Abstract
Mitochondrial Ca2+ uptake is thought to provide an important signal to increase energy production to meet demand but, in excess, can also trigger cell death. The mechanisms defining the relationship between total Ca2+ uptake, changes in mitochondrial matrix free Ca2+, and the activation of the mitochondrial permeability transition pore (PTP) are not well understood. We quantitatively measure changes in [Ca2+]out and [Ca2+]mito during Ca2+ uptake in isolated cardiac mitochondria and identify two components of Ca2+ influx. [Ca2+]mito recordings revealed that the first, MCUmode1, required at least 1 µM Ru360 to be completely inhibited, and responded to small Ca2+ additions in the range of 0.1 to 2 µM with rapid and large changes in [Ca2+]mito. The second component, MCUmode2, was blocked by 100 nM Ru360 and was responsible for the bulk of total Ca2+ uptake for large Ca2+ additions in the range of 2 to 10 µM; however, it had little effect on steady-state [Ca2+]mito. MCUmode1 mediates changes in [Ca2+]mito of 10s of μM, even in the presence of 100 nM Ru360, indicating that there is a finite degree of Ca2+ buffering in the matrix associated with this pathway. In contrast, the much higher Ca2+ loads evoked by MCUmode2 activate a secondary dynamic Ca2+ buffering system consistent with calcium-phosphate complex formation. Increasing Pi potentiated [Ca2+]mito increases via MCUmode1 but suppressed [Ca2+]mito changes via MCUmode2. The results suggest that the role of MCUmode1 might be to modulate oxidative phosphorylation in response to intracellular Ca2+ signaling, whereas MCUmode2 and the dynamic high-capacity Ca2+ buffering system constitute a Ca2+ sink function. Interestingly, the trigger for PTP activation is unlikely to be [Ca2+]mito itself but rather a downstream byproduct of total mitochondrial Ca2+ loading.
INTRODUCTION
Ca2+ is the central signaling molecule for cardiac excitation-contraction coupling, and its uptake by mitochondria plays a fundamental role in the regulation of ATP production (McCormack et al., 1990; Demaurex and Distelhorst, 2003; Maack and O’Rourke, 2007; Szabadkai and Duchen, 2008). In addition, the extent of mitochondrial Ca2+ uptake during metabolic stress determines whether the myocyte lives or dies, owing to the activation of the permeability transition pore (PTP; Crompton, 1999). Although PTP activation is unequivocally linked to total mitochondrial Ca2+ load, it is presently unclear how much Ca2+ is free or bound in the mitochondrial matrix during PTP activation, as well as how much Ca2+ is required to maintain energy balance.
Mitochondrial matrix free Ca2+ ([Ca2+]mito) is determined by the balance between Ca2+ uptake, extrusion, and buffering. Ca2+ uptake is driven by the electrochemical Ca2+ gradient and is mediated by a mitochondrial Ca2+-selective uniporter (MCU; Gunter and Pfeiffer, 1990), a Ruthenium red (or its potent subcomponent, Ru360)–sensitive ion channel, whose selectivity is determined by nanomolar affinity Ca2+ binding to the pore, with an open probability that is voltage dependent (Kirichok et al., 2004). In single-channel mitoplast patch recordings of cardiac mitochondria, two different mitochondrial Ca2+ channels, mCU1 and mCU2, with different gating properties and Ru360 sensitivity, were observed (Michels et al., 2009). Two independent groups have recently reported that the molecular identity of the MCU pore is the protein product of the gene CCDC109A (Baughman et al., 2011; De Stefani et al., 2011). Knockdown of this gene suppresses the major component of mitochondrial Ca2+ uptake in cultured cells and in the liver (Baughman et al., 2011) and reconstitution of the protein forms Ca2+ channels in lipid bilayers (De Stefani et al., 2011). Nevertheless, in addition to the major component of MCU, other potential Ca2+ uptake pathways have been noted in mitochondria, including the rapid mode (RaM) of Ca2+ uptake (Sparagna et al., 1995; Buntinas et al., 2001) and the mitochondrial ryanodine receptor (mRyR; Beutner et al., 2001; Ryu et al., 2010). The relative importance of each Ca2+ uptake pathway, with respect to function, has not yet been determined.
Ca2+ extrusion in cardiac mitochondria is determined primarily by the mitochondrial Na+/Ca2+ exchanger (mNCE; Crompton et al., 1977). Its molecular identity is thought to be an ancestral member of the Na+/Ca2+ exchanger family, NCLX, a transporter capable of exchanging Ca2+ for either Na+ or Li+ (Palty et al., 2010). The mNCE plays an important role in modulating the steady-state balance between extra- and intramitochondrial Ca2+ in isolated mitochondria (Wei et al., 2011), and disruption of this equilibrium in conditions of cellular Na+ overload associated with cardiac disease can impact pyridine nucleotide redox and ROS balance in cardiac cells (Maack et al., 2006; Liu and O’Rourke, 2008; Kohlhaas et al., 2010). A minor component of mitochondrial Ca2+ efflux is also contributed by the mitochondrial PTP (Wei et al., 2011), which has been proposed to serve as a Ca2+ relief valve for mitochondria (Gunter and Pfeiffer, 1990). Disruption of this mechanism can have diverse functional consequences in vivo (Elrod et al., 2010).
The least well understood, but perhaps most important, determinant of [Ca2+]mito is the Ca2+ buffering system of the mitochondrial matrix, consisting of both fixed and dynamic buffering mechanisms. The rapid formation of complexes between Ca2+ and phosphate (Pi) in mitochondria, described almost 50 yr ago, has been shown to be of major importance in determining how much Ca2+ can be accumulated into mitochondria, as well as how much [Ca2+]mito changes in response to total Ca2+ taken up (Nicholls and Chalmers, 2004; Chinopoulos and Adam-Vizi, 2010). In fact, in the presence of Pi, but not acetate, as the accompanying permeant anion, mitochondria are capable of sequestering enormous amounts of total Ca2+ (>1 M), while maintaining [Ca2+]mito in the low micromolar range, as determined in isolated mitochondria loaded with matrix free Ca2+ indicators (Davis et al., 1987; Lukács and Kapus, 1987; Gunter et al., 1988; Lukács et al., 1988; Saavedra-Molina et al., 1990; Walajtys-Rhode et al., 1992; Chalmers and Nicholls, 2003). Interestingly, this range of [Ca2+]mito, <5 µM, appears to be the range over which key Ca2+-sensitive mitochondrial enzymes are regulated (Denton et al., 1980; McCormack et al., 1990; Walajtys-Rhode et al., 1992).
In cardiac mitochondria, although many studies have investigated rates of Ca2+ uptake, efflux, and PTP activation in isolated mitochondria and intact cells using various methods, the dynamics and quantitative determinants of changes in [Ca2+]mito are incompletely understood. Here, we use matrix-loaded fura-FF, a ratiometric Ca2+ indicator with an intermediate Ca2+-binding affinity, to quantitatively examine changes in [Ca2+]mito while simultaneously monitoring extramitochondrial Ca2+ and ΔΨm in isolated guinea pig heart mitochondria. The results indicate that two components of mitochondrial Ca2+ uptake are present: one which mediates a large, rapid change in [Ca2+]mito in response to small additions of extramitochondrial Ca2+ (MCUmode1), and another which mediates the stereotypical slow, lower affinity, Ca2+ uptake pathway (MCUmode2) which is capable of taking up large amounts of Ca2+ but leads to relatively small changes in [Ca2+]mito. Although MCUmode2 is completely blocked by 100 nM Ru360, MCUmode1 has a lower inhibitor affinity, requiring 1 µM Ru360 for complete inhibition. The differential responses of [Ca2+]mito to Ca2+ entry via the two different pathways can be explained by a two component buffer system comprised of both static Ca2+ buffers and dynamic Ca2+ buffering by Pi, which enters in parallel with large amplitude Ca2+ influx. The findings have important implications with respect to the physiological regulation of oxidative phosphorylation by Ca2+, as well as for the activation of the PTP, which, remarkably, is shown to be uncorrelated with [Ca2+]mito.
