In typical cultured animal cells, mitochondria exist in shapes ranging from long interconnected tubules, often clustered in the perinuclear region of the cell, to small spherical forms in the cell periphery. However, mitochondrial appearance at any given moment in time is only a snapshot of dynamic change of morphology, which may or may not be the same in future observations dependent on the stimuli/stresses encountered. Fission and fusion are the main processes for changing mitochondrial morphology, governed by dynamin-related large GTPases: DLP1/Drp1 for fission, and Mfn1/2 and OPA1 for fusion of the outer mitochondrial membrane and inner membrane, respectively. The requirement for energy in these processes along with their requisite roles in cell life and death through mitophagy and apoptosis emphasizes the critical function of mitochondrial fission and fusion. Indeed, pathological conditions such as the hereditary neuropathies Charcot–Marie–Tooth type 2A and optic atrophy type 1 arise from mutations in Mfn2 and OPA1, respectively (Alexander et al., 2000; Delettre et al., 2000; Züchner et al., 2004). Metabolic disease pathologies are also associated with progressive mitochondrial dysfunction and alterations in mitochondrial morphology. These pathologies include but are not limited to diabetic tissue damage, such as diabetic cardiomyopathy (DCM) and the commonly associated hepatic pathology of nonalcoholic fatty liver disease (NAFLD). This article discusses the current knowledge connecting mitochondrial form and function in relation to metabolic disease, highlighting a plausible mechanistic linkage of mitochondrial morphology and metabolic disease.
Bioenergetics and mitochondrial morphology
Mitochondria are responsible for the bulk of cellular ATP production through oxidative phosphorylation (OXPHOS). ATP production is driven by the proton motive force, the result of electron transport chain (ETC) components pumping out protons across the inner membrane. The rate of ATP production is greatly dependent on the inner mitochondrial membrane potential (ΔΨm) in coupled respiration. Therefore, strict coupling and maintenance of ΔΨm are necessary for efficient energy production through OXPHOS. Interplay between mitochondrial bioenergetics and morphology has been suspected since observations in the late 1960s by Hackenbrock (1966) noting that coupled ATP synthesis correlated with a condensed internal appearance in isolated mitochondria. In cultured cells, a change in mitochondrial morphology toward fragmentation was observed upon inhibition of complex I with rotenone (Benard et al., 2007). The influence of respiratory function on morphology was striking, as mitochondrial morphology was restored with recovery of complex I deficiency through genetic complementation (Koopman et al., 2005). Similar findings were reported with samples from both complex I and multi-complex–deficient patients (Benard et al., 2007). These observations indicate that changes in functional states of mitochondria lead to morphological alterations. This relationship turned out to be reciprocal, as inhibition of mitochondrial fission also resulted in deficiencies in respiration and ATP production (Parone et al., 2008). However, the mechanism of how mitochondrial function and morphology reciprocally influence each other remains ill defined.
Similarly, changes in the abundance of mitochondrial fusion proteins are associated with altered mitochondrial function (Bach et al., 2003; Olichon et al., 2003; Chen et al., 2005; Pich et al., 2005). The expression of Mfn2 is decreased in obesity, coincident with the suppressed oxidation of glucose and fatty acid, reduced membrane potential, and diminished expression of nuclear-encoded ETC components. Exogenous expression of full-length as well as truncated Mfn2 protein, lacking its transmembrane domain, reversed the phenotype and recovered membrane potential, suggesting that Mfn2 functions in an additional capacity in bioenergetics aside from the mitochondrial fusion activity. Silencing OPA1 also results in a loss of membrane potential, accompanied by the complete ablation of mitochondrial fusion, similar to observations in the double knockout of Mfn1 and Mfn2. However, the loss of respiratory capacity upon OPA1 silencing is more severe than that observed in Mfn double knockout cells, albeit with similar fusion deficiencies (Chen et al., 2005). Interestingly, overexpression of OPA1 maintains membrane potential and respiratory capacity despite inducing mitochondrial fragmentation, suggesting that the fragmented morphology per se is not the cause of mitochondrial dysfunction. Adding complexity to this relationship, different treatments that increase, decrease, or completely dissipate ΔΨm all result in similarly fragmented mitochondria (Gilkerson et al., 2000; Legros et al., 2002; Ishihara et al., 2003; Lyamzaev et al., 2004; De Vos et al., 2005). This raises questions as to whether a correlation between a functional state and mitochondrial morphology is a simple one-to-one relation and what underlying mechanisms make comparable morphological changes under differing energetic conditions and vice versa.
Mitochondrial morphology in cell maintenance
Mitochondrial function is essential for bioenergetic and redox homeostasis, calcium regulation, and ultimately cell life and death. These functions are not mutually exclusive, and an influence of mitochondrial morphology is observed within these processes.
Mitochondrial quality control and morphology.
