Abstract
Background: As antiretroviral treatment (ART) programmes in resource-limited settings mature, more patients are experiencing virological failure. Without resistance testing, deciding who should switch to second-line ART can be difficult. The consequences for second-line outcomes are unclear. In a workplace- and community-based multi-site programme, with 6-monthly virological monitoring, we describe outcomes and predictors of viral suppression on second-line, protease inhibitor-based ART.
Methods: We used prospectively collected clinic data from patients commencing first-line ART between 1/1/03 and 31/12/08 to construct a study cohort of patients switched to second-line ART in the presence of a viral load (VL) ≥400 copies/ml. Predictors of VL<400 copies/ml within 15 months of switch were assessed using modified Poisson regression to estimate risk ratios.
Results: 205 workplace patients (91.7% male; median age 43 yrs) and 212 community patients (38.7% male; median age 36 yrs) switched regimens. At switch compared to community patients, workplace patients had a longer duration of viraemia, higher VL, lower CD4 count, and higher reported non-adherence on first-line ART. Non-adherence was the reported reason for switching in a higher proportion of workplace patients. Following switch, 48.3% (workplace) and 72.0% (community) achieved VL<400, with non-adherence (17.9% vs. 1.4%) and virological rebound (35.6% vs. 13.2% with available measures) reported more commonly in the workplace programme. In adjusted analysis of the workplace programme, lower switch VL and younger age were associated with VL<400. In the community programme, shorter duration of viraemia, higher CD4 count and transfers into programme on ART were associated with VL<400.
Conclusion: High levels of viral suppression on second-line ART can be, but are not always, achieved in multi-site treatment programmes with both individual- and programme-level factors influencing outcomes. Strategies to support both healthcare workers and patients during this switch period need to be evaluated; sub-optimal adherence, particularly in the workplace programme must be addressed.
Introduction
As antiretroviral treatment (ART) programmes in resource-limited settings mature, patients are increasingly experiencing first-line, non-nucleoside reverse transcriptase inhibitor (NNRTI)-based, treatment failure necessitating a switch to second-line, protease inhibitor (PI)-based regimens [1]-[3]. Current rates of switching are low [4]-[5]; by the end of 2010 only 3% of patients in resource-limited settings (excluding South and Central Americas) had switched to second-line ART [1]. Low sensitivity of clinico-immunological definitions of treatment failure are partly responsible for low rates of switching. However programmes, such as those in South Africa which use virological monitoring, also report delays [4]. The reasons are likely to include lack of access to resistance tests to guide decisions, difficulties in excluding non-adherence as a cause of virological failure, and potentially concerns regarding cost and limited availability of subsequent regimens [6]-[7]. In the absence of resistance tests, deciding who has virological failure secondary to resistance is difficult. Studies from programmes which use routine virological monitoring have reported that the proportion of patients with no major drug resistance mutations is 9-60% on first raised viral load (300-1000 copies/ml) [8]-[12], 6-33% at confirmatory raised viral load (300-5000 copies/ml) [10], [13]-[15] and 12% at time of switching to second-line ART [16]; suggesting non-adherence is a major cause of viraemia at these time-points. Switching patients with no detectable resistance to second-line ART is arguably unnecessary, and potentially fails to address the underlying adherence issues. With limited regimen availability, unnecessary switching may compromise future treatment options for the individual, and drive up programme costs. In South Africa second-line ART is estimated to be 2.4 times more expensive per year in care than first-line ART [17].
The consequences of remaining on a virologically-failing first-line regimen include immunological and clinical progression and, with increasing duration of viraemia, accumulation of resistance [18]-[24]. For patients who eventually start second-line ART, the consequences of a switch strategy based on virological monitoring without resistance tests, on subsequent outcomes have not been fully described. Early reports of second-line outcomes appear promising with 78-87% of patients in care 12 months following switch, and 77-85% of those achieving viral suppression [25]-[27]. However, these reports are largely from academic or referral clinics, and it is unclear if the same outcomes will be seen under multi-site programmatic conditions.
This study aimed to describe second-line ART outcomes in a large workplace- and community-based multi-site programme, where, in line with South African national guidelines, 6-monthly viral load (VL) monitoring is standard of care. In addition we assessed whether co-variates available at the time of switch predict early viral suppression on second-line ART.
Methods
Study Design and Setting
This observational retrospective cohort analysis used prospectively-collected routine clinical data from the ART programmes of Aurum Institute, South Africa. These programmes, located within five provinces of South Africa (Gauteng, Free State, Limpopo, Mpumalanga and North West), comprise a workplace programme, with 56 clinics serving employees of predominantly mining companies; and a community programme, with 81 urban and peri-urban private general practitioner and non-government organization clinics serving patients with limited resources [28]-[29].
In the workplace, patients were eligible for ART (efavirenz [EFV] or nevirapine [NVP] with zidovudine [AZT], lamivudine [3TC] until 2008, then tenofovir/emtricitabine thereafter) if WHO stage IV, CD4≤250 cells/mm3, or CD4≤350 cells/mm3 plus WHO stage III. In the community programme, criteria for first-line ART (stavudine [d4T], 3TC, and NVP or EFV) were WHO stage IV or CD4≤200 cells/mm3. Similar criteria for switching to second-line ART were used in both programmes. Interventions to improve adherence were instigated following the first detectable VL, and VL was repeated 3-6 months later. A switch to second-line ART was recommended in patients with two raised VLs >1000-5000 in the presence of good adherence. Second-line ART comprised AZT, didanosine (ddI) and boosted lopinavir (bLPV); or abacavir (ABC), ddI, bLPV in the community and workplace programmes, respectively. Patients collected ART at 1-3 monthly intervals. All HIV-related treatment was free of charge.
CD4 count and VL were monitored at baseline, 6 weeks and 6 monthly intervals after commencing or switching ART. All community clinics were doctor-led; however some workplace clinics were nurse-led with doctors consulted for management of virological failure. Patients were offered adherence counseling at each attendance, with intensified counseling for those with suboptimal adherence.
Study Population
Patients were eligible for inclusion in the study if they (1) switched from first- to second-line ART between 1/1/2003 and 31/12/2008; (2) ≥15 years old at switch; and (3) VL >400 copies/ml at switch (regardless of whether criteria for switching, as per programme guidelines, were fulfilled). Data up to 31/3/2010 were included, allowing all patients 15 months potential follow-up.
Data Collection
At each visit, healthcare workers recorded data on symptoms, self-reported adherence, adverse events, prescriptions and reason for stopping or changing medication on standardized data collection forms. Before commencing ART, data were collected on patient’s self-reported previous exposure to ART. Reasons for leaving the programme, derived from patient or relative self-report, and active follow-up of patients missing appointments, were recorded on deregistration forms. Data capturers entered all forms into a central database with laboratory data transferred electronically from the central laboratory. Where civil identification numbers were available, deaths were identified through the National death register; and in the workplace, through employment records and hospital death registers. Where data were missing, clinic files were reviewed using a standardised data collection form. All community sites used a central off-site pharmacy. These dispensing records were used to confirm regimens and dates dispensed.
