Abstract
Background
Mitochondria dynamically buffer cytosolic Ca2+ in cardiac ventricular cells and this affects the Ca2+ load of the sarcoplasmic reticulum (SR). In sinoatrial-node cells (SANC) the SR generates periodic local, subsarcolemmal Ca2+ releases (LCRs) that depend upon the SR load and are involved in SANC automaticity: LCRs activate an inward Na+-Ca2+ exchange current to accelerate the diastolic depolarization, prompting the ensemble of surface membrane ion channels to generate the next action potential (AP).
Objective
To determine if mitochondrial Ca2+ (Ca2+ m), cytosolic Ca2+ (Ca2+ c)-SR-Ca2+ crosstalk occurs in single rabbit SANC, and how this may relate to SANC normal automaticity.
Results
Inhibition of mitochondrial Ca2+ influx into (Ru360) or Ca2+ efflux from (CGP-37157) decreased [Ca2+]m to 80±8% control or increased [Ca2+]m to 119±7% control, respectively. Concurrent with inhibition of mitochondrial Ca2+ influx or efflux, the SR Ca2+ load, and LCR size, duration, amplitude and period (imaged via confocal linescan) significantly increased or decreased, respectively. Changes in total ensemble LCR Ca2+ signal were highly correlated with the change in the SR Ca2+ load (r2 = 0.97). Changes in the spontaneous AP cycle length (Ru360, 111±1% control; CGP-37157, 89±2% control) in response to changes in [Ca2+]m were predicted by concurrent changes in LCR period (r2 = 0.84).
Conclusion
A change in SANC Ca2+ m flux translates into a change in the AP firing rate by effecting changes in Ca2+ c and SR Ca2+ loading, which affects the characteristics of spontaneous SR Ca2+ release.
Introduction
Mitochondrial Ca2+ flux plays a fundamental role buffering cytosolic Ca2+ (Ca2+ c) in cardiac ventricular myocytes (VM) in normal and pathological conditions [1]. Ca2+ c enters mitochondria through the mitochondrial uniporter and is extruded by the mitochondrial Na+-Ca2+ exchanger [2]. Mitochondrial Ca2+ buffering modulates both cell and sarcoplasmic reticulum (SR) Ca2+ load [1], [3], and on this basis affects VM contractility. In response to β-adrenergic stimulation, shifts in VM mitochondrial Ca2+ flux mediate an increase in energy supply required for increased contractility.
Sinoatrial nodal cells (SANC) initiate each heart beat by generating spontaneous APs that emanate from the sinoatrial node. Numerous studies over the last decade indicate a crucial role for SR cycling in SANC normal automaticity, giving rise to a coupled-clock system which explains the basis of robust regulation of SANC function [4], [5], [6] : Intracellular Ca2+ cycling comprises a “Ca2+ clock” within the coupled-clock system and the ensemble of surface membrane electrogenic molecules comprises the system’s membrane clock. Unlike VM, even in the absence of β-adrenergic stimulation in SANC, high levels of basal cAMP and cAMP-mediated protein kinase A-dependent (PKA) and Ca2+/calmodulin-dependent protein kinase II phosphorylation of Ca2+ cycling-proteins of both clocks enables the SR (Ca2+ clock) to generate spontaneous rhythmic local, subsarcolemmal Ca2+ releases (LCRs) via SR ryanodine receptors. LCRs activate an inward Na+-Ca2+ exchange current that accelerates the rate of diastolic depolarization, prompting the membrane clock to generate the next action potential (AP).
We hypothesized that changes in Ca2+ flux into and out of mitochondria in SANC will directly affect SR Ca2+ loading, and thus indirectly affect SR Ca2+ release, and therefore that a change in mitochondrial Ca2+ (Ca2+ m) cycling will modulate the normal automaticity of the coupled-clock system.
Changing the external electrical stimulation rate in VM is a convenient approach to induce changes in cross-talk between mitochondrial and SR Ca2+ cycling. But, this method can not be explored in SANC, which fire spontaneous AP’s. Therefore, to test our hypothesis that mitochondrial-SR Ca2+ crosstalk exists and links to SANC AP firing, we measured [Ca2+]c, [Ca2+]m, SR Ca2+ load and Ca2+ releases, and the spontaneous AP firing rate in intact, single, isolated SANC in response to specific inhibitors of Ca2+ flux into (Ru360) and Ca2+ flux from (CGP-37157) mitochondria.
Results
Selective Quenching of Cytosolic Indo-1 by Mn2+ Permits Assessment of [Ca2+]m in SANC
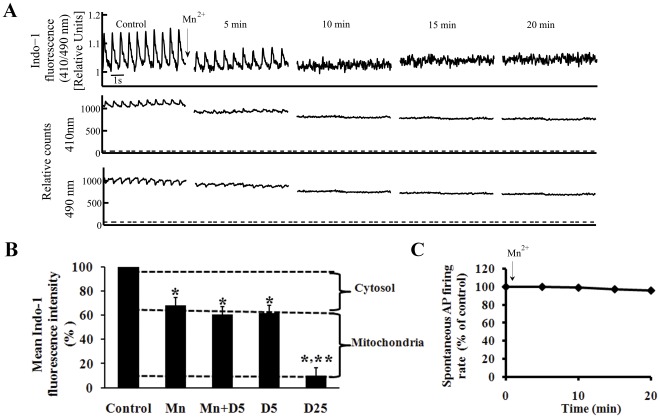
Ca2+ m was indexed by selective Mn2+ quenching of the cytosolic fluorescence of the Ca2+ probe, Indo-1 [7]. Application of 50 µM MnCl2 to Indo-1-AM loaded SANC selectively quenched the cytosolic Indo-1 fluorescence ratio 410/490 in a time dependent manner (11±2 min), as evidenced by the disappearance of the AP-induced cytosolic Ca2+ transient (Fig. 1A). The change in Indo-fluorescence intensity at each wavelength is illustrated in lower panels in Fig. 1A. Note that following 15-min of exposure to Mn2+, the remaining fluorescence was stable. Importantly, 20 min of exposure to Mn2+ caused only a small, non-significant decrease in the spontaneous AP firing rate compared to control (Fig. 1C). Since our prior studies [4], [5], [6] have demonstrated a tight relationship between Ca2+ c and normal automaticity the lack of an appreciable effect of Mn2+ on SANC AP firing rate suggests that at this concentration Mn2+ has minimal effects on [Ca2+]c or SR Ca2+ cycling.
Figure 1. Validation of mitochondrial Ca2+ measurements.
(A) Mn2+ (50 µmol/L) quenching of the cytosolic Indo-1 fluorescence ratio 410/490 (top panel), and fluorescence emission at wavelengths of 410 (middle panel), and 490 nm (lower panel). Dashed line under recordings of flourecence emission at individual wavelengths indicates background cell autofluorescence (the fluorescence without Indo-1 loading). (B) Permeabiliziation of the sarcolemma by 5 µmol/L digitonin alone (D5; n = 6) or together with Mn2+ to quench cytosolic Indo-1 (n = 6). Note that Mn2+ plus digitonin decreases the Indo fluorescence to the same level as Mn2+ alone (n = 10). A higher digitonin concentration (25 µmol/L D25; n = 4), permeabilizes the mitochondrial membrane in addition to the sarcolemma, and markedly depletes the cell fluorescence. (C) Application of Mn2+ does not alter the spontaneous AP firing rate. *p<0.05 vs. drug control, **p<0.05 vs. Mn2+.
To define the compartmental Indo-quenching, we quenched Indo-1 fluorescence using varying concentrations of digitonin: a low concentration of digitonin (5 µM) or saponin (25 µg/ml), which permeabilizes only the sarcolemma (Fig. S1 A-B); and a higher concentration of digitonin (25 µM), which also permeabilizes mitochondrial membranes (Fig. S1 C-D) [8], [9]. Figure 1B shows that Mn2+ treatment decreased the fluorescence to 68±2% of its control value. A low concentration of digitonin alone, or together with Mn2+ to quench cytosolic Indo-1, decreased the fluorescence to 63±4 and 61±3% control, respectively, i.e., to the same level as Mn2+ alone (p = 0.1 and 0.7, respectively). Saponin (25 µg/ml) reduced the fluorescence to 64±5% (n = 5), i.e. to the same level as permeabilizing of sarcolemma with the lower concentration of digitonin. Thus, when the SANC plasma membrane was selectively permeablized, the Indo fluorescence decreased to the same level as it did in cells with an intact surface membrane in the presence of Mn2+. In contrast, a higher concentration of digitonin, which also permeabilizes mitochondrial membranes, decreased the fluorescence to 10±4% control (p = 0.001, compared to the Mn2+ treated group). These results provide evidence that the Mn2+-resistant fluorescence of Indo-1 loaded cells is derived from Indo-1 within the mitochondrial compartment. Note, that in the time-control experiment protocol without Mn2+ application, Indo-1 fluorescence was only slightly quenched (−7±3%).
