The heart is nearly unique in the body in that it has a constant workload well beyond the normal maintenance of cellular integrity. In addition, the heart of a large animal such as man also has the capacity to increase its workload by nearly 10-fold for considerable amounts of time. A high steady state and peak work demand require the heart to use the space- and weight-efficient mitochondria to provide an adequate energy conversion rate to support these activities. The heart’s energy conversion results in remarkably high metabolite turnover rates (i.e., time to turnover the entire metabolite pool), with values ranging from resting to maximum workloads of 10–2 s for ATP, 170–40 ms for ADP, and 250–70 ms for mitochondrial nicotinamide adenine dinucleotide (NADH) (Mootha et al., 1997; Balaban, 2002). With this requirement for a constant workload coupled to large sustained changes in dynamic range, it is not surprising that cardiac energy conversion is highly specialized. As seen from the metabolic pool turnover estimates above, a slight mismatch between energy conversion rates and workload could severely impact metabolite levels in seconds. Thus, tight temporal coupling between mitochondrial energy conversion and heart workload is an absolute requirement of the metabolic control network. In addition, because oxygen extraction from the coronary vasculature is nearly complete, a tight coupling of coronary blood flow control to heart workload is important. Taking these data into consideration, it is not surprising that there is a linear relationship between the rate of mitochondrial respiration, blood flow, and workload (Starling and Visscher, 1927; Gutterman and Cowley, 2006).
Remarkably, the heart is able to accomplish this over a wide workload range with very little change in the steady-state concentrations of ATP, ADP, and NADH associated with this energy conversion process, despite the approximately100-ms turnover of some metabolite pools. Even the metabolites creatine phosphate (CrP) and creatine, which are associated with the creatine kinase system that is a temporal buffer of high energy phosphates (Funk et al., 1989), remain remarkably constant. Thus, the balance of energy conversion rates with ATP hydrolysis must be precisely orchestrated over very short time constants. This ability of energetic tissues to maintain the concentration of high energy phosphates during periods of increased use has been appreciated for many years. One of the first lengthy discussions and experimental insights was provided by A.V. Hill in his 1950 “Challenge to Biochemists” (Hill, 1950). Concerning the energetics of skeletal muscle, he stated, “Indeed, no change in the ATP has ever been found in living muscle except in extreme exhaustion, verging on rigor.” After a discussion of the “Lohmann reaction,” in which creatine kinase was believed to buffer ATP concentrations, he stated, “We should not, however, be so satisfied with the explanation of why no change in ATP is ever found in living muscle that we cease to look for it: for another possibility exists…Is it not possible that as stimulation proceeds a balance is reached at some intermediate level between breakdown and restoration?” Hill went on to propose using different animal models with different metabolic rates as part of his challenge. This proposal made by Hill will be the topic of this Perspective as it pertains to the heart. That is, I will review the current understanding of how heart mitochondria maintain a balance between the rate of ATP production (qATP) and hydrolysis while keeping the free energy available from ATP hydrolysis constant, and use some allometric relationships to contribute to the analysis.
As discussed by Hill for skeletal muscle, it has been appreciated for years that the cardiac concentration of the high energy phosphate molecules of energy metabolism, ATP and CrP, as well as the metabolic intermediates for their formation, ADP, inorganic phosphate (Pi), and creatine, are remarkably stable during physiological changes in workload. This was established in the heart using classical Wollenberger clamps or drill biopsy methods from the 1950s through the 1970s (Wollenberger, 1957; Boerth et al., 1969; Neely et al., 1972). Later, numerous nondestructive, more reliable in vivo cardiac phosphorus-31 nuclear magnetic resonance studies in animals (Balaban et al., 1986; Ligeti et al., 1987; Detre et al., 1990; Heineman and Balaban, 1990; Robitaille et al., 1990) and humans (Conway et al., 1988; Schaefer et al., 1992; Hudsmith et al., 2009) confirmed that CrP, ATP, Pi, calculated ADP, and creatine were essentially constant during physiological changes in workload. However, as maximum workloads were approached (Katz et al., 1989; Zhang et al., 1995; Lamb et al., 1997) or the tissue was metabolically compromised by ischemia, demand ischemia, different disease states, or hypoxia (Heineman and Balaban, 1990; Zhang et al., 1993, 2001), the ability to maintain these metabolite concentrations was compromised. Thus, under normal physiological conditions, the mammalian heart is capable of changing qATP with little or no change in the net concentrations of ADP and Pi, which are products of ATP hydrolysis and substrates for ATP formation in oxidative phosphorylation. This was a difficult concept to accept by many because of the logical dogma of oxidative phosphorylation being paced by the feedback of ATP hydrolysis products (Jacobus et al., 1982). This feedback mechanism does, apparently, come into play at high workloads (Zhang et al., 1995) or in response to artificial alterations of metabolic substrates (Kim et al., 1991), as well as in very young animals (Portman et al., 1989). The major limitation of the feedback system is the fundamentally limited dynamic range of using breakdown products as feedback while maintaining an adequate ΔGATP that is sufficient to support the potential energy requirements of the heart, including muscle contraction and calcium sequestration (Tian et al., 1998). The maintenance of energy metabolite concentrations and ΔGATP available for work during changes in workload has been termed metabolic homeostasis. Homeostasis is an appropriate term because the cardiac cell energy metabolic control network seems to be programmed to maintain ΔGATP at a remarkably constant value, much like cellular volume or pH. Herein, I discuss the efforts to understand the cellular and mitochondrial control network that orchestrates the metabolic homeostasis of the heart. This begins with a discussion of the distribution of cardiac contractile and energy conversion elements within the cell.