Outcomes
The primary outcome was viral suppression on second-line ART, which was defined as ever having achieved a VL<400 copies/ml between 2 weeks to 15 months of switching regimens. Secondary outcomes were defined as (1) alive and in care: no record of deregistration or loss to follow-up (no clinic contact for ≥6 months) by 15 months; (2) change in CD4 count: CD4 at 12 months (+/−3 months) minus CD4 count at switch (6 months before to 2 weeks after switch); (3) reported non-adherence: patient report of missing any second-line ART based on 7 day recall and/or healthcare worker recorded treatment interruption for non-adherence within 15 months of switch.
Risk Factors
Exposures on first-line ART (transfers into programme on ART, viral suppression, non-adherence), exposures at time of switch (duration and magnitude of viraemia, CD4 count, reason for switch, calendar year, number of new NRTIs in switch regimen) and demographic data (age, sex, programme) were considered as potential predictors of early virological suppression on second-line ART. An association between adherence on second-line ART and viral suppression on second-line ART was explored, however this variable was not included in our multivariable analysis as it was considered to lie on the causal pathway between our exposures of interest and the primary outcome.
Non-adherence on first-line ART was defined as patient report of missing any first-line ART based on 7 day recall and/or healthcare worker recorded treatment interruption for non-adherence at any time-point on first-line ART. Duration of viraemia was defined as the time period between the first VL >400 copies/ml following viral suppression to date of switch, where all interim VLs were >400 copies/ml. For patients with more than one episode of viraemia and re-suppression on first-line ART, only the viraemic period immediately preceding switch was considered. The variable was categorised as<12 months and ≥12 months. Not all patients were known to have achieved viral suppression on first-line ART therefore the following assumptions were made: (1) ART-naive patients with no evidence of viral suppression on first-line ART were considered viraemic since initiating ART; (2) patients who were transferred in with no subsequent viral suppression on first-line ART were categorised as viraemic for ≥12months.
Healthcare workers could document more than one reason for stopping the NNRTI-regimen. For the purposes of the analysis the primary reason for switch was defined as treatment failure, non-adherence or other e.g. toxicity. If, both non-adherence and treatment failure were documented, the primary reason for switch was defined as non-adherence; if treatment failure and other reasons were documented the primary reasons was defined as treatment failure.
Statistical Analysis
Modified Poisson regression with robust standard variance was used to estimate the association of exposures with viral suppression using the risk ratio [30]. This methodology was used, rather than logistic regression as the probability of the outcome was high and therefore the rare event assumption (odds and risk of an event are similar when the outcome is rare) did not hold true. By reporting risk ratios we avoided the possibility of the odds of an event being misinterpreted as risk and the strength of association being over-emphasized. A backwards stepwise approach was used whereby covariates associated with viral suppression (p≤0.2) in univariable analysis were considered for inclusion, and retained in the multivariable model if p≤0.2. Patients with missing outcome (died, left employment due to ill health, lost to follow-up or missing VL) were treated as failures; however patients who transferred out of clinic or left employment for reasons other than ill-health were excluded from the analysis. The Wald test was used to assess associations and, where appropriate, linearity and effect modification. Co-linearity was assessed by examining differences in standard errors between univariable and multivariable models.
Programme (community vs. workplace) was an effect modifier for multiple covariates (switch VL, transfers into programme on first-line ART, switch reason, age: p-value for interaction<0.05) therefore analyses are presented stratified by programme. Sensitivity analyses were performed by restricting analyses to patients who were ART-naive on initiating ART within the programme. Analyses were undertaken using STATA v11 (College Station, TX, USA).
Ethics
This study was approved by the research ethics committees of the University of KwaZulu Natal, South Africa and the London School of Hygiene and Tropical Medicine, UK.
Results
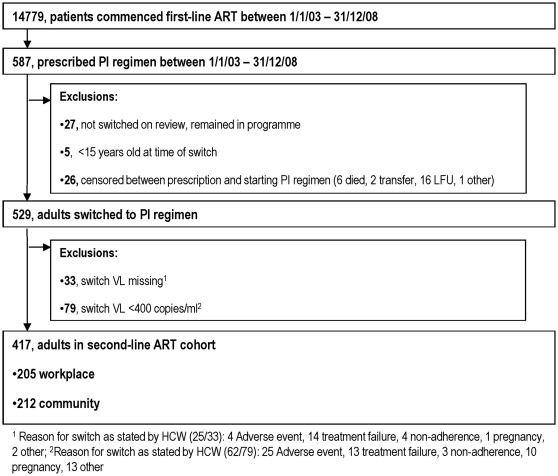
Of 14779 patients who commenced first-line ART, 555 adults were prescribed second-line ART, of which 26 left the programme before ART was dispensed. In total 417/529 adults (205 workplace and 212 community programme) had a documented VL ≥400 copies/ml at switch and were eligible for inclusion in the study (figure 1).
Figure 1. Study Flow diagram.
Selection of adults for analysis, from a cohort of patients initiating first-line, NNRTI-based, ART between the 1st January 2003 and 31st December 2008.
The characteristics of patients who switched to second-line ART are presented in table 1. Compared to the community, patients in the workplace were older, more likely to be male, commenced first-line ART at a higher CD4 count and less advanced clinical stage, were more likely to be ART-naive when initiating first-line ART in the programme (10.8% vs. 52.1%) and have a longer duration on first-line prior to switch. Non-adherence on first-line ART was reported in a higher proportion of patients in the workplace vs. community programme. In both programmes, of the 62 patients classified as non-adherent on first-line ART, 21% of patients self-reported non-adherence and 84% had ART modified or interrupted for non-adherence by healthcare workers. More patients in the workplace programme were prescribed a second-line regimen consistent with programme guidelines (90.7% vs. 59.0% in the community programme); however 87.7% of community patients did modify at least one of the NRTI backbone drugs in addition to receiving a bPI.