MATERIALS AND METHODS
Mitochondria were isolated from adult guinea pig hearts on ice using a homogenization method previously described (Aon et al., 2010). In brief, the heart tissue was homogenized in 75 mM sucrose, 225 mM mannitol, 1 mM HEPES, and 1 mM EGTA, pH 7.4, with 0.1 mg/ml bacterial proteinase type XXIV (Sigma-Aldrich). The homogenate was centrifuged at 480 g for 5 min at 4°C, and the supernatant was centrifuged at 7,700 g for 10 min. The mitochondrial pellet was washed twice at 7,700 g for 5 min and resuspended in sucrose-based isolation solution with 20 µM EGTA to ∼10–20 mg mitochondrial protein/ml (protein concentration was determined by BCA assay). During the experiments, ∼0.6 mg of isolated mitochondria was suspended in 2 ml potassium-based buffer solution composed of: 137 mM KCl, 20 µM EGTA, 20 mM HEPES, and 5 mM glutamate/malate (G/M), with or without 2 mM KH2PO4 at pH 7.15.
Multiple mitochondrial parameters, including mitochondrial inner membrane potential (ΔΨm) extra- and intramitochondrial Ca2+ concentrations, and mitochondrial light scattering, were simultaneously monitored in a stirred cuvette using a fluorometer (QuantaMaster; Photon Technology International) at room temperature. ΔΨm was monitored by the dual-excitation ratiometric method with the fluorescent probe tetramethylrhodamine methyl ester (TMRM; 300 nM) at excitations of 546 and 573 nm and emission at 590 nm (Scaduto and Grotyohann, 1999). Mitochondrial 90° light scattering was monitored at 540 nm excitation. The fluorescent Ca2+ indicator Calcium green 5N (CaGreen; Invitrogen) was used to monitor extramitochondrial Ca2+ ([Ca2+]out), with excitation and emission wavelengths at 505 and 535 nm. Quantitative measurements of [Ca2+]mito were made with the dual-excitation ratiometric Ca2+ indicator fura-FF, loaded into the isolated mitochondria as the fura-FF-AM form (20 µM for 30 min at room temperature; washed two to three times with sucrose-based isolation buffer). The fura-FF signal was calibrated in mitochondria treated with the Ca2+ ionophore 4-bromo-A23187 (2 µM; Deber et al., 1985; Nicholls and Chalmers, 2004) in the presence of 5 µg/ml oligomycin and 5 µM FCCP to allow equilibration between intra- and extramitochondrial calcium (Wan et al., 1989; Chalmers and Nicholls, 2003; Andrienko et al., 2009; Fig. 1). The calibration curve was established according to the following equation (Grynkiewicz et al., 1985):
where R is the ratio of 510-nm emission intensities for excitation at 340 and 380 nm. Kd′ is the apparent Ca-fura-FF dissociation constant, and β is the fluorescence intensity ratio for Ca2+-free and Ca2+-saturated fura-FF excited at 380 nm. Rmax and Rmin are R values for Ca2+-saturated and Ca2+-free fura-FF. The calibration was done for fura-FF–loaded mitochondria for each day’s experiment (example shown in Fig. 1). Ratios of background-uncorrected and background-corrected fura-FF fluorescence signal at 340 and 380 nm excitation were fitted to the Grynkiewicz equation (Fig. 1, B and C). Autofluorescence values, and their changes upon Ca2+ addition, were relatively small compared with the fura-FF fluorescence intensities (Fig. 1 A, teal and magenta traces) and [Ca2+]mito calculated from calibration curves with or without subtraction of autofluorescence showed no significant differences (Fig. 1 D). Therefore, autofluorescence corrections were not necessary, and were not made, in the subsequent experiments. The method for calibrating the extramitochondrial calcium signal (CaGreen) was as described previously (Wei et al., 2011).
Figure 1.
Calibration of fura-FF signals. (A) Fluorescence recordings of mitochondria in the presence or absence of fura-FF loading (340:510 nm ex:em and 380:510 nm FuraFF or control, respectively) along with [Ca2+]out (CaGreen: 505:535 nm ex:em). The Ca2+ signals were calibrated with multiple additions of 5 µM Ca2+ in the presence of the ionophore, 2 µM 4-bromo-A23187, 5 µg/ml oligomycin, and 5 µM FCCP. (B) The excitation ratio for fura-FF was calculated with (red) or without (black) correction for autofluorescence. (C) [Ca2+]mito was determined after fitting the calibration curve to the equation [Ca2+] = Kd′β (R − Rmin)/(Rmax − R). The values obtained were Rmax = 3.2, Rmin = 0.5, Kd′ = 10.7, β = 2.5 without autofluorescence correction (black); Rmax = 3.7, Rmin = 0.5, Kd′ = 12.7, β = 2.75 for corrected ratio(red). (D) [Ca2+]mito in intact mitochondria after multiple additions of Ca2+ (15.3 µM first addition, 5.7 µM for subsequent additions) show that autofluorescence correction had little effect on the calculated [Ca2+]mito.
In the Results and Figure Legends, Ca2+ additions to the cuvette are those calculated using the online version of WEBMAXC (http://maxchelator.stanford.edu/webmaxc/webmaxcE.htm). Taking into account the presence of 20 µM EGTA in the experimental solution, this corresponded to free Ca2+ changes in the cuvette of 0.011, 0.2, 0.55, 2, 5.7, and 15.3 µM for additions of 1, 10, 15, 20, 25, and 35 µM Ca2+, respectively. Because other low-affinity Ca2+ buffers in the solution, such as glutamate and malate, were not included in this calculation, these numbers tend to overestimate the free Ca2+ change for larger Ca2+ additions, explaining discrepancies in the direct measurements of initial [Ca2+]out using calibrated CaGreen signals. Mitochondrial matrix calcium buffering, i.e., the relationship between the total Ca2+ taken up by mitochondria and the change in [Ca2+]mito, was expressed as the ratio of bound/free Ca2+ and was examined in the presence of different anions, including Pi (0.1–10 mM), arsenate (Asi; 2 mM), or vanadate (Vi; 2 mM). Cyclosporin A (CsA; 1 µM) was used to shift the threshold for activation of the PTP to a higher Ca2+ range to study Ca2+ dynamics over a wide range of mitochondrial Ca2+ loads. The figures show representative responses for each experiment; however, every experiment was repeated at least three times with a similar result (see Fig. S1 for an example of the range of statistical variability of the response).
Online supplemental material
Fig. S1 shows the mean and standard error of the response of [Ca2+]mito to large Ca2+ additions (n = 4 experiments). Fig. S2 shows the lack of a ryanodine effect on mitochondrial Ca2+ for a single Ca2+ addition. Fig. S3 shows Na+/Ca2+ exchanger effects on mitochondrial Ca2+ with different concentrations of Na+ and in the presence of the inhibitor CGP37157. Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201210784/DC1.
