Abstract
Heat shock proteins (Hsps) are a set of molecular chaperones involved in cellular repair. They provide protective mechanisms that allow cells to survive potentially lethal insults, In response to a conditioning stress their expression is increased. Here we examined the connection between Hsps and Aβ42, the amyloid peptide involved in the pathological sequence of Alzheimer’s disease (AD). Extracellular Aβ42 associates with neuronal cells and is a major constituent of senile plaques, one of the hallmarks of AD. Although Hsps are generally thought to prevent accumulation of misfolded proteins, there is a lack of mechanistic evidence that heat shock chaperones directly modulate Aβ42 toxicity. In this study we show that neither extracellular Aβ42 nor Aβ42/PrPC trigger the heat shock response in neurons. To address the influence of the neuroprotective heat shock response on cellular Aβ42, Western analysis of Aβ42 was performed following external Aβ42 application. Five hours after a conditioning heat shock, Aβ42 association with CAD cells was increased compared to control neurons. However, at forty-eight hours following heat shock Aβ42 levels were reduced compared to that found for control cells. Moreover, transient transfection of the stress induced Hsp40, decreased CAD levels of Aβ42. In contrast to CAD cells, hippocampal neurons transfected with Hsp40 retained Aβ42 indicating that Hsp40 modulation of Aβ42 proteostasis is cell specific. Mutation of the conserved HPD motif within Hsp40 significantly reduced the Hsp40-mediated Aβ42 increase in hippocampal cultures indicating the importance of this motif in regulating cellular Aβ42. Our data reveal a biochemical link between Hsp40 expression and Aβ42 proteostasis that is cell specific. Therefore, increasing Hsp40 therapeutically with the intention of interfering with the pathogenic cascade leading to neurodegeneration in AD should be pursued with caution.
Introduction
Alzheimer’s disease (AD), an age-dependent neurodegenerative disease that is estimated to affect 35 million people worldwide is characterized by amyloid deposits, neurofibrillary tangles, selective neuronal loss, cognitive decline and memory loss [1], [2]. Multiple lines of evidence suggest an imbalance between the production and clearance of Aβ1−42, a 42 residue long β amyloid protein that spontaneously self aggregates into dimers, oligomers, protofibrils, and fibrils and initiates a toxic sequence of events leading to synaptic dysfunction and dementia [3]. Aβ42 as well as Aβ40 are derived from the amyloid precursor protein (APP) by the sequential proteolytic processing of α, β and γ secretases (reviewed: [4], [5]. Following proteolysis, the peptides can be secreted or transferred to the endosomal/lysosomal system. Intraneuronal Aβ42 is comprised of both uptake of Aβ42 from the extracellular space as well as intracellular cleavage of APP [6]. Synaptic activity increases levels of secreted, extracellular Aβ peptides while reducing intracellular levels [7].
Why do the physiological mechanisms that under normal circumstances tightly regulate Aβ42 production, cell association and clearance fail? Deficiencies in cellular chaperone systems are one possibility. Molecular chaperones maintain protein homeostasis by assisting nascent polypeptides to fold, protecting mature proteins from stresses and eliminating misfolded proteins. Protein quality control mechanisms are critical to neural function and defects in proteolytic pathways are widely held to lead to neurodegeneration [8]. The cellular level of chaperones might affect the toxicity of Aβ42. In fact, enhancement of the cellular quality control machinery, has been proposed to prevent or delay the cascade of misfolding in conformational diseases [9], [10]. In addition to maintenance of protein homeostasis (proteostasis) by constitutive chaperones, in response to a number of stressful stimuli, there is an induction of stress-induced chaperones (eg Hsp40, Hsp90, Hsp70 and Hsp27). Understanding the biochemical sequence of events that underlies Aβ42-mediated neurodegeneration requires a clear understanding of the role(s) that chaperones play in the AD pathogenic cascade.
A number of chaperones are implicated in Aβ42 proteostasis [11]. Several chaperones have been found both in association with senile plaques [12]–[14] as well as endogenous Aβ42 [15]. These reports have given rise to the notion that molecular chaperones are suppressors of toxic Aβ42 conformations leading to AD. This idea is consistent with the observations that heat shock genes appear to be induced poorly late in life and that the principal risk factor for AD is age [9]. Further support for this view has come from reports demonstrating that in experimental models, Hsp70 [16], [17], Hsp27 [18] and Hsp90 [16] protect against the toxic effects of Aβ42. Also, Hsp70 is reported to suppress cognitive deficits and pathological phenotypes in AD mice [19]. In vitro Hsp70/40 and Hsp90 suppressed early stages of Aβ42 assembly into aggregates but had no effect on fibrils [16]. Still, many questions remain unanswered regarding the chaperone folding paths for Aβ42. For example, Mearow and colleagues have shown that heat shock of neonatal rat cortical cultures increases the detrimental effects of Aβ42 on cell survival while overexpressing Hsp27 protects against Aβ42 [18]. In mice models of Alzheimer’s disease overall content of the chaperone αβ crystallin is reduced [20]. However, in contrast to the concept of therapeutic rescue by chaperones, several molecular chaperones actually support the formation of the toxic Aβ42 oligomeric species [21]–[23]. This promotion of Aβ42 oligomerization by select chaperones has similar features to that observed in response to general anesthesia [24], [25].
In addition to cellular chaperones, cellular prion protein (PrPC)/Aβ42 association could influence Aβ42 quality control. Aβ42 in contrast to Aβ40, associates rapidly with neuronal cells [26]. Two distinct Aβ42, oligomeric conformations accumulate on the surface of living cells [27]. Exposure of Aβ42 to pH = 6 for 24 hours to mimic endosomal conditions increases Aβ42 binding to PC12 cells [28]. The cellular prion protein has been shown to act as a functional high affinity receptor for Aβ42 [29]–[31] Strittmatter and colleagues report that association of PrPC with Aβ42 mediates downstream Aβ42-impairement of hippocampal long term potentiation and that AD transgenic mice lacking PrPC accumulate Aβ42 but have normal survival and test normal for learning and memory [29], [31]. Furthermore, transgenic overexpression of PrPC is shown to enhance amyloid plaque formation in an AD mouse model [32]. Along these lines, pathological levels of Aβ42 have been shown to disrupt PrPC modulation of NMDA (N-Methyl-d-aspartate) receptor activity [33]. However, in contrast, Balducci et al report that Aβ42 impairs consolidation of long-term recognition memory in mice independent of PrPC, raising questions regarding the role of Aβ42/PrPC association in AD progression [30]. Additionally, other molecules (eg STI1) are recognized to bind PrPC, but whether these agents compete with Aβ42 for binding is unknown [34]. Following cell association, insoluble Aβ42 aggregates localize to endosome/lysosome compartments [35], [36]. Curiously, one report reveals that PrPC inhibits β-secretase cleavage of amyloid precursor protein and reduces Aβ42 levels [37].