Table 1. Baseline characteristics of patients receiving second-line ART, according to programme.
| Workplace | Community | ||
| N = 205 (N, %) | N = 212 (N, %) | ||
| Age, years (median, IQR) | 43 (37-49) | 36 (31-42) | |
| Sex, male | 188 (91.7) | 82 (38.7) | |
| Start of first-line ART | |||
| WHO clinical stage III or IV, N = 152/190 | 108 (71.0) | 164 (86.3) | |
| CD4 at start of first-line, cells/mm3 (median, IQR), N = 195/193 | 166 (91-221) | 122 (43-195) | |
| Transfers into programme on ART, N = 185/192 | 20 (10.8) | 100 (52.1) | |
| On first-line ART | |||
| Duration on first-line pre-switch, days (median, IQR) | 695 (447-1019) | 517 (310-754) | |
| Reported non-adherence | 54 (26.3) | 8 (3.8) | |
| Viral suppression,<400 copies/ml, N = 190/151 | 130 (68.4) | 108 (71.5) | |
| At switch to second-line ART | |||
| Documented reason for switch, N = 180/192 | |||
| Treatment failure | 147 (81.7) | 160 (83.3) | |
| Non-adherence | 14 (7.8) | 1 (0.5) | |
| Other e.g. toxicity, pregnancy | 19 (10.6) | 31 (16.1) | |
| Year of switch | |||
| ≤2005 | 43 (21.0) | 11 (5.2) | |
| 2006-2007 | 57 (27.8) | 84 (39.6) | |
| 2008 | 105 (51.2) | 117 (55.2) | |
| Number of new NRTIs in switch regimen | |||
| None | 14 (6.8) | 26 (12.3) | |
| 1 | 7 (3.4) | 46 (21.7) | |
| ≥2 | 184 (89.8) | 140 (66.0) | |
| Duration of viraemia at switch 1 , N = 205/207 | 82 (40.0) | 95 (45.9) | |
| <12 months | |||
| ≥12 months | 123 (60.0) | 112 (54.1) | |
| Duration of viraemia between viral suppression and switch1a, days (median, IQR), N = 129/108 | 365 (173-538) | 218 (115-394) | |
| Duration of viraemia in ART-naïve patients without viral suppression1b, days (median, IQR), N = 60/43 | 538 (330-766) | 368 (114-544) | |
| VL(log10) at switch (median, IQR) | 4.6 (4.1-5.1) | 4.3 (3.7-4.6) | |
| CD4 at switch, cells/mm3 (median, IQR) | 169 (97-235) | 187 (95-270) | |
Duration of viraemia was defined as (a) Patients with viral suppression on first-line ART: date of first viral load >400 copies/ml following viral suppression to date of switch, N = 237 (57.5%)1a; (b) ART-naive patients with no viral suppression on first-line ART: date of commencing first-line ART to date of switch, N = 103 (25.0%)1b; (c) Patients with ART-experienced pre-programme who did not achieve viral suppression on first-line ART: assumed to be ≥12months, N = 72 (17.4%). Abbreviations: IQR, inter-quartile range; VL, viral load; NRTI, nucleoside reverse transcriptase inhibitor.
A longer median duration of viraemia was observed amongst patients in the workplace vs. community programme; 365 days (IQR 173-538) vs. 218 days (IQR 115-394) in patients with viral suppression on first-line ART. In both programmes there was a median of 3 detectable VLs prior to switch (range: workplace 1-13, community 1-10). At switch, compared to the community, patients in the workplace programme had a higher median log10 VL (4.6 [IQR 4.1-5.1] vs. 4.3 [IQR 3.7-4.6]) and a lower median CD4 count (169 cells/mm3 [IQR 97–235] vs. 187 [IQR 95–270]).
Reasons for Switching
In both programmes treatment failure was the commonest documented reason for switching regimens (workplace 82.2% [148/180 patients with recorded reason] vs. community 83.8% [161/192]). Non-adherence was recorded as a reason for switch in 7.8% (n = 14) of the workplace vs. 0.5% (n = 1) of the community programme. 10.6% [19/180] of patients in the workplace vs. 16.1% [31/192] in the community had other reported reasons for switching e.g. toxicity, although all were viraemic at the time of switching.
The two VLs prior to switch were ≥1000 copies/ml in 80.6% (336/417) of patients switched to second-line ART; in 16.1% (n = 67) the VL at switch was ≥1000 copies/ml with the preceding measurement 400-999 copies/ml or missing; and in 3.3% (n = 14) the switch VL was 400-999 copies/ml with the preceding measurement ≥400 copies/ml or missing.
Clinical outcomes on Second-Line Art
Outcomes stratified by programme are presented in Table 2. 73.7% (N = 179) of patients in the workplace and 84.4% (N = 151) in the community programme were alive and in care (p<0.01) at 15 months, with 48.3% (N = 98) vs. 72.0% (N = 152), respectively, having achieved viral suppression (p<0.01) by 15 months. Patients in both programmes had a median of 5 VLs following switch, with 87.3% (workplace) and 88.7% (community) with ≥1 measurement. Of the 250 patients who achieved viral suppression, 19.2% had no further VL measurements within the follow-up period. Of those with further measurements, 35.6% (26/73) of patients in the workplace vs. 13.2% (17/129) of those in the community experienced a subsequent episode of viral rebound to ≥400 copies/ml (p<0.01; median 3 measurements [range 2-8 workplace, 2-5 community]). At 12 months (+/−3 months), of the patients who were still in care, 46.8% (59/126) of workplace and 72.0% (116/161) of community programme had a VL<400 copies/ml.
Table 2. Outcomes at 15 months of second-line ART.
| Workplace | Community | |||
| N = 205 (N, %) | N = 212 (N, %) | pa | ||
| Clinical outcomes at 15 months | ||||
| Alive and in care | 151 (73.7) | 179 (84.4) | <0.01 | |
| Diedb | 12 (5.8) | 12 (5.7) | - | |
| Lost to follow-up | 29 (14.0) | 15 (7.1) | - | |
| Transfer out | - | 5 (2.4) | - | |
| Other e.g. left employment | 13 (6.3) | 1 (0.5) | - | |
| Non-adherence reported on second-line ART | 37 (17.9) | 3 (1.4) | <0.01 | |
| Change in CD4 count from switch to 12m following switch, range 9-15m (mean, 95% CI), N = 127/162 | +68 (40-95) | +127 (101-154) | <0.01 | |
| VL<400 within 15m of regimen start, range 2wks-15m, c N = 203/211 | 98 (48.3) | 152 (72.0) | <0.01 | |
| Viral rebound (≥400) following initial viral suppression, d N = 73/129 | 26 (35.6) | 17 (13.2) | <0.01 | |
Chi2 was used for comparison of proportions; paired t-test was used for comparison of mean CD4 count increase;
cause of death was available for 19/24 patients: 12 "natural causes" not further specified, 3 pneumonia, 1 tuberculosis, 1 cryptococcal meningitis, 1 gastroenteritis, 1 cerebro-vascular accident;
Patients with missing outcome who transferred out of programme or left employment for reasons other than ill-health were excluded from the analysis (N = 2 workplace, N = 1 community). All other patients with missing outcome were treated as failures (N = 11 workplace, N = 12 community);
Patients with ≥1 VL measurement following initial viral suppression (VL<400) on second-line ART.