RESULTS
In Fig. 2 (top), simultaneous recordings of [Ca2+]out (green traces), [Ca2+]mito (black traces), and ΔΨm (red traces) are shown superimposed for large (Fig. 2, A–D) or small (Fig. 2, E–H) Ca2+ additions to the cuvette (from a starting [Ca2+]out of ∼0.100 µM before the first addition). Immediately evident is the fact that two components of the mitochondrial Ca2+ response are present. For the series of larger Ca2+ additions in Fig. 2 A, although the first Ca2+ addition (15.3 µM) evoked a large increase in [Ca2+]mito to ∼50 µM, subsequent additions (5.7 µM each) caused a transient increase (amplitude 10–20 µM) in [Ca2+]mito, followed by a decline back to the same 50 µM steady-state matrix concentration. Moreover, after the sixth Ca2+ addition, this steady-state [Ca2+]mito begins to decrease after each pulse (to ∼20 µM), suggesting an increase in the matrix Ca2+ buffer capacity (changes in matrix volume, as assessed by light scattering, could not account for these changes in [Ca2+]mito, compare Fig. 8). ΔΨm depolarized by 5–10 mV with each Ca2+ addition and recovered back to 180 mV as extramitochondrial Ca2+ (and MCU flux, which follows the electrochemical gradient for Ca2+) returned to a low level. Comparing the change in matrix free Ca2+ (expressed as nmols/mg mitochondrial protein assuming 1 µl/mg mitochondrial volume; Fig. 2 B) to the total amount of Ca2+ taken up from the bath (ΣCa2+uptake; Fig. 2 C) allowed us to calculate the ratio of bound/free Ca2+ (Fig. 2 D), illustrating how the buffer capacity dramatically increases after the first Ca2+ addition. The initial rapid jump in [Ca2+]mito (MCUmode1), which reached completion well before the end of the major slow uptake phase of the uniporter (MCUmode2), prompted us to explore the [Ca2+]mito response to a much lower range of Ca2+ concentrations.
Figure 2.
[Ca2+]mito (black), [Ca2+]out (green), and ΔΨm (red) responses to large or small Ca2+ additions in isolated guinea pig heart mitochondria. (A–D) Ca2+ additions were as follows: 15.3 µM Ca2+ first addition, 5.7 µM for each subsequent addition. (E–H) Ca2+ additions were as follows: 0.2 µM Ca2+ first addition, 0.011 µM for each subsequent addition. Corresponding calculated values of free Ca2+mito expressed in nmol/mg, total ∑Ca2+ taken up, and the bound/free Ca2+ ratio are shown for large (A–D) or small (E–H) additions. [Ca2+]mito and [Ca2+]out were monitored with Calcium green and fura-FF simultaneously. Experimental solution was the standard KCl-based buffer with 5 mM NaCl, 5 mM G/M, 1 mM KH2PO4, 20 µM EGTA, and 1 µM CsA. 300 nM TMRM was used to monitor ΔΨm ratiometrically.
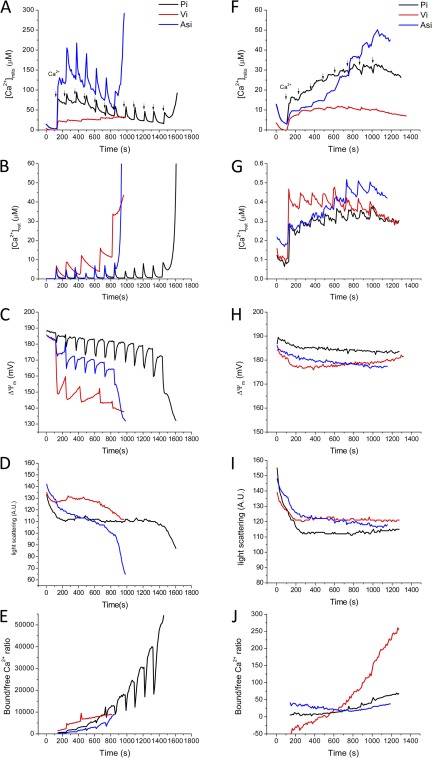
Figure 8.
Effects of Pi, (black), arsenate (Asi, blue), or vanadate (Vi, red) anions on the regulation of mitochondrial Ca2+. The buffer solution contained 2 mM Pi, Asi, or Vi in the presence of 1 µM of CsA. (A–E) Large Ca2+ additions (first Ca2+ addition was 15.3 µM, subsequent additions 5.7 µM each) were made until mitochondrial PTP opening occurred, as indicated by ΔΨm collapse (C), swelling (D), and uncontrolled Ca2+ efflux (A). (F–J) Smaller Ca2+ additions (first Ca2+ addition 0.2 µM, subsequent 0.011 µM each) evoked similar [Ca2+]mito responses without significantly disrupting ΔΨm or activating PTP.
Fig. 2 E shows that adding 0.2 µM Ca2+ first allows one to resolve the kinetics of the initial high-affinity Ca2+ uptake. [Ca2+]mito increased over the course of 100 s (as compared with the <10-s increase for the 15.3-µM addition in Fig. 2 A) and responded in a stepwise fashion for two subsequent additions of 0.01 µM each. Six more 0.01 µM Ca2+ additions evoked transient increases in [Ca2+]mito but little change in the steady-state [Ca2+]mito until the ninth addition, when the steady state actually began to decline. ΔΨm decreased by only 3 mV (188 to 185mV) after the first Ca2+ addition but did not change for subsequent additions (Fig. 2 E). Interestingly, despite the 100-fold lower amount of total Ca2+ loaded into the mitochondria in the small (Fig. 2 G) versus large (Fig. 2 C) Ca2+ addition experiments, the steady-state [Ca2+]mito, in both cases, settled into a range between 15 and 20 µM, demonstrating that matrix Ca2+ buffering dynamically responds over a very wide range. This is clearly evident when one compares the changes in matrix free Ca2+ (Fig. 2, B and F) with the total Ca2+ taken up by the mitochondria (Fig. 2, C and G), expressed as the ratio of bound/free Ca2+ (Fig. 2, D and H). This ratio spanned from ∼3 to 30,000 over the range of Ca2+ additions examined.
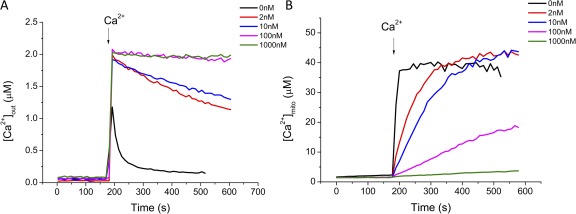
We next examined the properties of MCUmode1 in greater detail for an initial Ca2+ addition of 2 µM. CGP-37157, cyclosporin A, and thapsigargin were also included in the experimental solution to inhibit possible Ca2+ fluxes through the mNCE, PTP, and the sarcoplasmic reticular Ca2+ ATPase, respectively. Ru360 is a derivative of Ruthenium red that potently inhibits mitochondrial Ca2+ uptake with a KD of ∼1.3 nM (Ying et al., 1991). In the absence of Ru360, [Ca2+]mito (Fig. 3 B, black trace) increased rapidly after the Ca2+ addition and then reached a plateau, even though extramitochondrial Ca2+ decreased continuously over 50–100 s (Fig. 3 A, black trace). At Ru360 concentrations of 2 and 10 nM (Fig. 3 B, red and blue traces), the rate of rise of MCUmode1 was slowed but its plateau was unchanged. In contrast, MCUmode2 was markedly inhibited (the initial rate of decline of [Ca2+]out decreased by almost 95%) at 10 nM Ru360 (Fig. 3 A, blue trace) and was completely blocked by 100 nM Ru360 (Fig. 3 A, magenta trace). MCUmode1, in contrast, was only partially inhibited by 100 nM Ru360, and 1 µM Ru360 was required for complete inhibition (Fig. 3 B).
Figure 3.
Concentration dependence of Ru360 inhibition of mitochondrial Ca2+ uptake. Fura-FF–loaded mitochondria were preincubated with 0 (black), 2 (red), 10 (blue), 100 (magenta), or 1,000 nM (green) Ru360 in the standard KCl-based buffer with 2 mM KH2PO4, 5 mM G/M, 5 mM NaCl, 10 µM CGP37157, 1 µM CsA, and 2.5 µM thapsigargin. 2 µM free Ca2+ was added to initiate mitochondrial Ca2+ uptake and [Ca2+]out (A) and [Ca2+]mito (B) were monitored simultaneously.