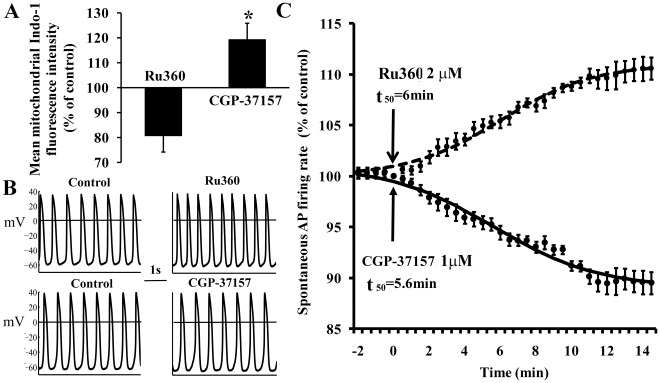
Ru360 and CGP-37157, specific inhibitors of Ca influx into and efflux from mitochondria, respectively, were used to perturb [Ca2+]m. Figure 2A shows that Ru360 (2 µM) decreased mean [Ca2+]m to 80±8% of control (from 210±19 to 171±19 nM) of the Mn2+ quenched control; conversely, CGP-37157 (1 µM) increased [Ca2+]m to 119±7% of control (from 210±19 to 270±13 nM) (p<0.05 Ru360 vs. CGP-37157, see Fig. S2 for representative examples). Thus, Ru360 and CGP-37157 affect Ca2+ m comparably in SANC as in previous studies in ventricular myocytes [10], [11].
Figure 2. The effect of inhibition of mitochondrial fluxes on mitochondrial Ca2+ and spontaneous AP firing rate.
(A) Mean mitochondrial fluorescence intensity in response to specific inhibition of mitochondrial Ca2+ influx or efflux (n = 10 for each drug), (B) AP recordings (before and after 15 min exposure to each of the drugs), and (C) average time-dependent change in the rate of AP induced contractions in the presence of CGP-37157 (n = 12) or Ru360 (n = 11). **p<0.05 vs. Ru360.
Perturbations of Ca2+ m Cycling Affect the SANC Spontaneous AP Firing Rate
The main question of our study is whether a change in Ca2+ cycling into and out of mitochondria can modulate normal automaticity. Figure 2B shows representative examples of the effect of a 15-min exposure of SANC to specific inhibitors of Ca2+ influx into or efflux from mitochondria on spontaneous SANC AP firing. The average time-courses of the change in AP firing rate in response to perturbations of mitochondrial Ca2+ efflux or influx are illustrated in Fig. 2C. Inhibition of Ca2+ influx into mitochondria by Ru360 (2 µM) caused, over a 10-min period, a progressive increase in the spontaneous SANC AP firing rate to 111±1% control (from 2.7±0.25 to 3±0.3 Hz). Conversely, inhibition of Ca2+ efflux from mitochondria by CGP-37157 (1 µM) progressively reduced the spontaneous AP firing rate to 89±2% control (from 2.65±0.2 to 2.3±0.2 Hz) over a 10-min period. Note that both effects evolved and saturated with roughly the same time course. These drug effects were not reversible even after 10-min wash. The changes in other AP parameters are summarized in Table 1.
Table 1. Average characteristics of SANC spontaneous APs in response to pharmacological perturbations (n = 9 in each group).
| Control | Ru360 | Control | CGP-37157 | |
| Frequency (Hz) | 2.7±0.25 | 3±0.3* | 2.65±0.2 | 2.3±0.2* , ** |
| Action potential amplitude (mV) | 90±2 | 91±2 | 91±4 | 93±5 |
| Overshoot (mV) | 31±1 | 31±2 | 33±3 | 35±3 |
| Maximal diastolic potential (mV) | −59±1 | −60±1 | −61±2 | −62±2 |
| Max rate of diastolic depolarization, dV/dt (mV/s) | 6.2±1 | 6.8±1 | 6±1 | 5.8±1 |
p<0.05 vs. drug control.
p<0.05 vs. Ru360.
Perturbations of SANC Ca2+ m Cycling Affect the SANC Ca2+ c Characteristics
To determine how Ca2+ buffering by the mitochondria affects [Ca2+]c we loaded cells with Indo-1AM. Changes in spontaneous AP firing rate by inhibition of mitochondrial Ca2+ influx or efflux were determined in Indo-1/AM loaded cells (see representative examples in Fig. S2). Table 2 lists the average characteristics of the AP-induced Ca2+ c transients measured by Indo-1 and Table 3 list the average characteristics of the AP-induced Ca2+ c transients in the presence of Ru360 or CGP-37157. Inhibition of Ca2+ m influx significantly increased peak systolic Ca2+ from 380±23 to 415±28 nM (p = 0.001) and decreased the 90% decay time of cytosolic [Ca2+] (T-90c) from 302±13 to 285±11 ms (p = 0.04). The diastolic Indo fluorescence (p = 0.6), time to peak Ca2+ (T-Pc; p = 0.07), and 50% decay time of cytosolic Ca2+ (T-50c; p = 0.2) were not significantly altered. Conversely, inhibition of Ca2+ m efflux significantly decreased peak systolic [Ca2+] from 369±13 to 342±13 nM (p = 0.02) and increased T-90c from 301±14 to 325±12 ms (p = 0.04). The diastolic Indo fluorescence (p = 0.12), T-Pc (p = 0.3), and T-50c (p = 0.2) were not significantly altered. Therefore, the effects of inhibition of [Ca2+]m influx or efflux on [Ca2+]c in SANC are similar to their effects in prior studies in guinea pig ventricular cardiac myocytes [12].
Table 2. Average characteristics of SANC spontaneous AP induced Ca2+ transients (n = 43).
| Peak systolic Ca2+ (nmol/L) | 374±8 |
| Minimum diastolic Ca2+ fluorescence (nmol/L) | 130±4 |
| Ca2+ transient amplitude (systolic-diastolic) (nmol/L) | 244±8 |
| T-Pc (ms) | 87±3 |
| T-50c (ms) | 182±5 |
| T-90c (ms) | 301±8 |
| Frequency (Hz) | 2.3±0.1 |
Table 3. Average characteristics of SANC spontaneous AP induced Ca2+ transients in response to pharmacological perturbations.
| % control | Ru360 (n = 10; 2 µM) | CGP-37157 (n = 10; 1 µM) | CPA (n = 8; 5 µM) |
| Peak Systolic Ca2+ | 10±1* (p = 0.001) | −10±3* (p = 0.03) | −14±3* (p = 0.001) |
| Diastolic Ca2+ fluorescence | 2±2 (p = 0.59) | −6±4 (p = 0.12) | −1±4 (p = 0.4) |
| Ca2+ transient amplitude (systolic-diastolic) | 13±3* (p = 0.001) | −10±5* (p = 0.001) | −28±7* (p = 0.003) |
| T-Pc | −6±5 (p = 0.2) | 2±4 (p = 0.3) | 10±5 (p = 0.14) |
| T-50c | −4±3 (p = 0.08) | 3±3 (p = 0.2) | 8±5* (p = 0.02) |
| T-90c | −6±2* (p = 0.03) | 7±3* (p = 0.04) | 19±7* (p = 0.04) |
| Frequency | 10±2* (p = 0.004) | −10±3* (p = 0.02) | −33±4* (p<0.001) |
| Mean mitochondrial fluorescence intensity | 80±8* (p = 0.02) | 119±7* (p = 0.03) | 86±8* (p = 0.045) |
p<0.05 vs. drug control.
