Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated during normal physiological processes are highly reactive with cellular lipids, DNA, and proteins. Superoxide and nitric oxide are the primary ROS and RNS, respectively, produced in cells, and both species react with other molecules and each other to form a diverse array of additional ROS and RNS (e.g., hydrogen peroxide, hydroxyl radical, peroxynitrite, hyperchlorite, singlet oxygen). ROS/RNS were originally thought to represent noxious species produced during oxidative stress that are primarily destructive to cells. Indeed, high levels of ROS and RNS have long been known to promote cell damage and death. However, recent evidence indicates that the production of low to moderate levels of ROS/RNS is critical for the proper regulation of many essential cellular processes including gene expression, signal transduction, and muscle adaptation to endurance exercise training (Reid, 2001; Dröge, 2002; Powers et al., 2011).
Cellular levels of ROS reflect a delicate balance between ROS production and detoxification. Cellular production of ROS in skeletal muscle, with superoxide as the primal species, originates from three principal sources: (1) membrane-associated NADPH oxidase, (2) cytosolic xanthine and xanthine oxidase, and (3) the mitochondrial electron transport chain (ETC). Cellular RNS levels are generated primarily by nitric oxide synthase (to produce nitric oxide) or its subsequent reaction with superoxide to produce peroxynitrite. ROS detoxification involves several cellular antioxidant defense systems including superoxide dismutase (SOD; converting superoxide to H2O2), catalase (breaking down H2O2 to oxygen and water), thioredoxin reductase/thioredoxin (catalyzing the formation/reduction of protein disulfide bonds), glutathione peroxidase (catalyzing reduced glutathione and H2O2 to oxidized glutathione and water), and various non-enzymatic antioxidants (such as reduced glutathione). Despite the existence of such well-coordinated cellular ROS detoxification systems, when uncontrolled ROS production overwhelms these defense mechanisms, excessive ROS stress can trigger irreversible cell damage that contributes to the pathogenesis of a wide variety of disorders including cancer, neurodegenerative diseases, cardiovascular diseases, and muscular dystrophies (Andersen, 2004; Paravicini and Touyz, 2006; Haigis and Yankner, 2010; Lawler, 2011; Khan, 2012).
An unmet need for direct measurement of mitochondrial superoxide dynamics
Superoxide is the primary oxygen free radical produced in mitochondria and is highly unstable, being rapidly dismutated to H2O2 by Mn-SOD. Mitochondria are a major source of superoxide production, which plays a critical role in maintaining the proper redox status of both the organelle and cell. Superoxide is produced in mitochondria by slippage of an electron from the ETC to molecular oxygen during oxidative phosphorylation, the source of aerobic cellular ATP production. Neurodegeneration, cardiomyopathy, and perinatal death result from increased ROS stress caused by ablation of mitochondrial Mn-SOD (Li et al., 1995; Lebovitz et al., 1996). Therefore, characterizing the properties and regulation of mitochondrial superoxide production and detoxification is of central importance to understanding proper cellular redox regulation and the impact of its dysregulation on various pathologies.
The absence of a suitably targeted, specific, and readily reversible sensor for mitochondrial superoxide production has severely limited progress toward this important objective. The most commonly used ROS detectors are MitoSOX-red, H2DCF, and the protein-based redox probe, roGFP. MitoSOX-red is mitochondrial targeted and considered to be a relatively superoxide-specific fluorescent dye at certain excitation wavelengths (e.g., 396 nm). However, superoxide-induced changes in MitoSOX-red fluorescence are irreversible, and its signal is contaminated by DNA binding when using non-optimal excitation wavelengths. H2DCF is normally used to measure cellular levels of ROS, as it is not specifically targeted to mitochondria. In addition, H2DCF fluorescence is also irreversible and dependent on several cellular processes, and thus, does not provide an accurate direct readout of dynamic changes in ROS (Karlsson et al., 2010). Although roGFP can be targeted to the mitochondrial matrix, it is a general redox sensor and does not directly measure levels of ROS or superoxide (Hanson et al., 2004).
Discovery of mitochondrial superoxide flash (mSOF) activity using a reversible, GFP-based superoxide biosensor
“Flashes,” stunning discrete bursts of fluorescence within the mitochondrial matrix, were first observed using a CCD camera in epifluorescence experiments of quiescent skeletal myotubes expressing the mitochondrial-targeted Ca2+-sensitive probe, ratiometric pericam (mt-pericam). However, these flashes were quickly deduced to not be caused by changes in matrix Ca2+ because they were observed only for one of the two Ca2+-sensitive excitation wavelengths of mt-pericam (i.e., 490 nm, but not 405 nm). This conclusion was confirmed in experiments in which flash activity was unaffected after deletion of the calmodulin and calmodulin-binding domains of mt-pericam (termed mt-cpYFP). Subsequent work revealed that mt-cpYFP is a superoxide-specific ROS sensor (Wang et al., 2008).
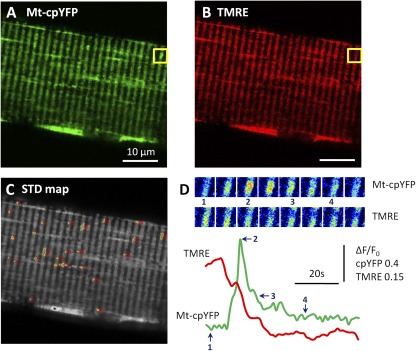
Using mt-cpYFP in conjunction with confocal microscopy, discrete large fluorescence flashes (Fig. 1, A and D) that occur along with a depolarization of the mitochondrial membrane potential (i.e., coincident with a decrease in mitochondrial-targeted tetramethylrhodamine ethyl ester [TMRE] fluorescence; Fig. 1, B and D) are observed within either single or restricted clusters of interconnected mitochondria across a wide variety of cell types (Wang et al., 2008; Pouvreau, 2010; Wei et al., 2011). The depolarization reflects the transient opening of a large conductance pore because the events coincide with a sustained loss of mitochondrial-loaded Rhod-2 fluorescence (Wang et al., 2008) and significant mitochondrial swelling (Ma et al., 2011). Flash events are stochastic and quantal in nature, exhibiting a relatively well-conserved magnitude (0.5 ΔF/F), time to peak (∼5 s), and τdecay (∼8 s), although the frequency of these events varies widely across different cell types and experimental conditions.