Patients in the workplace had a lower mean CD4 count increase at 12 months of second-line ART than those in the community programme (p<0.01). Non-adherence was reported in a higher proportion of patients in the workplace, compared to the community programme (17.9% [workplace] vs. 1.4% [community]). In both programmes, of the 40 patients classified as non-adherent on second-line ART, 19% were identified through patient self-report and 83% through healthcare workers modification or interruption of ART for non-adherence.
Predictors of Viral Suppression on Second-Line Art
Unadjusted and adjusted analysis of variables associated with viral suppression in the workplace and community programme are summarised in tables 3 and 4. In adjusted analysis of the workplace programme, a lower log10 VL (adjusted risk ratio [aRR] 1.59 [95% CI: 1.09-2.34] for<4 vs. ≥5) and younger age (aRR 0.87 [95% CI: 0.79-0.95]/5 year increase) at switch were the strongest predictors of viral suppression. In addition, our data suggests an association between switch for non-adherence vs. treatment failure (aRR 0.45 [95% CI: 0.17-1.16]) and lack of viral suppression on second-line ART. While the association did not reach statistical significance, the effect size was large. Duration of viraemia was not associated with viral suppression on second-line ART.
Table 3. Predictors of early viral suppression (viral load<400 copies/ml) on second-line ART in the workplace programme.
| Viral suppression | Univariable, N = 203 | Multivariable, N = 178 | ||||
| N/at risk (%) | RR (95% CI) | pa | aRR (95% CI) | pa | ||
| Transfers into programme on ART, N = 184 | ||||||
| Yes | 7/20 (35.0) | 0.69 (0.37-1.28) | ||||
| No | 83/164 (50.6) | 1 | 0.24 | |||
| Viral suppression, first-line ART, N = 189 | ||||||
| Yes | 66/130 (50.8) | 1 | 0.53 | |||
| No | 27/59 (45.8) | 0.90 (0.65-1.25) | ||||
| Reported non-adherence, first-line ART | ||||||
| Yes | 23/54 (42.6) | 0.85 (0.60-1.20) | ||||
| No | 75/149 (50.3) | 1 | 0.35 | |||
| Reason for switch, N = 178 | ||||||
| Treatment failure | 76/145 (52.4) | 1 | 1 | |||
| Other | 8/19 (42.1) | 0.80 (0.46-1.39) | 0.44 | 0.81 (0.49-1.34) | 0.41 | |
| Non-adherence | 3/14 (21.4) | 0.41 (0.15-1.13) | 0.08 | 0.45 (0.17-1.16) | 0.1 | |
| Year of switch | ||||||
| ≤2007 | 55/100 (55.0) | 1 | 0.06 | 1 | 0.12 | |
| 2008 | 43/103 (41.8) | 0.76 (0.57-1.01) | 0.79 (0.59-1.07) | |||
| Duration of viraemia | ||||||
| <12 months | 42/82 (51.2) | 1.11 (0.83-1.47) | ||||
| ≥12 months | 56/121 (46.3) | 1 | 0.49 | |||
| VL(log10) at switch | ||||||
| ≥5 | 22/58 (37.9) | 1 | <0.01 | 1 | <0.01 | |
| 4-4.99 | 45/103 (43.7) | 1.15 (0.77-1.71) | 0.87 (0.58-1.33) | |||
| <4 | 31/42 (73.8) | 1.95 (1.34-2.83) | 1.59 (1.09-2.34) | |||
| CD4 at switch | ||||||
| <100 | 22/54 (40.7) | 1 | 0.17b | |||
| 100-199 | 36/74 (48.7) | 1.19 (0.80-1.78) | ||||
| ≥200 | 40/75 (53.3) | 1.31 (0.89-1.93) | ||||
| New NRTIs in switch regimen | ||||||
| ≤1 | 11/21 (52.4) | 1.10 (0.71-1.69) | ||||
| ≥2 | 87/182 (47.8) | 1 | 0.68 | |||
| Age at switch, per 5 years increase | 98/203 (48.3) | 0.86 (0.79-0.94) | 0.01b | 0.87 (0.79-0.95) | <0.01b | |
| Gender | ||||||
| Male | 87/186 (46.8) | 1 | 0.10 | |||
| Female | 11/17 (64.7) | 1.38 (0.94-2.03) | ||||
| Reported non-adherence, second-line ARTc | ||||||
| Yes | 12/36 (33.3) | 0.47 (0.22-1.00) | ||||
| No | 86/167 (51.5) | 1 | 0.05 | |||
Wald test; b test for linear trend with no evidence of departure from linearity (CD4, p = 0.84; Age, p = 0.47); c not included in the multivariable model as considered to be on the causal pathway between exposures at time of switch and viral suppression on second-line ART.
Table 4. Predictors of early viral suppression (viral load<400 copies/ml) on second-line ART in the community programme.
| Viral suppression | Univariable, N = 211 | Multivariable, N = 191 | ||||
| N/at risk (%) | RR (95% CI) | pa | aRR (95% CI) | pa | ||
| Transfers into programme on ART, N = 191 | ||||||
| Yes | 82/100 (82.0) | 1.33 (1.10-1.61) | 1.33 (1.11-1.61) | |||
| No | 56/91 (61.5) | 1 | <0.01 | 1 | <0.01 | |
| Virological suppression, first-line ART, N = 150 | ||||||
| Yes | 75/108 (69.4) | 1 | 0.56 | |||
| No | 27/42 (64.3) | 0.93 (0.71-1.20) | ||||
| Reported non-adherence, first-line ART | ||||||
| Yes | 5/8 (62.5) | 0.86 (0.50-1.49) | ||||
| No | 147/203 (72.4) | 1 | 0.60 | |||
| Reason for switch,b N = 190 | ||||||
| Treatment failure | 114/159 (71.7) | 1 | 0.77 | |||
| Other | 23/31 (74.2) | 1.03 (0.82-1.30) | ||||
| Year of switch | ||||||
| ≤2007 | 74/95 (77.9) | 1 | 0.08 | |||
| 2008 | 78/116 (67.2) | 0.86 (0.73-1.02) | ||||
| Duration of viraemia, N = 206 | ||||||
| <12 months | 72/94 (76.6) | 1.11 (0.94-1.32) | 1.22 (1.03-1.44) | |||
| ≥12 months | 77/112 (68.7) | 1 | 0.21 | 1 | 0.02 | |
| VL(log10) at switch | ||||||
| ≥5 | 20/29 (69.0) | 1 | 0.59 | |||
| 4-4.99 | 80/114 (70.2) | 1.02 (0.77-1.34) | ||||
| <4 | 52/68 (76.5) | 1.11 (0.84-1.46) | ||||
| CD4 at switch | ||||||
| <100 | 32/54 (59.3) | 1 | 0.01 | 1 | 0.02 | |
| 100-199 | 56/67 (83.6) | 1.41 (1.10-1.80) | 1.37 (1.05-1.78) | |||
| ≥200 | 64/90 (71.1) | 1.20 (0.93-1.55) | 1.16 (0.88-1.52) | |||
| New NRTIs in switch regimen | ||||||
| ≤1 | 54/72 (75.0) | 1.06 (0.90-1.26) | ||||
| ≥2 | 98/139 (70.5) | 1 | 0.48 | |||
| Age at switch | ||||||
| <35 | 65/90 (72.22) | 1.07 (0.83-1.38) | ||||
| 35-44 | 62/84 (73.81) | 1.09 (0.84-1.41) | ||||
| ≥45 | 25/37 (67.57) | 1 | 0.80 | |||
| Gender | ||||||
| Male | 60/82 (73.2) | 1 | 0.77 | |||
| Female | 92/129 (71.3) | 0.97 (0.82-1.16) | ||||
| Reported non-adherence, second-line ART c | ||||||
| Yes | 1/3 (33.33) | 0.19 (0.02-2.12) | ||||
| No | 151/208 (72.60) | 1 | 0.16 | |||
Wald test; b only one patient switched regimens for non-adherence in the community programme. This patient has therefore been excluded as it would not be possible to assess a potential association); c not included in the multivariable model as considered to be on the causal pathway between exposures at time of switch and viral suppression on second-line ART.
