Abstract
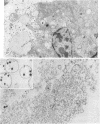
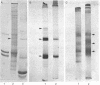
Preparations enriched in apparently intact secretory vesicles were isolated from homogenates of lactating rat and bovine mammary tissue by differential and density gradient centrifugation in isoosmotic media. Morphologically these preparations consisted nearly entirely of vesicles of varying sizes, at least some of which contained casein micelles. Endoplasmic reticulum vesicles, Golgi apparatus cisterna and dictyosomes, mitochondria, peroxisomes, lysosomes, and nuclei were not observed in secretory vesicle-rich fractions. Vesicle preparations were enriched in lactose relative to total membrane fractions from mammary gland. The galactosyltransferase of lactose synthase (UDPgalactose: D-glucose 4 beta-galactosyl-transferase, EC 2.4.1.22) was also present in secretory vesicle preparations, alphas1- and beta-caseins, alpha-lactalbumin, and beta-lactoglobulin, the major secretory proteins of differentiated mammary epithelial cells, were identified as constituents of vesicle-rich fractions from bovine mammary gland. These observations suggest that the major carbohydrate and major proteins of milk are compartmentalized into secretory vesicles and are secreted by exocytotic fusion of secretory vesicles with the apical plasma membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARGMANN W., FLEISCHHAUTER K., KNOOP A. [On the morphology of milk secretion. II. With a criticism of the scheme of secretion morphology]. Z Zellforsch Mikrosk Anat. 1961;53:545–568. [PubMed] [Google Scholar]
- Baumrucker C. R., Keenan T. W. Membranes of mammary gland. X. Adenosine triphosphate dependent calcium accumulation by Golgi apparatus rich fractions from bovine mammary gland. Exp Cell Res. 1975 Feb;90(2):253–260. doi: 10.1016/0014-4827(75)90314-6. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushway A. A., Keenan T. W. 5-thio-D-glucose is an acceptor for UDP-galactose : D-glucose 1-galactosyltransferase. Biochem Biophys Res Commun. 1978 Mar 30;81(2):305–309. doi: 10.1016/0006-291x(78)91533-4. [DOI] [PubMed] [Google Scholar]
- Castle J. D., Jamieson J. D., Palade G. E. Secretion granules of the rabbit parotid gland. Isolation, subfractionation, and characterization of the membrane and content subfractions. J Cell Biol. 1975 Jan;64(1):182–210. doi: 10.1083/jcb.64.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet I. R., Goode J. A., Hamon M. H., Laurie M. S., Linzell J. L., Peaker M. Secretory activity of goat mammary glands during pregnancy and the onset of lactation. J Physiol. 1975 Oct;251(3):763–773. doi: 10.1113/jphysiol.1975.sp011120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Lüder M. R., Kartenbeck J., Zerban H., Keenan T. W. Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol. 1976 Apr;69(1):173–195. doi: 10.1083/jcb.69.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE L. J., HIRS C. H., PALADE G. E. On the protein composition of bovine pancreatic zymogen granules. J Biol Chem. 1963 Jun;238:2054–2070. [PubMed] [Google Scholar]
- Groves M. L., Kiddy C. A. Polymorphism of gamma-casein in cow's milk. Arch Biochem Biophys. 1968 Jul;126(1):188–193. doi: 10.1016/0003-9861(68)90573-0. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J., Cheetham R. D. Lactose synthesis by a golgi apparatus fraction from rat mammary gland. Nature. 1970 Dec 12;228(5276):1105–1106. doi: 10.1038/2281105a0. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J. Glycosyltransferases: do they exist on the surface membrane of mammalian cells? FEBS Lett. 1975 Jul 15;55(1):8–13. doi: 10.1016/0014-5793(75)80944-6. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The topography of lactose synthesis. Biochem J. 1975 Apr;148(1):77–84. doi: 10.1042/bj1480077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Mechanism of milk secretion. Physiol Rev. 1971 Jul;51(3):564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- Mather I. H., Keenan T. W. Studies on the structure of milk fat globule membrane. J Membr Biol. 1975 Apr 23;21(1-2):65–85. doi: 10.1007/BF01941062. [DOI] [PubMed] [Google Scholar]
- Mather I. H., Weber K., Keenan T. W. Membranes of mammary gland. XII. Loosely associated proteins and compositional heterogeneity of bovine milk fat globule membrane. J Dairy Sci. 1977 Mar;60(3):394–402. doi: 10.3168/jds.S0022-0302(77)83878-2. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Jamieson J. D., Palade G. E. Composition of cellular membranes in the pancreas of the guinea pig. I. Isolation of membrane fractions. J Cell Biol. 1971 Apr;49(1):109–129. doi: 10.1083/jcb.49.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt W. D., Morré D. J. A glycosyl transferase of high specific activity in secretory vesicles from isolated Golgi apparatus of rat liver. Biochim Biophys Acta. 1973 Apr 28;304(2):397–407. doi: 10.1016/0304-4165(73)90259-6. [DOI] [PubMed] [Google Scholar]
- Morre J., Merlin L. M., Keenan T. W. Localization of glycosyl transferase activities in a Golgi apparatus-rich fraction isolated from rat liver. Biochem Biophys Res Commun. 1969 Nov 20;37(5):813–819. doi: 10.1016/0006-291x(69)90964-4. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Patton S., Fowkes F. M. The role of the plasma membrane in the secretion of milk fat. J Theor Biol. 1967 Jun;15(3):274–281. doi: 10.1016/0022-5193(67)90137-3. [DOI] [PubMed] [Google Scholar]
- Patton S., Keenan T. W. The milk fat globule membrane. Biochim Biophys Acta. 1975 Oct 31;415(3):273–309. doi: 10.1016/0304-4157(75)90011-8. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Keenan T. W. Membranes of mammary gland. XV. 5-Thio-D-glucose decreases lactose content and inhibits secretory vesicle maturation in lactating rat mammary gland. Exp Cell Res. 1978 Feb;111(2):413–425. doi: 10.1016/0014-4827(78)90186-6. [DOI] [PubMed] [Google Scholar]
- Silcock W. R., Patton S. Correlative secretion of protein, lactose and K + in milk of the goat. J Cell Physiol. 1972 Feb;79(1):151–154. doi: 10.1002/jcp.1040790118. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Brew K. Isolation and characteristics of galactosyltransferase from Golgi membranes of lactating sheep mammary glands. J Biol Chem. 1977 Oct 25;252(20):7294–7299. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- VanDerWoude W. J., Morré D. J., Bracker C. E. Isolation and characterization of secretory vesicles in germinated pollen of Lilium longiflorum. J Cell Sci. 1971 Mar;8(2):331–351. doi: 10.1242/jcs.8.2.331. [DOI] [PubMed] [Google Scholar]
- WELLINGS S. R., DEOME K. B. Milk protein droplet formation in the Golgi apparatus of the C3H/Crgl mouse mammary epithelial cells. J Biophys Biochem Cytol. 1961 Feb;9:479–485. doi: 10.1083/jcb.9.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whitney R. M., Brunner J. R., Ebner K. E., Farrell H. M., Jr, Josephson R. V., Morr C. V., Swaisgood H. E. Nomemclature of the proteins of cow's milk: fourth revision. J Dairy Sci. 1976 May;59(5):795–815. doi: 10.3168/jds.s0022-0302(76)84280-4. [DOI] [PubMed] [Google Scholar]