Figure 1.
mSOFs are coincident with depolarization of the mitochondrial membrane potential. (A and B) Representative mt-cpYFP (A) or TMRE (B) confocal images of an adult skeletal muscle fiber obtained from a muscle-specific, mt-cpYFP–expressing transgenic mouse co-labeled with TMRE, a mitochondrial membrane potential indicator; boxed region indicates an area containing an mSOF. (C) A standard deviation (STD) map generated from a stack of time-lapsed images from the same fiber shown in A and B (1.24 s/frame, 100 frames), using a custom-developed program (Flash Collector) for automated mSOF detection and analysis. Detected mSOF events are outlined in yellow and numbered in red (event no. 25 corresponds to the boxed flash shown in A and D). (D) Time course of mt-cpYFP and TMRE fluorescence within the boxed region shown in A (mt-cpYFP) and B (TMRE). (Top) Series of pseudo color time-lapse mt-cpYFP (top) and TMRE (bottom) images within the boxed regions. (Bottom) Time course of simultaneously recorded mt-cpYFP (green) and TMRE (red) fluorescence for the individual flash event shown in A and B. Numbers and arrows (1–4) indicate times where the corresponding pseudo color images were taken.
The transient events of mt-cpYFP fluorescence were termed “mitochondrial superoxide flashes” (mSOFs) because their frequency is increased after knockdown of mitochondrial SOD2 (Huang et al., 2011), inhibited by ROS scavengers (tiron), SOD mimetics (MnTMPyP) (Wang et al., 2008) and mitochondrial-targeted antioxidants (mito-TEMPO) (Huang et al., 2011), and abolished under conditions of complete anoxia (Huang et al., 2011). In addition, an irreversible increase in MitoSOX-red fluorescence occurs during each mSOF event (Pouvreau, 2010), consistent with a burst of superoxide production during a flash. Importantly, the in vitro fluorescence of purified recombinant cpYFP is significantly increased by superoxide (produced by xanthine and xanthine oxidase in the presence of oxygen), but not by other ROS/RNS species including hydrogen peroxide, hydroxyl radical, nitric oxide, peroxynitrite, or even across a wide redox potential range (from −319 to −7 mV). The fluorescence of purified cpYFP is also not altered by millimolar concentrations of Ca2+, ATP, ADP, NAD(P)+, or NAD(P)H (Wang et al., 2008). However, as cpYFP fluorescence, like other GFP-based biosensors, is pH sensitive (with a pKa of ∼8.5; Wang et al., 2008), flashes could reflect a complex combination of changes in matrix superoxide and pH (see Controversy 3 below).
Several observations support the stochastic nature of mSOF activity. First, mSOF events occur randomly distributed in space and time, with no apparent connection to one another (Wang et al., 2008). Individual mSOF events occur spontaneously and abruptly and do not propagate to adjacent mitochondria, and repetitive activity from a given site is rarely observed in healthy cells during the time course sampled (Wang et al., 2008; Wei et al., 2011; Li et al., 2012). For example, in adult skeletal muscle fibers, only 3.6 ± 0.5% of all mSOF events exhibit a second event during 2 min of continuous recording. Moreover, a random distribution of individual noncoupled events continues to be observed even when sampling the same region of the cell for several scans. Finally, for cells with two or more flashes, the interval between consecutive flashes in time is well described by an exponential function that depends on the basal mSOF frequency, consistent with flash production being a stochastic Poisson process with a variable rate (Li et al., 2012).
The spatial morphology of mSOF events is highly heterogeneous (see the standard deviation map of detected flash events shown in Fig. 1 C). In skeletal muscle fibers, flashes are observed as being either punctuate, rod-shaped, U-shaped, or string-like either along or across the Z-line. In addition, large clusters or patches that can span several sarcomeres are also observed (Pouvreau, 2010; Fang et al., 2011; Wei et al., 2011). Comparison of mitochondrial localization observed by electron microscopy (Wei et al., 2011) with mSOF dimensions observed in confocal measurements in isolated skeletal muscle fibers or in vivo xyzt 4-D muscle imaging (Fang et al., 2011) indicates that the heterogeneous morphologies of mSOF events observed in skeletal muscle reflects an extensive and highly complex underlying mitochondrial network.
Relationship between mSOF activity and prior studies of mitochondrial membrane potential flickers and ROS generation
The characteristic that mSOF events occur concurrently with a transient depolarization of the mitochondrial membrane is reminiscent of previously described spontaneous transient depolarizations in the mitochondrial membrane potential (“flickers”) that in some cases exhibit similar spatial-temporal properties (Duchen et al., 1998; Hüser et al., 1998; Buckman and Reynolds, 2001; O’Reilly et al., 2003). However, although a transient depolarization of the mitochondrial membrane potential occurs during every mSOF event, similar flickers often occur in the absence of mSOF activity (Wang et al., 2008; Pouvreau, 2010; Wei et al., 2011). In addition, flickers are often repetitively generated at the same site and have been linked to a wide range of stimuli including mitochondrial Ca2+ uptake (Duchen et al., 1998), proton entry through the ATP synthase (Buckman and Reynolds, 2001), and the opening of inner membrane anoin channels (O’Rourke, 2000; Aon et al., 2003). On the other hand, flickers associated with mSOF activity are rarely repetitive, do not require mitochondrial Ca2+ uptake (Wang et al., 2008), and are not blocked by inner membrane anoin channel inhibitors (Pouvreau, 2010). Thus, transient depolarizations of the mitochondrial membrane potential observed during mSOF events appear to reflect a specific subpopulation of the spontaneous flickers reported previously.