In adjusted analysis of the community programme, shorter duration, but not magnitude of viraemia, predicted viral suppression (<12 months aRR 1.22 [95% CI: 1.03–1.44] vs. ≥12 months). Patients who were transferred into the programme on ART, and those switched at a higher CD4 count were more likely to suppress following switch. Sensitivity analyses of both programmes, restricting to ART-naïve patients, resulted in similar models (data not presented).
Discussion
We have demonstrated, in a community ART programme delivered by a network of private general practitioners and non-government organisations, outcomes on second-line ART, both in terms of remaining in programme and achieving viral suppression, which are comparable to those reported from academic referral clinics [25]-[26]. In contrast, in the workplace programme, over a quarter of patients were no longer alive and in care by 15 months of second-line ART and less than half achieved viral suppression.
The differences in outcomes by programme are of concern and are surprising given that both programmes use similar switch guidelines. We hypothesise that variations in healthcare workers’ switching practices, together with both individual and programme factors, may explain these outcomes.
Although guidelines were similar for both programmes, differences in switching practices were evident; patients in the workplace were switched at a more advanced stage of immune-suppression with a higher log10 VL, lower CD4 count and longer duration of viraemia. This was not explained by baseline characteristics at initiation of first-line ART; patients in the community initiated ART at a more advanced stage of HIV than in the workplace programme. Although prolonged viraemia in the presence of drug pressure is associated with NRTI cross-resistance [22]-[24], we do not believe that resistance is an adequate explanation for the different virological outcomes observed between programmes. Firstly, in settings without prior exposure to boosted PIs, given the potency of these drugs, high rates of early viral suppression are expected even in patients with extensive thymidine analogue mutations [26], [31]-[34]. Secondly, although the duration of viraemia was shorter in the community programme, over half of the patients were viraemic for more than 12 months and are thus likely to also have resistance.
We hypothesise that differences in healthcare workers’ implementation of switch guidelines, and the extent to which non-adherence is excluded prior to switching regimens, will influence early virological outcomes on second-line ART. Current guidelines give little indication of how best to manage patients who are believed to be non-adherent and who continue to experience virological failure despite intensified adherence interventions. Perceived non-adherence has been shown to influence healthcare workers decisions regarding ART prescribing [35].
In the community programme a longer duration of viraemia and lower CD4 count at switch predicted failure to achieve viral suppression on second-line ART. A longer duration of viraemia may be acting as a marker of non-adherence on first-line ART (albeit that drug resistance mutations could be accumulating) with these patients requiring a longer period to address adherence issues before the regimen is switched. However, patients considered adherent are switched quickly; this is consistent with these individuals being more likely to achieve viral suppression.
In the workplace programme, switch VL<10000 was one of the strongest predictors of viral suppression. While patients switched at higher VLs may take longer to suppress, the great majority should have achieved viral suppression by 15 months. An alternative explanation is that a high VL reflects non-adherence [12], [36]. Undisclosed non-adherence can result in healthcare worker misclassification of the aetiology of viraemia. Healthcare worker documented reason for switch will therefore only partially adjust for non-adherence. In the workplace cohort 14 patients had their NNRTI-regimen stopped for non-adherence and were switched to second-line ART; in multivariable analysis there is a suggestion that these patients were less likely to achieve viral suppression than those switched for treatment failure alone. This finding is consistent with results from other studies which report low rates of viral suppression amongst patients switched to second-line ART in the presence of wild-type virus [26], [37]-[39].
Not only were patients in the workplace programme less likely to achieve viral suppression on second-line ART, they were also more likely to be lost to the programme and to experience viral rebound following initial suppression. On univariable analysis of data from the workplace programme non-adherence on second-line ART was associated with failure to achieve viral suppression. In the community programme the analysis was underpowered to assess an association due to low levels of reported non-adherence. While it is possible that failure to achieve viral suppression and viral rebound is due to emergence of early PI resistance we feel this is unlikely; other studies in resource-limited settings indicate that early second-line failure is more likely to be due to non-adherence, with PI mutations rarely seen and low PI concentrations reported [40]-[42]. We believe that these early viral rebounds are secondary to failure to sustain improved adherence behaviour which resulted from adherence interventions implemented at the time of switch. This could be due to contextual factors influencing patients’ adherence behaviour or failure of the health-care system to adequately support patients at high risk of non-adherence.
Although predictors of viral suppression differed between programmes, overall the findings are consistent with other studies; duration and magnitude of viraemia [37], [43], CD4 count at switch [38], [43], recent calendar year [44], older age [38], adherence [25], [31], [37]-[38] and prior-ART [25] have all been shown to be associated with second-line virological outcomes.
Other studies in this setting have also highlighted differences in switching rates and first- and second-line outcomes by site [40], [45]-[49]; Pujades-Rodriguez et al. report differences in switching rates between urban and rural sites, while others have found clinic type to be associated with second-line virological failure [40], [45]. The underlying reasons are multi-factorial with patient, health-system and community factors contributing [47]-[51].
The observed differences in programme outcomes may in part be due to the different patient populations, both in terms of individuals and the community, and the healthcare systems. The workplace population was older, predominantly male, comprised largely of migrants living in close proximity to their site of work, with access to only one major healthcare provider. In contrast patients accessing the community programme were younger, mostly female, and while potentially migrants, had a choice of healthcare provider.