That said, which molecular chaperones directly regulate Aβ42 protestasis and/or toxicity remain to be established. In this study we have monitored the association of Aβ42 with cultured neural cells following induction of the heat shock. Our findings demonstrate that heat shock initially increases Aβ42 association with CAD neuroblastoma cells but is followed by a decline in cellular Aβ42 levels at 48 hours. Transient transfection experiments revealed that Hsp40 decreased cellular levels of Aβ42 in CAD cell but increased cellular levels of Aβ42 in hippocampal cultures. We evaluated the influence of exogenously applied soluble PrPC on cellular uptake/processing of Aβ42 to test the hypothesis that soluble PrPC would bind to Aβ42 and reduce association with cell anchored PrPC, thereby blocking an early event in the Aβ42 pathogenic cascade. Here we document that PrPC failed to decrease cellular association of Aβ42. Our data reveal a biochemical link between cellular levels of Hsp40 and Aβ42 that is cell line specific. This raises the possibility that Hsp40 is involved in the pathogenic cascade leading to dementia and neurodegeneration in AD.
Results and Discussion
Extracellular Aβ42 does not Trigger the Heat Shock Response
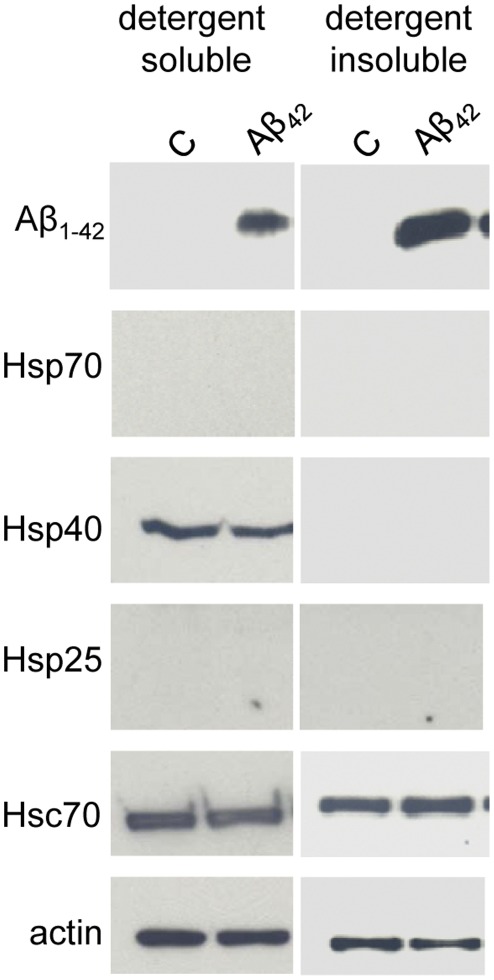
Heat shock chaperones are induced in response to a number of cell stressors such temperature, ischemia and heavy metals. During aging when heat shock genes are thought to be induced poorly, humans are susceptible to AD. To gain insight into the involvement of the heat shock response in Aβ42 pathogenic cascades, we carried out biochemical studies to establish whether the treatment of neuroblastoma cells with Aβ42 triggers the expression of the stress-induced chaperones. Mouse CAD neuroblastoma cells were incubated with a high concentration (25 µM) of Aβ42 for 48 hours, rinsed in PBS and solublized. 30 µg of supernatant (1% TX-100/0.1% SDS soluble proteins) and 10 µl of total pellet (1% TX-100/0.1% SDS insoluble proteins) were subjected to Western analysis. Figure 1 demonstrates that Aβ42 was clearly found in both soluble and insoluble CAD cell fractions. The expression of cellular Hsp70 (heat shock protein of 70 kDa), Hsp25 (Heat shock protein of 25 kDa) and Hsp40 (Heat shock protein of 40 kDa) in CAD cells did not change following treatment with Aβ42 for 48 hours. Hsp70 and Hsp25 were not detectable in either the presence or absence of Aβ42. Moreover, Hsp40 showed modest expression in control CAD cells as previously described [38], and no change was observed in response to Aβ42 treatment. The expression levels of the constitutive chaperone Hsc70 (Heat shock cognate protein of 70 kDa) also did not change in response to Aβ42. Actin is shown as a loading control.
Figure 1. Aβ42 does not trigger the heat shock response in CAD neural cells.
CAD cells were incubated with 25 µM Aβ42 for 48 hours, washed in PBS and lysed. 30 µg of soluble protein or 10 µl of the insoluble fraction were resolved by SDS-PAGE and the indicated proteins were evaluated by Western blot analysis. β-actin is shown as a loading control. Data are representative of 3 separate experiments.
Aβ42 does not Block the Heat Shock Response
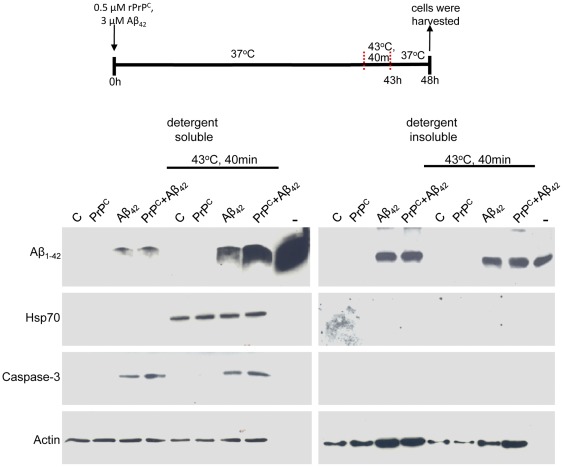
The heat shock response is a highly conserved cell survival program that enhances cell survival to subsequent insults [9]. Since interference with the heat shock response would be expected to reduce protein surveillance and triage mechanisms and downstream cell survival, we next tested whether Aβ42 altered induction of the heat shock chaperones. CAD cells were incubated with 3 µM Aβ42 then heat shocked at 43°C for 40 min and allowed to recover for 5 hours prior to lysis and Western analysis. Figure 2 clearly shows that Hsp70 is induced by heat shock and that Aβ42 does not alter the induction of this stress inducible chaperone. As expected, Aβ42 treatment of CAD cells triggers apoptotic pathways as shown by activation of caspase 3, a marker of programmed cell death. The Aβ42 activation of caspase 3 was not altered by heat shock. Heat shock treatment increased cellular levels of soluble Aβ42 ∼3.5 fold, suggesting that following heat shock neurons are more prone to the cellular toxicity of Aβ42 ( Figure 2 ). This observation is in line with a previous study demonstrating that Aβ42 decreases cortical neuron cell survival and that heat shock renders neurons more vulnerable to Aβ42 treatment [18]. Taken together, our data demonstrate that, although a large number of stressors activate the neuroprotective heat shock response, Aβ42 failed to increase the expression of the heat shock chaperones. Moreover, removal/disruption of the protective effects of a conditioning heat shock against cell death is not part of the Aβ42 pathogenic cascade.
Figure 2. Heat shock initially increases Aβ42 association with CAD neural cells.
In four independent experiments CAD cells were incubated with 3 µM Aβ42 and/or 0.5 µM bovine rPrPC, after ∼42 hours cells were subjected to heat-shock at 43°C for 40 minutes and then allowed to recover for 5 hours. The cells were rinsed in PBS and lysed. 30 µg of the soluble protein or 10 µl of the insoluble fraction was resolved by SDS-PAGE and subjected to Western blot analysis. Data are representative of 4 separate experiments.