Zorov et al. (2000) reported that intense focal laser photoactivation can experimentally trigger a local depolarization of the mitochondrial membrane potential that is coincident with a corresponding discrete increase in mitochondrial ROS production. Moreover, under conditions of substrate deprivation (Romashko et al., 1998) or continued intense laser irradiation (Zorov et al., 2000; Aon et al., 2003; Brady et al., 2004), local events of mitochondrial membrane potential depolarization and ROS generation can lead to propagating, cell-wide oscillations in membrane potential, ROS, and NADH. The potential role of mitochondrial permeability transition pore (mPTP) activity in both discrete mitochondrial membrane potential flickers (Hüser et al., 1998; Zorov et al., 2000; Buckman and Reynolds, 2001; Jacobson and Duchen, 2002; Aon et al., 2003) and propagated global metabolic oscillations (Romashko et al., 1998; Aon et al., 2003; Brady et al., 2004) has been debated. Thus, in some respects (e.g., coincidence of a flicker with an ROS burst, ETC dependence, and debate regarding mPTP involvement), mSOF events are related to these findings. Indeed, it is possible that mSOFs may serve as fundamental building blocks for triggered local ROS-induced ROS release and propagating cell-wide waves of metabolic oscillation. Specifically, propagating waves of mitochondrial depolarization and ROS production may arise from the coordination and summation of discrete mSOF events, analogous to how propagating global Ca2+ waves can result from the spatial and temporal summation of Ca2+ sparks (Cheng et al., 1996). Nevertheless, the discovery of mSOF activity represents a major advance for the field because their identification demonstrates that individual mitochondria in quiescent (unstimulated) cells produce discrete quanta of superoxide over time that do not normally propagate into cell-wide events. As such, mSOF activity reflects a constant, ongoing physiological process of mitochondrial bioenergetics that uses the fundamental core elements and machinery shown previously to also mediate mitochondrial flickers and cell-wide metabolic oscillations.
mSOF events as biomarkers of cellular metabolic activity
Similar to localized Ca2+ release events in muscle, termed “Ca2+ sparks,” mSOF activity is also stochastic and quantal in nature, exhibiting relatively uniform amplitude, spatial, and temporal properties, whereas the frequency of mSOF events varies across cell types, metabolic activity, and pathological conditions. Importantly, mSOF activity reflects the cellular aerobic metabolic state, as flash frequency is strongly dependent on mitochondrial ETC, ATP synthase, and adenine nucleotide translocase (ANT) functionality (Wang et al., 2008; Fang et al., 2011; Wei et al., 2011). mSOF frequency in skeletal muscle is increased early during repetitive tetanic stimulation and decreased after prolonged repetitive stimulation (Wei et al., 2011). Moreover, mSOF activity in adult ventricular cardiomyocytes is abolished during complete anoxia (Huang et al., 2011) and markedly increased during reperfusion after either hypoxia (Wang et al., 2008) or chemical anoxia (Huang et al., 2011). In addition, mSOF activity is significantly increased when skeletal muscle fibers are exposed to media rich in mitochondrial substrates (Pouvreau, 2010; Wei et al., 2011) and after glucose injection in live animals (Fang et al., 2011). Finally, mSOF activity is also increased in several pathological models of enhanced oxidative stress including cardiac ischemic reperfusion injury (Wang et al., 2008), malignant hyperthermia (Wei et al., 2011), and ROS-induced apoptosis (Ma et al., 2011).
Proposed mechanism of mSOF generation
Although the precise sequence of events responsible for initiation and termination of an mSOF event remains unclear, the mechanism must explain the following fundamental observations: (a) mSOFs are stochastic all-or-none events with relatively uniform spatiotemporal properties; (b) mSOF frequency is regulated by the cellular metabolic state; (c) mSOF activity depends on ETC, ATP synthase, and ANT functionality; and (d) mSOF events are coincident with the opening of a large conductance pore that depolarizes the mitochondria membrane potential (Fig. 1 D).
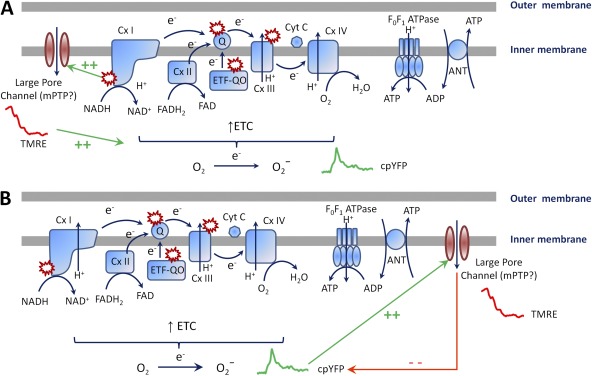
To account for these fundamental observations, Wang et al. (2008) proposed that constitutive low-level superoxide production from the ETC triggers the opening of a large pore within the mitochondrial inner membrane (e.g., mPTP) that results in depolarization, mitochondrial swelling, and alterations in the molecular components of the ETC machinery. The depolarization stimulates ETC activity to increase extrusion of protons from the mitochondrial matrix to restore the mitochondrial membrane potential. This acceleration in ETC activity, coupled with alterations in the molecular components of the ETC machinery that reduce efficiency of electron flux, is proposed to lead to an increase in electron slippage and superoxide production from several specific sites within the ETC (see red “explosions” in Fig. 2), leading to a burst in superoxide production (Fig. 2 A). Consistent with this general proposed mechanism, prior studies in intact cells indicate that brief openings of the mPTP result in both mitochondrial depolarization and increased ROS production (Zorov et al., 2000; Batandier et al., 2004). The reason for flash termination in this mechanism is not entirely clear but could result from either auto-inhibition of the ETC by superoxide or other downstream ROS/RNS species (Brookes et al., 2004), proton influx and matrix acidification inhibiting mPTP activity (Nicolli et al., 1993), local depletion of ETC substrates, or deceleration in ETC activity upon restoration of the mitochondrial membrane potential.