Higher levels of non-adherence were reported amongst patients on first- and second-line ART in the workplace, compared to the community programme. While differences in non-adherence between programmes may be due to differences in reporting, studies conducted in this workplace setting have demonstrated multiple barriers to maintaining adherence including lack of social support, uncertainty about ART’s health benefits, belief in traditional medicine and patient-provider language barriers [48], [52]. While not unique to this setting [53], these barriers may be more prevalent amongst patients in a workplace as compared to a community setting. Indeed higher levels of non-adherence have been reported amongst patients enrolled in one of the workplace clinics vs. a government public clinic [48]. Patients within the workplace were older than those in the community programme and within the workplace programme older patients were less likely to achieve viral suppression. In many studies older age is associated with better adherence and superior outcomes [54]-[59], however this association may not be generalisable to the workplace setting where older age is a perceived barrier to adherence [52]. In resource-limited settings male gender has been associated with later initiation of ART [60], defaulting [59], [61]-[62], non-adherence [63]-[64] and mortality [64]-[68]. The association between gender and viral suppression varies; some studies report an association between female gender and first-line viral suppression [69]-[70] others between male gender and second-line viral suppression [71], These studies are from different settings, from urban townships to rural programmes; while biological characteristics may contribute, the association with gender is likely to be influenced by societal determinants specific to each setting. Within the community programme we did not find any association between gender and second-line virological suppression, and we were unable to assess the role of gender within the workplace as the majority of patients were male. Finally approximately half of the patients on second-line ART in the community programme were transfers into care, in some cases these were patients who self-funded first-line ART but could not afford more expensive second-line regimens (personal communication, S Charalambous). This was the strongest predictor of viral suppression in the community cohort. We believe patients who transfer between healthcare providers are likely to be highly motivated individuals [25], [48]. Fox et al. report similar findings; patients switched after only one VL, who were considered to be transfers into care on ART, were more likely to achieve viral suppression on second-line ART [25].
This study included large patient numbers across multiple sites. Extensive efforts were made to limit measurement bias and reduce effects of missing data by reviewing clinic notes, verification of switch date by cross-checking with pharmacy data, and ascertainment of deaths through multiple sources (linkage to national death register, company employment records and hospital death register).
There are limitations to our analysis. It was based on routinely collected programme data; clinic- and contextual level covariates e.g. clinic staffing levels or patients’ migrant status which could influence outcomes were not available. Due to lack of resistance data, incomplete programme reporting of non-adherence and inaccuracy of self-report as a measure of adherence, we were unable to fully explore the respective roles of resistance and adherence in early second-line virological outcomes. In addition, as programme acted as an effect modifier for multiple covariates, our analysis was stratified by programme. We were therefore unable to quantify the effect of programme (workplace vs. community) adjusted for potential confounders. Other limitations include that, for pragmatic purposes, our definition of duration of viraemia was a composite measure; for patients who did not achieve viral suppression on first-line ART, duration of viraemia was dependant on knowledge of pre-programme ART exposure and duration in programme. While our definition was subject to measurement error we do not believe it has resulted in bias; there was no evidence of co-linearity between ART exposure and duration of viraemia in the community programme multivariable model, and using an alternative definition based on duration of observed viraemia while in programme, similar associations were found. Also, with no difference in frequency of virological monitoring between programmes it is unlikely that detection bias would explain the differences in duration of viraemia, nor indeed virological outcomes. Finally in both second-line cohorts, the majority of patients were cared for by four clinics and the results may therefore be biased towards practices in these larger clinics. It is possible that the programmatic differences we have described in this study relate more to the individual clinics, rather than the programmes themselves. In a larger study looking at predictors of switching to second-line ART we found switching varied markedly by clinic. Programme, however was not associated with switching, nor did it account for clinic-level clustering [49]. As the majority of clinics contributed only one to two patients to this second-line analysis, clustering by clinic was not adjusted for.
Conclusion
The results from this study reflect the real-life dilemmas encountered in managing virological failure and switching to second-line ART in a resource-constrained setting. We demonstrate that it is possible to achieve high levels of viral suppression on second-line ART in multi-site programmes; however this is not true of all settings with both individual- and programme-level factors influencing outcomes. Despite similar guidelines, switching practices differed between programmes. With no access to resistance tests and imperfect adherence assessment tools, deciding who is failing therapy and might benefit from switching is difficult. The factors driving sub-optimal adherence, particularly in the workplace programme, need addressed and strategies to support switch decisions, such as targeted resistance tests, which may be cost-neutral, warrant further investigation [72].
Acknowledgments
We would like to thank the patients and healthcare teams at the participating sites, and the staff at The Aurum Institute for their assistance with this study. These data were presented previously at 18th Conference on Retroviruses and Opportunistic Infections, Boston 2011.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding for this study was provided through a Wellcome Trust Clinical PhD Programme (VJ; Grant number 087261/Z/08/Z). The community programme was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR; Grant number 5U2GPS000811) and the workplace programme by the employers. The content of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of these funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization, UNAIDS, UNICEF. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access: Progress Report 2011. 2011;5 Available: http://www.who.int/hiv/pub/progressreports/en/index.html. Accessed 2012 Jan. [Google Scholar]
