140:267–274 10.1016/0003-9861(70)90031-7 [DOI] [PubMed] [Google Scholar]
- Fain G.L., Matthews H.R., Cornwall M.C., Koutalos Y. 2001. Adaptation in vertebrate photoreceptors. Physiol. Rev. 81:117–151 [DOI] [PubMed] [Google Scholar]
- Franke R.R., Sakmar T.P., Oprian D.D., Khorana H.G. 1988. A single amino acid substitution in rhodopsin (lysine 248—leucine) prevents activation of transducin. J. Biol. Chem. 263:2119–2122 [PubMed] [Google Scholar]
- Futterman S., Hendrickson A., Bishop P.E., Rollins M.H., Vacano E. 1970. Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors. J. Neurochem. 17:149–156 10.1111/j.1471-4159.1970.tb02195.x [DOI] [PubMed] [Google Scholar]
- Gross A.K., Xie G., Oprian D.D. 2003. Slow binding of retinal to rhodopsin mutants G90D and T94D. Biochemistry. 42:2002–2008 10.1021/bi020612r [DOI] [PubMed] [Google Scholar]
- Hárosi F.I. 1975. Absorption spectra and linear dichroism of some amphibian photoreceptors. J. Gen. Physiol. 66:357–382 10.1085/jgp.66.3.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.J. 1995. Light adaptation and the rising phase of the flash photocurrent of salamander retinal rods. J. Physiol. 487:441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.J., Crouch R.K., Wiggert B., Cornwall M.C., Chader G.J. 1989. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc. Natl. Acad. Sci. USA. 86:9606–9610 10.1073/pnas.86.23.9606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.J., Fein A., MacNichol E.F., Jr, Cornwall M.C. 1993. Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation. J. Gen. Physiol. 102:483–502 10.1085/jgp.102.3.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.J., Cornwall M.C., Fain G.L. 1996. Equivalence of background and bleaching desensitization in isolated rod photoreceptors of the larval tiger salamander. J. Gen. Physiol. 108:333–340 10.1085/jgp.108.4.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T.D., Pugh E.N., Jr 1992. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449:719–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T.D., Pugh E.N., Jr 2004. Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 23:307–380 10.1016/j.preteyeres.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Liu X., Garriga P., Khorana H.G. 1996. Structure and function in rhodopsin: correct folding and misfolding in two point mutants in the intradiscal domain of rhodopsin identified in retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 93:4554–4559 10.1073/pnas.93.10.4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNichol E.F., Jr 1978. A photon-counting microspectrophotometer for the study of single vertebrate photoreceptor cells. In Frontiers of Visual Science. Cool S.J., Smith E.L., Springer-Verlag, New York: 194–208 [Google Scholar]
- Mariani A.P. 1986. Photoreceptors of the larval tiger salamander retina. Proc. R. Soc. Lond. B Biol. Sci. 227:483–492 10.1098/rspb.1986.0035 [DOI] [PubMed] [Google Scholar]
- Molday R.S., MacKenzie D. 1983. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry. 22:653–660 10.1021/bi00272a020 [DOI] [PubMed] [Google Scholar]
- Noy N., Xu Z.J. 1990. Kinetic parameters of the interactions of retinol with lipid bilayers. Biochemistry. 29:3883–3888 10.1021/bi00468a013 [DOI] [PubMed] [Google Scholar]
- Okajima T.I., Pepperberg D.R., Ripps H., Wiggert B., Chader G.J. 1990. Interphotoreceptor retinoid-binding protein promotes rhodopsin regeneration in toad photoreceptors. Proc. Natl. Acad. Sci. USA. 87:6907–6911 10.1073/pnas.87.17.6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K., Jäger S., Buczyłko J., Crouch R.K., Bredberg D.L., Hofmann K.P., Asson-Batres M.A., Saari J.C. 1994. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 33:13741–13750 10.1021/bi00250a027 [DOI] [PubMed] [Google Scholar]
- Papermaster D.S., Dreyer W.J. 1974. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 13:2438–2444 10.1021/bi00708a031 [DOI] [PubMed] [Google Scholar]
- Pepperberg D.R., Cornwall M.C., Kahlert M., Hofmann K.P., Jin J., Jones G.J., Ripps H. 1992. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis. Neurosci. 8:9–18 10.1017/S0952523800006441 [DOI] [PubMed] [Google Scholar]
- Pepperberg D.R., Jin J., Jones G.J. 1994. Modulation of transduction gain in light adaptation of retinal rods. Vis. Neurosci. 11:53–62 10.1017/S095252380001110X [DOI] [PubMed] [Google Scholar]
- Pugh E.N., Lamb T.D. 2000. Phototransduction in vertebrate rods and cones: Molecular mechanisms of amplification, recovery, and light adaptation. In Molecular Mechanisms in Visual Transduction. Stavenga D.G., DeGrip W.J., Pugh E.N., Elsevier Science, Amsterdam/New York: 183–255 [Google Scholar]
- Redmond T.M., Wiggert B., Robey F.A., Nguyen N.Y., Lewis M.S., Lee L., Chader G.J. 1985. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 24:787–793 10.1021/bi00324a038 [DOI] [PubMed] [Google Scholar]
- Reeves P.J., Callewaert N., Contreras R., Khorana H.G. 2002. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. USA. 99:13419–13424 10.1073/pnas.212519299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renk G., Grover T., Crouch R., Mao B., Ebrey T.G. 1981. A spin-labeled retinal pigment analogue of the purple membrane. Photochem. Photobiol. 33:489–494 10.1111/j.1751-1097.1981.tb05450.x [DOI] [Google Scholar]
- Shichida Y., Imai H. 1998. Visual pigment: G-protein-coupled receptor for light signals. Cell. Mol. Life Sci. 54:1299–1315 10.1007/s000180050256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfuss J., Xie G., Edwards P.C., Burghammer M., Oprian D.D., Schertler G.F. 2007. Crystal structure of a thermally stable rhodopsin mutant. J. Mol. Biol. 372:1179–1188 10.1016/j.jmb.2007.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfuss J., Edwards P.C., D’Antona A., Fransen M., Xie G., Oprian D.D., Schertler G.F. 2011. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature. 471:656–660 10.1038/nature09795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuts E.Z., Harosi F.I. 1991. Solubility of retinoids in water. Arch. Biochem. Biophys. 287:297–304 10.1016/0003-9861(91)90482-X [DOI] [PubMed] [Google Scholar]
- Wu Q., Chen C., Koutalos Y. 2006. All-trans retinol in rod photoreceptor outer segments moves unrestrictedly by passive diffusion. Biophys. J. 91:4678–4689 10.1529/biophysj.106.086728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G., Gross A.K., Oprian D.D. 2003. An opsin mutant with increased thermal stability. Biochemistry. 42:1995–2001 10.1021/bi020611z [DOI] [PubMed] [Google Scholar]