Because of the tight coupling between energy conversion and constant cardiac contraction, is there an optimal relationship between cytosolic volume devoted to energy conversion (i.e., mitochondria) versus contractile elements (i.e., myofibrils)? Each cardiac myocyte is “designed” to meet its work, or contractile force, requirements at maximum workloads. This concept has been termed “symmorphosis” by Weibel et al. (1991), logically suggesting that physiological maximum demands determine the capacity of all of the elements in a physiological system, with little or no “wasted” reserve capacity. Using this approach, the cell has a fixed requirement for the cross-sectional area of the trans-cellular contractile elements relative to its maximum power requirements. Related to this, if mitochondria have a fixed ability for qATP per unit mitochondrial volume, the mitochondrial volume required to support this activity will be fixed relative to the myofibril content. An interesting way to evaluate this relationship between contractile element and mitochondria cellular content in the mammalian left ventricle is to look across an allometric series of animals where the peak power does not change dramatically, but the resting rate can vary over an order of magnitude (Weibel and Hoppeler, 2004). Hoppeler et al. (1984) performed this analysis and remarkably found that the fractional volume of mitochondria was fixed from mouse to horse at ∼21% of the total heart mass. This was recently confirmed when the amount of cytochrome a,a3 or total number of cytochrome chains per gram of heart was found to be essentially identical across a similar allometric series (Phillips et al., 2012). Thus, the relationship between the cytosolic volume used for energy conversion is fixed as a function of animal size, implying an optimized ratio of cross-sectional area of muscle fibers to mitochondrial volume to support the constant metabolic needs of the mammalian heart. Surprisingly, the ratio of mitochondrial volume, or cytochrome chains, to muscle mass is also the same in the left and right heart from the same animals, even though the pressure volume work of the hearts is nearly fivefold higher in the left ventricle compared with the right chamber (Phillips et al., 2011b). Phillips et al. (2011b) suggested that the rate of ATP use per gram of the right and left heart actually approaches the same value at maximum workload based on the slope of the respiratory rate versus rate pressure product (RPP) for the left and right ventricles. The right ventricle has a much steeper relationship between RPP and oxygen consumption than the left ventricle, which reveals very similar oxygen consumption levels when extrapolated to the maximum workload. Thus, the cytosolic fraction devoted to energy conversion and contractile apparatus of the mammalian right and left chambers are identical, and this is likely related to the same peak work requirements of the two chambers per gram of myocardium. These authors suggested that because the heart cell was already optimized with regard to mitochondrial content and contractile elements, the only way the heart can respond to chronic increases in workload and maintain metabolic homeostasis is to increase the number of cells or total cell cytoplasm, resulting in hypertrophy. This was a requirement because remodeling the energetically “balanced” cardiac cell cytoplasm would not improve efficiency or sustained power. The steep relationship between RPP and metabolic stress in the right ventricle may also explain why this chamber is more prone to hypertrophy than the left ventricle. These results on the volume distribution of cardiac mitochondria suggest that across the mammalian allometric series, and even between the left and right heart of a given animal, the fraction volume of the mitochondria and contractile elements is fixed, and it is likely optimized to provide appropriate qATP to match ATP hydrolysis by the contractile elements, and Ca2+ SR recycling maintains metabolic homeostasis during normal and near maximal workloads.
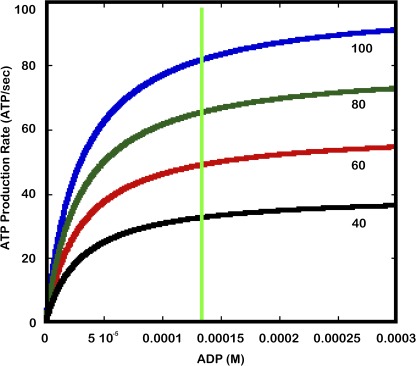
Given that the mitochondrial volume fraction of the mammalian cardiac myocyte is fixed, how can the mitochondria match qATP over the wide range of ATP hydrolysis without changing the kinetic or thermodynamic driving forces for ATP production? The simplest method, or Occam’s razor, to accomplish this task is to simply change the maximum velocity ATP production (qATPmax) in proportion to the ATP production demand. This remarkably simple mechanism is illustrated in Fig. 1, where a simple Michaelis–Menten model for ADP driving ATP production with a Km of 30 µM with variable qATPmax values generates a series of [ADP] versus qATP curves. As seen from the green line, qATP can be altered by simply changing the qATPmax of the reaction while keeping ADP constant. It should be pointed out that the mitochondrial ATP production reaction is also a function of [Pi] and the inner mitochondrial membrane proton motive force (ΔΨH), which are ignored in this simplification but will be discussed further below. It is important to note that for this mechanism to work during dynamic changes in cardiac workload, qATPmax cannot be simply a function of [E] or mitochondrial F1F0ATPase content, as in the classical Michaelis–Menten system where Vmax = [E]. Because [F1F0ATPase] is constant in the heart with workload, the only way to alter qATPmax is to change enzyme kinetics via posttranslational modifications (PTMs), assuming no changes in the kinetic and thermodynamic driving forces. For the purposes of this discussion, I will refer to the theoretical qATPmax (qATPmaxt) as the maximum rate of qATP only limited by [F1F0ATPase], whereas the observed qATPmax (qATPmaxo) is the capacity of the enzyme as modulated by potential PTM events.