Soluble PrPC does not Block Cell Association of Aβ42
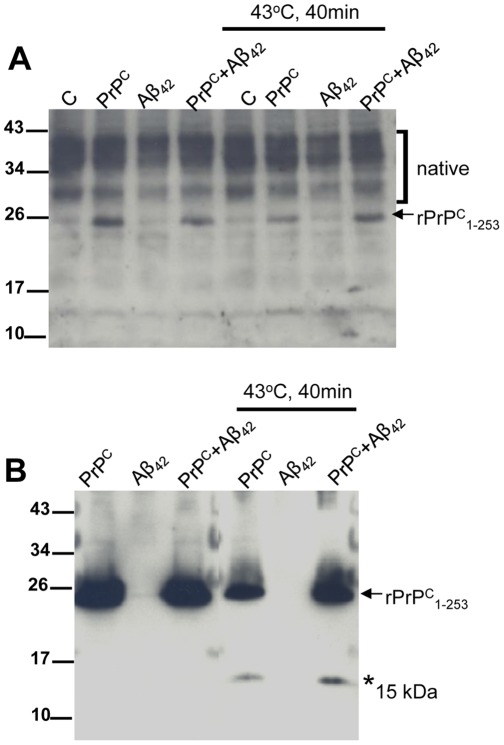
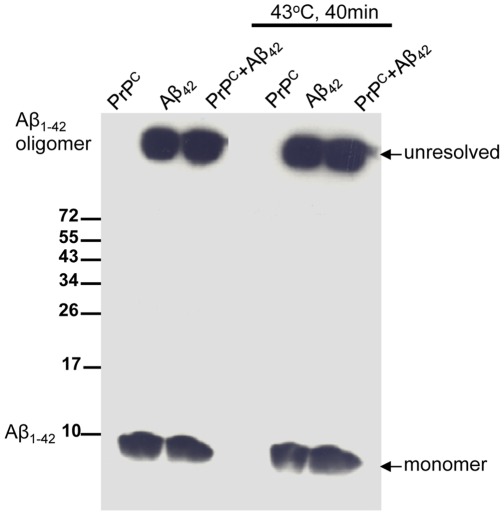
PrPC has been proposed to act as a functional high affinity receptor for Aβ42 [29]–[31]. Based on these reports, we wanted to determine how PrPC may impact the cellular association of Aβ42. Therefore, we examined the possibility that soluble recombinant PrPC would competitively displace Aβ42 from CAD cells thereby reducing the association of Aβ42 with the neuronal cell culture. Treatment with 0.5 µM recombinant PrPC did not induce Hsp70 or activation of caspase 3. Also, PrPC failed to alter the heat shock induction of Hsp70 or the Aβ42 induced activation of caspase 3. No difference in cellular Aβ42 association was observed between Aβ42 and PrPC/Aβ42 treated control (no-heat shock) cells. In fact, rather than inhibit cellular association of Aβ42, PrPC/Aβ42 co-treatment followed by heat shock revealed that PrPC increased Aβ42 levels in the soluble CAD cell fractions ( Figure 2 ). CAD cells express endogenous PrPC, a glycosylphosphatidyl (GPI) anchored plasma membrane protein [39], [40] ( Figure 3 ) that is subject to N-linked glycosylation and non-, mono- and di-glycosylated versions of PrPC simultaneously exist [41]. Recombinant bovine PrPC25−232 migrated further on SDS-PAGE then native unglycosylated PrPC therefore rendering cell association of exogenous recombinant PrPC distinguishable from endogenous PrPC. Exogenous recombinant PrPC was observed to associate with cells ( Figure 3 ). In the absence of CAD cells recombinant PrPC was observed to undergo partial breakdown following heat shock for 40 min at 43°C. Taken together our results show that exogenous PrPC does not block cellular Aβ42 association, in fact, following heat shock PrPC/Aβ42 co-treatment increased cell associated Aβ42.
Figure 3. PrPC associates with CAD cells.
(A) CAD cells were incubated with 3 µM Aβ42 and/or 0.5 µM bovine rPrPC, after ∼42 hours cells were subjected to heat-shock at 43°C for 40 minutes and then allowed to recover for 5 hours. The cells were rinsed in PBS and lysed. 30 µg of the soluble protein or 10 µl of the insoluble fraction was resolved by SDS-PAGE and cellular PrPC was determined by Western analysis. (B) Purified rPrPC was added to DMEM/F12 tissue culture media, subjected to heat-shock (43°C for 40 minutes) and then incubated at 37°C for 48 hours in the absence of cell lines and dissolved directly in sample buffer.
Heat Shock Promotes Time Dependent Clearance of Aβ42
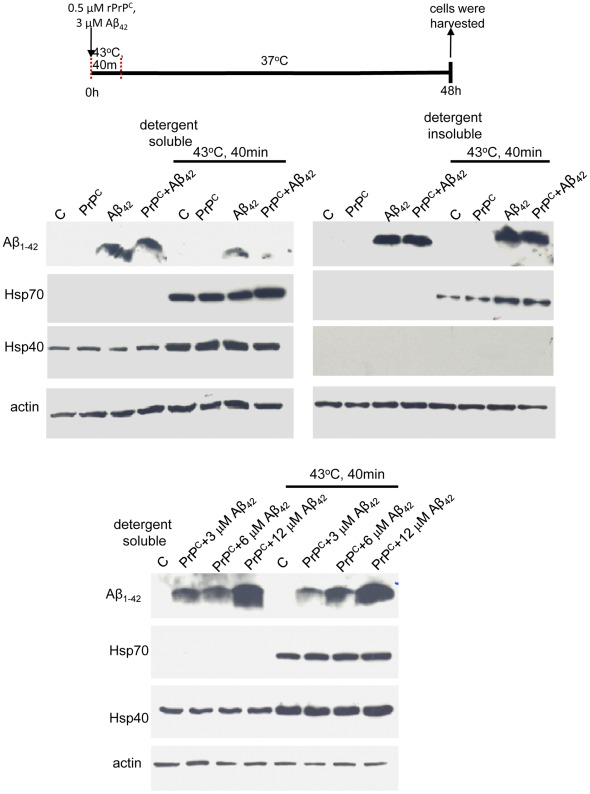
Although heat shock facilitated the pathogenicity of Aβ42 as measured by its increased cellular association, we speculated that this may be due to the physical effects of heat shock on membrane permeability rather than the conformational processing of PrPC by stress induced chaperones. To gain further insight into the relationship between cellular uptake/clearance of Aβ42 and the heat shock response, we carried out immunoblot analysis on CAD cells in which the heat shock was given at an earlier time point thereby increasing the time Aβ42 is exposed to the stress-induced chaperones. Figure 4 shows that when a 40 min heat shock was given starting at the time that Aβ42 was applied to CAD cells, the cellular levels of soluble Aβ42 at the 42 hour time point was reduced. Aβ42 does not always resolve as a discrete band by SDS-PAGE depending on abundance and the characteristic wide Aβ42 band is shown in Figure 4. Cellular levels of Hsp70 and Hsp40, which are elevated 3 hours following heat shock [38], remained elevated 48 hours following heat shock and at the 48 time point translocation of Hsp70 (but not Hsp40) to the detergent insoluble fraction was observed. Our observations demonstrate that while soluble Aβ42 is increased 5 hours following heat shock ( Figure 2 ), soluble Aβ42 is decreased 48 hours following heat shock ( Figure 4 ) indicating that with time heat shock chaperones increase cellular Aβ42 clearance. Geldanamycin-treatment of CAD cells which induces Hsp40 but not Hsp70 [38] was also observed to reduce cellular levels of Aβ42 (data not shown). Figure 4 (lower panel) clearly shows that CAD cell levels of Aβ42 increase in response to increasing Aβ42 concentrations. Again, PrPC, (either bovine upper panel or mouse lower panel Figure 4 ) did not reduce cell association of Aβ42 and did not alter the heat shock response.