The greater sensitivity of MCUmode2 to inhibition by Ru360 allowed us to investigate the Ca2+ dependence of MCUmode1 with MCUmode2 largely inhibited. Fig. 4 shows the [Ca2+]mito response to Ca2+ additions in the range of 0.2 to 5.7 µM Ca2+ in the absence (Fig. 4 A) and presence (Fig. 4 B) of 10 nM Ru360. With MCUmode2 almost completely inhibited by Ru360, as indicated by the near step-like changes in cuvette Ca2+ upon Ca2+ addition (Fig. 4 B, bottom), the rate of rise of MCUmode1 increased as a function of Ca2+ and the plateau level of [Ca2+]mito increased to ∼40 µM for additions of 2 and 5.7 µM (Fig. 4 B, top). Remarkably, although much more total Ca2+ uptake occurred with both MCUmode1 and MCUmode2 active in the absence of Ru360 (Fig. 4 A, bottom), the change in plateau [Ca2+]mito, presumably mediated only by MCUmode1, was very similar, reaching a plateau of ∼40 µM free Ca2+ (Fig. 4 A, top). The rate of rise of MCUmode1 was ∼10-fold faster in the absence of 10 nM Ru360; however, the Ca2+ dependence of the rate was similar. Steady-state ΔΨm was maintained at close to 185 mV during these experiments (unpublished data). The results indicate that MCUmode1 underlies the largest change in [Ca2+]mito after a Ca2+ addition to isolated cardiac mitochondria, whereas the large amount of Ca2+ taken up via MCUmode2 is almost entirely buffered.
Figure 4.
Calcium dependence of mitochondrial Ca2+ uptake in the absence (A) and presence (B) of 10 nM Ru360. [Ca2+]out and [Ca2+]mito were monitored during mitochondrial Ca2+ uptake initiated by Ca2+ additions ranging from 0.2 (black), to 0.55 (red), to 2 (blue), to 5.7 µM (green). 10 nM Ru360 largely eliminated MCUmode2 (see [Ca2+]out in bottom panels) but only partially inhibited MCUmode1 ([Ca2+]mito response in top panels). Standard KCl-based buffer with 2 mM KH2PO4, 5 mM G/M, 5 mM NaCl, 10 µM CGP, 1 µM CsA, and 2.5 µM thapsigargin.
The experiments shown in Fig. 5 were designed to test (1) whether the initial jump in [Ca2+]mito mediated by MCUmode1 could be reset and repeated if cuvette Ca2+ was lowered by EGTA addition, and (2) whether Ca2+ efflux from the compartment reported by fura-FF was mediated by the mNCE. Isolated mitochondria were first subjected to a single addition of Ca2+ (2 µM) to initiate mitochondrial Ca2+ uptake. EGTA was then added to decrease [Ca2+]out back to the nM range (∼100 nM) and a second Ca2+ addition was made (4 µM). In the absence of extramitochondrial Na+, [Ca2+]out declined with a time course similar to the experiments described in the previous paragraphs and [Ca2+]mito again showed a rapid increase via MCUmode1 to reach a plateau (Fig. 5 A). EGTA addition resulted in a rapid decrease in [Ca2+]out but [Ca2+]mito remained elevated (Fig. 5 A). A second Ca2+ addition effected a transient in [Ca2+]out similar to the first addition; however, instead of a stepwise increase in [Ca2+]mito the second pulse evoked a spike and decay response of [Ca2+]mito. Apparently, when [Ca2+]mito is already elevated, additional Ca2+ entering via MCU is buffered after a transient response. In contrast, in the presence of Na+, both [Ca2+]out and [Ca2+]mito decline after EGTA addition (Fig. 5 B). A second Ca2+ addition in this case evokes a [Ca2+]mito response similar to the first pulse, i.e., a rapid rise and plateau. Collectively, these results show that the rapid rise in [Ca2+]mito via MCUmode1 is best observed when [Ca2+]out is increased from the nanomolar to the micromolar range, and when the total mitochondrial Ca2+ load is low. Moreover, the Ca2+ compartment filled via MCUmode1 is emptied via mNCE, confirming that it is either the same compartment that is filled by MCUmode2, or it has Ca2+ efflux pathways identical to the latter.
Figure 5.
Resetting of [Ca2+]mito (black) for two Ca2+ additions. 2 µM Ca2+ was first added to initiate mitochondrial Ca2+ uptake. 25 µM EGTA was added to decrease the extramitochondrial calcium concentration ([Ca2+]out, green) to the submicromolar range, and another 4 µM Ca2+ was added to evoke a second [Ca2+]mito response. A decrease in [Ca2+]mito upon lowering [Ca2+]out occurred only in the presence of Na+. Conditions: (A) zero NaCl; (B) 15 mM NaCl; (C) 15 mM NaCl plus 100 nM Ru360; (D) 15 mM NaCl plus 2 mM MgATP.
The same two-pulse protocol evoked two increases in [Ca2+]mito that plateaued after ∼100 s with MCUmode2 completely blocked with 100 nM Ru360 (Fig. 5 C). This residual uptake was likely to be mediated by MCUmode1, whose kinetics were slowed by 100 nM Ru360, in accordance with the data shown in Fig. 3 B. Finally, the same protocol was applied in a more physiological condition in the presence of 2 mM MgATP (free Mg2+ was calculated to be 0.467 mM, which is close to that present in the cytoplasm). In the presence of MgATP, the rates of mitochondrial Ca2+ uptake via MCUmode1 and MCUmode2 were both decreased (Fig. 5 D), but the Na+-dependent Ca2+ efflux rate and qualitative responses to the sequential pulses were similar to those in the absence of MgATP (compare Fig. 5 B).
The slowing of the Ca2+ uptake rate with MgATP could be attributed to the increased Mg2+ present in the solution (Fig. 6). The physiological Mg2+ concentration in cytosol is ∼0.1–0.8 mM (Szanda et al., 2009) and Mg2+ is known to have inhibitory effects on mitochondrial Ca2+ uptake (Crompton et al., 1975; Bragadin et al., 1979). The effects of Mg2+ (from 0.1 to 1.0 mM) on mitochondrial Ca2+ uptake and [Ca2+]mito were compared for both small and large Ca2+ additions. The mitochondrial Ca2+ uptake rate and [Ca2+]mito were minimally affected for additions of 15 µM Ca2+ (Fig. 6, A–C); however, with smaller Ca2+ additions (first Ca2+ addition, 0.2 µM; subsequent, 0.011 µM each), Mg2+ slowed mitochondrial Ca2+ uptake (Fig. 6, D and E). Decreased uptake was indicated by higher [Ca2+]out and lower [Ca2+]mito in the presence of high Mg2+. The finding that Mg2+ inhibition can be overcome at high Ca2+ suggests that Mg2+ is competitive with respect to Ca2+ flux through the MCU.
Figure 6.
Effect of Mg2+ on the regulation of mitochondrial Ca2+. (A–C) Large Ca2+ additions (Ca2+ additions were 15.3 µM each) in the presence of zero (black), 0.1 (blue), or 1 mM (red) MgCl2. (D–F) Small Ca2+ additions (first Ca2+ addition 0.2 µM, subsequent 0.011 µM each) with 0 (black), 0.1 (green), 0.5 (blue), and 1 mM (red) MgCl2.