Perturbation of [Ca2+]m Cycling Affects SR Ca2+ Load
Numerous studies of SANC indicate that the characteristics of SR Ca2+ cycling are determined, in part, by the SR Ca2+ load. Perturbations of mitochondrial Ca2+ flux might affect SR Ca2+ loading if these two organelles were located substantially close to each other. To determine the proximity of mitochondria to SR we employed dual immunolabeling to SANC with anti-SERCA2 antibody and Mitotracker orange to visualize the SR and mitochondrial membrane, respectively. SANC of varying size exhibit robust uniform SERCA2 immunolabeling (Fig. S3 left panels) and robust mitochondrial labeling (Fig. S3 middle panels). The merged images (Fig. S3 right panels) indicate that a substantial part of the mitochondrial mass is colocalized with that of SERCA2. This colocalization would highly facilitate rapid mitochondrial-SR Ca2+ cycling.
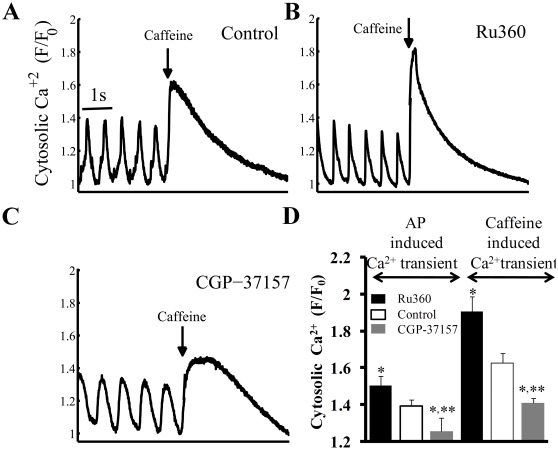
Changes in AP triggered Ca2+ transient in response to perturbing Ca2+ m flux (Fig. 2C) could change the SR Ca2+ loading. Specifically, inhibition of Ca2+ m influx, while reducing [Ca2+]m (Fig. 2A), might result in more Ca2+ within the cytosol available for pumping into the SR; conversely, inhibition of Ca2+ m efflux to increase [Ca2+]m might “steal” Ca2+ from the cytosol, resulting in reduced Ca2+ pumping into the SR. To estimate changes in the SR content, brief rapid applications of caffeine were applied (“spritzed”) onto the cell following drug application to perturb Ca2+ m flux. Representative examples and average data are presented in Figures 3A–C and Fig. 3D (right bars), respectively. Blocking Ca2+ influx into mitochondria increased the SR Ca2+ load (assessed by change in amplitude of the Ca2+ transient induced by a brief rapid application of caffeine) by 17±5% of control (1.6±0.05 to 1.9±0.08 F/F0; p = 0.01); conversely inhibition of Ca2+ efflux from mitochondria reduced the SR Ca2+ load by 13±2% of control (1.6±0.05 to 1.4±0.03 F/F0; p = 0.03) (Fig. 3D, right bars). Although CGP-37157 reduced the rate of decay of the caffeine-evoked response compared to Ru360, this decrease was not significant. The change in the AP-induced Ca2+ transient amplitude by Ru360 or CGP-37157 prior to caffeine application (Fig. 3D, left bars) is similar to the trend measured by Indo-1 (Table 3). Note that the relative drug-induced changes in the caffeine and AP-induced cytosolic Ca2+ transient amplitudes and changes in Ca2+ m and cycle length are all roughly equivalent. Thus, the changes in the amplitude of systolic Ca2+ c transient due to manipulation of Ca2+ m flux, could, in part at least, be due to changes in the SR Ca2+ load. Although, Ru360 and CGP-37157 tended to increase and decrease, respectively, the PLB phosphorylation at Serine-16 (PKA-dependent site), these effects were not statistically significant (Fig. S4). Note, however, that isoproterenol, employed as a positive control, markedly increases PLB phosphorylation.
Figure 3. SR load estimation from rapid caffeine application.
Effects of a rapid application (“spritz”) of caffeine (indicated by the arrow) onto SANC (A) in control, or (B) in the presence of Ru360 or (C) CGP-37157. (D) Average effects of Ru360 or CGP-37157 on peak AP-induced cytosolic Ca2+ prior to a caffeine spritz (left), and the subsequent caffeine-induced cytosolic Ca2+ transient (right) (n = 12, for each group). (The caffeine response can be usually measured only once in a given SANC, because following caffeine application a prolonged period is required for AP firing rate to return to the control AP firing rate. Therefore, the effects of caffeine before (i.e. control) and following application of drugs that affect Ca2+ m flux were measured in different cells).
Perturbation of [Ca2+]m Cycling Affects Spatiotemporal Characteristics of Rhythmic, Spontaneous SR-generated Local Ca2+ Releases (LCRs) via Ryanodine Receptors
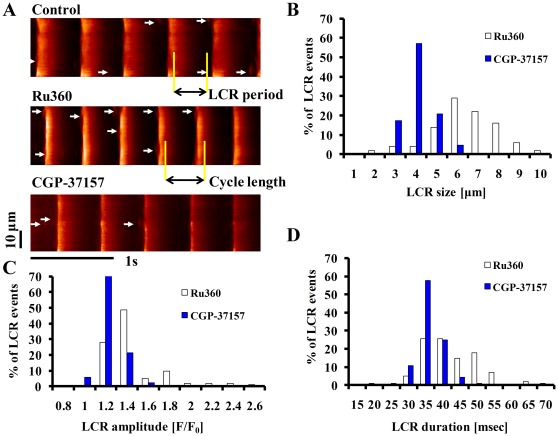
Prior studies have shown that changes in SR Ca2+ loading in SANC affect spontaneous diastolic LCRs [5], [13]. To determine how a perturbation of Ca2+ m cycling affects spatiotemporal characteristics of LCRs, we measured LCRs in Fluo-4 AM loaded SANC, and imaged its Ca2+-dependent fluorescence by confocal microscopy. Representative examples (Figure 4A) show that the spontaneous AP firing rate, measured via confocal line scan imaging increased (by Ru360) or decreased (by CGP-37157). Figures 4B-4D depict histograms of LCR size, amplitude, and duration in the presence of Ru360 or CGP-37157. Ru360 shifted distribution of LCR amplitudes, spatial widths, and durations to larger values. On average, Ru360 increased LCR size from 4.2±0.1 to 6.1±0.2 µm (p<0.001), the LCR amplitude from 1.24±0.02 to 1.36±0.04F/Fo (p = 0.04), and the LCR duration from 35±0.8 to 41±0.8ms (p<0.001). In contrast to Ru360, CGP-37157 decreased the average LCR size from 4.3±0.1 to 2.6±0.1 µm (p<0.001), the average LCR amplitude from 1.22±0.02 to 1.14±0.01F/Fo (p = 0.02), and the average LCR duration from 35±0.5 to 29±0.6ms (p<0.001).
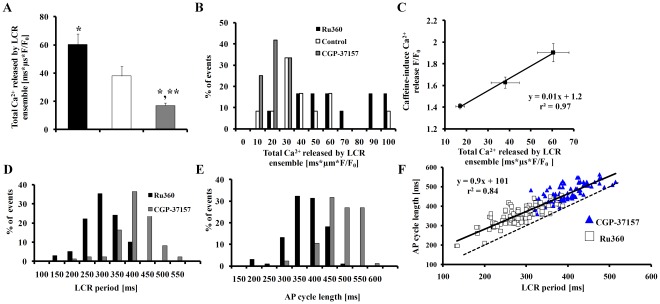
Figure 4. Specific inhibition of Ca influx into or efflux from mitochondria in intact SANC modifies spatiotemporal characteristics of LCRs.
(A) Confocal line scan Ca2+ images of a representative SANC before and following exposure to 2 µmol/L Ru360 or 1 µmol/L CGP-37157. LCRs are indicated by arrowheads. The LCR period is defined as the time from the peak of the prior AP-induced Ca2+ transient to the LCR onset. Histograms of LCR (B) size (full width at half-maximum amplitude), (C) amplitude (F/F0), and (D) duration (full duration at half-maximum amplitude) in the presence of Ru360 (n = 12; 102 LCRs) and CGP-37157 (n = 12; 92 LCRs).