Figure 4. Aβ42 levels are reduced 48 hours following induction of the cellular heat shock response.
CAD cells were incubated with 3 µM Aβ42 and/or 0.5 µM bovine rPrPC. Immediately following addition of Aβ42 or rPrPC, the cells were subjected to heat-shock at 43°C for 40 minutes, allowed to recover for 48 hours, and washed in PBS prior to lysis. 30 µg of soluble cellular protein or 10 µl of the insoluble fraction was resolved by SDS-PAGE and subjected to Western blot analysis. Lower panel: CAD cells were incubated with the indicated increasing concentrations of Aβ42 and/or 0.5 µM mouse (L42) rPrPC. Data are representative of 3 separate experiments.
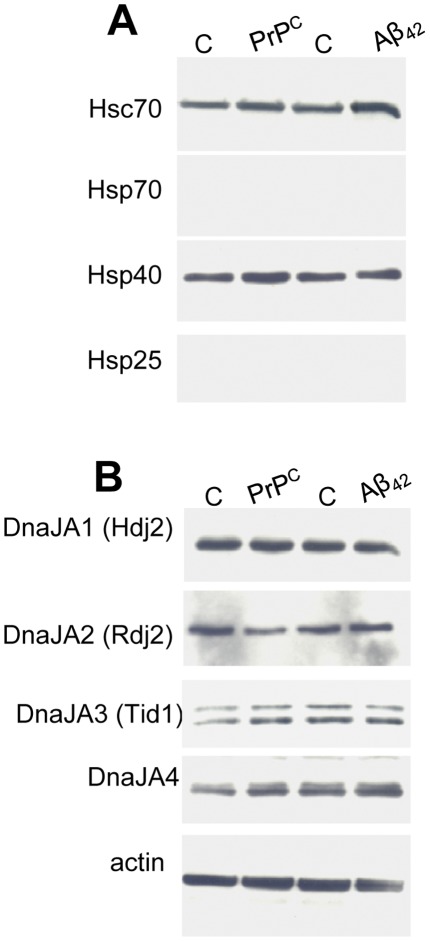
Figure 5 shows that in the absence of cells, heat shock per se does not cause degradation or oligomerization of Aβ42. Also, PrPC does not initiate any shifts in the molecular weight of Aβ42 indicative of proteolysis or SDS-resistant oligomerization. In contrast a 15 kDa breakdown of PrPC was observed following heat shock. Figure 6 shows that neither PrPC nor Aβ42 were found to alter cellular levels of the constitutive chaperones DnaJA1/Hdj2, DnaJA2/Rdj2, DnaJA3/Tid1, DnaJA4, Hsc70 or the stress induced chaperones Hsp70, Hsp40 and Hsp25, indicating that a generalized reduction in these molecular chaperone levels is not an underlying mechanism of Aβ42 induced neurodegeneration.
Figure 5. Aβ42 is not directly altered by heat shock.
(A) Purified Aβ42 was added to DMEM/F12 tissue culture media, subjected to heat-shock (43°C for 40 minutes) and then incubated at 37°C for 48 hours in the absence of cell lines and dissolved directly in sample buffer.
Figure 6. Aβ42 or bovine rPrPC do not alter expression of the J proteins DnaJA1(Hdj2), DnaJA2(Rdj2), DnaJA3 (Tid1) or DnaJA4 in neural cells.
CAD cells were incubated with 3 µM Aβ42 or 0.5 µM bovine rPrPC for 48 hours and washed in PBS prior to lysis. (A) Western analysis of heat shock proteins (B) The J proteins; DnaJA1(Hdj2), DnaJA2(Rdj2), DnaJA3 (Tid1) or DnaJA4 were detected by Western blot analysis. β-actin is shown as a loading control. Data are representative of 4 separate experiments.
Taken together, these results indicate that heat shock chaperones have a role in clearance of cellular Aβ42, and may perhaps be involved in reducing Aβ42 pathogenesis.
Modulation of Aβ42 by Hsp40 is Cell Line Specific
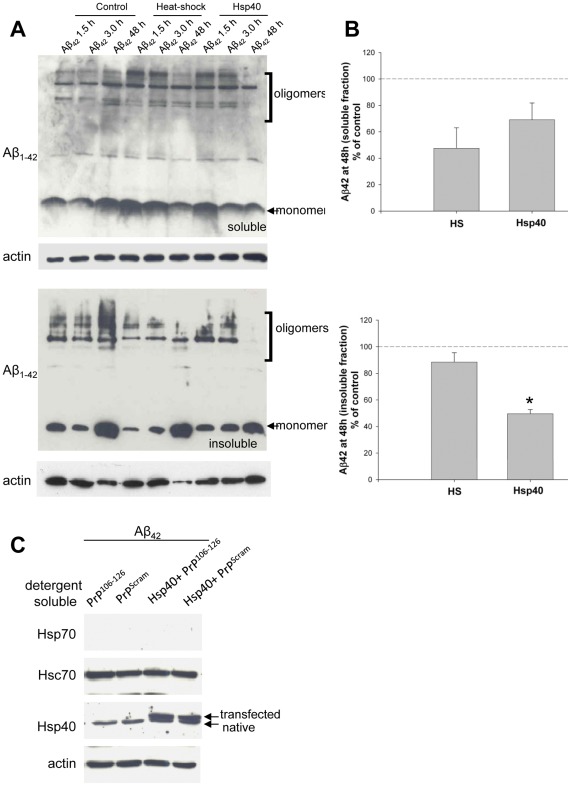
To further investigate the role that specific inducible chaperones play in heat shock induced reduction of Aβ42, CAD cells were transfected with the stress induced J protein Hsp40 and then challenged with the toxic Aβ42 ( Figure 7 ). Both heat shock and Hsp40 transfection reduced soluble and insoluble Aβ42 (monomer) at 48 hours. Quantitative immunoblotting uncovered a 50% ±3 Hsp40-mediated reduction compared to a smaller heat shock-mediated decreases 88% ±6 in insoluble monomeric Aβ42. Soluble Aβ42 monomer was found to decrease to 67% ±12 following Hsp40 transfection and 48% ±15 following heat shock. Hsp40 and heat shock both caused changes in Aβ42 oligomerization, initially increasing the Aβ42 72 kDa oligomer followed by a decrease at 48 hrs ( Figure 7 ). These experiments clearly establish Hsp40 as a chaperone that influences cellular clearance of Aβ42. Transfection does not induce the stress response ( Figure 7C ). Likewise, residues encoding amino acids 106–126 of PrPC as well as a scrambled control do not induce Hsp70 or increase Hsp40 levels.
Figure 7. Transient transfection of Hsp40 reduces cellular Aβ42.