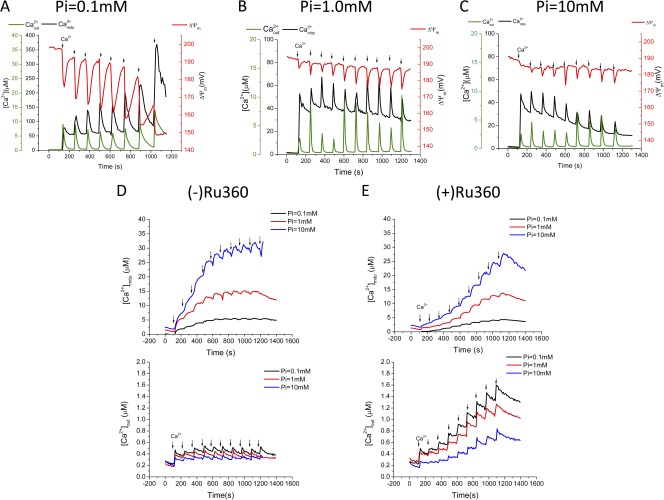
The rapid buffering of [Ca2+]mito during large increases in total mitochondrial Ca2+ evident in the preceding experiments prompted us to investigate the effects of changing the anion concentration on matrix Ca2+ dynamics. Because the formation of calcium phosphate complexes is thought to be the major mitochondrial Ca2+ buffering reaction (Nicholls and Chalmers, 2004), we examined the effects of varying Pi over the range of 0.1 to 10 mM for both large and small Ca2+ additions. In 0.1 mM Pi, the first large Ca2+ addition (15.3 µM) increased [Ca2+]mito immediately to ∼50 µM (Fig. 7 A; black trace), and subsequent Ca2+ additions (5.7 µM each) induced transients in [Ca2+]mito with increasing amplitudes of >100 µM. Nevertheless, [Ca2+]mito returned to a matrix setpoint of 50 µM after each transient. Ca2+ additions in the presence of 0.1 mM Pi evoked deeper and more prolonged depolarizations in ΔΨm (Fig. 7 A; red trace) than those observed for 1 mM (Fig. 7 B) or 10 mM (Fig. 7 C) Pi, increasing in amplitude from 20 to 40 mV with each subsequent addition. The overall rate of mitochondrial Ca2+ uptake was accelerated in 1 mM (Fig. 7 B) and 10 mM (Fig. 7 C) Pi, as compared with the rate in 0.1 mM Pi, and the rate of return of [Ca2+]mito to the matrix Ca2+ setpoint was accelerated. Moreover, the [Ca2+]mito setpoint achieved was lowered to 30 µM in 1 mM Pi and to 11 µM in 10 mM Pi by the end of the protocol. ΔΨm was well maintained in both 1 and 10 mM Pi (Fig. 7, B and C). Thus, the Ca2+ buffering action of Pi facilitates the regulation of [Ca2+]mito down to low levels in the face of very high mitochondrial Ca2+ loads and preserves the energy state of the mitochondria.
Figure 7.
Inorganic phosphate (Pi) dependence of mitochondrial Ca2+ uptake for large or small Ca2+ additions. Isolated mitochondria were incubated in 0.1 (A), 1 (B), or 10 (C) mM Pi with 5 mM NaCl, 5 mM G/M, and 1 µM CsA present. Ca2+ additions: First Ca2+ addition was 15.3 µM, subsequent additions 5.7 µM each (A–C); red line represents mitochondrial membrane potential ΔΨ (D–E). First Ca2+ addition 0.2 µM, subsequent 0.011 µM each. The black, red, blue lines represent 0.1, 1.0, and 10.0 mM Pi, respectively. (E) 10 nM Ru360-treated mitochondria.
For small Ca2+ additions (0.2 µM first, with subsequent additions of 0.011 µM each), Pi had a different effect on [Ca2+]mito (Fig. 7, D and E). In this case, increasing Pi from 0.1 to 1 to 10 mM markedly enhanced the rise in [Ca2+]mito associated with each Ca2+ addition and [Ca2+]mito was not regulated down to the low levels observed after larger Ca2+ additions. The enhanced Ca2+ uptake at high Pi was indirectly confirmed by the fact that [Ca2+]out was also observed to be lower in 10 mM Pi (Fig. 7 D, bottom). With MCUmode2 inhibited with 10 nM Ru360, the potentiating effect of Pi on mitochondrial Ca2+ uptake for the high-affinity uptake pathway was still present (Fig. 7 E). These results illustrate the complexity of the mitochondrial matrix Ca2+ buffering system, which depends on the amplitudes and rates of entry of both Ca2+ and Pi.
Finally, the effect of varying the chemical nature of the buffering anion was explored by comparing the effects of Pi with those of the Pi analogues, vanadate (Vi), or arsenate (Asi; Fig. 8). For large Ca2+ additions (Fig. 8, A–E), the most obvious effect of substituting Pi was that the total Ca2+ load at which PTP was triggered was markedly lower with Vi or Asi, as indicated by loss of the ability to retain matrix Ca2+ (Fig. 8, A and B), ΔΨm depolarization (Fig. 8 C), and rapid mitochondrial swelling (Fig. 8 D). Interestingly, this PTP sensitization could not be directly attributed to differences in [Ca2+]mito; Vi substitution lowered [Ca2+]mito and substantially decreased the rate of mitochondrial Ca2+ uptake, whereas Asi potentiated [Ca2+]mito without having a significant effect on the uptake rate. Matrix Ca2+ buffering, as indicated by the bound/free Ca2+ ratio (Fig. 8 E), was more effective for Vi and less effective for Asi for the first few Ca2+ additions, as compared with Pi, although just before the activation of PTP in Vi and Asi, bound/free Ca2+ was similar for all three anions. Striking differences in the extent of ΔΨm depolarization during Ca2+ uptake were evident when Pi was substituted with Asi or Vi (Fig. 8 C). Vi substitution had the largest effect, causing a ΔΨm depolarization of >30 mV after the first Ca2+ addition, whereas the depolarization was only 2 mV in Pi. With smaller Ca2+ additions, there was little effect of anion substitution on the ΔΨm (Fig. 8 H) or light scattering responses (Fig. 8 I), but just as for the larger Ca2+ pulses, [Ca2+]mito was lower in Vi.
DISCUSSION
In the present work, we used calibrated Ca2+-sensitive fluorescent indicators to quantitatively measure changes in [Ca2+]out and [Ca2+]mito (as well as ΔΨm and light scattering in some experiments) during mitochondrial Ca2+ uptake in isolated mitochondria. This method allowed us to identify two components of Ca2+ influx that have: (1) very different effects on matrix free [Ca2+], (2) different sensitivities to inhibition by Ru360, (3) different affinities for Ca2+, and (4) different responses to a change in Pi. MCUmode1 required at least 1 µM Ru360 to be completely inhibited, and responded to small Ca2+ additions in the range of 0.1 to 2 µM with rapid and large changes in [Ca2+]mito. MCUmode2 was blocked by <100 nM Ru360, was responsible for the bulk of total Ca2+ uptake for large Ca2+ additions in the range of 2 to 15 µM, and took >100 s to reach steady state. The finding that the MCUmode1 could mediate changes in [Ca2+]mito of 10 s of μM, even in the presence of 100 nM Ru360, indicates that at low total Ca2+ loads there is a finite degree of Ca2+ buffering in the matrix; however, at the much higher Ca2+ loads supported by MCUmode2, a secondary dynamic Ca2+ buffering system involving Pi is engaged. The results suggest that the role of MCUmode1 might be to modulate oxidative phosphorylation in response to intracellular Ca2+ signaling, whereas MCUmode2, and the dynamic high-capacity Ca2+ buffering system constitutes a Ca2+ sink function. Interestingly, the trigger for PTP activation is unlikely to be [Ca2+]mito itself but rather a downstream byproduct of matrix Ca2+ loading.