Figure 2.
Potential mechanisms for mSOF generation. (A) Mechanism 1: Pore opening triggers mSOF events. A small increase in constitutive ROS production opens a large pore channel to cause depolarization of the mitochondrial membrane potential, which subsequently stimulates the ETC to produce a burst in superoxide production. (B) Mechanism 2: Pore opening terminates mSOF events. A mild hyperpolarization of the mitochondrial inner membrane potential results in a dramatic increase in superoxide production from complex I, which triggers the opening of a large pore channel that depolarizes the mitochondrial membrane potential. The pore opening dissipates the electrical and proton gradient across the inner membrane to terminate ETC-dependent superoxide production from complex I (see text for details). ETC, electron transport chain; ETF-QO, electron transferring flavoprotein-quinone oxidoreductase; ANT, adenine nucleotide translocase; mPTP, mitochondrial permeability transition pore; Cyt C, cytochrome C; Q, Q cycle; Cx I, II, III, IV, complex I, II, III, IV; TMRE, tetramethylrhodamine ethyl ester. Red “explosion” symbols indicate places where electron leak and superoxide production occur. Green arrows indicate stimulatory effects, and red arrows indicate inhibitory effects.
Although this proposed mechanism for mSOF generation accounts for the fundamental observations outlined above (quantal nature of flash properties, metabolic state of the cell, ETC/synthase/ANT dependence, and depolarization via the opening of a large pore channel), several notable controversies and unanswered issues remain to be resolved. These issues include the identity of the large pore channel, the temporal relationship between channel opening and flash generation, and the relative importance of changes in matrix superoxide and pH during a flash. These hotly debated controversies and their implications for future work are considered in detail below.
Controversy 1: Identity of the large pore channel
The finding that mSOF activity is coincident with a transient depolarization of the mitochondrial inner membrane potential, rapid equilibration of small molecules (e.g., Rhod-2) between the matrix and cytosol (Wang et al., 2008), and mitochondrial swelling (Ma et al., 2011) demonstrates that mSOF events are coincident with the opening of a large conductance channel within the mitochondrial inner membrane. However, a consensus regarding the identity of the channel involved and precisely how it is activated has not been reached. Initial studies conducted in cardiac myocytes (Wang et al., 2008) and subsequently in HeLa cells (Ma et al., 2011) implicated the mPTP, a high-conductance Ca2+/ROS-sensitive pore capable of permeating molecules up to 1.5 kD. These studies found that mSOF frequency is reduced both by the addition of 1 µM cyclosporin A (CsA) and after siRNA-mediated knockdown of cyclophilin D (CypD). In addition, 20 µM atractyloside (an mPTP activator and ANT inhibitor) increases mSOF frequency in neonatal rat ventricular cardiomyocytes (Wang et al., 2008). However, a role for CsA/CypD-dependent mPTP activity in mSOF activity was not confirmed in adult skeletal muscle fibers where mSOF activity is unaltered by 1–5 µM CsA (Pouvreau, 2010; Wei et al., 2011) and is normal in muscle fibers isolated from CypD knockout mice (Wei et al., 2011). In addition, 1 µM carboxyatractylaside decreases both mSOF frequency and amplitude in adult skeletal muscle fibers, presumably as a result of an inhibition of ANT activity because a similar effect is also observed for the ANT inhibitor bongkreric acid (20 µM) (Wei et al., 2011).
Although tissue-specific factors may underlie some differences observed between mSOF activity in cardiac and skeletal muscle cells, the observation that mSOF activity is normal in skeletal muscle fibers from CypD-null mice indicates that CypD-dependent mPTP activity is not an essential requirement for mSOF generation. As one possible explanation, a CypD-independent mechanism for mPTP activation could be involved. Although the mPTP in CypD-deficient cells shows a striking reduction in Ca2+ sensitivity of activation (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005), mPTP responsiveness to ubiquinone 0, depolarization, pH, adenine nucleotides, and oxidative stress is unaffected by CypD ablation (Basso et al., 2005). Thus, high levels of superoxide produced during a flash could trigger depolarization by opening nearby mPTP channels in a CsA/CypD-independent manner, although this mechanism would place mPTP activation and mitochondrial depolarization downstream of superoxide production (see below). Alternatively, it will be important for future studies to consider the potential role of other large conductance channels present within the mitochondrial inner membrane. For example, two separate large pore, high-conductance translocase of inner membrane (TIM) channels (∼1 nS) coordinate the transfer of polypeptides from the mitochondrial intermembrane space into the matrix (TIM22 and TIM23) (Grigoriev et al., 2004). Thus, an intriguing possibility that warrants future consideration is to determine if mSOF events are initiated by a transient depolarization of the mitochondrial membrane potential produced during polypeptide import into the mitochondrial matrix through TIM22/23 channels.