(A) CAD cells were transiently transfected with Hsp40 as indicated for 24 hours prior to the addition of 3 µM Aβ42. Immediately following addition of Aβ42, indicated cells were subjected to heat shock at 43°C for 40 minutes and allowed to recover. At the indicated times cells were washed in PBS, lysed separated into soluble and insoluble fractions and cellular levels of Aβ42 were determined by Western analysis. Actin is shown as a loading control (B) Quantification of three independent experiments. *p<0.05. (C) Western analysis of CAD cells transfected with Hsp40 as indicated prior to incubation with 3 µM Aβ42 or 0.5 µM PrP106−126 or scrambled control. Data are representative of 4 separate experiments.
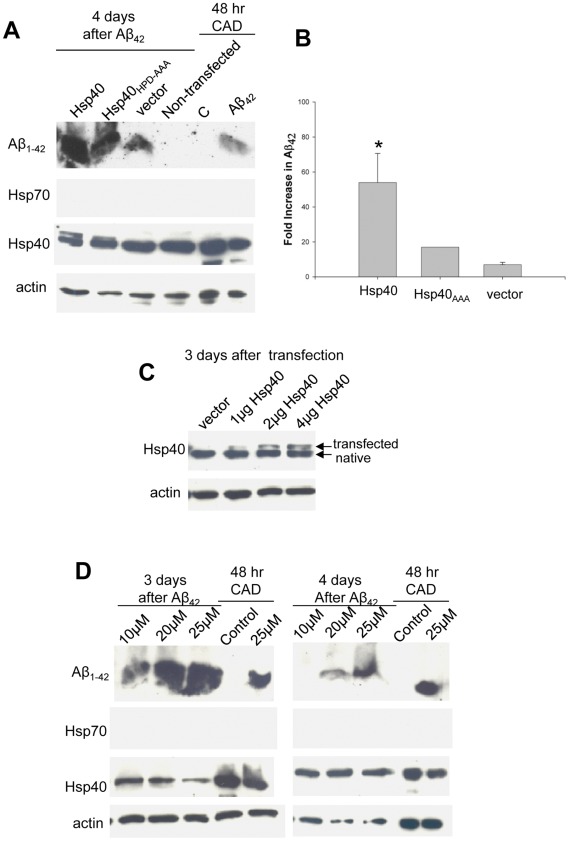
We then asked the question whether Hsp40 also regulates Aβ42 levels in primary hippocampal co-cultures of neurons and glia. Rat hippocampal neurons were isolated on postnatal day 0, transfected by electroporation with cDNA for myc-tagged Hsp40 or pCMV vector. Extensive neuritic outgrowth was found in both control and transfected cultures ( Figure 8 ). Bassoon (presynaptic-red) and neurofilament (green) expressing cells are apparent. DAPI staining is shown in blue. Healthy neurons are visible in both control and Aβ42–treated cultures. 3 days post-transfection cultures were treated with 10 µM Aβ42 and 4 days later cultures were washed in PBS and cellular (total) Aβ42 was determined by quantitative immunoblotting. Total lysates were prepared by direct cell lysis in TX100 lysis buffer followed by SDS sample buffer to ensure that all Aβ42 present was evaluated by Western blot analysis. Exposure of primary hippocampal cultures to extracellular Aβ42 resulted in rapid Aβ42 clearance. Figure 9 shows that 4 days following 10 µM Aβ42 exposure primary rat hippocampal cultures, which have high endogenous levels of Hsp40, reduce Aβ42 as does vector transfected neurons. To our surprise, in contrast to CAD neural cultures, transfection of hippocampal neurons with Hsp40 increased (59.3 fold) Aβ42 monomer levels over nontransfected cultures. These results reveal stark differences in neuronal processing of Aβ42 following Hsp40 transfection between CAD cell cultures and hippocampal cultures.
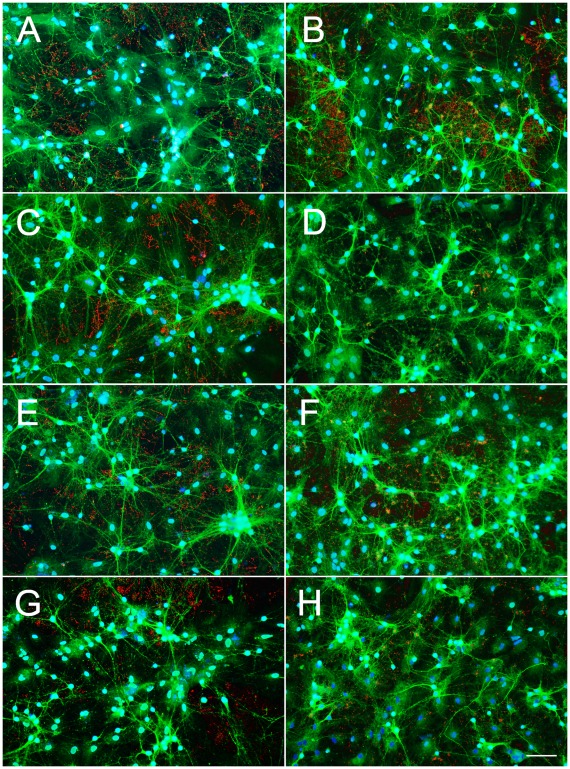
Figure 8. Effect of Aβ42 treatment on Hsp40 and Hsp40HPD-AAA-transfected neurons.
Rat hippocampal neurons were transfected by electroporation with 4 µg myc-tagged Hsp40, Hsp40HPD-AAA or vector alone and plated on silicon wafers (Silicon wafers, Silicon Quest, CA). Three days after transfection, neurons were incubated with 10 µM Aβ42 (panels B,D,F,H) and 7 days post transfection hippocampal cultures were fixed with 15% Picric Acid (Sigma, P6744), 4% PFA (Sigma, P6148), blocked in donkey serum/BSA and permeabilized with 0.1% Triton-X100 (Biorad, 161-0407) for 2 hours. Cultures were immunostained with Bassoon (red) (Enzo, ADI-VAM-PS003), and Neurofilament (green) (Millipore, A1991) and DAPI (blue) (Invitrogen, D1306) and examined by microscopy via an Olympus inverted scope (BX61WI) and water submergible 10x objective (UMplanF1). The panels are as follows A,B = controls; C,D transfected with pcDNA; E,F transfected with Hsp40 and G,H transfected with Hsp40HPD-AAA; A,C,E,G = no Aβ42; B,D,F,H = 10 µM of Aβ42. Neurons are healthy with axon and dendritic elaboration forming networks.
Figure 9. Transfection of hippocampal co-cultures with Hsp40 or Hsp40HPD-AAA elevates cellular Aβ42.
(A) Rat hippocampal neurons were transfected by electroporation with 4 µg myc-tagged Hsp40, Hsp40HPD-AAA or vector alone. Three days after transfection, neurons were incubated with 10 µM Aβ42 and 7 days post transfection hippocampal cultures were washed in PBS and cellular (total) Aβ42 was determined by quantitative immunoblotting. Actin is shown for reference. (B) Quantification of three independent experiments. *p<0.01 (C) CAD cells were transfected with 1, 2, 4 µg myc-tagged Hsp40 as indicated. Hsp40 and actin were determined 3 days following transfection. (D) Western analysis of hippocampal primary cultures (3 or 4 days following external Aβ42 application) or CAD cells treated with Aβ42 for 24 hours. Data are representative of 3 separate experiments.