Several earlier studies have used AM loading of Ca2+ indicators in isolated mitochondria to qualitatively assess matrix Ca2+ responses (Davis et al., 1987; Lukács and Kapus, 1987; Gunter et al., 1988; Lukács et al., 1988). A key question that one must first answer before interpretations about matrix Ca2+ can be made is whether the dye is localized exclusively in the matrix, or if a certain fraction of the signal is coming from a nonmatrix compartment. Our evidence conclusively demonstrates that the fura-FF is reporting matrix Ca2+. Both the fast and slow components of the [Ca2+]mito signal can be completely blocked by Ru360, albeit with different sensitivities. In the case of MCUmode1 in particular, the kinetics were slowed by Ru360 in a concentration-dependent manner (Fig. 3 B). The initial rate of rise of MCUmode1 was approximately one order of magnitude slower in 100 nM Ru360 for a range of Ca2+ additions (Fig. 4). In addition, the depolarization of ΔΨm upon Ca2+ addition tracks the rapid component of the [Ca2+]mito response. This dissipation of the protonmotive force can occur only when Ca2+ current is flowing across the inner membrane. Fourth, when [Ca2+]out is lowered by EGTA addition to the cuvette, [Ca2+]mito remains high (Fig. 5 A) in the absence of Na+ because the mNCE is disabled, but it declines when mNCE is functional (Fig. 5 B). Thus, all of the evidence is consistent with matrix localization of the fura-FF.
Rapid effects of Ca2+ on mitochondrial function have been known since 1965 when Chance (1965) reported that Ca2+ was able to induce a “state 4 to state 3” respiratory transition in a manner similar to, but slightly faster than, ADP. Ca2+ addition (200 µM) by means of a rapid mixing device caused a cytochrome b oxidation response with a halftime of 70 ms. Territo et al. (2001) showed that the initial response time of mitochondrial Ca2+-stimulated respiration and light scattering changes is <300 ms for Ca2+ additions in the range of ∼60 to 2,000 nM and also reported that a 535-nM Ca2+ addition increased [Ca2+]mito by ∼700 nM (as measured using Rhod-2). Gunter and Pfeiffer (1990) and Sparagna et al. (1995) proposed that two pathways for mitochondrial Ca2+ uptake exist, a RaM which responds to fast transients of Ca2+ in the physiological range (100–500 nM) and the classical MCU which has a low affinity and slow kinetics. RaM-mediated uptake was complete in only 0.3 s and was inactivated when the prepulse steady-state Ca2+ exceeded 150 nM. Its amplitude could be fully reset after 1 min in <100 nM Ca2+ in cardiac mitochondria (Buntinas et al., 2001). Interestingly, RaM was found to be less sensitive to Ruthenium red than MCU (Sparagna et al., 1995; Buntinas et al., 2001). Hence, several aspects of MCUmode1 in the present study are similar to the properties of RaM, for example, a lower sensitivity to Ru360 and a higher Ca2+ affinity than MCUmode2. However, in the present study, MCUmode1 kinetics were slower to reach steady state. For example, in Fig. 2 E, there is a very rapid component of initial uptake for a Ca2+ addition of 200 nM, but [Ca2+]mito was still increasing 100 s after the addition and did not appear to be inactivated in the way that RaM would be when subsequent Ca2+ additions were made (Fig. 2 F).
Whether MCUmode1 and MCUmode2 are two modes of the same Ca2+ channel or are separate proteins remains to be determined. The recent discovery that knockdown of the product of the gene CCDC109A (renamed MCU) suppresses MCUmode2 (Baughman et al., 2011; De Stefani et al., 2011) might allow us to answer this question in future studies. Nevertheless, two Ca2+ channels (mCa1 and mCa2) with different gating and conductance properties have been observed recently in mitoplast patch clamp studies of cardiac mitochondria (Michels et al., 2009). MCa1 was inhibited by 200 nM Ru360, whereas mCa2, which had a lower conductance, required 10 µM Ru360 to be suppressed, providing some evidence to suggest that two independent Ca2+ channels with different Ru360 sensitivities exist in the inner membrane. The skeletal muscle isoform of the ryanodine receptor has also been proposed as an alternative mitochondrial Ca2+ uptake protein. We did not observe any effect of 100 µM ryanodine on Ca2+ uptake kinetics in our experiments (Fig. S2).
The remarkable finding of the present study was that small amounts of Ca2+ uptake lead to large changes in [Ca2+]mito, whereas large Ca2+ additions give only a transient increase in [Ca2+]mito with no change or an even a lower steady-state Ca2+. Two possible explanations could account for the paradoxical behavior of [Ca2+]mito; either the Ca2+ efflux rate from the mitochondria increases to match the Ca2+ entering for large Ca2+ loads, or Ca2+ buffering in the matrix is markedly increased in parallel with Ca2+ entry. The former explanation can be ruled out because we simultaneously measured [Ca2+]out, which confirmed that the Ca2+ taken up was retained inside the mitochondria. Under zero Na+ conditions, or in the presence of CGP-37157, an inhibitor of mNCE, [Ca2+]mito, was minimally affected (Fig. S3), although there was an effect of Na+ on the extramitochondrial Ca2+ steady-state concentration, as we have previously described (Wei et al., 2011). The only remaining explanation is that above a certain threshold of Ca2+ uptake, the Ca2+ buffer capacity increases rapidly to counteract MCUmode2-mediated uptake. This behavior is similar to that reported by Chalmers and Nicholls (2003) for rat liver and brain mitochondria. They noted that, over a wide range of mitochondrial Ca2+ loads (from 10 to 500 nmol/mg), mitochondria were capable of maintaining [Ca2+]mito within a narrow range between 1 and 5 µM, whereas <10 nmol/mg [Ca2+]mito was more linearly related to the magnitude of the uptake. Because the dynamic buffering occurred in the presence of Pi, but not when acetate was the anion, they interpreted the buffering mechanism to be related to the formation of calcium-phosphate complexes, in accordance with other studies (David, 1999; Nicholls, 2005; Warashina, 2006). Our data are consistent with this interpretation but differ in the quantitative details. For small Ca2+ additions, [Ca2+]mito increased in a stepwise manner from a baseline of ∼2.5 µM up to ∼25 µM before the secondary buffering took effect and a plateau was reached (Fig. 2 E). This corresponded to an increase in calcium-phosphate buffering occurring after a total Ca2+ load of 0.5 nmol/mg (Fig. 2 G). This threshold is likely to vary depending on how the Ca2+ addition is made. For example, the larger additions evoked only a single rapid jump in [Ca2+]mito to ∼60 µM (Fig. 2 A) at a total load of 25 nmol/mg, followed by more and more efficient buffering up to 100 nmol/mg (Fig. 2 C). The bound/free Ca2+ ratio increased continuously to a level >30,000 as ΣCa2+uptake increased. Increasing Pi over a range from 0.1 to 10 mM did, in fact, result in a lower steady-state [Ca2+]mito for large Ca2+ additions (Fig. 7, A–C). In addition, at 0.1 mM Pi, the rate of Ca2+ uptake through MCUmode2 was markedly depressed, ΔΨm depolarization was deeper and slower to recover, and [Ca2+]mito rose dramatically for the later Ca2+ additions. The slower kinetics of MCUmode2 in 0.1 mM Pi could be related to a decreased rate of calcium-phosphate formation, which would limit the influx rate by decreasing the electrochemical driving force for Ca2+ entry, or it could be a result of the Ca2+ influx rate being limited by the slow kinetics of the Pi transport process because anion flux would be required to maintain electroneutrality during movement of the divalent cation. In contrast to the Pi effect on large Ca2+ additions, Pi enhanced the rise in [Ca2+]mito with each small Ca2+ addition. This was the result of enhanced total Ca2+ uptake rather than a change in matrix buffering, as indicated by lower [Ca2+]out at high Pi (Fig. 7, D and E). Facilitation of MCUmode1 by Pi was even present when MCUmode2 was blocked by Ru360.