Figure 1.
Michaelis–Menten model for ADP driving ATP production at maximum Pi values. Each colored line represents a different Vmax for the reaction. The Vmax values are listed at the end of each curve. The green line represents a constant homeostatic ADP concentration.
Returning to the allometric series in cardiac energy metabolism discussed above, is there evidence here that qATPmax is modulated? As mentioned earlier, the “resting” heart rate and workload are roughly 10 times higher in the rat than in larger animals, mostly because of its higher resting heart rate. As also discussed above, the mitochondrial or [F1F0ATPase] contents are nearly identical using a variety of proteomic methods (Phillips et al., 2012). Thus, if qATPmax was also constant, the [ADP] content would have to be much higher in smaller animals than in larger animals to maintain qATP equal to hydrolysis. However, the estimated in vivo [ADP] concentration in dog (∼55 µM) (Katz et al., 1989) or human (estimated by Chacko et al., 2000) is similar to that in mouse (∼50 µM) (Chacko et al., 2000), or even much lower (∼13 µM) (Himmelreich and Dobson, 2000). These data are inconsistent with ADP or Pi driving resting respiratory rate in large animals and small rodents. Chacko et al. (2000) realized this after making these observations in the mouse and stated, “It is also tempting to speculate that a relatively constant cardiac PCr/ATP ratio and [ADP] across species with 10-fold differences in heart rate is consistent with the hypothesis that ADP does not control cardiac energy turnover under typical physiological conditions across mammalian species.” These data imply that qATPmaxo is lower than qATPmaxt at rest in larger animals, and qATPmaxo may approach qATPmaxt at rest in active small rodents, providing the higher qATP required. Consistent with this notion, Phillips et al. (2012) demonstrated that the extracted molar activity of the F1F0ATPase was higher in smaller animals compared with larger animals. These authors suggested that PTMs modulate the activity of the mitochondrial oxidative phosphorylation complexes depending on the metabolic stress (qATP/qATPmaxt) of a given heart or tissue. These data seem to support that qATPmax is differentially modulated at rest in various species.
What is the evidence for dynamic regulation of qATPmaxo? For this mechanism to work, qATPmaxo must be modulated after acute changes in workload. Wan et al. (1993) found a net depolarization of ΔΨH, the primary driving force for qATP, whereas ADP and Pi remained essentially constant during cardiac work increases. These results agree with Kauppinen (1983), who also observed a small depolarization ΔΨH with workload. These data are consistent with nearly routine human clinical data. Using Tc-sestamibi as a monitor of mitochondrial membrane potential (Piwnica-Worms et al., 1994), clinicians often observe a decrease in Tc retention consistent with a depolarization ΔΨ at near maximum exercise in humans (Verzijlbergen et al., 1994). Wan et al. (1993) concluded, “Based on these findings it was proposed that during increased cardiac work, the capacity of the mitochondrial ATP synthase was increased.” Here, the “capacity of the mitochondrial ATP synthase” is equivalent to qATPmaxo. Again, this conclusion is based on the observation that qATP increases with greater work without increases in ADP, Pi, or ΔΨH. Wan et al. (1993) also attempted to measure alterations in the activity of extracted F1F0ATPase, but these were not elaborated on. However, much earlier, Das and Harris (1989, 1990) found that with increases in cardiac cell work, substantial increases in the reverse ATPase reaction of the F1F0ATPase could be detected in rapidly extracted submitochondrial particles (SMPs). This reaction is of interest because the ATP hydrolysis reaction requires the complex rotation of the γ subunit, much like the ATP synthetic reaction. Thus, alterations of the hydrolysis reaction could be reflecting events that affect the synthetic reaction. Interestingly, these authors also found that these effects were apparently dependent on the increase in matrix Ca2+ (Das and Harris, 1990). This basic observation was repeated in heart in vivo, where Scholz and Balaban (1994) showed that reversible activity increases in extracted F1F0ATPase could be detected in small biopsies collected from the beating heart that were stimulated with dobutamine. In those studies, they noted that preparation speed was critical in demonstrating the effect; it took only seconds to sonicate the biopsy to generate the SMPs and perform assays in the absence of detergents. These effects were expanded on by Phillips et al. (2012), who not only evaluated the effect of animal size but also the effect of work on the in vivo porcine heart and perfused rabbit heart using native gel techniques to monitor F1F0ATPase activity instead of SMPs. These authors also found work-related alterations in extracted F1F0ATPase activity that was very sensitive to preparation time and detergents. Thus, a very labile modification of F1F0ATPase ATPase activity seems related to the activity of the state of the tissue just before extraction, which is consistent with an increase in qATPmaxo with workload via a dynamic PTM process.