A mechanistic connection between mitochondrial morphology control and bioenergetics was first observed in the requirement of the membrane potential for mitochondrial fusion (Legros et al., 2002; Ishihara et al., 2003, 2006). The loss of membrane potential with carbonyl cyanide m-chlorophenyl hydrazone treatment promotes OPA1 cleavage, which blocks fusion, resulting in fragmented mitochondria. Inhibited fusion was fully reversible upon carbonyl cyanide m-chlorophenyl hydrazone washout and membrane potential recovery followed by new expression of OPA1. In contrast to mitochondrial fusion that requires the proper membrane potential, mitochondrial fission results in depolarization in one of the daughter mitochondria during the majority of fission events in pancreatic β cells (Twig et al., 2008). As mentioned, depolarization would inhibit the ability of these mitochondria to fuse and, as such, these are selectively targeted for removal through mitophagy (Twig et al., 2008). This selective fusion of energetically active mitochondria along with the segregation of depolarized mitochondria through fission connects the mitochondrial shape control to mitophagy, constituting a mitochondrial quality–control mechanism. Twig et al. (2008) observed fusion–fission-paired occurrences in which the fusion event is followed by fission and mitochondria remain mostly in the post-fission state. Mitochondrial fusion is required for the exchange of mitochondrial components, and presumably fission segregates damaged components through a yet to be defined mechanism. In this context, complete inhibition of mitochondrial fission is detrimental to properly functioning mitochondria, causing the accumulation of oxidative damage (Twig et al., 2008). Therefore, misregulation of not only mitophagy but also fission/fusion could in turn allow for the enhanced production of toxic reactive oxygen species (ROS) from damaged mitochondria.
Two recent studies reported a different aspect of mitochondrial morphology in autophagic degradation of mitochondria (Gomes et al., 2011; Rambold et al., 2011). In these studies, mitochondria were shown to become elongated under nutrient starvation through Drp1 phosphorylation/dephosphorylation. Elongated mitochondria during starvation are spared from mitophagy and continue to produce ATP by enhancing ATP synthase oligomerization and cristae density, serving as a protective mechanism. Conversely, in fusion-deficient cells, fragmented mitochondria persist even in starvation and deplete cellular energy, leading to starvation-driven cell death (Gomes et al., 2011). These results are congruent with the prior study identifying stress-induced mitochondrial hyperfusion (Tondera et al., 2009). The interconnected networks of mitochondria induced by a variety of stress stimuli increased ATP production. These observations demonstrate that changing mitochondrial morphology through fission and fusion can affect mitochondrial function. In this manner, mitochondrial morphogenesis is intimately associated with adaptations to promote cell survival in response to extracellular stimuli. Disruption of this fine balance would then result in deleterious consequences for cell maintenance and survival.
Mitochondrial fission/fusion and apoptosis.
Tissue and organ maintenance requires elimination of dysfunctional cells through apoptosis. By retaining and releasing death regulatory factors, mitochondria are central to regulating the progression of intrinsic apoptosis. Mitochondrial fragmentation is prevalent in apoptotic cell death and precedes caspase activation. In fact, mitochondrial morphology appears to play a role in apoptosis, as suppressing mitochondrial fission or promoting fusion delays apoptotic cell death (Frank et al., 2001; Sugioka et al., 2004). Bcl-2 family proteins are regulators of apoptosis, and given mitochondria fragmentation in apoptosis, it is not surprising that interactions between mitochondrial fission/fusion machinery and Bcl-2 proteins have been identified (James et al., 2003; Brooks et al., 2007; Li et al., 2008; Liu and Shio, 2008; Shroff et al., 2009). Mfn1 and Mfn2 were demonstrated to physically interact with Bak under normal conditions; however, in apoptotic cells, Bak dissociates from Mfn2 but enhances the Mfn1 interaction (Brooks et al., 2007). DLP1/Drp1 has also been shown to colocalize with Bax and Bak at the mitochondrial outer membrane, which promotes the DLP1 sumoylation in apoptosis (Karbowski et al., 2002; Wasiak et al., 2007). Recently, DLP1/Drp1 has been reported to promote apoptosis independently of its role as a GTPase by inducing membrane hemifusion, which stimulates Bax oligomerization and insertion into the outer mitochondrial membrane (Montessuit et al., 2010). Bax/Bak pore formation then would allow cytochrome c release and progression through apoptotic cell death. Another process required for cytochrome c release is cristae remodeling that is coordinated with the OPA1 processing independently of Bax-mediated outer membrane pore formation (Cipolat et al., 2006). Therefore, mitochondrial fission/fusion proteins are likely to contribute to multiple independent processes in concert to permit the progression of apoptosis. Given that knowledge of the observed interactions is rudimentary between mitochondrial fission/fusion machinery and apoptosis-regulating Bcl-2 proteins, it is still a matter of debate whether the progression of apoptosis and mitochondrial remodeling are interdependent processes. Components comprising mitochondrial remodeling machinery are still expanding. The mechanistic control of diverse interactions within such a macromolecular complex remains to be defined in different contexts of normal and apoptotic conditions. Not unlike mitophagy, the underlying purpose of apoptosis is survival although in a larger scale. Improper regulation of the process could then allow the propagation of tissue dysfunction and eventually organ failure. It follows then the same premise that disruption of proper maintenance of mitochondrial morphology and the machinery necessary for these changes could influence the progression of cumulative insult pathologies, such as metabolic diseases.
Calcium regulation and mitochondrial morphology.