The total Ca2+ of the LCR ensemble shifted to lower values in response to CGP-37157, and to higher values in response to Ru360 (Fig. 5A–B). Figure 5A shows that, interestingly, Ru360 increased the ensemble LCR Ca2+ from 38±6 to 61±7 ms*µm*F/F0 (p<0.001) and CGP-37157 decreased it from 35±3 to 17±2 ms*µm*F/F0 (p = 0.003). The Ru360-induced increase, and the CGP-37157-induced decrease in the total LCR ensemble Ca2+ paralleled the effects of Ru360 and CGP-37157 on the amplitude of caffeine induced Ca2+ release into the cytosol (Fig. 5C). This suggests that changes in spontaneous LCRs elicited by Ru360 or CGP-37157 are linked to concomitant changes in SR Ca2+ load effected by these drugs.
Figure 5. Relationships among the Ca2+ release of the LCR ensemble, LCR period, SR Ca2+ load and AP firing rate.
The total LCR Ca2+ ensemble (A) average data and (B) histogram in the presence of Ru360 or CGP-37157. (C) The relationship between caffeine-induced Ca2+ release F/F0 and the total LCR Ca2+ ensemble. Specific inhibition of Ca2+ influx into or efflux from mitochondria in intact SANC shifts the LCR period and AP cycle length. (D) LCR period and (E) AP cycle length in the presence of Ru360 (n = 12; 102 LCRs) or CGP-37157 (n = 12; 92 LCRs). (F) The change in LCR period in response to perturbing mitochondrial Ca2+ flux predicts the concomitant change in AP cycle length. The Ru360-induced decrease in the spontaneous cycle length, and CGP-37157-induced increase in spontaneous cycle length, are both predicted by their effects on the LCR period. The dashed line is the line of identity. *p<0.05 vs. control, **p<0.05 vs. Ru360.
The LCR period, defined as the time from the peak Ca2+ transient (the onset SR Ca2+ release triggered by the prior AP) to an LCR onset (as illustrated in Fig. 4A) was reduced by Ru360 from 317±5 to 274±6ms (Fig. 5D, p<0.001), and increased by CGP-37157 to 389±7ms (Fig. 5D, p<0.001). Changes in the spontaneous AP cycle length occurred concomitantly with changes in the LCR period by specific inhibition of Ca2+ influx into or efflux from the mitochondria (Fig. 5E); change in LCR period (from 274±6 (Ru360) to 389±7ms (CGP-37157)) predicted the concomitant change in the spontaneous SANC AP cycle length from 349±5 to 462±6ms (Fig. 5F, r2 = 0.84, p<0.001). The relationship between the change in LCR period and AP cycle length by perturbations of Ca2+ m flux is quantitatively strikingly similar to that which occurs in response to other interventions which affect the LCR period and AP cycle length of SANC [14]. Interestingly, simultaneous application of Ru360 and CGP-37157 neither significantly altered Cam, the spontaneous AP firing rate, nor LCR characteristics (Fig. 6). Thus, when both drugs are present the decrease in Ca2+ influx into the mitochondria by Ru360 can be compensated for by the decrease in Ca2+ efflux from the mitochondria by CGP-37157. Note, that this result only indicates that blocking both Ca2+ influx and efflux does not change the mitochondrial buffering capacity, and does not indicate that under physiological conditions mitochondrial fluxes do not play a role in modulating SR Ca2+ loading.
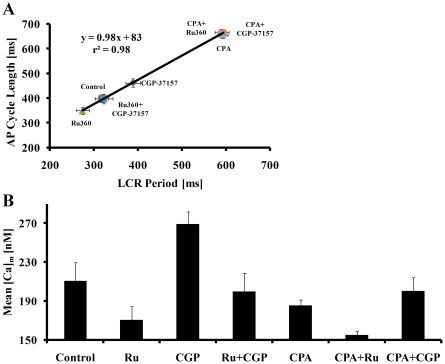
Figure 6. The effect of CPA on AP cycle length, LCR period and mitochondrial Ca2+.
(A) The effect of CPA on the change in LCR period predicts the concurrent prolongation of AP cycle length. Note that this effect of CPA on LCR period and AP cycle length form a continuum with the effects of Ru360 and CGP-37157 on LCR period and AP cycle length. (B) In the presence of CPA neither Ru360 nor CGP-37157 affect the LCR period or AP cycle length, and [Ca2+]m decreased. The combination of Ru360 plus CGP-37157 does not change [Ca2+]m, AP cycle length or the LCR period.
Inhibition of SR Ca2+ Cycling Abolishes the Effect of Mitochondrial Ca2+ Cycling on the SANC Spontaneous AP Firing Rate
To prove that a change in SR load is a necessary component in the feedback mechanism between [Ca2+]m and the AP firing rate, we reduced the SR load by blocking SR Ca2+ pumping with Cyclopiazonic acid (CPA) (5 µM) [15]. Both the LCR period (from 347±8 to 565±24 ms; p<0.001) and AP cycle length (from 413±7 to 594±20 ms; p<0.001) were markedly prolonged by CPA (Fig. 6A) and Ca2+ m decreased (Table 3). Note that changes in the spontaneous AP cycle length induced by CPA alone or by inhibition of mitochondrial Ca2+ flux, and changes in the LCR period form a continuum (Fig. 6A). Note also in Figure 6A, that in the presence of CPA the effects of Ru360 or CGP-37157 on the AP cycle length are eliminated. Moreover, the difference in [Ca2+]m between Ru360 and CGP-37157 (Fig. 6B) is reduced in the presence of CPA, and therefore the effects of these drugs on mitochondrial Ca2+ buffering are reduced (Fig. 6B). To further test the idea that an increase in mitochondrial Ca2+ buffering decreases the SR Ca2+ load, we employed a sufficient concentration of CPA to reduce SR Ca2+ load to a similar extent as CGP-37157, and compared its effect on AP cycle length to those of CGP-37157. CPA (0.5 µM) decreased the spontaneous AP firing rate by 15±3% (n = 12, from 2.4±0.1 to 2±0.07 Hz; p = 0.003) and reduced the SR Ca2+ load by 17±3% (n = 12 in each group; from 1.5±0.09 to 1.2±0.05 F/F0; p = 0.003). This decrease in SR load by CPA is comparable to decreases in both spontaneous AP firing rate and SR Ca2+ load effected by CGP-37157. These results are consistent with our interpretation that the changes in AP cycle length due to manipulation of [Ca2+]m content are linked to changes in the SR Ca2+ load.
Blocking the “funny” Current, If, does not Affect the Mitochondrial Ca2+ Cycling Effect on the SANC Spontaneous AP Firing Rate
It had previously been thought that If is the main regulator of SANC spontaneous AP firing rate [16]. To determine the extent to which If effectively influences the effect of perturbing Ca2+ m cycling on the SANC spontaneous AP firing rate, we employed CsCl to inhibit If. Figure S5 shows that application of CsCl plus Ru360 increased the beating rate, over a 15-min period, to 111±1% control (from 2.5±0.1 to 2.74±0.1 Hz; p = 0.003). Application of CsCl plus CGP-37157 decreased the beating rate, over a 15-min period, to 88±1% control (from 2.5±0.1 to 2.26±0.1 Hz; p = 0.01). These effects of If inhibition plus inhibition of mitochondrial Ca2+ fluxes neither quantitatively nor qualitatively differed from those when inhibitors of mitochondrial Ca2+ flux alone were applied without If inhibition. Therefore If does not have a role in the effects of Ru360 or CGP-37157 on AP firing rate (compare Fig. S5 with Fig. 2).
Novel Numerical Model Simulations Predict the Changes in SANC Membrane Ionic Currents, SR and Mitochondrial Ca2+ Fluxes, and AP Cycle Length in Response to Ru360 or CGP-37157
To quantitatively simulate SR and mitochondrial dynamics and the AP firing rate when Ca2+ m is perturbed, and to simulate how surface membrane currents change when SR and mitochondrial Ca2+ fluxes are perturbed, we extended the SANC coupled-clock numerical model [17] to include mitochondrial Ca2+ fluxes. For details of the model and simulations see in Text S1 (the model parameters are in Table S1 and Table S2).