These F1F0ATPase assays are interesting because they involve the rotation of the γ subunit within the complex; however, the effect on ATP synthesis can only be speculated. Regrettably, the only way to monitor ATP synthetic activity is to have a ΔΨH across the membrane to drive the reaction forward. Thus, this assay is only practically conducted in isolated cardiac mitochondria. The extensive purification and artificial methods of ΔΨH used in reconstituted systems is not likely to be feasible with this apparently labile system. In a series of studies, Territo et al. (2000, 2001) and Balaban et al. (2003) determined the effective qATPmaxo by monitoring the qATP via oxygen consumption in the presence of saturating [ADP] and [Pi] while monitoring ΔΨH or NADH redox state to estimate the driving force for ATP production. These studies clearly demonstrated that the cardiac mitochondria qATPmax can be dynamically modulated. They showed that alterations in matrix Ca2+ can be a major regulator of qATPmax by modulating F1F0ATPase activity independently of the classical Ca2+-dependent dehydrogenase activation (McCormack et al., 1990). These results were consistent with studies by Moreno-Sánchez (1985; Moreno-Sánchez and Hansford, 1988) and Panov and Scaduto (1996). Phillips et al. (2012) also showed that pretreating mitochondria with Ca2+ increased F1F0ATPase hydrolytic activity in native gels, supporting the link between in vitro ATPase assays and synthetic activity within mitochondria. Again, these activity differences were dependent on rapid sample preparation and the detergents used. From these isolated mitochondria studies, it is apparent that qATPmax can be modulated within isolated mitochondria and that the cytosolic signaling molecule regulating this process might be Ca2+, which is involved in activating muscle contraction, and Ca2+ sequestration in the SR is also a major energetic requirement of the cardiac cell (Tian et al., 1998; Balaban, 2002).
The regulation of energy metabolism in the cardiac cell by Ca2+ is complex. Ca2+ release from the SR activates muscle contraction but also must be actively pumped back into the SR before the next contraction. The energetic cost of Ca2+ pumping is estimated at 10–30% of the total energy cost of muscle contraction. Some cardiac mitochondria are located in close proximity to the SR, and a local release of Ca2+ in these regions likely results in a high local delivery of Ca2+ to the mitochondria (Rizzuto and Pozzan, 2006), increasing the kinetics driving force for Ca2+ entry into the matrix. Within the matrix, Ca2+ clearly activates several dehydrogenases via dephosphorylation, pyruvate dehydrogenase (PDH), or poorly defined substrate affinity mechanisms (Denton, 2009). This increase in dehydrogenase activity could increase the net driving force for ATP production by increasing [NADH] and subsequently the ΔΨH. Indeed, many authors have suggested that the activation of the dehydrogenases is adequate to support the observed metabolic homeostasis in the heart with workload. Can the simple increase in delivery of NADH via the dehydrogenases activated by Ca2+ explain the balancing of qATP with hydrolysis in the absence of changes in ADP and Pi? The potential energy in NADH is delivered to the F1F0ATPase via the generation of ΔΨH. If NADH alone was driving ATP production higher, ΔΨH would need to increase to drive qATP faster when [ADP], [Pi], and ΔGATP are fixed. How big a change in ΔΨH would have to occur to increase qATP three- to fourfold under these energy homeostatic conditions? The relationship between ΔΨH and qATP is linear over physiological rates, with a slope change of ∼1.3% in ATP production/mV ΔΨ (Territo et al., 2000). This suggests that increasing the heart rate by threefold would require approximately an additional 150 mV over the resting value of 180 mV, taking ΔΨ to >300 mV. Thus, driving respiration through NADH alone is very inefficient with regard to ΔΨ. Indeed, as discussed above, most of the current evidence suggests that ΔΨ decreases with increasing workload, which would decrease the driving force for ATP production, not increase it. Thus, a model that only uses alterations in NADH and/or ΔΨ caused by modulation of dehydrogenase activity is not consistent with experimental results. Clearly, the activation of NADH generation by Ca2+ must be coupled to other “downstream” events in oxidative phosphorylation to be effective in maintaining metabolic homeostasis. Many studies have suggested that Ca2+ is key in maintaining cardiac metabolic homeostasis. The best piece of evidence is the observation that blocking Ca2+ entry into mitochondria disrupts metabolic homeostasis in working tissues (Katz et al., 1988; Unitt et al., 1989; Liu and O’Rourke, 2008). Thus, if Ca2+ is the key cytosolic signaling molecule linking workload and mitochondria energy conversion, it is likely that it acts on more than just dehydrogenase reactions (Balaban, 2002; Glancy and Balaban, 2012).