Controversy 2: Chicken or egg: Temporal relationship between large pore channel opening and mSOF activation
Prior studies demonstrated a clear depolarization of the mitochondrial membrane potential (i.e., transient reduction in TMRE fluorescence) coincident with every mSOF event (Wang et al., 2008; Pouvreau, 2010; Fang et al., 2011; Ma et al., 2011; Wei et al., 2011). Although in some cases the TMRE fluorescence returns to baseline during the decay in mt-cpYFP fluorescence, in other cases the return is significantly delayed (e.g., tens of seconds). However, within the temporal resolution of these experiments, the time course of the reduction in TMRE fluorescence overlaps with the rise time in mt-cpYFP fluorescence during an mSOF event. In addition, potential kinetic differences between the TMRE and mt-cpYFP reporters further limit the ability to determine which of the two events occurs first. Thus, definitive evidence is lacking with regard to whether large pore channel opening and mitochondrial depolarization precede or are the consequence of the corresponding mSOF event.
Because transient depolarizations of the mitochondrial membrane potential in intact cells were shown to stimulate ROS production from the ETC (Zorov et al., 2000; Batandier et al., 2004), Wang et al. (2008) proposed that openings of a large pore channel (e.g., mPTP) in the mitochondrial inner membrane trigger an ETC-dependent burst in superoxide production (Fig. 2 A). Consistent with this idea, the reduction of mSOF activity by inhibition of mPTP activity (Wang et al., 2008; Ma et al., 2011), the rapid equilibration of small molecules (Wang et al., 2008), and mitochondrial swelling (Ma et al., 2011) during a mSOF event support the involvement of the opening of a large conductance channel within the mitochondrial inner membrane during each mSOF event. However, although all mSOF events occur together with a simultaneous reduction in TMRE fluorescence (i.e., mitochondrial depolarization), many similar reductions in TMRE fluorescence occur in the absence of an mSOF (Wang et al., 2008). Thus, if a flash event reflects a depolarization-induced burst in ETC-dependent superoxide production, it is not entirely clear why this would be observed for only a subset of these depolarizations. In addition, the application of tiron, a superoxide scavenger, similarly reduces the frequency of both mSOF events and TMRE flickers in adult skeletal muscle fibers (Pouvreau, 2010). These two observations (i.e., TMRE reductions without mSOFs and tiron similarly reducing both mSOF and TMRE frequency) are most consistent with large pore channel openings residing downstream of (and potentially activated by) bursts in superoxide produced by the ETC.
Results from isolated mitochondria demonstrate a very steep nonlinear relationship between the mitochondrial membrane potential and ROS produced by complex I (Hansford et al., 1997; Votyakova and Reynolds, 2001; Miwa and Brand, 2003). Specifically, the rate of superoxide production from complex I during reverse electron transport is exquisitely sensitive to changes in the proton motive force (Lambert and Brand, 2004; Lambert et al., 2010), such that superoxide production by complex I is markedly reduced by even mild uncoupling (Brand et al., 2004). Thus, an alternate mechanism for mSOF generation and termination is that mild hyperpolarization of the mitochondrial membrane potential produces a burst of superoxide produced at complex I that is then terminated by superoxide activating nearby CypD-independent mPTP channels to open, and thus, depolarize the mitochondrial membrane potential, dissipate the proton motive force, and reduce superoxide production from complex I (Fig. 2 B). Indeed, Pouvreau (2010) reported that transient mitochondrial hyperpolarization is sometimes observed just before flash generation. According to this model, CypD-independent mPTP activation is located downstream of ETC-dependent superoxide production, and its activation by superoxide serves to terminate the mSOF event by rapidly dissipating the mitochondrial proton motive force, and thus, superoxide production from complex I (Fig. 2 B).
Clearly, additional work is required to more definitively determine the temporal relationship between large pore channel opening, depolarization of the mitochondrial inner membrane potential, and mSOF generation. Resolution of this central issue will significantly impact proper refinement of the fundamental mechanism for mSOF generation and termination.
Controversy 3: Do flashes reflect oscillations in mitochondrial superoxide, ATP, and/or pH?
As discussed above, the following lines of evidence support the conclusion that mt-cpYFP flash events reflect a transient elevation of superoxide within the mitochondrial matrix: (a) purified cpYFP fluorescence is increased by superoxide produced by xanthine and xanthine oxidase and reversed by the addition of Cu/Zn-SOD (Wang et al., 2008); (b) purified cpYFP fluorescence is not altered by other ROS/NOS, Ca2+, ATP, ADP, NAD(P)H/NAD(P)+, or redox potential (Wang et al., 2008); (c) flash frequency is inhibited by superoxide scavengers (e.g., tiron), SOD mimetics (e.g., MnTMPyP), and mitochondrial-targeted antioxidants (e.g., mito-TEMPO) (Wang et al., 2008; Pouvreau, 2010; Huang et al., 2011); (d) flash activity is enhanced by agents that increase superoxide production (e.g., menadione) (Huang et al., 2011); (e) flash activity is enhanced after siRNA-mediated knockdown of mitochondrial Mn-SOD (Huang et al., 2011); (f) flash events are coincident with an irreversible increase in MitoSOX-red fluorescence (Pouvreau, 2010); and (g) flash activity is reduced during severe hypoxia, abolished under conditions of complete anoxia, and increased immediately upon reperfusion with oxygenated media (Wang et al., 2008; Huang et al., 2011).