Under normal conditions, increased mitochondrial Ca2+ activates tricarboxylic acid cycle dehydrogenases (Denton et al., 1972, 1978) and ATP synthase (Jouaville et al., 1999), indicating a critical role of Ca2+ in mitochondrial bioenergetics. Important to the discussion of oxidative stress in metabolic disease, Ca2+ also activates the antioxidant enzyme manganese superoxide dismutase (Hayashi et al., 2009). However, large mitochondrial Ca2+ influx induces the permeability transition and eventual outer membrane permeabilization leading to apoptosis. The calcium uniporter constitutes the main mitochondrial Ca2+ uptake mechanism. Although cytosolic Ca2+ levels are orders of magnitude lower than the reported Km of the calcium uniporter (Rizzuto et al., 1993, 1998), the close proximity between the ER and mitochondria allows Ca2+ concentrations at several hundred micromolar at the microdomains between the ER at regions containing IP3 receptors and mitochondria. The physical association of the two organelles has been known for some time and was estimated to be quite substantial, 5–20% of the ER in contact with mitochondria (Rizzuto et al., 1998; Csordás et al., 2006). The tight interaction has been reported to involve the fusion protein Mfn2 tethering mitochondria to the ER, and the knockdown of Mfn2 resulted in impaired mitochondrial Ca2+ uptake (de Brito and Scorrano, 2008). This ER–mitochondria association should play a significant role in not only energy production but also Ca2+ overload–induced apoptosis. Recently, using electron microscopy and tomography, Friedman et al. (2011) visualized the ER wrapping around the mitochondrial tubule. Further studies revealed that these contacts are independent of the aforementioned Mfn2-mediated ER–mitochondrial tethering. Interestingly, these ER contacts were found to be sites of future mitochondrial fission. Fission proteins, DLP1/Drp1 and the mitochondrial fission factor Mff, were observed in these ER–mitochondria contacts, although the contacts occurred independently of these proteins. In support of the microdomain hypothesis for mitochondrial Ca2+ influx, this enveloping contact creates a previously unrecognized large surface area contact; however, the role of these novel contacts in the ER–mitochondria Ca2+ microdomain remains to be tested. Intriguingly, elevated levels of Ca2+ induce both mitochondrial (Szabadkai et al., 2006; Hom et al., 2007; Yu et al., 2011) and ER fragmentation (Subramanian and Meyer, 1997). Possibly, the proximity of the two organelles may serve to control mitochondrial morphology through regulation of Ca2+ levels. In the case of glucose-stimulated Ca2+ increase and resultant mitochondrial fragmentation, Ca2+ influx across the plasma membrane was required (Yu et al., 2011). High flux of Ca2+ across the plasma membrane may induce ER fragmentation as noted previously (Subramanian and Meyer, 1997), which could alter the organelle’s Ca2+-buffering capacity and subsequently mitochondrial Ca2+ contents. In addition, Ca2+ has been shown to regulate mitochondrial fission by phosphorylation or dephosphorylation of DLP1, which is discussed later.
As mentioned above, mitochondrial Ca2+ plays an important role in bioenergetics. However, too much Ca2+ is detrimental, causing cell injury through ROS production and the mitochondrial permeability transition. ROS also regulate OXPHOS coupling and cellular Ca2+ contents, and excess levels of ROS result in oxidative damage. Thus, Ca2+ and ROS must be regulated in a delicate balance, influencing one another for proper bioenergetic activity (Brookes et al., 2004). As discussed in this section, mitochondrial morphology is directly and indirectly associated with Ca2+ regulation, apoptosis/mitophagy, and bioenergetics. In metabolic excess, it is likely that the tight regulatory balance for normal bioenergetics goes awry, and resulting ROS overproduction and cell injury contribute to downstream pathology.
Altered metabolic flux and mitochondrial form/function
In vivo observations.
Hepatic mitochondrial dysfunction is well documented in the progression of metabolic disease, with impairments in ETC complexes (Pérez-Carreras et al., 2003) and a subsequent depression of ATP levels in the progression of NAFLD to nonalcoholic steatohepatitis (Cortez-Pinto et al., 1999). Changes in mitochondrial function are accompanied by alterations in their morphology in this pathological condition. These include the altered cristae structure, crystalline inclusion, and abnormally shaped mitochondria referred to as “megamitochondria” (Caldwell et al., 1999; Jayakumar et al., 2011). Because of the nature of these clinical observations only at the diagnosis stage, the progression of NAFLD with respect to mitochondrial structure and function is not well documented. ETC deficiencies have been shown in all five respiratory complexes (Pérez-Carreras et al., 2003), and up-regulation of β oxidation in exposure to excess free fatty acid (FFA) has also been reported (Miele et al., 2003). An increase in oxidative stress in fatty liver disease is conceivably the result of the convergence of these two factors.
Clinical observations correlating disrupted mitochondrial form and function are also apparent in DCM. The pathological condition of DCM has been associated with morphologically aberrant swollen and round mitochondria, with a loss of discernable cristae (Regan et al., 1977). Atrial samples from diabetic patients displayed deficiencies in utilization of fatty acid and glutamate as substrate in permeabilized myofibrils, in correlation with enhanced ROS production with all substrates assessed (Anderson et al., 2009). Enhanced oxidative stress was also observed, along with an increased propensity for the opening of the permeability transition pore with exogenous Ca2+ treatment (Anderson et al., 2011).