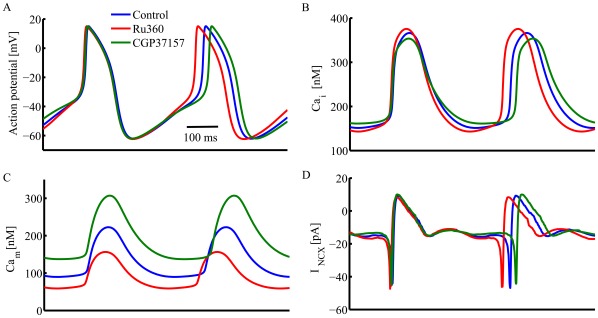
The extended coupled clock numerical simulations reproduce the effect of manipulation of Ca2+ m on spontaneous AP firing rate (Fig. 7A) and Ca2+ c (Fig. 7B). The model simulations also predict that manipulation of mitochondrial Ca2+ flux perturbs the junctional and network SR Ca2+ fluxes, as observed experimentally (Fig. S6). This finding is in accord with our experimental observations that manipulation of mitochondrial Ca2+ flux affects the SR Ca2+ content. While our experimental data permit quantification of the average cytosolic and diastolic [Ca2+]m only (see above), the extended coupled clock numerical simulations also predict both systolic and diastolic levels of [Ca2+]m, under basal conditions, and when mitochondrial Ca2+ flux is inhibited (Fig. 7C). Inhibition of mitochondrial Ca2+ influx by Ru360 decreased the maximum diastolic [Ca2+]m (from 89.8 to 60 nM) and the systolic [Ca2+]m (from 222.7 to 160 nM). Inhibition of mitochondrial Ca2+ efflux by CGP-37157, however, increased both diastolic [Ca2+]m (to 137.2 nM) and systolic [Ca2+]m (to 308 nM). Note, that manipulation of Ca2+ m affected the cycle length without affecting If kinetics, which is also consistent with our experimental result. Moreover, the model predicts that sarcolemmal Na+-Ca2+ exchanger current kinetics are altered indirectly due to change in LCR characteristics (Fig. 7D) when Ca2+ m flux is perturbed.
Figure 7. Simulations of the coupled clock numerical model, extended to include mitochondrial Ca2+ fluxes.
The simulated effects of specific inhibition of Ca influx into or efflux from mitochondria on (A) membrane potential, (B) cytosolic Ca2+, (C) mitochondrial Ca2+, and (D) sarolemmal Na+-Ca2+ exchanger current in intact SANC.
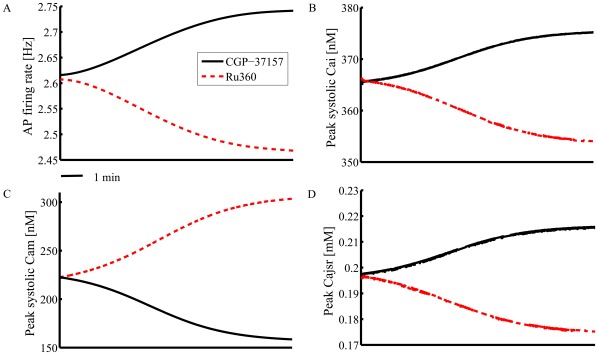
Since it is not possible experimentally to implement long recordings in isolated, single SANC (∼15 min) to measure the kinetics of the changes in intracellular Ca2+ dynamics and AP firing we used a numerical model to predict the inhibition of Ca2+ influx or efflux affects on cytosolic Ca2+, SR Ca2+ load, and AP firing rate. Model simulations predict that inhibition of mitochondrial Ca2+ efflux by CGP-37157 increases peak [Ca2+]m over a 10-min time course and concomitantly decreases peak cytosolic Ca2+, peak jSR Ca2+ and spontaneous AP firing rate (Fig. 8). Simulation of inhibition of mitochondrial Ca2+ influx by Ru360 yielded a mirror image of the effect of CGP-37157. Thus these numerical simulations support the idea that when mitochondrial Ca2+ flux is perturbed, the changes in mitochondrial Ca2+, cytosolic Ca2+, SR Ca2+ loading and AP firing rate occur with the same kinetics.
Figure 8. The extended coupled clock numerical model simulations of kinetics.
The simulation of the change in (A) AP firing rate, (B) peak systolic cytosolic Ca2+, (C) peak systolic mitochondrial Ca2+, and (D) peak Ca2+ in junctional SR in response to specific inhibition of Ca2+ flux into or flux from mitochondria.
In summary, we conclude that the coupled clock system of intracellular Ca2+ cycling proteins and surface membrane electrogenic molecules that regulates SANC normal automaticity includes SR-mitochondrial Ca2+ cross-talk.
Discussion
The present results show, for the first time, that mitochondrial-SR Ca2+ cross talk, previously demonstrated in ventricular myocytes [18], also occurs within SANC. A most important novel finding of our study is that changes in Ca2+ cycling into and out of mitochondria in SANC, effected by using Ru360 and CGP-37157, modulate basal coupled-clock system automaticity via an impact on Ca2+ c, SR loading and indirectly on Ca2+ release characteristics: blocking mitochondrial Ca2+ efflux increases [Ca2+]m, decreases the SR load, decreases the amplitude of the AP triggered cytosolic Ca2+ transient and total Ca2+ released spontaneously by the LCR ensemble, and reduces the AP firing rate. Conversely, reducing mitochondrial Ca2+ influx decreases [Ca2+]m, increases SR Ca2+ loading, increases the amplitude of the AP triggered cytosolic Ca2+ transient and total Ca2+ released spontaneously by the LCR ensemble, and increases the AP firing spontaneous rate (Fig. 3, 4, 5, Table 3). That interfering with mitochondrial Ca2+ flux modulates the spontaneous AP firing rate via effects on Ca2+ c and SR Ca2+ loading and release supports the idea that, in the absence of these mitochondrial Ca2+ flux inhibitors, mitochondrial buffering of Ca2+ c modulates SR Ca2+ cycling and AP firing. Since basal Ca2+-cAMP-PKA signaling within SANC also regulates ATP supply-demand matching, Ca2+ m-Ca2+ c cross-talk indirectly links to ATP supply and demand mechanisms.
Mitochondrial-SR Ca2+ cross-talk observed in our experiments in SANC also occurs in other cell types. Observation in non-excitable cells indicate that the pattern and frequency of spontaneous cytosolic Ca2+ oscillations changes in the presence of CGP-37157, and it was argued, therefore, that mitochondrial Ca2+ flux may modulate cytosolic Ca2+ oscillations in these cells [19]. Also, the amplitude of spontaneous Ca2+ waves in Cajal cells originating from the mitochondrial area are attenuated by Ru360, while CGP-37157 suppressed the initial Ca2+ rise that reduced Ca2+ wave frequency [20]. In VM, differences in the kinetics of mitochondrial Ca2+ influx and efflux have been noted among species [12], [18], [21]. For example, Maack et al, similar to the present study, observed changes of [Ca2+]c and [Ca2+]m in guinea-pig cardiomyocytes when mitochondrial Ca2+ fluxes were perturbed by Ru360 or CGP-37157 [12]. In rat cardiomyocytes under basal conditions, in contrast to guinea-pig, application of Ru360 decreases Ca2+ m, but does not alter Ca2+ c [11], [22]. Also of note, in isolated perfused guinea pig hearts, CGP-37157 has only a non-significant trend to reduce heart rate [3], whereas in the present study, the AP firing rate is significantly reduced by CGP-37157. Differences between the prior study and our study likely not only reflect species differences, but also the different experimental preparations employed: i.e., isolated single pacemaker cells in the present study vs. intact hearts in the prior study.
Whether mitochondrial Ca2+ buffers cytosolic Ca2+ on a beat-to-beat base in VM is a controversial issue related to uncertainty with respect to the extent and kinetics of both uptake and release of Ca2+ into and out the mitochondria [23], [24] (for a thoughtful review see [25]). To estimate the mitochondrial influx rate in VM, Ca2+ m was measured in response to different pacing rates (or during stimulation compared to quiescent mode). External stimulation in SANC is not feasible, however, because SANC beat spontaneously, and it is challenging to over-drive the spontaneous SANC AP firing rate, while maintaining a normal rhythm (Lakatta et al. unpublished observation). Beat-to-beat Ca2+ m flux into or out of the mitochondria of SANC in the present study can be roughly estimated, however assuming that Ru360 or CGP-37157 reduces the influx or efflux by 30%, respectively (based on our numerical model), and assuming a linear time-course of drug effects to inhibit their targets. Given the experimentally determined time course of the drug-induced changes in [Ca2+]m, and the average number of spontaneous AP cycles over this time, a rough estimation of drug-induced changes in Ca2+ flux from or into the mitochondria per AP cycle is on the order of 0.5 nM/beat.