Despite this considerable evidence, the suggestion that mt-cpYFP flashes reflect transient bursts of superoxide production within the mitochondrial matrix has been challenged. Because flash events are tightly coupled to respiration and blocked by agents that inhibit ATP synthesis, including oligomycin and antimycin A, the latter of which actually enhances steady-state superoxide production from purified mitochondria (Muller et al., 2004; Anderson and Neufer, 2006), Muller (2009) suggested that the probe may actually report changes in mitochondrial ATP rather than superoxide. However, in contrast to this suggestion, purified cpYFP exhibits no sensitivity to concentrations of ATP up to 10 mM, flash activity is absent in glycolytic ρ0 cells, and mSOF frequency transiently peaks immediately upon reperfusion after anoxia, whereas ATP recovery during this time is quite slow as the adenine nucleotide deficit is replenished (Wang et al., 2008; Huang et al., 2011).
A recent study in Arabidopsis mitochondria observed stochastic bursts in mt-cpYFP fluorescence with amplitude and spatiotemporal properties similar to those reported previously for mSOF activity, but concluded that the events reflected transient alkalinization of the mitochondrial matrix rather than a change in superoxide (Schwarzländer et al., 2011). Indeed, like other GFP-based probes, purified cpYFP is pH sensitive, with an increase in fluorescence observed upon alkalinization (Wang et al., 2008). Schwarzländer et al. (2011) reported that mt-cpYFP exhibits a pKa of ∼8.6 in lysed Arabidopsis mitochondria (we have confirmed a similar pKa for the probe in intact adult mouse skeletal muscle fibers using an in vitro K+/nigericin calibration approach). Thus, mt-cpYFP fluorescence is clearly sensitive to both superoxide ions and pH.
Schwarzländer et al. (2011) reported that global mt-cpYFP fluorescence in purified Arabidopsis mitochondria was increased upon the application of substrates (e.g., succinate with rotenone, pyruvate plus malate, and thiamine pyrophosphate). Moreover, this increase in global probe fluorescence was not altered by conditions expected to either enhance (e.g., menadione, Mn-SOD knockdown) or reduce (tiron, tempol) superoxide production. Thus, the observed increase in global mt-cpYFP fluorescence during the addition of mitochondrial substrates is consistent with ETC-dependent proton flux promoting matrix alkalinization. Unfortunately, however, effects of superoxide-modifying interventions on dynamic flash activity in Arabidopsis mitochondria were not determined. The proposal that mt-cpYFP flashes in Arabidopsis mitochondria reflect transient events of matrix alkalinization was based on the observation that flashes were eliminated by nigericin, a K+–H+ antiporter that abolishes the mitochondrial pH gradient without altering membrane potential (Schwarzländer et al., 2011). However, mt-cpYFP flashes in this study were obtained from purified mitochondria in the presence of 20 µM rotenone, a potent complex I inhibitor that abolishes mSOF activity in intact cells (Wang et al., 2008; Wei et al., 2011). Thus, the relevance of these events to those reported in cells with intact complex I functionality is unclear. More importantly, nigericin treatment will also inhibit ETC-dependent superoxide production because ROS generation from complex I depends strongly on the presence of the pH gradient across the mitochondrial inner membrane (Lambert and Brand, 2004; Lambert et al., 2010).
Mitochondria are highly dynamic organelles that constantly undergo oxidative phosphorylation to produce ATP needed to support a diverse array of cellular energy requirements. Because the ETC pumps protons out of the matrix and the resulting proton gradient is used to drive ATP production by the F1F0 ATPase, fluctuations in matrix pH in actively respiring mitochondria are not unexpected. Indeed, mitochondrial alkalinization transients in quiescent cells have been reported using mito-SypHer, a mitochondrial-targeted ratiometric pH-sensitive probe (Azarias and Chatton, 2011; Santo-Domingo, J., and N. Demaurex. 2011. 65th Annual Meeting of The Society of General Physiologists. Abstr. 34). In astrocytes, mitochondrial alkalinization transients are coincident with mitochondrial Na+ spikes (increase in CoroNa Red fluorescence), mitochondrial depolarizations (decrease in TRME fluorescence), and bursts in superoxide production (increase in MitoSOX-red fluorescence) (Azarias and Chatton, 2011). These results are consistent with the model in Fig. 2 A, as the opening of a large conductance channel would: (a) provide a pathway for Na+ influx down its electrochemical gradient, (b) depolarize the mitochondrial membrane potential, and (c) stimulate a burst in ETC activity that pumps protons across the inner membrane to alkalinize the matrix. Because mt-cpYFP fluorescence increases in response to both superoxide ions and alkalinization, it is conceivable that flashes represent a combination of changes in both superoxide and pH. However, a critical piece of evidence that argues strongly against matrix alkalinization occurring during a flash is that these events are coincident with the opening of a large pore channel (e.g., CypD-independent mPTP activation) (Wang et al., 2008; Pouvreau, 2010; Ma et al., 2011; Wei et al., 2011). Given the significant pH gradient and highly negative membrane potential across the mitochondrial inner membrane, the opening of a large pore channel would result in significant proton influx and matrix acidification. Simultaneous measurement of matrix pH during mSOF activity would help to address this issue.