To begin to address the mechanism of the Hsp40-mediated increase in Aβ42 hippocampal co-cultures were transfected with a mutated form of Hsp40 predicted to bind to client proteins but not have chaperone activity. Hsp40 interacts with and activates the ATPase Hsp70/Hsc70 via its J domain. An HPD (histidine-proline-aspartic acid) motif located within the J domain of Hsp40 is required for harnessing Hsc70/Hsp70 for conformational work. By mutating the histidine-proline-aspartic acid motif of Hsp40 to alanines (Hsp40HPD-AAA) an Hsp40 that binds client protein but does not activate Hsc70/Hsp70 ATPase is generated. Transfection of hippocampal neurons with Hsp40HPD-AAA increased Aβ42 monomer (15.3 fold) compared to vector control (7.9 fold) ( Figure 9A&B ). Although Hsp40HPD-AAA increased cellular Aβ42 levels over untreated control cells (no transfection, no extracellular Aβ42) and vector control cultures (transfection with vector alone followed by application of extracellular Aβ42), the Hsp40HPD-AAA-mediated increase (15.3 fold) was smaller compared to Hsp40 (59.3 fold) indicating that the HPD motif within the J domain of Hsp40 impacts directly on cell processing of Aβ42. Figure 9C &D show that transfection efficiency is lower in co cultures compared to CAD cells. Like that seen for CAD cells, Aβ42 did not induce the heat shock response in hippocampal neurons. PrPC did not block the association/accumulation of Aβ42 in hippocampal neurons (data not shown). Figure 9D compares total cellular hippocampal cultures exposed to 10, 20, 25 µM Aβ42 at the time of transfection (left panel) and three days following transfection (right panel) to CAD cells treated for 24 hours with 25 µM Aβ42.
Taken together, these results demonstrate that modulation of Aβ42 by Hsp40 is cell line specific.
In summary we have found that Hsp40 is able to influence cellular levels of Aβ42. Neural processing of extracellular Aβ42 is dynamic. Overproduction and impaired clearance of Aβ42, are implicated in AD [8]. Accumulation of Aβ oligomers can lead to synaptic dysfunction and disruption in neural plasticity [42]–[45], however, details of the molecular cascade(s) that underlie Aβ42 neuronal toxicity remain unclear. Understanding how cells regulate cellular Aβ42 levels requires identification of the cellular chaperone machinery involved in processing the neural amyloid pool. In this study we provide mechanistic evidence that Hsp40 regulates association/accumulation of extracellular Aβ42 with neurons and that Hsp40-mediated regulation is cell specific.
Hsp40 is an evolutionarily ancient and widely expressed chaperone that almost certainly targets multiple client proteins. In neurons, Hsp40 is found to be concentrated in postsynaptic densities [46], in lipid rafts [47] and in association with presynaptic chaperones [38]. Furthermore, Hsp40 is linked to neurite outgrowth [48]. That said, its precise role in synaptic transmission and neurodegeneration is not yet known. Hsp40 is a member of the J protein family [49]. All J proteins have a tetrahelical Hsp70/Hsc70-interacting domain called a J domain. Via their J domain, J proteins target a wide array of cellular proteins to the ATPases Hsc70/Hsp70 for conformational work. Although our observation that Hsp40 alters Aβ42 cellular turnover in a cell culture specific manner is in contrast to the notion that heat shock chaperones rid the cell of toxic proteins in all cells, it is possible that in disease conditions Hsp40 may protect a toxic protein (eg Aβ42) from triage resulting in acceleration of disease progression. In fact, the J protein family determines which chaperone pathway is pursued by Hsc70/Hsp70 [50]. For example, DnaJB2(HSJ1) stimulates ubiquitination and sorting of substrates to the proteosome [51], while DnaJC6(auxilin) stimulates recycling of clathrin from clathrin coated vesicles [52] and DnaJB6 (Mrj) regulates keratin turnover [53]. Further experimentation is required to understand how chaperones like Hsp40 either aid in folding and maintenance or lead to degradation.
Cellular levels of Hsp40 routinely rise and fall as part of the heat shock program. Levels of constitutive Hsp40 vary drastically among neural cell lines [38] and classes of neurons in the adult rat brain [54] but how this correlates with Aβ42 levels remains to be established. Although the heat shock response is a highly conserved cellular program that confers transient cyto-protection via the induction and translocation of stress induced chaperones, differences in the threshold for the induction of the heat shock response, the complement of heat shock proteins induced as well as the constitutive expression of heat shock proteins are observed among neurons. For example, Hsp27 is induced by heat shock in primary hippocampal cultures but not primary cortical cultures [18]. Also, some neurons (eg motor neurons) have a high threshold for inducing the heat shock response [55]. The cellular variations in either constitutive expression or heat shock expression of Hsp40 may well lead to selective vulnerability of neurons. Other heat shock proteins have been shown to protect against Aβ42 neuronal toxicity. Expression of Hsp27 in cortical neuronal cultures prepared from postnatal day 1 rats protects against Aβ42-associated toxicity [18]. Also, Hsp70 overexpressing mice have reduced Aβ [19]. Our data show that extracellular Aβ42 does not elicit a heat shock response in neurons nor does it alter induction of the heat shock response. Moreover we demonstrate that while heat shock initially increases neural Aβ42, with time in culture Aβ42 levels are reduced in neurons following heat shock compared to control cultures.
The basis of the cell specific responses to Hsp40 is likely due to differences in the chaperone networks between the cultured cells. The elaborate chaperone machinery that is present in cells rids the cell of toxic proteins often via assembly of chaperones into active chaperone complexes. Levels of chaperones and chaperone complexes may differ between CAD neuroblastoma cells and primary hippocampal cultures. Further experimentation is required to develop a detailed and comprehensive overview of differences in chaperones among neurons. In conclusion we have shown that (1) Hsp40 modulates processing of extracellular Aβ42. (2) Hsp40 modulation of Aβ42 is cell specific and dependent on the conserved HPD motif within Hsp40’s J domain. (3) Aβ42 does not trigger the heat shock response or alter the threshold for induction of the heat shock response. (4) Soluble PrPC does not block association of extracellular Aβ42. Overall these results elucidate an important link between Hsp40 and cellular levels of Aβ42,which has not been illustrated previously.
Materials and Methods
Reagents and Chemicals
Amyloid- β42 (Aβ42) was from rPeptide. Recombinant bovine PrPC (rPrPC) was from Prionics. Anti-Aβ 6E10 monoclonal antibody and DnaJA4 monoclonal antibody were from Cedarlane Laboratories. Anti-Hsp40 rabbit polyclonal, anti-Hsp70 mouse monoclonal and anti-Hsp25 mouse monoclonal were from Stressgen. Anti-Hsc70 mouse monoclonal and anti-β-actin mouse monoclonal were from Sigma. Geldanamycin was from Calbiochem. Anti-Rdj2 mouse monoclonal and anti-DnaJA3 (Tid1) polyclonal antibody were from Abnova. Caspase-3 (8G10) Rabbit monoclonal antibody was from Cell Signaling Technology. DnaJ1 (Hdj2) polyclonal antibody was from USBiological. Peroxidase-conjugates AffiniPure Goat Anti-Mouse IgG (H+L) was from Cedarlane.