How are other matrix elements involved in the oxidative generation of ATP dynamically modified by Ca2+ or other transducers? The role of PTM in the regulation of NADH generation is well established, especially for the PDH reaction. Clearly, the effects of workload on the extracted F1F0ATPase (Das and Harris, 1990; Scholz and Balaban, 1994; Phillips et al., 2012) and the effects of Ca2+ on isolated mitochondria qATPmax (Territo et al., 2000) independent of dehydrogenase activation point to a role for a dynamic PTM system regulating the elements of ATP production well downstream of NADH generation. However, the specific mechanisms for this apparently labile PTM modulation of F1F0ATPase activity, as well as other elements in oxidative phosphorylation, remain obscure. Over the last several years, hundreds of PTM sites including phosphorylation, acylation, oxidation, and nitrosylation have been found in the oxidative phosphorylation complexes. The significance of these sites with regard to the mole fraction of protein affected and the functional consequences of modification are also poorly defined. The phosphorylation events on Complex IV (Acin-Perez et al., 2011) and cytochrome c (Pecina et al., 2010) have been reasonably correlated with functional alterations, but the exact understanding of the kinase and phosphatase systems and their relationship with cardiac workload is unknown. The mitochondrial matrix kinase–phosphatase system is particularly puzzling. The evidence that many classical cytosolic protein kinases are present and recruited to the mitochondrial interspace is compelling (Antico Arciuch et al., 2009; Means et al., 2011). However, the evidence that any of these are in the matrix space is less than convincing at this stage, although 32P-labeling studies of mitochondria clearly show matrix kinase activity (Aponte et al., 2009). Interestingly, the PDH and branched-chain dehydrogenase kinases that are clearly present in the matrix have more sequence homology with bacterial histidine kinases than with their cytoplasmic functional relatives (Popov et al., 1992). Several properties of the bacterial phosphorylation system are apparently present in the matrix protein phosphorylation system. These include autophosphorylation (Phillips et al., 2011a), pH sensitivity (Pressman, 1964; Aponte et al., 2009), and highly labile nature (discussed above). The presence of these bacterial systems in mammalian tissues is gaining recognition (Besant et al., 2003; Jung and Jung, 2009) and should be more seriously considered in the mitochondria, where remnants of most of the bacterial systems remain key elements in their functionality (Pallen, 2011).
In summary, a very specific relationship between mitochondria and myofibril content of the mammalian heart exists across species and between heart chambers. This specific relationship likely reflects the necessity of a constant supply of ATP to the myofibrils at maximum workloads, locking in the relationship between mitochondrial and myofibril volumes. It has been appreciated for years that the heart is capable of altering qATP with ATP hydrolysis without large swings in [NADH], [ATP], [ADP], and [Pi], but small alterations in ΔΨ. This results in a metabolic homeostasis for most of the driving forces for ATP production over a wide range of physiological workloads. The simplest hypothesis for explaining these results is that qATPmax is dynamically modulated through reversible PTMs, including the regulation of dehydrogenases and other elements of oxidative phosphorylation and especially F1F0ATPase. A small drop in ΔΨ likely drives reducing equivalents to the high gain cytochrome oxidase system, which supports the required increase reducing equivalent removal by oxygen, but also reflects the lack of an increase in driving force for ATP production by simply increasing NADH production. It is important to note that these results are not consistent with a simple alteration in dehydrogenase activity to explain the observed metabolic homeostasis. A significant modification of qATPmax kinetics is still required. The evidence that qATPmax modulated by PTMs, which are potentially regulated by matrix Ca2+, is intriguing but still indirect through monitoring the driving forces of the reaction or from extracted biopsy data evaluating ATP hydrolysis activity. Although the alterations in reaction kinetics are consistent with PTM events, the definitive identification of these sites remains elusive. Growing evidence suggests that a closer look at the potential role of unconventional bacterial PTMs, which are much more difficult to detect than conventional PTM sites, might illuminate new mechanisms for the dynamic matrix regulatory events that contribute to the metabolic homeostasis of the heart.
This Perspectives series includes articles by Sheu et al., Zhang et al., Santo-Domingo and Demaurex, Wei and Dirksen, O-Uchi et al., Nowikovsky et al., and Galloway and Yoon.
Acknowledgments
I thank the present and past members of my laboratory, including Darci Phillips, Vamsi Mootha, Paul Territo, Andrew Arai, Rachael Hopper, Thor Johnson, David Chess, Brian Glancy, Fred Heineman, and Raul Covian, for helpful discussions and experimental contributions to this topic. Colleagues outside of the Laboratory of Cardiac Energetics who influenced this article include Alan Koretsky, Robert Harris, William Stanley, George Radda, and Brian O’Rourke. I would especially like to thank the late Peter Hochachka and Britton Chance for their numerous discussions concerning and contributions to many of the concepts presented here. I will miss their contributions in the future.
This work was supported by National Heart, Lung and Blood Institute, Division of Intramural Research.
Shing-Shey Sheu served as guest editor.