As discussed, given the intimate relationship between mitochondrial morphology and bioenergetics, altered control of mitochondrial morphology must then be considered as a potential underlying pathological mechanism in these metabolic diseases. However, metabolic stimuli driving the changes in the form/function of mitochondria are complex in obesity and insulin resistance associated with NAFLD. There is a high correlation of incidence of type 2 diabetes and NAFLD consistent with an environment of hyperglycemia, hyperinsulinemia, and elevated levels of triglyceride in circulation. In such circumstances, insulin resistance allows persistent hyperglycemia while uncontrolled lipolysis produces elevated levels of FFAs in circulation. Therefore, in vitro studies with combinatorial insult may more closely represent the in vivo milieu of metabolic disease associated with diabetes, whereas studies of individual insult may allow for interpretation of relative contributions to the in vivo situation.
In vitro studies.
At the cellular level, alterations in metabolic flux are sufficient to induce mitochondrial form alterations. Previous studies demonstrated that elevated levels of glucose were sufficient to rapidly induce mitochondrial fragmentation (Yu et al., 2006, 2008). These changes were transient and were not dependent on cell type, as hepatic and cardiovascular-derived cells exhibited similar phenotypes. Coincident with mitochondrial fragmentation was the appearance of enhanced ROS. The ROS elevation requires glucose metabolism through mitochondria, as both l-glucose and inhibition of mitochondrial pyruvate transport suppressed the ROS increase. Importantly, inhibition of mitochondrial fission eliminates the ROS increase, which places alterations in mitochondrial morphology upstream of ROS production, supportive of form dictating function. Collectively, the combination of mitochondrial fission and enhanced metabolic input creates a state conducive to electron slippage and ROS production in the ETC. The underlying mechanism for the role of mitochondrial morphology in the ROS increase is still unresolved, but the effect on ETC component organization such as supercomplex formation is plausible. Elevated ROS also correlated with increased apoptosis, which was also suppressed with fission inhibition (Yu et al., 2008). These observations suggest a potential benefit of controlling fission on hyperglycemic cell injury by affecting ROS production, apoptosis, or both.
In addition to glucose, metabolic insult by elevated FFAs has been demonstrated to increase ROS levels in hepatic cell lines and primary hepatocytes (Feldstein et al., 2004; Malhi et al., 2006; Nakamura et al., 2009; Zhang et al., 2010). Altering mitochondrial ETC complex activities and mitochondrial FFA uptake alleviated increases in ROS levels suggestive of their mitochondrial origin (Nakamura et al., 2009). In the context of metabolic disease, the enhanced ROS would not only increase mitochondrial and cellular damage but additionally they cause insulin resistance in hepatic cells by a JNK-induced IRS-2 phosphorylation that inhibits insulin signaling. Although mitochondrial morphology was not evaluated in these studies, membrane potential loss and cytochrome c release were observed, consistent with the mitochondrial permeability transition and apoptosis.
Endocrine signaling from the pancreas is vital in regulating whole organism metabolism. Pancreatic β cells exposed to FFA (palmitate) exhibited suppression of OXPHOS, as indicated by reduced membrane potential, ATP contents, and oxygen consumption (Las et al., 2011). Deficiencies in the mtDNA-encoded complex IV subunits were noted in the absence of changes of nuclear-encoded subunits, suggestive of compartmentalized damage within the mitochondria, possibly through elevated ROS from palmitate incubation. The authors reported that decreased cellular ATP levels in palmitate insult impair autophagy through failure to acidify the lysosomal compartment. This palmitate-induced β-cell mitochondrial dysfunction was accompanied by mitochondrial fragmentation. Mitochondrial fragmentation was also observed in β cells exposed to glucolipotoxic insult, a treatment that induced β-cell apoptosis (Molina et al., 2009). Mitochondrial fragmentation occurred relatively rapidly within 4 h of treatment and required the fission protein Fis1, a putative DLP1 receptor. Fis1 depletion inhibited the apoptosis in this condition, suggesting that mitochondrial fission is required for apoptotic cell death in pancreatic β cells under glucolipotoxic insult. Interestingly, although inhibition of mitochondrial fission was sufficient to circumvent apoptotic cell death, it failed to recover impaired insulin secretion. Although speculative, these observations suggest that cellular/mitochondrial function may be irreversibly impaired before the progression to apoptosis, and that this fragmentation is apoptosis associated and consequential from mitochondrial dysfunction. More detailed studies will be necessary to determine when mitochondrial fragmentation occurs relative to the mitochondrial dysfunction and apoptotic cell death in this metabolic insult.
What comes first, misshape or dysfunction?