CAD (CNS Catecholaminergic Derived) Mouse Neuroblastoma Cells [38], [56], [57]
CAD (CNS catecholaminergic derived) mouse neuroblastoma cells were seeded into 6 well plates and grown in DMEM/F12 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin as previously described. Cells were lysed in 40 mM Tris (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 0.1% SDS, 1% TX-100, 0.5 mM PMSF and protease inhibitor (Sigma) at 4°C for 1 hour. Lysates were centrifuged at 15000×g for 5 minutes at 4°C and the supernatant (soluble fraction) and pellet (insoluble fraction) was collected and stored at −70°C. For transient transfection, CAD cells were washed in PBS and transiently transfected with c-myc tagged rat Hsp40 DNA using Lipofectamine-2000 (Invitrogen) in Opti-MEM. For heat shock experiments cells were incubated at 43°C for 40 minutes and then returned to 37°C. Amyloid-β1-42-TFA was dissolved in 1% NH4OH as recommended by the supplier (rPeptide) to a concentration of 1 mg/mL. The resulting solution was sonicated for 1 minute, aliquoted and stored at −70°C. With this preparation, Aβ42 will be mainly in the monomeric form (∼95%) [58]. Recombinant bovine Prion Protein (Prionics, amino acids PrPC25-242) was suspended in H20, aliquoted and stored at −70°C. PrPC106-126 and the scrambled PrPC106-126 (Anaspec) were resuspended in DMSO to a final stock concentration of 1 mM and stored at −20°C [59]. Recombinant mouse His-tagged L42 prion protein was prepared as previously described [60], [61]. Aliquots were diluted in culture media immediately prior to treatment of cells. Protein concentration of the soluble CAD cell fraction was determined by Bradford assay (BioRad). The 1% TX-100/0.1% SDS insoluble cell fraction was dissolved directly in sample buffer.
Primary Hippocampal Cell Culture [62], [63]
Dissociated primary co-cultures of neurons and glia were isolated by dissection from Sprague Dawley rats (Charles River) at postnatal day 0 as previously described [62]. Animals were anesthetized on ice and sacrificed by decapitation. Hippocampi were removed and incubated in 40 µl/ml of papain. After 30 minutes cells were washed three times in fresh Eagle’s basal media (GIBCO-Invitrogen) supplemented with B-27, penicillin, streptomycin, L-glutamine and 4% feta bovine serum. Cells were triturated using three decreasing calibers of pipettes and the cell solution was then transfected by electroporation (Bio Rad Gene Pulsor Xcell:settings 150 volts, pulse length 25, 5 pulses, pulse interval 0.1 and cuvete size 4 mm) with cDNA of either myc-tagged Hsp40 or Hsp40HPD-AAA or vector control (pCDNA3.1) and then plated. For immunostaining, transfected cells were plated on silicon wafers (Silicon wafers, Silicon Quest, CA). Co-cultures were grown for 7 days in a 5% CO2 incubator. After transfection, Aβ42 was added to the culture and 3 days later the cells were washed in PBS, lysed in 40 mM Tris (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 0.1% SDS, 1% TX-100, 0.5 mM PMSF and protease inhibitor (Sigma) at 4°C for 1 hour. Total cell lysates were solubilized directly in sample buffer and evaluated by Western blot analysis. The University of Calgary Conjoint Faculties Research Ethics Board specifically approved this study (protocol number M09008).
Immunoblotting
Proteins were electrotransferred from polyacrylamide gels to 0.2 µm nitrocellulose membrane in 20 mM Tris, 150 mM glycine and 12% methanol. Membranes were blocked with in PBS with 0.1% Tween 20, 4% milk and incubated with primary antibody overnight at 4°C. The membranes were washed and incubated with horseradish peroxidase-coupled secondary antibody. The signal was developed using West Pico reagent (Pierce Biotechnology Inc.) and exposed to Kodak film. Bound antisera were quantitated by Biorad Fluor-S MultiImager Max and QuantityOne 4.2.1 software.
Acknowledgments
We are grateful to Dr. Frank Jirik and Dr. Pankaj Tailor for supplying PrPC106-126 and PrPCscrambled peptides and for scientific discussions during the course of these experiments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by funding from the Canadian Institute of Health Research MOP#74713 (Dr. Braun) and MOP#7944 (Dr. Colicos)and the Alberta Prion Research Institute. Dr. Braun is a Senior Scholar of the Alberta Heritage Foundation for Medical Research. Dr. Colicos is a Scholar of the Alberta Heritage Foundation for Medical Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. J Biol Chem. 2010;285:12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Abeta oligomers. FEBS J. 2010;277:1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed A, Posse dC. Int J Alzheimers Dis 2011: 127984; 2011. Abeta internalization by neurons and glia.127984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tampellini D, Rahman N, Lin MT, Capetillo-Zarate E, Gouras GK. Impaired beta-Amyloid Secretion in Alzheimer’s Disease Pathogenesis. J Neurosci. 2011;31:15384–15390. doi: 10.1523/JNEUROSCI.2986-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingol B, Sheng M. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron. 2011;69:22–32. doi: 10.1016/j.neuron.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 10.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 11.Magrane J, Querfurth HW. Heat Shock Proteins, Unfolded Protein Response Chaperones and Alzheimer’s Disease. In: Asea AA, Brown IR, editors. Heat Shock Proteins and the Brain: Implications for Neurodegenerative Diseases and Neuroprotection. Springer; 2008. pp. 25–50. [Google Scholar]
- 12.Perez N, Sugar J, Charya S, Johnson G, Merril C, et al. Increased synthesis and accumulation of heat shock 70 proteins in Alzheimer’s disease. Brain Res Mol Brain Res. 1991;11:249–254. doi: 10.1016/0169-328x(91)90033-t. [DOI] [PubMed] [Google Scholar]
- 13.Hamos JE, Oblas B, Pulaski-Salo D, Welch WJ, Bole DG, et al. Expression of heat shock proteins in Alzheimer’s disease. Neurology. 1991;41:345–350. doi: 10.1212/wnl.41.3.345. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara H, Inaguma Y, Goto S, Inagaki T, Kato K. Alpha B crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer’s disease. J Neurol Sci. 1993;119:203–208. doi: 10.1016/0022-510x(93)90135-l. [DOI] [PubMed] [Google Scholar]
- 15.Fonte V, Kapulkin V, Taft A, Fluet A, Friedman D, et al. Interaction of intracellular beta amyloid peptide with chaperone proteins. Proc Natl Acad Sci U S A. 2002;99:9439–9444. doi: 10.1073/pnas.152313999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans CG, Wisen S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J Biol Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 17.Magrane J, Rosen KM, Smith RC, Walsh K, Gouras GK, et al. Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response. J Neurosci. 2005;25:10960–10969. doi: 10.1523/JNEUROSCI.1723-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King M, Nafar F, Clarke J, Mearow K. The small heat shock protein Hsp27 protects cortical neurons against the toxic effects of beta-amyloid peptide. J Neurosci Res. 2009;87:3161–3175. doi: 10.1002/jnr.22145. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino T, Murao N, Namba T, Takehara M, Adachi H, et al. Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci. 2011;31:5225–5234. doi: 10.1523/JNEUROSCI.5478-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojha J, Karmegam RV, Masilamoni JG, Terry AV, Cashikar AG. Behavioral defects in chaperone-deficient Alzheimer’s disease model mice. PLoS ONE. 2011;6:e16550. doi: 10.1371/journal.pone.0016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stege GJ, Renkawek K, Overkamp PS, Verschuure P, van Rijk AF, et al. The molecular chaperone alphaB-crystallin enhances amyloid beta neurotoxicity. Biochem Biophys Res Commun. 1999;262:152–156. doi: 10.1006/bbrc.1999.1167. [DOI] [PubMed] [Google Scholar]