The validity of our interpretations of the present results assumes that the method used to measure Ca2+ m does not substantially affect [Ca2+]m itself, or affect other functions within SANC that are related to the AP firing rate. Because our major goal was to explore whether perturbations of mitochondrial Ca2+ cycling modulates the basal AP firing rate of SANC, neither cell permeabilization [26] which abolishes AP firing, nor dialysis of rhod-2 [12] is suitable to achieve this goal (Dialysis is a method to measure [Ca2+]m that requires acute rupture of a membrane patch, often creating a small leak current, that notably affects the balance of ionic currents during diastolic depolarization and spontaneous AP frequency of SANC [27]). We selected the Mn2+ quench approach to assess [Ca2+]m in SANC because measurements of [Ca2+]m in VM by cell permeabilization and Mn2+ quench are in close agreement [26]. The ability of Mn2+ to selectively quench Ca2+ c fluorescence, without causing marked changes in Ca2+ m, is based on its slower quenching of the mitochondrial fluorescence [7], [28]. Important requirements of this method are: 1) that the lowest concentration of Mn2+ that rapidly quenches cytosolic Ca2+but has minimal effects on sarcolemmal or mitochondrial Ca2+ ion transport be employed and 2) that during Ca2+ c quenching the temperature be maintained at a near physiological level. We employed 50 µM Mn2+ to quench Ca2+ c fluorescence, which at this concentration has no effect on our major functional endpoint, the spontaneous AP firing rate. Specifically, since we previously have shown [4], [5], [6] a tight relationship between cell cycling Ca2+ and AP firing rate, the lack of a sustained effect of Mn2+ on the SANC spontaneous AP firing rate strongly suggests that there is no functionally significant inhibition of sarcolemmal Ca2+ channels (i.e. L-type channels) by 50 µM Mn2+. Another concern about the Mn2+ quench technique is that Mn2+ can inhibit oxidative phosphorylation. The lack of a sustained effect of Mn2+ on the basal spontaneous AP firing rate again suggests that Mn2+ does not perturb the matching of ATP supply to demand, since we have previously shown that this matching is closely linked to the AP firing rate [29].
The selectivity of drugs employed to perturb Ca2+ m flux is another important issue that merits consideration in interpreting the present results. We employed Ru360 at concentration of 2 µM to selectively block Ca2+ influx into the mitochondria, i.e. a concentration similar to that used in prior studies in VM [11], [12] to effectively block Ca2+ influx to the mitochondria and to decrease [Ca2+]m. In rat VM, in which Ca2+ c is not substantially affected by changes in Ca2+ m, this concentration of Ru360 does not affect SR Ca2+ uptake or L-type current [11]. Moreover, under basal conditions in rat VM Ru360 neither affects mitochondrial membrane potential nor ROS production [30]. Further, CPG-37157 does not likely increase the probability of mPTP opening, which generates ROS, because even during cardiac glycoside toxicity, application of CGP-37157 together with ouabain reduces oxidative species production that is effected by ouabain itself, even though Ca2+ m is higher during ouabain application than during control conditions [3].
We employed CGP-37157 at concentration of 1 µM because it selectively blocks the mitochondrial Na+-Ca2+ exchanger (IC50 = 0.5 µM) [31] and increases [Ca2+]m, but has only minor effects on sarolemmal Na+-Ca2+ exchanger (IC50 = 13 µM), or Ca2+ uptake by SR (IC50 = 10 µM) or on ryanodine receptor open probability (IC50 = 9.5 µM) [32]. L-type Ca2+ current is a crucial element of SANC automaticity [14]. Although CGP-37157 has been reported to affect L-type current in atrial cells (IC50 = 0.3 µM) [33], in rat even 10 µM Ru360 VM does not affect L-type current [11]. Our experimental evidence (Fig. 6) indicates that simultaneous application of Ru360 and CGP-37157 in SANC cancel each other’s effect on automaticity, suggesting that CGP-37157 does not have a substantial effect on L-type current in SANC. Moreover, the lack of effect of Ru360 or CGP-37157 on SANC AP parameter characteristics (Table 1) strongly suggests that there is no significant functional inhibition of any sarcolemmal channels by either of these drugs applied as in our study.
Our novel numerical model simulations reproduce our experimental findings and validate our interpretation of these findings: changes in mitochondrial Ca2+ flux affect the spontaneous AP firing rate of SANC via changes in SR Ca2+ loading and release. Furthermore, while our experimental method to measure [Ca2+]m does not permit evaluation of systolic and diastolic [Ca2+]m, our model predicts that under basal conditions both diastolic and systolic [Ca2+]m are lower than the diastolic and systolic [Ca2+]c, respectively. However, the systolic [Ca2+]m is higher than diastolic [Ca2+]c.
Summary
In summary, the present study shows, for the first time, that SANC mitochondria and SR are closely associated, i.e., that Ca2+ cycling into and out of the mitochondria acts as a dynamic buffer of cytosolic Ca2+, thus affecting Ca2+ availability for SR Ca2+ loading and release. When mitochondrial Ca2+ influx or Ca2+ efflux is selectively blocked, SR Ca2+ cycling and Ca2+ load are altered. This leads to changes in spontaneous AP triggered SR Ca2+ release that produce changes in both global AP induced cytosolic Ca2+ transients and to spontaneous, localized submembrane diastolic SR Ca2+ release. Therefore, under basal conditions mitochondrial Ca2+ fluxes modulate SR Ca2+ cycling via an effect on SR Ca2+ loading, and on this basis mitochondrial Ca2+ flux is indirectly linked to the SANC spontaneous AP firing rate.
Materials and Methods
Single SANC Isolation, Cell Contraction and Electrophysiological Recordings
Single, spontaneously beating SANC were isolated from New Zealand White rabbit hearts as previously described [13], using protocols approved by the Animal Care and Use Committee of the National Institutes of Health (protocol #034LCS2013). The cell contraction rate and Ca2+ cycling were studied in Tyrode solution at 35±0.5°C, with the following composition (in mM): 140 NaCl, 5.4 KCl, 2 MgCl2, 5 HEPES, 1.8 CaCl2, and 5.5 Glucose, and was titrated to pH 7.4 with NaOH. Cells were imaged with an LSM-510 inverted confocal microscope using a 63×/1.4 N.A. oil immersion lens (Carl Zeiss). Linescan images (using 633 nm He-Ne laser excitation, 512×1 pixels at 21.5 pixel/µm and 0.8 ms/line), were recorded with a scan line oriented along the short axis of the cell to quantify the spontaneous AP induced contraction rate. Cell contraction measurements were recorded for 15 min under control conditions and following drug application for times specified. Spontaneous action potentials were measured via a perforated patch-clamp technique with 35 µM β-escin (Sigma) added to the pipette solution that contained (in mM): 120 K-gluconate, 5 NaCl, 5 MgATP, 5 NaATP, 5 HEPES and 20 KCl, pH 7.2 with KOH. Action potentials were recorded using an Axopatch-200 B patch-clamp amplifier (Axon Instruments, Foster City, CA). For both cell contraction and electrophysiological recordings cells from at least 5 rabbits were used.
Calibration and Measurements of Ca2+-Dependent Fluorescence Signals
Intracellular Ca2+ was measured with calibrated Indo-1 fluorescence to assess cytosolic Ca2+ signals. SANC were placed in a chamber on the inverted fluorescence microscope stage (Zeiss IM-35) and were loaded with 14 µM Indo-1 AM (Molecular Probes) for 15-min at room temperature, and subsequently superfused with Tyrode’s solution for 20-min to remove excess indicator and allow full de-esterification as the bath temperature slowly was increased to 35±0.5°C. The apparatus to detect Indo-1 fluorescence is as described previously [9], except that a 63x/1.4 N.A. oil UV fluorglycerin-immersion objective (Zeiss) was used. The Indo signals were corrected for background and autofluorescence by subtracting averaged signals from cells not loaded with Indo-1 (n = 5), determined in every experiment. [Ca2+]i was calculated according to the equation [Ca2+]i = βxKdx(R-Rmin)/(Rmax-R), using a Kd of 844 nM. The average Rmin (minimal ratio), Rmax (maximal ratio), and β (the ratio of maximal and minimal I490) for the fluorescence system were determined by sequential exposure of SANC to a high potassium, zero- Ca2+ solution (in mM: 132 KCl, 10 Hepes, 2 MgCl2, pH 7.2 with KOH) containing metabolic inhibitors (10 mM 2-deoxyglucose and 100 µM 2,4-dinitrophenol) (2), the same solution with 1 mM EGTA and 20 µM ionmycin (for Rmin). Measurements were taken when the fluorescence at both wavelengths reached stable values, and (3) high Ca2+ Tyrode’s solution (5 mM Ca2+ instead of EGTA) was used for determining Rmax. Average Rmax, Rmin and β were 1.9±0.16, 0.9±0.01, and 2.1±0.6, respectively (n = 10). The method limitation derived from: 1) a change in pH from the calibrated solution (pH = 7.4 in bath solution compare to 7.2 see above), 2) imprecision of Kd of Indo-1 and 3) partial quenching of mitochondrial Ca2+ by Mn2+ affect the calculated [Ca2+]m. For Ca2+ recordings cells from at least 5 rabbits were used.