Implications and future perspectives
Mitochondria have long been known to represent a primary source for energy and superoxide production in the cell. The recent discovery of stochastic mSOF activity in quiescent cells and the tight linkage of these events to ETC/ANT activity, large pore channel opening, and the cellular metabolic state have provided intriguing new insights into the intimate relationship between mitochondrial energy production and ROS signaling. As highlighted in this Perspective, several unanswered questions and pressing controversies regarding mSOF activity remain to be resolved. For example, details regarding the precise mechanism of mSOF generation/termination and their temporal relationship with several other simultaneously occurring mitochondrial processes (e.g., changes in mPTP activity, membrane potential, pH, Na+, O2 consumption, ATP production, etc.) still need to be fully worked out. Potentially, many of the studies discussed in this Perspective may actually be measuring different aspects of a common fundamental bioenergetic process in individual mitochondria. In this context, mSOF events reflect a constant, ongoing physiological process in quiescent cells that is designed to produce quanta of mitochondrial superoxide, the frequency of which is modulated by the particular cellular context and metabolic state. However, additional work is needed to fully characterize the cellular mechanisms that regulate the frequency of mSOF production. For example, the frequency of mSOF activity may be regulated by many cellular signaling processes including mitochondrial Ca2+ uptake during cytoplasmic Ca2+ signaling (Wei et al., 2011) or mitochondrial PKCε translocation during ischemic preconditioning (Budas and Mochly-Rosen, 2007). Finally, it will be important for future studies to determine the degree to which altered mSOF activity contributes to ROS overproduction and metabolic dysfunction in a wide range of mitochondrial diseases and oxidative stress related disorders.
This Perspectives series includes articles by Sheu et al., Zhang et al., Balaban, Santo-Domingo and Demaurex, O-Uchi et al., Nowikovsky et al., and Galloway and Yoon.
Acknowledgments
We would like to thank Drs. Heping (Peace) Cheng, Shey-Shing Sheu, and Wang Wang for many helpful and stimulating discussions regarding this work.
This research is supported by a National Institutes of Health Grant (AR44657 to R.T. Dirksen) and an Academia Dei Lincea Fellowship (to L. Wei).
Shey-Shing Sheu served as guest editor.
Footnotes
Abbreviations used in this paper:
- ANT
- adenine nucleotide translocase
- CsA
- cyclosporin A
- CypD
- cyclophilin D
- ETC
- electron transport chain
- mPTP
- mitochondrial permeability transition pore
- mSOF
- mitochondrial superoxide flash
- mt-pericam
- ratiometric pericam
- RNS
- reactive nitrogen species
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- TIM
- translocase of inner membrane
- TMRE
- tetramethylrhodamine ethyl ester
References
- Anderson E.J., Neufer P.D. 2006. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am. J. Physiol. Cell Physiol. 290:C844–C851 10.1152/ajpcell.00402.2005 [DOI] [PubMed] [Google Scholar]
- Andersen J.K. 2004. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. 10:S18–S25 10.1038/nrn1434 [DOI] [PubMed] [Google Scholar]
- Aon M.A., Cortassa S., Marbán E., O’Rourke B. 2003. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 278:44735–44744 10.1074/jbc.M302673200 [DOI] [PubMed] [Google Scholar]
- Azarias G., Chatton J.Y. 2011. Selective ion changes during spontaneous mitochondrial transients in intact astrocytes. PLoS ONE. 6:e28505 10.1371/journal.pone.0028505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., et al. 2005. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 434:658–662 10.1038/nature03434 [DOI] [PubMed] [Google Scholar]
- Basso E., Fante L., Fowlkes J., Petronilli V., Forte M.A., Bernardi P. 2005. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 280:18558–18561 10.1074/jbc.C500089200 [DOI] [PubMed] [Google Scholar]
- Batandier C., Leverve X., Fontaine E. 2004. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J. Biol. Chem. 279:17197–17204 10.1074/jbc.M310329200 [DOI] [PubMed] [Google Scholar]
- Brady N.R., Elmore S.P., van Beek J.J., Krab K., Courtoy P.J., Hue L., Westerhoff H.V. 2004. Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization. Biophys. J. 87:2022–2034 10.1529/biophysj.103.035097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M.D., Affourtit C., Esteves T.C., Green K., Lambert A.J., Miwa S., Pakay J.L., Parker N. 2004. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 37:755–767 10.1016/j.freeradbiomed.2004.05.034 [DOI] [PubMed] [Google Scholar]
- Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.-S. 2004. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287:C817–C833 10.1152/ajpcell.00139.2004 [DOI] [PubMed] [Google Scholar]
- Buckman J.F., Reynolds I.J. 2001. Spontaneous changes in mitochondrial membrane potential in cultured neurons. J. Neurosci. 21:5054–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas G.R., Mochly-Rosen D. 2007. Mitochondrial protein kinase Cepsilon (PKCepsilon): emerging role in cardiac protection from ischaemic damage. Biochem. Soc. Trans. 35:1052–1054 10.1042/BST0351052 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer M.R., Lederer W.J., Cannell M.B. 1996. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 270:C148–C159 [DOI] [PubMed] [Google Scholar]
- Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47–95 [DOI] [PubMed] [Google Scholar]
- Duchen M.R., Leyssens A., Crompton M. 1998. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J. Cell Biol. 142:975–988 10.1083/jcb.142.4.975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., Chen M., Ding Y., Shang W., Xu J., Zhang X., Zhang W., Li K., Xiao Y., Gao F., et al. 2011. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 21:1295–1304 10.1038/cr.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev S.M., Muro C., Dejean L.M., Campo M.L., Martinez-Caballero S., Kinnally K.W. 2004. Electrophysiological approaches to the study of protein translocation in mitochondria. International Review of Cytology. Academic Press, New York: 227–274 [DOI] [PubMed] [Google Scholar]