- 2.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 3.Boulle A, Bock P, Osler M, Cohen K, Channing L, et al. Antiretroviral therapy and early mortality in South Africa. Bull World Health Organ. 2008;86:678–687. doi: 10.2471/BLT.07.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keiser O, Tweya H, Boulle A, Braitstein P, Schecter M, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keiser O, Orrell C, Egger M, Wood R, Brinkhof MW, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5:e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Li PC, Kumarasamy N, Boyd M, Chen YM, et al. Deferred modification of antiretroviral regimen following documented treatment failure in Asia: results from the TREAT Asia HIV Observational Database (TAHOD). HIV Med. 2010;11:31–39. doi: 10.1111/j.1468-1293.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent C, Bourgeois A, Mpoudi-Ngole E, Ciaffi L, Kouanfack C, et al. Tolerability and effectiveness of first-line regimens combining nevirapine and lamivudine plus zidovudine or stavudine in Cameroon. AIDS Res Hum Retroviruses. 2008;24:393–399. doi: 10.1089/aid.2007.0219. [DOI] [PubMed] [Google Scholar]
- 9.Barth RE, Wensing AM, Tempelman HA, Moraba R, Schuurman R, et al. Rapid accumulation of nonnucleoside reverse transcriptase inhibitor-associated resistance: evidence of transmitted resistance in rural South Africa. AIDS. 2008;22:2210–2212. doi: 10.1097/QAD.0b013e328313bf87. [DOI] [PubMed] [Google Scholar]
- 10.Messou E, Chaix ML, Gabillard D, Minga A, Losina E, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Cote d'Ivoire. J Acquir Immune Defic Syndr. 2011;56:356–364. doi: 10.1097/QAI.0b013e3182084b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zyl GU, van der Merwe L, Claassen M, Zeier M, Preiser W. Antiretroviral resistance patterns and factors associated with resistance in adult patients failing NNRTI-based regimens in the western cape, South Africa. J Med Virol. 2011;83:1764–1769. doi: 10.1002/jmv.22189. [DOI] [PubMed] [Google Scholar]
- 12.Marconi VC, Sunpath H, Lu Z, Gordon M, Koranteng-Apeagyei K, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46:1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bussmann H, Wester CW, Thomas A, Novitsky V, Okezie R, et al. Response to zidovudine/didanosine-containing combination antiretroviral therapy among HIV-1 subtype C-infected adults in Botswana: two-year outcomes from a randomized clinical trial. J Acquir Immune Defic Syndr. 2009;51:37–46. doi: 10.1097/QAI.0b013e31819ff102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orrell C, Walensky RP, Losina E, Pitt J, Freedberg KA, et al. HIV type-1 clade C resistance genotypes in treatment-naive patients and after first virological failure in a large community antiretroviral therapy programme. Antivir Ther. 2009;14:523–531. [PMC free article] [PubMed] [Google Scholar]
- 15.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;53:480–484. doi: 10.1097/QAI.0b013e3181bc478b. [DOI] [PubMed] [Google Scholar]
- 16.Sigaloff KC, Ramatsebe T, Viana R, Wit TF, Wallis CL, et al. Accumulation of HIV Drug Resistance Mutations in Patients Failing First-Line Antiretroviral Treatment in South Africa. AIDS Res Hum Retroviruses. 2011;28(2):171–175. doi: 10.1089/aid.2011.0136. [DOI] [PubMed] [Google Scholar]
- 17.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24:915–919. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 18.Nicastri E, Chiesi A, Angeletti C, Sarmati L, Palmisano L, et al. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76:153–160. doi: 10.1002/jmv.20352. [DOI] [PubMed] [Google Scholar]
- 19.Seyler C, Adje-Toure C, Messou E, Dakoury-Dogbo N, Rouet F, et al. Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa. AIDS. 2007;21:1157–1164. doi: 10.1097/QAD.0b013e3281c615da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, et al. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008;22:2097–2106. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keiser O, Tweya H, Braitstein P, Dabis F, MacPhail P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15:251–258. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantor R, Shafer RW, Follansbee S, Taylor J, Shilane D, et al. Evolution of resistance to drugs in HIV-1-infected patients failing antiretroviral therapy. AIDS. 2004;18:1503–1511. doi: 10.1097/01.aids.0000131358.29586.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta RK, Hill A, Sawyer AW, Cozzi-Lepri A, von Wyl V, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:409–417. doi: 10.1016/S1473-3099(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 24.Cozzi-Lepri A, Phillips AN, Martinez-Picado J, Monforte A, Katlama C, et al. Rate of accumulation of thymidine analogue mutations in patients continuing to receive virologically failing regimens containing zidovudine or stavudine: implications for antiretroviral therapy programs in resource-limited settings. J Infect Dis. 2009;200:687–697. doi: 10.1086/604731. [DOI] [PubMed] [Google Scholar]
- 25.Fox MP, Ive P, Long L, Maskew M, Sanne I. High rates of survival, immune reconstitution, and virologic suppression on second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;53:500–506. doi: 10.1097/QAI.0b013e3181bcdac1. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinipour MC, Kumwenda JJ, Weigel R, Brown LB, Mzinganjira D, et al. Second-line treatment in the Malawi antiretroviral programme: high early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med. 2010;11:510–518. doi: 10.1111/j.1468-1293.2010.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pujades-Rodriguez M, O'Brien D, Humblet P, Calmy A. Second-line antiretroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS. 2008;22:1305–1312. doi: 10.1097/QAD.0b013e3282fa75b9. [DOI] [PubMed] [Google Scholar]
- 28.Charalambous S, Grant AD, Day JH, Pemba L, Chaisson RE, et al. Establishing a workplace antiretroviral therapy programme in South Africa. AIDS Care. 2007;19:34–41. doi: 10.1080/09500340600677872. [DOI] [PubMed] [Google Scholar]
- 29.Russell EC, Charalambous S, Pemba L, Churchyard GJ, Grant AD, et al. Low haemoglobin predicts early mortality among adults starting antiretroviral therapy in an HIV care programme in South Africa: a cohort study. BMC Public Health. 2010;10:433. doi: 10.1186/1471-2458-10-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 31.Murphy RA, Sunpath H, Lu Z, Chelin N, Losina E, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 2010;24:1007–1012. doi: 10.1097/QAD.0b013e3283333639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunupuradah T, Chetchotisakd P, Munsakul W, Jirajariyavet S, Kantipong P, et al. [#584]. Presented at 18th Conference on Retroviruses and Opportunistic Infections Boston, USA, February 27, 2011; 2011. Second-line LPV/r Monotherapy Was Inferior to TDF/3TC/LPV/r in Patients who Failed NNRTI Regimen: HIV STAR Study. [Google Scholar]
- 33.Bartlett J, Aga E, Ribaudo H, Wallis C, Katzenstein D, et al. [#583] Presented at 18th Conference on Retroviruses and Opportunistic Infections; Boston, USA, February 27, 2011; 2011. A Pilot Study of LPV/r Monotherapy following Virologic Failure of First-line NNRTI-containing Regimens in Resource-limited Settings: The Week-24 Primary Analysis of ACTG 5230. [Google Scholar]
- 34.Manosuthi W, Thongyen S, Nilkamhang S, Manosuthi S, Sungkanuparph S. Long-term treatment outcomes of ritonavir-boosted lopinavir monotherapy among HIV-infected patients who experienced NRTI and NNRTI failure. AIDS Res Ther. 2012;9:8. doi: 10.1186/1742-6405-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimmel AD, Daniels N, Betancourt TS, Wood R, Prosser LA. AIDS Care; 2012. Decision maker priorities for providing antiretroviral therapy in HIV-infected South Africans: a qualitative assessment. doi: 10.1080/09540121.2011.630366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prosperi MC, Mackie N, Di Giambenedetto S, Zazzi M, Camacho R, et al. Detection of drug resistance mutations at low plasma HIV-1 RNA load in a European multicentre cohort study. J Antimicrob Chemother. 2011;66:1886–1896. doi: 10.1093/jac/dkr171. [DOI] [PubMed] [Google Scholar]