Footnotes
Abbreviations used in this paper:
- CrP
- creatine phosphate
- NADH
- nicotinamide adenine dinucleotide
- PDH
- pyruvate dehydrogenase
- Pi
- inorganic phosphate
- PTM
- posttranslational modification
- qATP
- rate of ATP production
- qATPmax
- maximum qATP
- qATPmaxo
- observed qATPmax
- qATPmaxt
- theoretical qATPmax
- RPP
- rate pressure product
- SMP
- submitochondrial particle
References
- Acin-Perez R., Gatti D.L., Bai Y., Manfredi G. 2011. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 13:712–719 10.1016/j.cmet.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antico Arciuch V.G., Alippe Y., Carreras M.C., Poderoso J.J. 2009. Mitochondrial kinases in cell signaling: Facts and perspectives. Adv. Drug Deliv. Rev. 61:1234–1249 10.1016/j.addr.2009.04.025 [DOI] [PubMed] [Google Scholar]
- Aponte A.M., Phillips D., Hopper R.K., Johnson D.T., Harris R.A., Blinova K., Boja E.S., French S., Balaban R.S. 2009. Use of (32)P to study dynamics of the mitochondrial phosphoproteome. J. Proteome Res. 8:2679–2695 10.1021/pr800913j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R.S. 2002. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J. Mol. Cell. Cardiol. 34:1259–1271 10.1006/jmcc.2002.2082 [DOI] [PubMed] [Google Scholar]
- Balaban R.S., Kantor H.L., Katz L.A., Briggs R.W. 1986. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 232:1121–1123 10.1126/science.3704638 [DOI] [PubMed] [Google Scholar]
- Balaban R.S., Bose S., French S.A., Territo P.R. 2003. Role of calcium in metabolic signaling between cardiac sarcoplasmic reticulum and mitochondria in vitro. Am. J. Physiol. Cell Physiol. 284:C285–C293 [DOI] [PubMed] [Google Scholar]
- Besant P.G., Tan E., Attwood P.V. 2003. Mammalian protein histidine kinases. Int. J. Biochem. Cell Biol. 35:297–309 10.1016/S1357-2725(02)00257-1 [DOI] [PubMed] [Google Scholar]
- Boerth R.C., Covell J.W., Seagren S.C., Pool P.E. 1969. High-energy phosphate concentrations in dog myocardium during stress. Am. J. Physiol. 216:1103–1106 [DOI] [PubMed] [Google Scholar]
- Chacko V.P., Aresta F., Chacko S.M., Weiss R.G. 2000. MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am. J. Physiol. Heart Circ. Physiol. 279:H2218–H2224 [DOI] [PubMed] [Google Scholar]
- Conway M.A., Bristow J.D., Blackledge M.J., Rajagopalan B., Radda G.K. 1988. Cardiac metabolism during exercise measured by magnetic resonance spectroscopy. Lancet. 2:692 10.1016/S0140-6736(88)90510-7 [DOI] [PubMed] [Google Scholar]
- Das A.M., Harris D.A. 1989. Reversible modulation of the mitochondrial ATP synthase with energy demand in cultured rat cardiomyocytes. FEBS Lett. 256:97–100 10.1016/0014-5793(89)81725-9 [DOI] [PubMed] [Google Scholar]
- Das A.M., Harris D.A. 1990. Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc. Res. 24:411–417 10.1093/cvr/24.5.411 [DOI] [PubMed] [Google Scholar]
- Denton R.M. 2009. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 1787:1309–1316 10.1016/j.bbabio.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Detre J.A., Koretsky A.P., Williams D.S., Ho C. 1990. Absence of pH changes during altered work in the in vivo sheep heart: a 31P-NMR investigation. J. Mol. Cell. Cardiol. 22:543–553 10.1016/0022-2828(90)90956-3 [DOI] [PubMed] [Google Scholar]
- Funk C., Clark A., Jr, Connett R.J. 1989. How phosphocreatine buffers cyclic changes in ATP demand in working muscle. Adv. Exp. Med. Biol. 248:687–692 10.1007/978-1-4684-5643-1_76 [DOI] [PubMed] [Google Scholar]
- Glancy B., Balaban R.S. 2012. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 51:2959–2973 10.1021/bi2018909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman D.D., Cowley A.W., Jr 2006. Relating cardiac performance with oxygen consumption: historical observations continue to spawn scientific discovery. Am. J. Physiol. Heart Circ. Physiol. 291:H2555–H2556 10.1152/classicessays.00044.2006 [DOI] [PubMed] [Google Scholar]
- Heineman F.W., Balaban R.S. 1990. Phosphorus-31 nuclear magnetic resonance analysis of transient changes of canine myocardial metabolism in vivo. J. Clin. Invest. 85:843–852 10.1172/JCI114511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V. 1950. A challenge to biochemists. Biochim. Biophys. Acta. 4:4–11 10.1016/0006-3002(50)90003-5 [DOI] [PubMed] [Google Scholar]
- Himmelreich U., Dobson G.P. 2000. Detection and quantification of free cytosolic inorganic phosphate and other phosphorus metabolites in the beating mouse heart muscle in situ. NMR Biomed. 13:467–473 10.1002/nbm.664 [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Lindstedt S.L., Claassen H., Taylor C.R., Mathieu O., Weibel E.R. 1984. Scaling mitochondrial volume in heart to body mass. Respir. Physiol. 55:131–137 10.1016/0034-5687(84)90018-5 [DOI] [PubMed] [Google Scholar]
- Hudsmith L.E., Tyler D.J., Emmanuel Y., Petersen S.E., Francis J.M., Watkins H., Clarke K., Robson M.D., Neubauer S. 2009. (31)P cardiac magnetic resonance spectroscopy during leg exercise at 3 Tesla. Int. J. Cardiovasc. Imaging. 25:819–826 10.1007/s10554-009-9492-8 [DOI] [PubMed] [Google Scholar]