Confocal Imaging for AP Triggered Spontaneous Local Ca2+ Releases
Ca2+ cycling into and out of the cytosol was measured with Flou-4 AM (Molecular Probes) to assess AP triggered Ca2+ release, spontaneous LCRs, and caffeine induced SR Ca2+ release. SANC were loaded with 5 µM Flou-4 AM for 20 min at room temperature, and subsequently superfused with Tyrode’s solution at 35±0.5°C. The Ca2+ fluorescence was imaged by a LSM510 confocal microscope (see above) using a 40x/1.3 N.A. oil immersion lens. Cells were excited with the 488 nm laser line of an Ar laser, and fluorescence emission was collected with LP 505 nm, with the pinhole set to obtain no more than 5 µm optical slice (512×1 pixels at 14.9 pixel/µm and 2 ms/line). All images were recorded with a scan line oriented along the long axis of the cell, close to the sarcolemmal membrane, and processed with IDL software or Matlab.
The AP induced Ca2+ transient was expressed as a peak value (F) normalized to minimal fluorescence (F0). The amplitude of individual LCRs was also expressed as a peak value (F) normalized to minimal fluorescence (F0). LCR spatial size (FWHM) was indexed as the full width at half-maximum amplitude. LCR duration (FDHM) was characterized as the full duration at half-maximum amplitude. The number of LCRs was normalized per 100 µm of the linescan image and during a 1s time interval. The integral of Ca2+ of an individual LCR was estimated as follows: FWHMxFDHMx(F/F0-1)/2. The total Ca2+ released by LCR ensemble was calculated as the sum of the signal masses of diastolic LCRs that occurred between AP-induced Ca2+ transients. The SR Ca2+ content was estimated by the amplitude of the Ca2+ transient induced by a brief rapid application of caffeine (20 mM, 1s) onto the cell by pressure-ejection via a nearby pipette, as described previously [5]. For Ca2+ recordings cells from at least 5 rabbits were used.
Cell Permeabilization
SANC were permeabilized with 5 or 25 µM digitonin at 35±0.5°C with solution containing (in mM): 100 K aspartate, 25 KCl, 10 NaCl, 3 MgATP, 0.8 MgCl2, (free [Mg2+] ∼1 mM), 20 HEPES, 0.5 EGTA, 10 phosphocreatine, and 5 U/ml creatine phosphokinase; pH 7.2 with KOH.
Immunostaining
SANC were incubated with 500 nM Mitotracker Orange (MT; Molecular Probes), for 1 h at 37°C, fixed with 4% formaldehyde in phosphate buffer (PBS) and permeabilized with 1% Triton X-100/PBS. Nonspecific cross-reactivity was blocked by incubating the samples overnight in solution containing: 2% BSA/PBS, 5% donkey serum, 0.01% NaN3, and 0.2% Triton. SANC were then incubated with anti-SERCA2 antibody (1∶100, IgG1, clone IID8; Affinity BioReagents, Golden, CO) at 4°C overnight. This was followed by labeling with a Cy5-conjugated secondary antibody (1∶1000, anti-mouse, Jackson ImmunoResearch) incubation. The fluorescence was imaged by a LSM510 confocal microscope (see above) using a 40x/1.3 N.A oil immersion lens. The Cy5 and MT fluorophores were excited with 633 and 543 nm He-Ne lasers, respectively.
Western Blot of Phospholamban
SANC suspensions were divided equally into 4 aliquots: the first aliquot was treated with 2 µM Ru360, the second with 1 µM CGP-37157, the third with 1 µM isoproterenol; and the forth was used as a control. Cells were incubated at 36°C for 10 min. After the treatment, samples were centrifuged and the pellets were frozen in liquid nitrogen. Pelleted cells were resuspended in RIPA lysis buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris, with pH adjusted to 8) that also included a standard mammalian protease inhibitor cocktail (Sigma) and phosphatase inhibitors 1 mM NaF, 2 mM Na3VO4. Total protein concentration was determined using a standard, commercially available protein quant kit (GE Healthcare, 2D protein Quant kit). The accuracy of protein quantification was verified on the 4–12% Bis-Tris polyacrylamide SDS-PAGE gel (Invitrogen) labeled with the fluorescent Sypro Ruby gel stain (Invitrogen). The total integral of fluorescence intensities for each protein lane was calculated using ImageQuant5 software (GE Healthcare). 5-15 µg of proteins from each aliquot were mixed with the NuPage sample buffer and DTT (10 mM final), and resolved on 4–12% Bis-Tris polyacrylamide SDS-PAGE gel using standard MES/SDS running buffer. Proteins were transferred for 25 min to the activated PVDF membrane (0.45 µm, Molecular Probes) using standard semidry transfer unit (Bio-Rad) in NuPage transfer buffer (Bicine 25 mM, Bis-tris 25 mM EDTA 1.0 mM, Methanol 20%, pH 7.2, Invitrogen). PVDF membranes with transferred proteins were blocked overnight in 5% nonfat milk (Bio-Rad), and resuspended in TBS-T buffer (50 mM Tris-HCl; 500 mM NaCl, 1% Tween, pH 7.5).
To detect total phospholamban the first membrane was incubated over-night with a total anti-PLB monoclonal phospholamban antibody (1∶7000, Badrilla) and incubated 1 h at room temperature with goat anti-mouse conjugated antibodies (1∶5000, Dako). To detect phospholamban phosphorylation, the second and the third membranes were incubated over-night with anti-P-Ser-16 rabbit polyclonal antibody (1∶2000, Badrilla) or the anti- P-Thr-17 rabbit antibody (1∶2000, Badrilla), and incubated for 1 h at room temperature with polyclonal goat anti-rabbit secondary antibody (1∶5000, Dako). To detect actin, the membranes were incubated over-night with anti-actin goat polyclonal antibodies (1∶3000, Santa Cruz), and incubated for 1 h at room temperature with donkey anti-goat-IgG-HRP secondary antibody (1∶5000, Santa Cruz). Membranes were developed for 5 min using SuperSignal West Pico (or Dura) Chemiluminescent Substrate (Thermo Fisher Scientific) and visualized on the HyBlot CL autoradiography film using a series of sequential exposure times (Denville Scientific Inc.) to obtain an optimum signal intensity.
Drugs
CGP-37157, a mitochondrial Na+-Ca2+ exchanger inhibitor, and Ru360, an inhibitor of Ca2+ flux into the mitochondria, were purchased from EMD Chemicals; Isoproterenol, digitonin, saponin, ionmycin, cesium chloride, cyclopiazonic acid, caffeine and MnCl2 were purchased from Sigma.
Statistical Analysis
Data are presented as mean±SEM. When the means of paired samples were to be compared, a paired t-test was employed. For multiple pharmacologic treatments, a linear mixed-effects model with Dunnett’s method to adjust p-values was used. This model accounts for repeated measurements on the same preparation while allowing testing for differences among pharmacological perturbations. P<0.05 was taken to indicate statistical significance.
Supporting Information
Validation of compartmentatal indo-quenching. (A–B) Low concentration of digitonin (5 µmol/L) does not permeabilize mitochondrial membrane, however high concentration of digitonin (25 µmol/L) does permeabilize mitochondrial membrane (C–D) visualized by 125 nmol/L tetramethylrhodamine methyl ester (TMRM).
(TIF)
Representative examples of Indo-1 fluorescence. Mn2+ (50 µmol/L) quenching of the cytosolic indo-1 fluorescence ratio 410/490 in the presence of Ru360 (upper panel) and CGP-37157 (lower panel).