- 37.Murphy R, Sunpath H, Castilla C, Ebrahim S, Court R, et al. [#582]. Presented at 18th Conference on Retroviruses and Opportunistic Infections; Boston, February 27, 2011; 2011. Adherence to Second-line ART and Long-term Virologic Outcomes in South Africa. [Google Scholar]
- 38.Pujades-Rodriguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010;304:303–312. doi: 10.1001/jama.2010.980. [DOI] [PubMed] [Google Scholar]
- 39.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, et al. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 40.El-Khatib Z, Ekstrom AM, Ledwaba J, Mohapi L, Laher F, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. AIDS. 2010;24:1679–1687. doi: 10.1097/QAD.0b013e32833a097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, et al. Low lopinavir plasma or hair concentrations explain second line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56:333–339. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. AIDS Res Treat 2011; 2011. Protease Inhibitor Resistance Is Uncommon in HIV-1 Subtype C Infected Patients on Failing Second-Line Lopinavir/r-Containing Antiretroviral Therapy in South Africa. doi: 10.1155/2011/769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lodwick R, Costagliola D, Reiss P, Torti C, Teira R, et al. Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med. 2010;170:410–419. doi: 10.1001/archinternmed.2009.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cambiano V, Lampe FC, Rodger AJ, Smith CJ, Geretti AM, et al. Use of a prescription-based measure of antiretroviral therapy adherence to predict viral rebound in HIV-infected individuals with viral suppression. HIV Med. 2010;11:216–224. doi: 10.1111/j.1468-1293.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- 45.Pujades-Rodríguez M, Ferreyra C, Calmy A, Balkan S. [THE0126]. Presented at XVIII International AIDS Conference; Vienna, Austria, July 18th, 2010; 2010. Failure on First Line Therapy and Inequalities in Switching to Second Line in Adults Treated in Urban and Rural ART Programs: Multicentric Analysis in 28 MSF-Supported African and Asian Sites. [Google Scholar]
- 46.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS ONE. 2010;5:e12888. doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fielding KL, Charalambous S, Stenson AL, Pemba LF, Martin DJ, et al. Risk factors for poor virological outcome at 12 months in a workplace-based antiretroviral therapy programme in South Africa: a cohort study. BMC Infect Dis. 2008;8:93. doi: 10.1186/1471-2334-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahab M, Charalambous S, Karstaedt AS, Fielding KL, Hamilton R, et al. Contrasting predictors of poor antiretroviral therapy outcomes in two South African HIV programmes: a cohort study. BMC Public Health. 2010;10:430. doi: 10.1186/1471-2458-10-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston V, Fielding K, Charalambous S, Churchyard G, Phillips A, et al. [Abstract L-119]. Presented at 19th Conference on Retroviruses and Opportunistic Infections; Seattle, USA, 5th March, 2012; 2012. Outcomes Following Virological Failure and Predictors of Switching to Second-line Antiretroviral Therapy in a Multi-site Programme in South Africa. [Google Scholar]
- 50.Wouters E, Van Damme W, Van Loon F, van Rensburg D, Meulemans H. Public-sector ART in the Free State Province, South Africa: Community support as an important determinant of outcome. Soc Sci Med. 2009;69(8):1177–1185. doi: 10.1016/j.socscimed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 51.Braitstein P, Daggy J, Easterbrook P, Cohen C, Braithwaite R, et al. [Abstract 1015]. Presented at 18th Conference on Retroviruses and Opportunistic Infections; Boston, USA, February 27th, 2011; 2011. Retaining adults in HIV care: impact of key program characteristics on patient loss to follow-up (LTFU) in the IeDEA East Africa Consortium. [Google Scholar]
- 52.Dahab M, Charalambous S, Hamilton R, Fielding K, Kielmann K, et al. "That is why I stopped the ART": patients' & providers' perspectives on barriers to and enablers of HIV treatment adherence in a South African workplace programme. BMC Public Health. 2008;8:63. doi: 10.1186/1471-2458-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grabar S, Kousignian I, Sobel A, Le Bras P, Gasnault J, et al. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18:2029–2038. doi: 10.1097/00002030-200410210-00007. [DOI] [PubMed] [Google Scholar]
- 55.Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007;167:684–691. doi: 10.1001/archinte.167.7.684. [DOI] [PubMed] [Google Scholar]
- 56.Kamya MR, Mayanja-Kizza H, Kambugu A, Bakeera-Kitaka S, Semitala F, et al. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 57.Ahoua L, Guenther G, Pinoges L, Anguzu P, Chaix ML, et al. Risk factors for virological failure and subtherapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern Uganda. BMC Infect Dis. 2009;9:81. doi: 10.1186/1471-2334-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17:1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 59.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS ONE. 2010;5:e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keiser O, Anastos K, Schechter M, Balestre E, Myer L, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kranzer K, Lewis JJ, Ford N, Zeinecker J, Orrell C, et al. Treatment interruption in a primary care antiretroviral therapy program in South Africa: cohort analysis of trends and risk factors. J Acquir Immune Defic Syndr. 2010;55:e17–23. doi: 10.1097/QAI.0b013e3181f275fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14:722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rougemont M, Stoll BE, Elia N, Ngang P. Antiretroviral treatment adherence and its determinants in Sub-Saharan Africa: a prospective study at Yaounde Central Hospital, Cameroon. AIDS Res Ther. 2009;6:21. doi: 10.1186/1742-6405-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen SC, Yu JK, Harries AD, Bong CN, Kolola-Dzimadzi R, et al. Increased mortality of male adults with AIDS related to poor compliance to antiretroviral therapy in Malawi. Trop Med Int Health. 2008;13:513–519. doi: 10.1111/j.1365-3156.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 65.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 66.MacPherson P, Moshabela M, Martinson N, Pronyk P. Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg. 2009;103:588–593. doi: 10.1016/j.trstmh.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 68.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kipp W, Alibhai A, Saunders LD, Senthilselvan A, Kaler A, et al. Gender differences in antiretroviral treatment outcomes of HIV patients in rural Uganda. AIDS Care. 2010;22:271–278. doi: 10.1080/09540120903193625. [DOI] [PubMed] [Google Scholar]
- 70.DART Virology Group and Trial Team. Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS. 2006;20:1391–1399. doi: 10.1097/01.aids.0000233572.59522.45. [DOI] [PubMed] [Google Scholar]
- 71.Levison JH, Orrell C, Losina E, Lu Z, Freedberg KA, et al. Early outcomes and the virological effect of delayed treatment switching to second-line therapy in an antiretroviral roll-out programme in South Africa. Antivir Ther. 2011;16:853–861. doi: 10.3851/IMP1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosen S, Long L, Sanne I, Stevens WS, Fox MP. The net cost of incorporating resistance testing into HIV/AIDS treatment in South Africa: a Markov model with primary data. J Int AIDS Soc. 2011;14:24. doi: 10.1186/1758-2652-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]