Substitution of Pi with Asi or Vi had striking effects on [Ca2+]mito and the threshold for PTP activation. Interestingly, Asi and Vi had opposite effects on [Ca2+]mito for multiple pulses but they both sensitized the mitochondria to PTP activation. Although Asi appeared to substitute reasonably well for Pi for the first six Ca2+ additions with respect to the overall kinetics of MCUmode2, Vi substitution slowed Ca2+ uptake and inhibited the rise in [Ca2+]mito. Even though the total Ca2+ uptake was lower in Vi, the PTP activation threshold was the lowest of the three anions. In accord with the conclusions of Chalmers and Nicholls (2003), PTP activation was, in all cases, independent of [Ca2+]mito. Therefore, an alternative hypothesis is required to explain why the PTP is activated at a reproducible total mitochondrial Ca2+ load. One possible mechanism could be related to the formation of Ca2+-phosphate species or Ca2+-polyphosphate complexes (Pavlov et al., 2005; Abramov et al., 2007). If this is the case, both Asi and Vi appear to be adequate (or better) substitutes for Pi in terms of triggering PTP. Recently, Basso et al. (2008) reported that the desensitizing effect of CsA on PTP activation by Ca2+ is absent when Asi or Vi are substituted for Pi. Our results confirm this finding and argue against the potential explanation that differences in Ca2+ buffering and [Ca2+]mito could be behind the Asi and Vi effects.
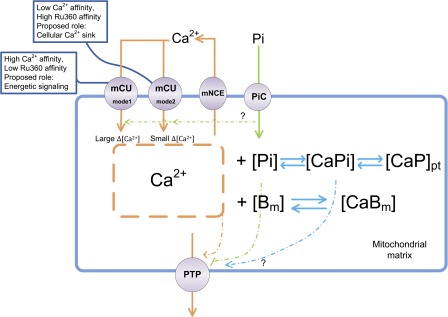
Based on the experimental observations, we propose that mechanisms of mitochondrial Ca2+ uptake and matrix Ca2+ buffering involve two stages (summarized in Fig. 9): (1) Ca2+ uptake mediated by MCUmode1 for extramitochondrial Ca2+ changes in the physiological range of cytoplasmic Ca2+ that lead to a rise in [Ca2+]mito as the fixed Ca2+ buffers become saturated, and (2) Ca2+ uptake mediated by MCUmode2 that is accompanied by rapid buffering via calcium-phosphate complex formation with little change in [Ca2+]mito. Importantly, the MCUmode1 pathway would be accelerated by nearby Ca2+ release into a local extramitochondrial microdomain (Hajnóczky et al., 2000), explaining observations of rapidly rising mitochondrial Ca2+ transients during cardiac excitation-contraction coupling (Maack et al., 2006). The magnitude of changes in [Ca2+]mito brought about by MCUmode1 would fulfill the role of a signal to increase oxidative phosphorylation and ATP production (Bell et al., 2006), as they span the range of activation of the Ca2+ dependent dehydrogenases, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and isocitrate dehydrogenase (McCormack et al., 1990), and/or Ca2+-dependent allosteric regulatory sites on the respiratory chain complexes (Territo et al., 2000). In contrast, MCUmode2, along with the rapid dynamic Ca2+ buffering mechanism, fulfills the role of a protective Ca2+ sink for the cell, enabling the cell to survive in the presence of rather large total Ca2+ loads, for example, those that might be encountered in the heart during ischemia-reperfusion, tachycardia, or maximal sympathetic stimulation. In this scenario, the major mitochondrial Ca2+ efflux pathway, mNCE, would serve as a modulator of the balance between cytoplasmic Ca2+ and matrix Ca2+ because its capacity to counteract large MCU fluxes is limited (and would tend to cause cytoplasmic Ca2+ overload if its Vmax was fast enough to match MCU-mediated influx). PTP activation can be clearly dissociated from [Ca2+]mito overload by itself but does correlate well with a reproducible threshold of total mitochondrial Ca2+ loading. This suggests that a downstream byproduct of Ca2+, perhaps a Ca2+-phosphate species itself, triggers permeabilization of the inner membrane. How cyclophilin d modulates this trigger remains a mystery because it seems that substitution of Pi with Asi or Vi bypasses the inhibition by cyclosporin A.
Figure 9.
Summary of the mechanisms governing mitochondrial Ca2+ dynamics. Mitochondrial Ca2+ uptake occurs through MCUmode1 with a high Ca2+ affinity, but low Ru360 sensitivity, whereas the bulk of mitochondrial Ca2+ loading occurs through the low Ca2+ affinity, high Ru360-sensitive MCUmode2. Ca2+ extrusion occurs through the mNCE. Rapid Ca2+ entry via MCUmode1 saturates fixed mitochondrial Ca2+ buffers (Bm) to produce a large change in [Ca2+]mito, whereas larger amounts of Ca2+ entry, along with Pi uptake, result in reversible precipitation of calcium-phosphate (CaPpt) with little effect on steady-state [Ca2+]mito. Pi is transported into mitochondria through the phosphate carrier (PiC). Mitochondrial matrix contents are released when the mitochondrial PTP opens. PTP opening correlates with total mitochondrial Ca2+ load but not [Ca2+]mito, perhaps through the formation of calcium-phosphate complexes.
In conclusion, understanding mitochondrial Ca2+ dynamics requires quantitative assessment of not only Ca2+ influx and efflux rates across the mitochondria, but rates of matrix Ca2+ buffering at different Ca2+ loads and rates of entry. The future development of computational models that incorporate all of these factors will help us understand these interesting features that are vitally important for Ca2+ homeostasis, cell death, and cardiac disease.
Acknowledgments
This work was supported by National Institutes of Health grants R01HL101235 and R37HL54598 (to B. O’Rourke) and R01HL105216 (to R.L. Winslow).