Based on studies described in the previous sections, it is likely that form and function of mitochondria influence each other in both directions in metabolic insult. Defining the temporal sequence for functional and morphological alterations of mitochondria and dysregulation of other cellular processes in metabolic insult would provide useful information for cause-and-effect relationships of these pathological parameters. However, many of the studies with cultured cells used differing experimental time frames for assessing effects of FFA or glucose on cellular/mitochondrial processes, making the interpretation of the results complicated. Although most of the studies monitored mitochondrial function and morphology routinely at least 24 h after treatment, there also appears to be an acute response to metabolic insults. Hyperglycemic treatment has been shown to rapidly increase and then decrease mitochondrial fragmentation and ROS production within an hour, which repeats later with extended duration of the coinciding mitochondrial fragmentation and high ROS (Yu et al., 2006), indicating a cyclic fluctuation of mitochondrial morphology and function in response to metabolic insult. Therefore, it is possible that different mechanisms for the mitochondrial morphology change operate in immediate versus long-term responses to metabolic insult. For instance, 48-h treatment of hepatocytes with long-chain FFAs caused mitochondrial fragmentation with significantly suppressed Mfn2 expression (Zhang et al., 2011). Another study showed that treatment of human aortic endothelial cells with elevated levels of glucose for 24 h increased expression of both DLP1/Drp1 and Fis1 (Shenouda et al., 2011). These observations suggest that long-term metabolic insult can elicit alterations in cellular gene expression profiles to change mitochondrial morphology. In addition, the apoptosis-associated mitochondrial fragmentation could be related to the mitochondrial morphology change observed after a long-term insult, and so could the metabolic insult-induced mitochondrial dysfunction, as it is evident that mitochondrial dysfunction causes a morphological alteration. In contrast, immediate morphological response to metabolic insult is likely mediated by cellular signaling events at the posttranslational level. The high glucose-induced rapid mitochondrial fragmentation implicates an ERK1/2-mediated DLP1/Drp1 phosphorylation to increase fission (Yu et al., 2011). Although there is no direct evidence, because the high glucose insult evokes Ca2+ increase, it is possible that mitochondrial fission can be activated in this metabolic insult through Ca2+/calmodulin-dependent kinase Iα that phosphorylates DLP1 (Han et al., 2008), or the calcineurin-mediated DLP1 dephosphorylation (Cereghetti et al., 2008). In addition, mitochondrial depolarization can act as a bioenergetic signal that induces OPA1 cleavage preventing fusion (Duvezin-Caubet et al., 2006). Considering the distinct mechanisms for the mitochondrial shape control in acute and chronic conditions, further definition of the temporal execution of dynamic change of mitochondrial morphology under metabolic excess will be critical for understanding the correlation of mitochondrial shape with mitochondrial function and bioenergetic states in pathological progression. Furthermore, potential interplay between the early and late changes of mitochondrial morphology will be of interest regarding whether an early, often reversible, morphological/functional change in metabolic insult contributes to chronic, irreversible alterations in form and function of mitochondria in metabolic disease.
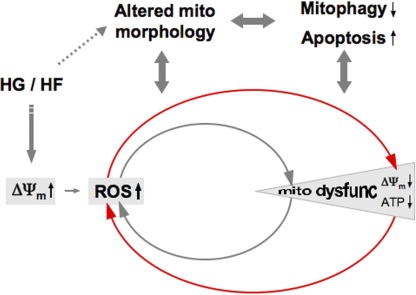
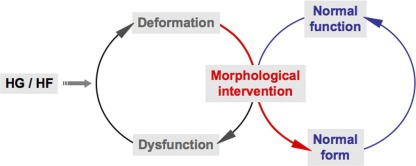
Many studies indicate the involvement of oxidative stress from increased ROS levels as a critical factor in pathological development of metabolic disease. From the mitochondrial bioenergetic standpoint, elevated levels of reducing equivalents of NADH and FADH2 from the catabolism of glucose and FFAs in metabolic excess overload the ETC, causing hyperpolarization and subsequent ROS overproduction. This hyperpolarization-induced ROS overproduction would continue until cumulative oxidative insult impairs the ETC complexes, causing mitochondrial depolarization and dysfunction. Thus, the next phase of pathological ROS production from dysfunctional mitochondria would be established, leading to further damage through the vicious amplifying cycle of mitochondrial dysfunction and ROS production (Fig. 1). In the therapeutic aspect, once mitochondria become dysfunctional, restoring mitochondrial function under continuous metabolic stress would be a challenging task. Therefore, defining the threshold for the pathological transition from the mitochondrial hyperpolarization-induced to dysfunction-induced ROS production in metabolic excess conditions would be important for an early intervention of the pathological progression. As discussed, morphological change of mitochondria is an important parameter associated with metabolic disease, as it is involved in ROS production and apoptosis/mitophagy, and evidence indicates that mitochondrial morphology can dictate mitochondrial functionality. Mitochondrial shape changes in response to metabolic insult occur through possibly differential mechanisms in early and late phases of the insult. Therefore, manipulating mitochondrial shape directly or through regulating an acute signal would be an attractive strategy for an early intervention of metabolic disease (Fig. 2).
Figure 1.
Mitochondrial morphology and function in metabolic excess. Increased metabolic input in the form of high glucose and/or fat (HG/HF) provides an increased entry of reducing equivalents into the ETC resulting in enhanced ΔΨm, favorable for electron slippage and ROS production. Metabolic signaling may alter mitochondrial morphology in HG/HF conditions. Altered mitochondrial morphology under metabolic excess plays a role in ROS production and subsequent mitochondrial dysfunction. Conversely, ROS inside mitochondria causes mitochondrial dysfunction, which may affect mitochondrial morphology. Accumulating damage inside of mitochondria through ROS progressively causes irreversible mitochondrial dysfunction for a new exacerbating cycle of ROS and mitochondrial dysfunction. Apoptosis and dysregulation of mitophagy are accompanied in the metabolic insult conditions, which also interface with mitochondrial morphology change.
Figure 2.