- Jacobus W.E., Moreadith R.W., Vandegaer K.M. 1982. Mitochondrial respiratory control. Evidence against the regulation of respiration by extramitochondrial phosphorylation potentials or by [ATP]/[ADP] ratios. J. Biol. Chem. 257:2397–2402 [PubMed] [Google Scholar]
- Jung K., Jung H. 2009. A new mechanism of phosphoregulation in signal transduction pathways. Sci. Signal. 2:pe71 10.1126/scisignal.296pe71 [DOI] [PubMed] [Google Scholar]
- Katz L.A., Koretsky A.P., Balaban R.S. 1988. Activation of dehydrogenase activity and cardiac respiration: a 31P-NMR study. Am. J. Physiol. 255:H185–H188 [DOI] [PubMed] [Google Scholar]
- Katz L.A., Swain J.A., Portman M.A., Balaban R.S. 1989. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am. J. Physiol. 256:H265–H274 [DOI] [PubMed] [Google Scholar]
- Kauppinen R.A. 1983. Proton electrochemical potential of the inner mitochondrial membrane in isolated perfused rat hearts, as measured by exogenous probes. Biochim. Biophys. Acta. 725:131–137 10.1016/0005-2728(83)90232-3 [DOI] [PubMed] [Google Scholar]
- Kim D.K., Heineman F.W., Balaban R.S. 1991. Effects of beta-hydroxybutyrate on oxidative metabolism and phosphorylation potential in canine heart in vivo. Am. J. Physiol. 260:H1767–H1773 [DOI] [PubMed] [Google Scholar]
- Lamb H.J., Beyerbacht H.P., Ouwerkerk R., Doornbos J., Pluim B.M., van der Wall E.E., van der Laarse A., de Roos A. 1997. Metabolic response of normal human myocardium to high-dose atropine-dobutamine stress studied by 31P-MRS. Circulation. 96:2969–2977 [DOI] [PubMed] [Google Scholar]
- Ligeti L., Osbakken M.D., Clark B.J., Schnall M., Bolinger L., Subramanian H., Leigh J.S., Chance B. 1987. Cardiac transfer function relating energy metabolism to workload in different species as studied with 31P NMR. Magn. Reson. Med. 4:112–119 10.1002/mrm.1910040203 [DOI] [PubMed] [Google Scholar]
- Liu T., O’Rourke B. 2008. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ. Res. 103:279–288 10.1161/CIRCRESAHA.108.175919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J.G., Halestrap A.P., Denton R.M. 1990. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70:391–425 [DOI] [PubMed] [Google Scholar]
- Means C.K., Lygren B., Langeberg L.K., Jain A., Dixon R.E., Vega A.L., Gold M.G., Petrosyan S., Taylor S.S., Murphy A.N., et al. 2011. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc. Natl. Acad. Sci. USA. 108:E1227–E1235 10.1073/pnas.1107182108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha V.K., Arai A.E., Balaban R.S. 1997. Maximum oxidative phosphorylation capacity of the mammalian heart. Am. J. Physiol. 272:H769–H775 [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R. 1985. Regulation of oxidative phosphorylation in mitochondria by external free Ca2+ concentrations. J. Biol. Chem. 260:4028–4034 [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hansford R.G. 1988. Dependence of cardiac mitochondrial pyruvate dehydrogenase activity on intramitochondrial free Ca2+ concentration. Biochem. J. 256:403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J.R., Denton R.M., England P.J., Randle P.J. 1972. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem. J. 128:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen M.J. 2011. Time to recognise that mitochondria are bacteria? Trends Microbiol. 19:58–64 10.1016/j.tim.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Panov A.V., Scaduto R.C., Jr 1996. Substrate specific effects of calcium on metabolism of rat heart mitochondria. Am. J. Physiol. 270:H1398–H1406 [DOI] [PubMed] [Google Scholar]
- Pecina P., Borisenko G.G., Belikova N.A., Tyurina Y.Y., Pecinova A., Lee I., Samhan-Arias A.K., Przyklenk K., Kagan V.E., Hüttemann M. 2010. Phosphomimetic substitution of cytochrome C tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry. 49:6705–6714 10.1021/bi100486s [DOI] [PubMed] [Google Scholar]
- Phillips D., Aponte A.M., Covian R.G., Balaban R.S. 2011a. Intrinsic protein kinase activity in mitochondrial oxidative phosphorylation complexes. Biochemistry. 50:2515–2529 10.1021/bi101434x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D., Aponte A.M., Covian R., Neufeld E., Yu Z.X., Balaban R.S. 2011b. Homogenous protein programming in the mammalian left and right ventricle free walls. Physiol. Genomics. 43:1198–1206 10.1152/physiolgenomics.00121.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D., Covian R., Aponte A.M., Glancy B., Taylor J.F., Chess D.J., Balaban R.S. 2012. Regulation of oxidative phosphorylation complex activity: effects of tissue specific metabolic stress within an allometric series and acute changes in workload. Am. J. Physiol. Regul. Integr. Comp. Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms D.P., Kronauge J.F., LeFurgey A., Backus M., Hockett D., Ingram P., Lieberman M., Holman B.L., Jones A.G., Davison A. 1994. Mitochondrial localization and characterization of 99Tc-SESTAMIBI in heart cells by electron probe X-ray microanalysis and 99Tc-NMR spectroscopy. Magn. Reson. Imaging. 12:641–652 10.1016/0730-725X(94)92459-7 [DOI] [PubMed] [Google Scholar]