- 22.Sakono M, Zako T, Ueda H, Yohda M, Maeda M. Formation of highly toxic soluble amyloid beta oligomers by the molecular chaperone prefoldin. FEBS J. 2008;275:5982–5993. doi: 10.1111/j.1742-4658.2008.06727.x. [DOI] [PubMed] [Google Scholar]
- 23.Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, et al. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1-42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 24.Carnini A, Lear JD, Eckenhoff RG. Inhaled anesthetic modulation of amyloid beta(1-40) assembly and growth. Curr Alzheimer Res. 2007;4:233–241. doi: 10.2174/156720507781077278. [DOI] [PubMed] [Google Scholar]
- 25.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Bateman DA, McLaurin J, Chakrabartty A. Requirement of aggregation propensity of Alzheimer amyloid peptides for neuronal cell surface binding. BMC Neurosci. 2007;8:29. doi: 10.1186/1471-2202-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bateman DA, Chakrabartty A. Two distinct conformations of Abeta aggregates on the surface of living PC12 cells. Biophys J. 2009;96:4260–4267. doi: 10.1016/j.bpj.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bateman DA, Chakrabartty A. Int J Alzheimers Dis 2011: 962352; 2011. Cell surface binding and internalization of abeta modulated by degree of aggregation.962352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarze-Eicker K, Keyvani K, Gortz N, Westaway D, Sachser N, et al. Prion protein (PrPc) promotes beta-amyloid plaque formation. Neurobiol Aging. 2005;26:1177–1182. doi: 10.1016/j.neurobiolaging.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 33.You H, Tsutsui S, Hameed S, Kannanayakal TJ, Chen L, et al. Abeta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2012;109:1737–1742. doi: 10.1073/pnas.1110789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caetano FA, Lopes MH, Hajj GN, Machado CF, Pinto AC, et al. Endocytosis of prion protein is required for ERK1/2 signaling induced by stress-inducible protein 1. J Neurosci. 2008;28:6691–6702. doi: 10.1523/JNEUROSCI.1701-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang AJ, Chandswangbhuvana D, Margol L, Glabe CG. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1-42 pathogenesis. J Neurosci Res. 1998;52:691–698. doi: 10.1002/(SICI)1097-4547(19980615)52:6<691::AID-JNR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Yang AJ, Chandswangbhuvana D, Shu T, Henschen A, Glabe CG. Intracellular accumulation of insoluble, newly synthesized abetan-42 in amyloid precursor protein-transfected cells that have been treated with Abeta1–42. J Biol Chem. 1999;274:20650–20656. doi: 10.1074/jbc.274.29.20650. [DOI] [PubMed] [Google Scholar]
- 37.Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, et al. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc Natl Acad Sci U S A. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibbs SJ, Barren B, Beck KE, Proft J, Zhao X, et al. Hsp40 couples with the CSPalpha chaperone complex upon induction of the heat shock response. PLoS ONE. 2009;4:e4595. doi: 10.1371/journal.pone.0004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 40.Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem. 1997;272:6324–6331. doi: 10.1074/jbc.272.10.6324. [DOI] [PubMed] [Google Scholar]
- 41.Haraguchi T, Fisher S, Olofsson S, Endo T, Groth D, et al. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch Biochem Biophys. 1989;274:1–13. doi: 10.1016/0003-9861(89)90409-8. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyszkiewicz JP, Yan Z. beta-Amyloid peptides impair PKC-dependent functions of metabotropic glutamate receptors in prefrontal cortical neurons. J Neurophysiol. 2005;93:3102–3111. doi: 10.1152/jn.00939.2004. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki T, Usuda N, Murata S, Nakazawa A, Ohtsuka K, et al. Presence of molecular chaperones, heat shock cognate (Hsc) 70 and heat shock proteins (Hsp) 40, in the postsynaptic structures of rat brain. Brain Res. 1999;816:99–110. doi: 10.1016/s0006-8993(98)01083-x. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR. Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J Neurosci Res. 2005;81:522–529. doi: 10.1002/jnr.20575. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi H, Kobayashi Y, Yoshihara T, Niwa J, Doyu M, et al. Hsp70 and Hsp40 improve neurite outgrowth and suppress intracytoplasmic aggregate formation in cultured neuronal cells expressing mutant SOD1. Brain Res. 2002;949:11–22. doi: 10.1016/s0006-8993(02)02568-4. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Braun AP, Braun JE. Biological Roles of Neural J Proteins. Cell Mol Life Sci. 2008;65:2385–2396. doi: 10.1007/s00018-008-8089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westhoff B, Chapple JP, van der Spuy J, Hohfeld J, Cheetham ME. HSJ1 is a neuronal shuttling factor for the sorting of chaperone clients to the proteasome. Curr Biol. 2005;15:1058–1064. doi: 10.1016/j.cub.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 52.Ungewickell E, Ungewickell H, Holstein SEH, Linder R, Prasad K, et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- 53.Watson ED, Geary-Joo C, Hughes M, Cross JC. The Mrj co-chaperone mediates keratin turnover and prevents the formation of toxic inclusion bodies in trophoblast cells of the placenta. Development. 2007;134:1809–1817. doi: 10.1242/dev.02843. [DOI] [PubMed] [Google Scholar]
- 54.Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12:51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, et al. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosales-Hernandez A, Beck KE, Zhao X, Braun AP, Braun JE. RDJ2 (DNAJA2) chaperones neural G protein signaling pathways. Cell Stress Chaperones. 2008;14:71–82. doi: 10.1007/s12192-008-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu F, Proft J, Gibbs S, Winkfein B, Johnson JN, et al. Quercetin targets cysteine string protein (CSPalpha) and impairs synaptic transmission. PLoS ONE. 2010;5:e11045. doi: 10.1371/journal.pone.0011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, et al. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- 59.Florio T, Thellung S, Amico C, Robello M, Salmona M, et al. Prion protein fragment 106-126 induces apoptotic cell death and impairment of L-type voltage-sensitive calcium channel activity in the GH3 cell line. J Neurosci Res. 1998;54:341–352. doi: 10.1002/(SICI)1097-4547(19981101)54:3<341::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 60.Eiden M, Buschmann A, Kupfer L, Groschup MH. Synthetic prions. J Vet Med B Infect Dis Vet Public Health. 2006;53:251–256. doi: 10.1111/j.1439-0450.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 61.Zahn R, von Schroetter C, Wuthrich K. Human prion proteins expressed in Escherichia coli and purified by high-affinity column refolding. FEBS Lett. 1997;417:400–404. doi: 10.1016/s0014-5793(97)01330-6. [DOI] [PubMed] [Google Scholar]
- 62.Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 63.Gutierrez RC, Hung J, Zhang Y, Kertesz AC, Espina FJ, et al. Altered synchrony and connectivity in neuronal networks expressing an autism-related mutation of neuroligin 3. Neuroscience. 2009;162:208–221. doi: 10.1016/j.neuroscience.2009.04.062. [DOI] [PubMed] [Google Scholar]