Morphological control to restore mitochondrial function in metabolic insult. Proper maintenance of mitochondrial morphology ensures proper bioenergetic activities and vice versa. Because of a reciprocal relationship between mitochondrial form and function, dysregulation of either mitochondrial morphology or function in metabolic excess causes a cycle of mitochondrial dysfunction and deformation, resulting in metabolic disease. Morphological intervention could put the brake on this vicious cycle and restore normal form of mitochondria, which leads to the recovery of normal function.
Conclusions
The cumulative evidence supports an intimate mitochondrial form–function relationship associated with the progression of metabolic diseases. A requisite bridge of this relationship is the bioenergetic status of the mitochondria, with shape influencing function and vice versa. In the context of metabolic disease, delineating the relative sequence of these changes remains an undefined variable in our understanding of their pathological progression. Regardless of temporal execution, the resultant damage to mitochondria must be reconciled to avoid the amplification of the insult. Mitochondrial fission and fusion have influence in mitophagy and apoptosis, with their disruption having detrimental consequences in cell and tissue maintenance. Dysregulation of mitochondrial fission/fusion therefore can exacerbate the metabolic insult-induced tissue damage through bioenergetic dysfunction while amplifying the effect through the misregulation of cellular maintenance mechanisms. Although it is clear that the total ablation of mitochondrial fission or fusion is detrimental to the cell, acute control at the appropriate times would be beneficial. As metabolic diseases are progressive, reliant upon the accumulation of insult, an early intervention could evade irreversible damage. Defining the underlying mechanisms controlling mitochondrial morphology in enhanced metabolic flux will define temporally appropriate targets of intervention in the metabolic disease progression.
This Perspectives series includes articles by Sheu et al., Zhang et al., Balaban, Santo-Domingo and Demaurex, Wei and Dirksen, O-Uchi et al., and Nowikovsky et al..
Acknowledgments
This work is supported by the National Institutes of Health (grants DK 078618 and DK061991 to Y. Yoon) and the American Heart Association (postdoctoral fellowship 12POST9430003 to C.A. Galloway).
Shey-Shing Sheu served as guest editor.
Footnotes
Abbreviations used in this paper:
- ΔΨm
- inner mitochondrial membrane potential
- DCM
- diabetic cardiomyopathy
- ETC
- electron transport chain
- FFA
- free fatty acid
- NAFLD
- nonalcoholic fatty liver disease
- OXPHOS
- oxidative phosphorylation
- ROS
- reactive oxygen species
References
- Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., et al. 2000. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26:211–215 10.1038/79944 [DOI] [PubMed] [Google Scholar]
- Anderson E.J., Kypson A.P., Rodriguez E., Anderson C.A., Lehr E.J., Neufer P.D. 2009. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 54:1891–1898 10.1016/j.jacc.2009.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.J., Rodriguez E., Anderson C.A., Thayne K., Chitwood W.R., Kypson A.P. 2011. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 300:H118–H124 10.1152/ajpheart.00932.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D., Pich S., Soriano F.X., Vega N., Baumgartner B., Oriola J., Daugaard J.R., Lloberas J., Camps M., Zierath J.R., et al. 2003. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 278:17190–17197 10.1074/jbc.M212754200 [DOI] [PubMed] [Google Scholar]
- Benard G., Bellance N., James D., Parrone P., Fernandez H., Letellier T., Rossignol R. 2007. Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 120:838–848 10.1242/jcs.03381 [DOI] [PubMed] [Google Scholar]
- Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. 2004. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287:C817–C833 10.1152/ajpcell.00139.2004 [DOI] [PubMed] [Google Scholar]
- Brooks C., Wei Q., Feng L., Dong G., Tao Y., Mei L., Xie Z.J., Dong Z. 2007. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc. Natl. Acad. Sci. USA. 104:11649–11654 10.1073/pnas.0703976104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell S.H., Swerdlow R.H., Khan E.M., Iezzoni J.C., Hespenheide E.E., Parks J.K., Parker W.D., Jr 1999. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J. Hepatol. 31:430–434 10.1016/S0168-8278(99)80033-6 [DOI] [PubMed] [Google Scholar]
- Cereghetti G.M., Stangherlin A., Martins de Brito O., Chang C.R., Blackstone C., Bernardi P., Scorrano L. 2008. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. USA. 105:15803–15808 10.1073/pnas.0808249105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chomyn A., Chan D.C. 2005. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280:26185–26192 10.1074/jbc.M503062200 [DOI] [PubMed] [Google Scholar]
- Cipolat S., Rudka T., Hartmann D., Costa V., Serneels L., Craessaerts K., Metzger K., Frezza C., Annaert W., D’Adamio L., et al. 2006. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 126:163–175 10.1016/j.cell.2006.06.021 [DOI] [PubMed] [Google Scholar]
- Cortez-Pinto H., Chatham J., Chacko V.P., Arnold C., Rashid A., Diehl A.M. 1999. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 282:1659–1664 10.1001/jama.282.17.1659 [DOI] [PubMed] [Google Scholar]
- Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnóczky G. 2006. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174:915–921 10.1083/jcb.200604016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito O.M., Scorrano L. 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 456:605–610 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- De Vos K.J., Allan V.J., Grierson A.J., Sheetz M.P. 2005. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr. Biol. 15:678–683 10.1016/j.cub.2005.02.064 [DOI] [PubMed] [Google Scholar]
- Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., et al. 2000. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 26:207–210 10.1038/79936 [DOI] [PubMed] [Google Scholar]
- Denton R.M., Randle P.J., Martin B.R. 1972. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem. J. 128:161–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R.M., Richards D.A., Chin J.G. 1978. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem. J. 176:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvezin-Caubet S., Jagasia R., Wagener J., Hofmann S., Trifunovic A., Hansson A., Chomyn A., Bauer M.F., Attardi G., Larsson N.G., et al. 2006. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J. Biol. Chem. 281:37972–37979 10.1074/jbc.M606059200 [DOI] [PubMed] [Google Scholar]
- Feldstein A.E., Werneburg N.W., Canbay A., Guicciardi M.E., Bronk S.F., Rydzewski R., Burgart L.J., Gores G.J. 2004. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 40:185–194 10.1002/hep.20283 [DOI] [PubMed] [Google Scholar]
- Frank S., Gaume B., Bergmann-Leitner E.S., Leitner W.W., Robert E.G., Catez F., Smith C.L., Youle R.J. 2001. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 1:515–525 10.1016/S1534-5807(01)00055-7 [DOI] [PubMed] [Google Scholar]
- Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. 2011. ER tubules mark sites of mitochondrial division. Science. 334:358–362 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkerson R.W., Margineantu D.H., Capaldi R.A., Selker J.M. 2000. Mitochondrial DNA depletion causes morphological changes in the mitochondrial reticulum of cultured human cells. FEBS Lett. 474:1–4 10.1016/S0014-5793(00)01527-1 [DOI] [PubMed] [Google Scholar]
- Gomes L.C., Di Benedetto G., Scorrano L. 2011. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13:589–598 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C.R. 1966. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J. Cell Biol. 30:269–297 10.1083/jcb.30.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.J., Lu Y.F., Li S.A., Kaitsuka T., Sato Y., Tomizawa K., Nairn A.C., Takei K., Matsui H., Matsushita M. 2008. CaM kinase Iα–induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 182:573–585 10.1083/jcb.200802164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Rizzuto R., Hajnoczky G., Su T.P. 2009. MAM: more than just a housekeeper. Trends Cell Biol. 19:81–88 10.1016/j.tcb.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom J.R., Gewandter J.S., Michael L., Sheu S.S., Yoon Y. 2007. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J. Cell. Physiol. 212:498–508 10.1002/jcp.21051 [DOI] [PubMed] [Google Scholar]
- Ishihara N., Jofuku A., Eura Y., Mihara K. 2003. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Commun. 301:891–898 10.1016/S0006-291X(03)00050-0 [DOI] [PubMed] [Google Scholar]
- Ishihara N., Fujita Y., Oka T., Mihara K. 2006. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 25:2966–2977 10.1038/sj.emboj.7601184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D.I., Parone P.A., Mattenberger Y., Martinou J.C. 2003. hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 278:36373–36379 10.1074/jbc.M303758200 [DOI] [PubMed] [Google Scholar]
- Jayakumar S., Guillot S., Argo C., Redick J., Caldwell S. 2011. Ultrastructural findings in human nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 5:141–145 10.1586/egh.11.9 [DOI] [PubMed] [Google Scholar]
- Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. 1999. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA. 96:13807–13812 10.1073/pnas.96.24.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M., Lee Y.J., Gaume B., Jeong S.Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C.L., Youle R.J. 2002. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159:931–938 10.1083/jcb.200209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman W.J., Visch H.J., Verkaart S., van den Heuvel L.W., Smeitink J.A., Willems P.H. 2005. Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am. J. Physiol. Cell Physiol. 289:C881–C890 10.1152/ajpcell.00104.2005 [DOI] [PubMed] [Google Scholar]
- Las G., Serada S.B., Wikstrom J.D., Twig G., Shirihai O.S. 2011. Fatty acids suppress autophagic turnover in β-cells. J. Biol. Chem. 286:42534–42544 10.1074/jbc.M111.242412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F., Lombès A., Frachon P., Rojo M. 2002. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell. 13:4343–4354 10.1091/mbc.E02-06-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen Y., Jones A.F., Sanger R.H., Collis L.P., Flannery R., McNay E.C., Yu T., Schwarzenbacher R., Bossy B., et al. 2008. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA. 105:2169–2174 10.1073/pnas.0711647105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.A., Shio H. 2008. Mitochondrial morphogenesis, dendrite development, and synapse formation in cerebellum require both Bcl-w and the glutamate receptor delta2. PLoS Genet. 4:e1000097 10.1371/journal.pgen.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamzaev K.G., Pletjushkina O.Y., Saprunova V.B., Bakeeva L.E., Chernyak B.V., Skulachev V.P. 2004. Selective elimination of mitochondria from living cells induced by inhibitors of bioenergetic functions. Biochem. Soc. Trans. 32:1070–1071 10.1042/BST0321070 [DOI] [PubMed] [Google Scholar]
- Malhi H., Bronk S.F., Werneburg N.W., Gores G.J. 2006. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 281:12093–12101 10.1074/jbc.M510660200 [DOI] [PubMed] [Google Scholar]