- Haigis M.C., Yankner B.A. 2010. The aging stress response. Mol. Cell. 40:333–344 10.1016/j.molcel.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R.G., Hogue B.A., Mildaziene V. 1997. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 29:89–95 10.1023/A:1022420007908 [DOI] [PubMed] [Google Scholar]
- Hanson G.T., Aggeler R., Oglesbee D., Cannon M., Capaldi R.A., Tsien R.Y., Remington S.J. 2004. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 279:13044–13053 10.1074/jbc.M312846200 [DOI] [PubMed] [Google Scholar]
- Huang Z., Zhang W., Fang H., Zheng M., Wang X., Xu J., Cheng H., Gong G., Wang W., Dirksen R.T., Sheu S.S. 2011. Response to “A critical evaluation of cpYFP as a probe for superoxide.” Free Radic. Biol. Med. 51:1937–1940 10.1016/j.freeradbiomed.2011.08.024 [DOI] [PubMed] [Google Scholar]
- Hüser J., Rechenmacher C.E., Blatter L.A. 1998. Imaging the permeability pore transition in single mitochondria. Biophys. J. 74:2129–2137 10.1016/S0006-3495(98)77920-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J., Duchen M.R. 2002. Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 115:1175–1188 [DOI] [PubMed] [Google Scholar]
- Karlsson M., Kurz T., Brunk U.T., Nilsson S.E., Frennesson C.I. 2010. What does the commonly used DCF test for oxidative stress really show? Biochem. J. 428:183–190 10.1042/BJ20100208 [DOI] [PubMed] [Google Scholar]
- Khan S.R. 2012. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol. Res. 40:95–112 10.1007/s00240-011-0448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A.J., Brand M.D. 2004. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 382:511–517 10.1042/BJ20040485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A.J., Buckingham J.A., Boysen H.M., Brand M.D. 2010. Low complex I content explains the low hydrogen peroxide production rate of heart mitochondria from the long-lived pigeon, Columba livia. Aging Cell. 9:78–91 10.1111/j.1474-9726.2009.00538.x [DOI] [PubMed] [Google Scholar]
- Lawler J.M. 2011. Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with Duchenne muscular dystrophy. J. Physiol. 589:2161–2170 10.1113/jphysiol.2011.207456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R.M., Zhang H., Vogel H., Cartwright J., Jr, Dionne L., Lu N., Huang S., Matzuk M.M. 1996. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA. 93:9782–9787 10.1073/pnas.93.18.9782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Zhang W., Fang H., Xie W., Liu J., Zheng M., Wang X., Wang W., Tan W., Cheng H. 2012. Superoxide flashes reveal novel properties of mitochondrial reactive oxygen species excitability in cardiomyocytes. Biophys. J. 102:1011–1021 10.1016/j.bpj.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., et al. 1995. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 11:376–381 10.1038/ng1295-376 [DOI] [PubMed] [Google Scholar]
- Ma Q., Fang H., Shang W., Liu L., Xu Z., Ye T., Wang X., Zheng M., Chen Q., Cheng H. 2011. Superoxide flashes: early mitochondrial signals for oxidative stress-induced apoptosis. J. Biol. Chem. 286:27573–27581 10.1074/jbc.M111.241794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S., Brand M.D. 2003. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem. Soc. Trans. 31:1300–1301 10.1042/BST0311300 [DOI] [PubMed] [Google Scholar]
- Muller F.L. 2009. A critical evaluation of cpYFP as a probe for superoxide. Free Radic. Biol. Med. 47:1779–1780 10.1016/j.freeradbiomed.2009.09.019 [DOI] [PubMed] [Google Scholar]
- Muller F.L., Liu Y., Van Remmen H. 2004. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 279:49064–49073 10.1074/jbc.M407715200 [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T., Tsujimoto Y. 2005. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 434:652–658 10.1038/nature03317 [DOI] [PubMed] [Google Scholar]
- Nicolli A., Petronilli V., Bernardi P. 1993. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry. 32:4461–4465 10.1021/bi00067a039 [DOI] [PubMed] [Google Scholar]
- O’Reilly C.M., Fogarty K.E., Drummond R.M., Tuft R.A., Walsh J.V., Jr 2003. Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys. J. 85:3350–3357 10.1016/S0006-3495(03)74754-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B. 2000. Pathophysiological and protective roles of mitochondrial ion channels. J. Physiol. 529:23–36 10.1111/j.1469-7793.2000.00023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini T.M., Touyz R.M. 2006. Redox signaling in hypertension. Cardiovasc. Res. 71:247–258 10.1016/j.cardiores.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Pouvreau S. 2010. Superoxide flashes in mouse skeletal muscle are produced by discrete arrays of active mitochondria operating coherently. PLoS ONE. 5:e13035 10.1371/journal.pone.0013035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S.K., Talbert E.E., Adhihetty P.J. 2011. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 589:2129–2138 10.1113/jphysiol.2010.201327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M.B. 2001. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don’t. J. Appl. Physiol. 90:724–731 [DOI] [PubMed] [Google Scholar]
- Romashko D.N., Marban E., O’Rourke B. 1998. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc. Natl. Acad. Sci. USA. 95:1618–1623 10.1073/pnas.95.4.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzländer M., Logan D.C., Fricker M.D., Sweetlove L.J. 2011. The circularly permuted yellow fluorescent protein cpYFP that has been used as a superoxide probe is highly responsive to pH but not superoxide in mitochondria: implications for the existence of superoxide ‘flashes.’ Biochem. J. 437:381–387 10.1042/BJ20110883 [DOI] [PubMed] [Google Scholar]
- Votyakova T.V., Reynolds I.J. 2001. DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 79:266–277 10.1046/j.1471-4159.2001.00548.x [DOI] [PubMed] [Google Scholar]
- Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., et al. 2008. Superoxide flashes in single mitochondria. Cell. 134:279–290 10.1016/j.cell.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Salahura G., Boncompagni S., Kasischke K.A., Protasi F., Sheu S.S., Dirksen R.T. 2011. Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB J. 25:3068–3078 10.1096/fj.11-187252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. 2000. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192:1001–1014 10.1084/jem.192.7.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]