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- MCU
- mitochondrial Ca2+-selective uniporter
- mNCE
- mitochondrial Na+/Ca2+ exchanger
- PTP
- permeability transition pore
- RaM
- rapid mode
References
- Abramov A.Y., Fraley C., Diao C.T., Winkfein R., Colicos M.A., Duchen M.R., French R.J., Pavlov E. 2007. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc. Natl. Acad. Sci. USA. 104:18091–18096 10.1073/pnas.0708959104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrienko T.N., Picht E., Bers D.M. 2009. Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes. J. Mol. Cell. Cardiol. 46:1027–1036 10.1016/j.yjmcc.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon M.A., Cortassa S., Wei A.C., Grunnet M., O’Rourke B. 2010. Energetic performance is improved by specific activation of K+ fluxes through K(Ca) channels in heart mitochondria. Biochim. Biophys. Acta. 1797:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso E., Petronilli V., Forte M.A., Bernardi P. 2008. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 283:26307–26311 10.1074/jbc.C800132200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 476:341–345 10.1038/nature10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.J., Bright N.A., Rutter G.A., Griffiths E.J. 2006. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J. Biol. Chem. 281:28058–28067 10.1074/jbc.M604540200 [DOI] [PubMed] [Google Scholar]
- Beutner G., Sharma V.K., Giovannucci D.R., Yule D.I., Sheu S.S. 2001. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 276:21482–21488 10.1074/jbc.M101486200 [DOI] [PubMed] [Google Scholar]
- Bragadin M., Pozzan T., Azzone G.F. 1979. Kinetics of Ca2+ carrier in rat liver mitochondria. Biochemistry. 18:5972–5978 10.1021/bi00593a033 [DOI] [PubMed] [Google Scholar]
- Buntinas L., Gunter K.K., Sparagna G.C., Gunter T.E. 2001. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim. Biophys. Acta. 1504:248–261 10.1016/S0005-2728(00)00254-1 [DOI] [PubMed] [Google Scholar]
- Chalmers S., Nicholls D.G. 2003. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 278:19062–19070 10.1074/jbc.M212661200 [DOI] [PubMed] [Google Scholar]
- Chance B. 1965. The energy-linked reaction of calcium with mitochondria. J. Biol. Chem. 240:2729–2748 [PubMed] [Google Scholar]
- Chinopoulos C., Adam-Vizi V. 2010. Mitochondrial Ca2+ sequestration and precipitation revisited. FEBS J. 277:3637–3651 10.1111/j.1742-4658.2010.07755.x [DOI] [PubMed] [Google Scholar]
- Crompton M. 1999. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341:233–249 10.1042/0264-6021:3410233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Palmieri F., Capano M., Quagliariello E. 1975. A kinetic study of sulphate transport in rat liver mitochondria. Biochem. J. 146:667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Künzi M., Carafoli E. 1977. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur. J. Biochem. 79:549–558 10.1111/j.1432-1033.1977.tb11839.x [DOI] [PubMed] [Google Scholar]
- David G. 1999. Mitochondrial clearance of cytosolic Ca(2+) in stimulated lizard motor nerve terminals proceeds without progressive elevation of mitochondrial matrix [Ca(2+)]. J. Neurosci. 19:7495–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H., Altschuld R.A., Jung D.W., Brierley G.P. 1987. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem. Biophys. Res. Commun. 149:40–45 10.1016/0006-291X(87)91602-0 [DOI] [PubMed] [Google Scholar]
- De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 476:336–340 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deber C.M., Tom-Kun J., Mack E., Grinstein S. 1985. Bromo-A23187: a nonfluorescent calcium ionophore for use with fluorescent probes. Anal. Biochem. 146:349–352 10.1016/0003-2697(85)90550-0 [DOI] [PubMed] [Google Scholar]
- Demaurex N., Distelhorst C. 2003. Cell biology. Apoptosis—the calcium connection. Science. 300:65–67 10.1126/science.1083628 [DOI] [PubMed] [Google Scholar]
- Denton R.M., McCormack J.G., Edgell N.J. 1980. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem. J. 190:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod J.W., Wong R., Mishra S., Vagnozzi R.J., Sakthievel B., Goonasekera S.A., Karch J., Gabel S., Farber J., Force T., et al. 2010. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 120:3680–3687 10.1172/JCI43171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450 [PubMed] [Google Scholar]
- Gunter T.E., Pfeiffer D.R. 1990. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258:C755–C786 [DOI] [PubMed] [Google Scholar]
- Gunter T.E., Restrepo D., Gunter K.K. 1988. Conversion of esterified fura-2 and indo-1 to Ca2+-sensitive forms by mitochondria. Am. J. Physiol. 255:C304–C310 [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Csordás G., Madesh M., Pacher P. 2000. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J. Physiol. 529:69–81 10.1111/j.1469-7793.2000.00069.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y., Krapivinsky G., Clapham D.E. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 427:360–364 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Kohlhaas M., Liu T., Knopp A., Zeller T., Ong M.F., Böhm M., O’Rourke B., Maack C. 2010. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 121:1606–1613 10.1161/CIRCULATIONAHA.109.914911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., O’Rourke B. 2008. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ. Res. 103:279–288 10.1161/CIRCRESAHA.108.175919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukács G.L., Kapus A. 1987. Measurement of the matrix free Ca2+ concentration in heart mitochondria by entrapped fura-2 and quin2. Biochem. J. 248:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukács G.L., Kapus A., Fonyó A. 1988. Parallel measurement of oxoglutarate dehydrogenase activity and matrix free Ca2+ in fura-2-loaded heart mitochondria. FEBS Lett. 229:219–223 10.1016/0014-5793(88)80831-7 [DOI] [PubMed] [Google Scholar]
- Maack C., O’Rourke B. 2007. Excitation-contraction coupling and mitochondrial energetics. Basic Res. Cardiol. 102:369–392 10.1007/s00395-007-0666-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack C., Cortassa S., Aon M.A., Ganesan A.N., Liu T., O’Rourke B. 2006. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ. Res. 99:172–182 10.1161/01.RES.0000232546.92777.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J.G., Halestrap A.P., Denton R.M. 1990. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70:391–425 [DOI] [PubMed] [Google Scholar]
- Michels G., Khan I.F., Endres-Becker J., Rottlaender D., Herzig S., Ruhparwar A., Wahlers T., Hoppe U.C. 2009. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 119:2435–2443 10.1161/CIRCULATIONAHA.108.835389 [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. 2005. Mitochondria and calcium signaling. Cell Calcium. 38:311–317 10.1016/j.ceca.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Nicholls D.G., Chalmers S. 2004. The integration of mitochondrial calcium transport and storage. J. Bioenerg. Biomembr. 36:277–281 10.1023/B:JOBB.0000041753.52832.f3 [DOI] [PubMed] [Google Scholar]
- Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., et al. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 107:436–441 10.1073/pnas.0908099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov E., Zakharian E., Bladen C., Diao C.T., Grimbly C., Reusch R.N., French R.J. 2005. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys. J. 88:2614–2625 10.1529/biophysj.104.057281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S.Y., Beutner G., Dirksen R.T., Kinnally K.W., Sheu S.S. 2010. Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 584:1948–1955 10.1016/j.febslet.2010.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Molina A., Uribe S., Devlin T.M. 1990. Control of mitochondrial matrix calcium: studies using fluo-3 as a fluorescent calcium indicator. Biochem. Biophys. Res. Commun. 167:148–153 10.1016/0006-291X(90)91743-C [DOI] [PubMed] [Google Scholar]
- Scaduto R.C., Jr, Grotyohann L.W. 1999. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 76:469–477 10.1016/S0006-3495(99)77214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparagna G.C., Gunter K.K., Sheu S.S., Gunter T.E. 1995. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J. Biol. Chem. 270:27510–27515 10.1074/jbc.270.46.27510 [DOI] [PubMed] [Google Scholar]
- Szabadkai G., Duchen M.R. 2008. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda). 23:84–94 10.1152/physiol.00046.2007 [DOI] [PubMed] [Google Scholar]
- Szanda G., Rajki A., Gallego-Sandín S., Garcia-Sancho J., Spät A. 2009. Effect of cytosolic Mg2+ on mitochondrial Ca2+ signaling. Pflugers Arch. 457:941–954 10.1007/s00424-008-0551-0 [DOI] [PubMed] [Google Scholar]
- Territo P.R., Mootha V.K., French S.A., Balaban R.S. 2000. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 278:C423–C435 [DOI] [PubMed] [Google Scholar]
- Territo P.R., French S.A., Dunleavy M.C., Evans F.J., Balaban R.S. 2001. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J. Biol. Chem. 276:2586–2599 10.1074/jbc.M002923200 [DOI] [PubMed] [Google Scholar]
- Walajtys-Rhode E., Zapatero J., Moehren G., Hoek J.B. 1992. The role of the matrix calcium level in the enhancement of mitochondrial pyruvate carboxylation by glucagon pretreatment. J. Biol. Chem. 267:370–379 [PubMed] [Google Scholar]
- Wan B., LaNoue K.F., Cheung J.Y., Scaduto R.C., Jr 1989. Regulation of citric acid cycle by calcium. J. Biol. Chem. 264:13430–13439 [PubMed] [Google Scholar]
- Warashina A. 2006. Mode of mitochondrial Ca2+ clearance and its influence on secretory responses in stimulated chromaffin cells. Cell Calcium. 39:35–46 10.1016/j.ceca.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Wei A.C., Liu T., Cortassa S., Winslow R.L., O’Rourke B. 2011. Mitochondrial Ca2+ influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporine A. Biochim. Biophys. Acta. 1813:1373–1381 10.1016/j.bbamcr.2011.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W.L., Emerson J., Clarke M.J., Sanadi D.R. 1991. Inhibition of mitochondrial calcium ion transport by an oxo-bridged dinuclear ruthenium ammine complex. Biochemistry. 30:4949–4952 10.1021/bi00234a016 [DOI] [PubMed] [Google Scholar]