- Miele L., Grieco A., Armuzzi A., Candelli M., Forgione A., Gasbarrini A., Gasbarrini G. 2003. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am. J. Gastroenterol. 98:2335–2336 10.1111/j.1572-0241.2003.07725.x [DOI] [PubMed] [Google Scholar]
- Molina A.J., Wikstrom J.D., Stiles L., Las G., Mohamed H., Elorza A., Walzer G., Twig G., Katz S., Corkey B.E., Shirihai O.S. 2009. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 58:2303–2315 10.2337/db07-1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montessuit S., Somasekharan S.P., Terrones O., Lucken-Ardjomande S., Herzig S., Schwarzenbacher R., Manstein D.J., Bossy-Wetzel E., Basañez G., Meda P., Martinou J.C. 2010. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 142:889–901 10.1016/j.cell.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Takamura T., Matsuzawa-Nagata N., Takayama H., Misu H., Noda H., Nabemoto S., Kurita S., Ota T., Ando H., et al. 2009. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 284:14809–14818 10.1074/jbc.M901488200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichon A., Baricault L., Gas N., Guillou E., Valette A., Belenguer P., Lenaers G. 2003. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 278:7743–7746 10.1074/jbc.C200677200 [DOI] [PubMed] [Google Scholar]
- Parone P.A., Da Cruz S., Tondera D., Mattenberger Y., James D.I., Maechler P., Barja F., Martinou J.C. 2008. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 3:e3257 10.1371/journal.pone.0003257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Carreras M., Del Hoyo P., Martín M.A., Rubio J.C., Martín A., Castellano G., Colina F., Arenas J., Solis-Herruzo J.A. 2003. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 38:999–1007 [DOI] [PubMed] [Google Scholar]
- Pich S., Bach D., Briones P., Liesa M., Camps M., Testar X., Palacín M., Zorzano A. 2005. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum. Mol. Genet. 14:1405–1415 10.1093/hmg/ddi149 [DOI] [PubMed] [Google Scholar]
- Rambold A.S., Kostelecky B., Elia N., Lippincott-Schwartz J. 2011. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. USA. 108:10190–10195 10.1073/pnas.1107402108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan T.J., Lyons M.M., Ahmed S.S., Levinson G.E., Oldewurtel H.A., Ahmad M.R., Haider B. 1977. Evidence for cardiomyopathy in familial diabetes mellitus. J. Clin. Invest. 60:885–899 10.1172/JCI108843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. 1993. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 262:744–747 10.1126/science.8235595 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 280:1763–1766 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- Shenouda S.M., Widlansky M.E., Chen K., Xu G., Holbrook M., Tabit C.E., Hamburg N.M., Frame A.A., Caiano T.L., Kluge M.A., et al. 2011. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 124:444–453 10.1161/CIRCULATIONAHA.110.014506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff E.H., Snyder C.M., Budinger G.R., Jain M., Chew T.L., Khuon S., Perlman H., Chandel N.S. 2009. BH3 peptides induce mitochondrial fission and cell death independent of BAX/BAK. PLoS ONE. 4:e5646 10.1371/journal.pone.0005646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K., Meyer T. 1997. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell. 89:963–971 10.1016/S0092-8674(00)80281-0 [DOI] [PubMed] [Google Scholar]
- Sugioka R., Shimizu S., Tsujimoto Y. 2004. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J. Biol. Chem. 279:52726–52734 10.1074/jbc.M408910200 [DOI] [PubMed] [Google Scholar]
- Szabadkai G., Simoni A.M., Bianchi K., De Stefani D., Leo S., Wieckowski M.R., Rizzuto R. 2006. Mitochondrial dynamics and Ca2+ signaling. Biochim. Biophys. Acta. 1763:442–449 10.1016/j.bbamcr.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Tondera D., Grandemange S., Jourdain A., Karbowski M., Mattenberger Y., Herzig S., Da Cruz S., Clerc P., Raschke I., Merkwirth C., et al. 2009. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 28:1589–1600 10.1038/emboj.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. 2008. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27:433–446 10.1038/sj.emboj.7601963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S., Zunino R., McBride H.M. 2007. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J. Cell Biol. 177:439–450 10.1083/jcb.200610042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Robotham J.L., Yoon Y. 2006. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA. 103:2653–2658 10.1073/pnas.0511154103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Sheu S.S., Robotham J.L., Yoon Y. 2008. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc. Res. 79:341–351 10.1093/cvr/cvn104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Jhun B.S., Yoon Y. 2011. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid. Redox Signal. 14:425–437 10.1089/ars.2010.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Seitz L.C., Abramczyk A.M., Chan C. 2010. Synergistic effect of cAMP and palmitate in promoting altered mitochondrial function and cell death in HepG2 cells. Exp. Cell Res. 316:716–727 10.1016/j.yexcr.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jiang L., Hu W., Zheng Q., Xiang W. 2011. Mitochondrial dysfunction during in vitro hepatocyte steatosis is reversed by omega-3 fatty acid-induced up-regulation of mitofusin 2. Metabolism. 60:767–775 10.1016/j.metabol.2010.07.026 [DOI] [PubMed] [Google Scholar]
- Züchner S., Mersiyanova I.V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E.L., Zappia M., Nelis E., Patitucci A., Senderek J., et al. 2004. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 36:449–451 10.1038/ng1341 [DOI] [PubMed] [Google Scholar]