- Popov K.M., Zhao Y., Shimomura Y., Kuntz M.J., Harris R.A. 1992. Branched-chain alpha-ketoacid dehydrogenase kinase. Molecular cloning, expression, and sequence similarity with histidine protein kinases. J. Biol. Chem. 267:13127–13130 [PubMed] [Google Scholar]
- Portman M.A., Heineman F.W., Balaban R.S. 1989. Developmental changes in the relation between phosphate metabolites and oxygen consumption in the sheep heart in vivo. J. Clin. Invest. 83:456–464 10.1172/JCI113904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B.C. 1964. Metabolic function of phosphohistidine. Biochem. Biophys. Res. Commun. 15:556–561 10.1016/0006-291X(64)90504-2 [DOI] [Google Scholar]
- Rizzuto R., Pozzan T. 2006. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86:369–408 10.1152/physrev.00004.2005 [DOI] [PubMed] [Google Scholar]
- Robitaille P.M., Merkle H., Lew B., Path G., Hendrich K., Lindstrom P., From A.H.L., Garwood M., Bache R.J., Uğurbil K. 1990. Transmural high energy phosphate distribution and response to alterations in workload in the normal canine myocardium as studied with spatially localized 31P NMR spectroscopy. Magn. Reson. Med. 16:91–116 10.1002/mrm.1910160110 [DOI] [PubMed] [Google Scholar]
- Schaefer S., Schwartz G.G., Steinman S.K., Meyerhoff D.J., Massie B.M., Weiner M.W. 1992. Metabolic response of the human heart to inotropic stimulation: in vivo phosphorus-31 studies of normal and cardiomyopathic myocardium. Magn. Reson. Med. 25:260–272 10.1002/mrm.1910250205 [DOI] [PubMed] [Google Scholar]
- Scholz T.D., Balaban R.S. 1994. Mitochondrial F1-ATPase activity of canine myocardium: effects of hypoxia and stimulation. Am. J. Physiol. 266:H2396–H2403 [DOI] [PubMed] [Google Scholar]
- Starling E.H., Visscher M.B. 1927. The regulation of the energy output of the heart. J. Physiol. 62:243–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo P.R., Mootha V.K., French S.A., Balaban R.S. 2000. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 278:C423–C435 [DOI] [PubMed] [Google Scholar]
- Territo P.R., French S.A., Balaban R.S. 2001. Simulation of cardiac work transitions, in vitro: effects of simultaneous Ca2+ and ATPase additions on isolated porcine heart mitochondria. Cell Calcium. 30:19–27 10.1054/ceca.2001.0211 [DOI] [PubMed] [Google Scholar]
- Tian R., Halow J.M., Meyer M., Dillmann W.H., Figueredo V.M., Ingwall J.S., Camacho S.A. 1998. Thermodynamic limitation for Ca2+ handling contributes to decreased contractile reserve in rat hearts. Am. J. Physiol. 275:H2064–H2071 [DOI] [PubMed] [Google Scholar]
- Unitt J.F., McCormack J.G., Reid D., MacLachlan L.K., England P.J. 1989. Direct evidence for a role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in the stimulated rat heart. Studies using 31P n.m.r. and ruthenium red. Biochem. J. 262:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijlbergen J.F., van Oudheusden D., Cramer M.J., Ascoop C.A., Zwinderman A.H., Niemeyer M.G., van der Wall E.E., Pauwels E.K. 1994. Quantitative analysis of planar technetium-99m Sestamibi myocardial perfusion images. Clinical application of a modified method for the subtracton of tissue crosstalk. Eur. Heart J. 15:1217–1226 [DOI] [PubMed] [Google Scholar]
- Wan B., Doumen C., Duszynski J., Salama G., Vary T.C., LaNoue K.F. 1993. Effects of cardiac work on electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am. J. Physiol. 265:H453–H460 [DOI] [PubMed] [Google Scholar]
- Weibel E.R., Hoppeler H. 2004. Modeling design and functional integration in the oxygen and fuel pathways to working muscle. Cardiovasc. Eng. 4:5–18 10.1023/B:CARE.0000025118.37085.45 [DOI] [Google Scholar]
- Weibel E.R., Taylor C.R., Hoppeler H. 1991. The concept of symmorphosis: a testable hypothesis of structure-function relationship. Proc. Natl. Acad. Sci. USA. 88:10357–10361 10.1073/pnas.88.22.10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberger A. 1957. Relation between work and labile phosphate content in the isolated dog heart. Circ. Res. 5:175–178 [DOI] [PubMed] [Google Scholar]
- Zhang J., Path G., Chepuri V., Xu Y., Yoshiyama M., Bache R.J., From A.H.L., Uğurbil K. 1993. Responses of myocardial high energy phosphates and wall thickening to prolonged regional hypoperfusion induced by subtotal coronary stenosis. Magn. Reson. Med. 30:28–37 10.1002/mrm.1910300106 [DOI] [PubMed] [Google Scholar]
- Zhang J., Duncker D.J., Xu Y., Zhang Y., Path G., Merkle H., Hendrich K., From A.H.L., Bache R.J., Uğurbil K. 1995. Transmural bioenergetic responses of normal myocardium to high workstates. Am. J. Physiol. 268:H1891–H1905 [DOI] [PubMed] [Google Scholar]
- Zhang J., Ugurbil K., From A.H., Bache R.J. 2001. Myocardial oxygenation and high-energy phosphate levels during graded coronary hypoperfusion. Am. J. Physiol. Heart Circ. Physiol. 280:H318–H326 [DOI] [PubMed] [Google Scholar]