(TIF)
Co-immunoelabeling of SERCA2 and part of the mitochondrial mass in SANC, visualized by anti-SERCA2 antibody and Mitotracker orange (MT) staining, respectively.
(TIF)
Western blots of phospholamban phosphorylation. (A) Representative examples of phospholamban phosphorylated at serine-16 site (PKA site) and total phospholamban in the basal state and following Ru360 (2 µmol/L), CGP-37157 (1 µmol/L) or isoproterenol (1 µmol/L). Actin is used as a protein loading control. (B) Fluorescent Sypro Ruby gel stain to validate the accuracy of protein loading. (C) Average phosphorylation ratio (n = 5) of phospholamban phosphorylated at serine-16 site to protein loading. *p<0.05 vs. control.
(TIF)
Average time-dependent change in the rate of AP induced contractions in the presence of CsCl and CGP-37157 (n = 7) or CsCl and Ru360 (n = 7).
(TIF)
Mitochondrial-SR numerical model. The extended coupled clock numerical model simulations of the effect of specific inhibition of Ca influx into or efflux from mitochondria in intact SANC on (A) Ca2+ in network SR, (B) Ca2+ in junctional SR, (C) Ca2+ release flux from the SR, and (D) Ca2+ in the sub membrane space.
(TIF)
(DOC)
(DOC)
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work was supported entirely by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T, Brown DA, O'Rourke B. Role of mitochondrial dysfunction in cardiac glycoside toxicity. J Mol Cell Cardiol. 2010;49:728–736. doi: 10.1016/j.yjmcc.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 5.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 6.Gao Z, Chen B, Joiner ML, Wu Y, Guan X, et al. I(f) and SR Ca(2+) release both contribute to pacemaker activity in canine sinoatrial node cells. J Mol Cell Cardiol. 2010;49:33–40. doi: 10.1016/j.yjmcc.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, et al. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 8.Aon MA, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spurgeon HA, Stern MD, Baartz G, Raffaeli S, Hansford RG, et al. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990;258:H574–586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths EJ, Wei SK, Haigney MC, Ocampo CJ, Stern MD, et al. Inhibition of mitochondrial calcium efflux by clonazepam in intact single rat cardiomyocytes and effects on NADH production. Cell Calcium. 1997;21:321–329. doi: 10.1016/s0143-4160(97)90120-2. [DOI] [PubMed] [Google Scholar]
- 11.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 12.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, et al. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008;102:761–769. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 14.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 15.Vinogradova TM, Brochet DX, Sirenko S, Li Y, Spurgeon H, et al. Sarcoplasmic reticulum Ca2+ pumping kinetics regulates timing of local Ca2+ releases and spontaneous beating rate of rabbit sinoatrial node pacemaker cells. Circ Res. 2010;107:767–775. doi: 10.1161/CIRCRESAHA.110.220517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 17.Maltsev VA, Lakatta EG. A novel quantitative explanation for the autonomic modulation of cardiac pacemaker cell automaticity via a dynamic system of sarcolemmal and intracellular proteins. Am J Physiol Heart Circ Physiol. 2010;298:H2010–2023. doi: 10.1152/ajpheart.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorn GW, 2nd, Scorrano L. Two close, too close: sarcoplasmic reticulum-mitochondrial crosstalk and cardiomyocyte fate. Circ Res. 2010;107:689–699. doi: 10.1161/CIRCRESAHA.110.225714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez-SanMiguel E, Vay L, Santo-Domingo J, Lobaton CD, Moreno A, et al. The mitochondrial Na+/Ca2+ exchanger plays a key role in the control of cytosolic Ca2+ oscillations. Cell Calcium. 2006;40:53–61. doi: 10.1016/j.ceca.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Hashitani H, Lang RJ, Suzuki H. Role of perinuclear mitochondria in the spatiotemporal dynamics of spontaneous Ca2+ waves in interstitial cells of Cajal-like cells of the rabbit urethra. Br J Pharmacol. 2010;161:680–694. doi: 10.1111/j.1476-5381.2010.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 22.Abdallah Y, Kasseckert SA, Iraqi W, Said M, Shahzad T, et al. Interplay between Ca(2+) cycling and mitochondrial permeability transition pores promotes reperfusion-induced injury of cardiac myocytes. J Cell Mol Med. 2010;15:2478–2485. doi: 10.1111/j.1582-4934.2010.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dedkova EN, Blatter LA. Mitochondrial Ca2+ and the heart. Cell Calcium. 2008;44:77–91. doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 24.O'Rourke B, Blatter LA. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chikando AC, Kettlewell S, Williams GS, Smith G, Lederer WJ. Ca2+ dynamics in the mitochondria - state of the art. J Mol Cell Cardiol. 2011;51:627–631. doi: 10.1016/j.yjmcc.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrienko TN, Picht E, Bers DM. Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes. J Mol Cell Cardiol. 2009;46:1027–1036. doi: 10.1016/j.yjmcc.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maltsev VA, Vinogradova TM, Stern MD, Lakatta EG. Letter to the editor: “Validating the requirement for beat-to-beat coupling of the Ca2+ clock and M clock in pacemaker cell normal automaticity”. Am J Physiol Heart Circ Physiol. 2011;300:H2323–2324. doi: 10.1152/ajpheart.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 29.Yaniv Y, Juhaszova M, Lyashkov AE, Spurgeon H, Sollott SJ, et al. Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand. J Mol Cell Cardiol. 2011;51:740–748. doi: 10.1016/j.yjmcc.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odagiri K, Katoh H, Kawashima H, Tanaka T, Ohtani H, et al. Local control of mitochondrial membrane potential, permeability transition pore and reactive oxygen species by calcium and calmodulin in rat ventricular myocytes. J Mol Cell Cardiol. 2009;46:989–997. doi: 10.1016/j.yjmcc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Cox DA, Conforti L, Sperelakis N, Matlib MA. Selectivity of inhibition of Na(+)-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J Cardiovasc Pharmacol. 1993;21:595–599. doi: 10.1097/00005344-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Neumann JT, Diaz-Sylvester PL, Fleischer S, Copello JA. CGP-37157 inhibits the sarcoplasmic reticulum Ca(2)+ ATPase and activates ryanodine receptor channels in striated muscle. Mol Pharmacol. 2011;79:141–147. doi: 10.1124/mol.110.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thu le T, Ahn JR, Woo SH. Inhibition of L-type Ca2+ channel by mitochondrial Na+-Ca2+ exchange inhibitor CGP-37157 in rat atrial myocytes. Eur J Pharmacol. 2006;552:15–19. doi: 10.1016/j.ejphar.2006.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of compartmentatal indo-quenching. (A–B) Low concentration of digitonin (5 µmol/L) does not permeabilize mitochondrial membrane, however high concentration of digitonin (25 µmol/L) does permeabilize mitochondrial membrane (C–D) visualized by 125 nmol/L tetramethylrhodamine methyl ester (TMRM).
(TIF)
Representative examples of Indo-1 fluorescence. Mn2+ (50 µmol/L) quenching of the cytosolic indo-1 fluorescence ratio 410/490 in the presence of Ru360 (upper panel) and CGP-37157 (lower panel).
(TIF)
Co-immunoelabeling of SERCA2 and part of the mitochondrial mass in SANC, visualized by anti-SERCA2 antibody and Mitotracker orange (MT) staining, respectively.
(TIF)
Western blots of phospholamban phosphorylation. (A) Representative examples of phospholamban phosphorylated at serine-16 site (PKA site) and total phospholamban in the basal state and following Ru360 (2 µmol/L), CGP-37157 (1 µmol/L) or isoproterenol (1 µmol/L). Actin is used as a protein loading control. (B) Fluorescent Sypro Ruby gel stain to validate the accuracy of protein loading. (C) Average phosphorylation ratio (n = 5) of phospholamban phosphorylated at serine-16 site to protein loading. *p<0.05 vs. control.
(TIF)
Average time-dependent change in the rate of AP induced contractions in the presence of CsCl and CGP-37157 (n = 7) or CsCl and Ru360 (n = 7).
(TIF)
Mitochondrial-SR numerical model. The extended coupled clock numerical model simulations of the effect of specific inhibition of Ca influx into or efflux from mitochondria in intact SANC on (A) Ca2+ in network SR, (B) Ca2+ in junctional SR, (C) Ca2+ release flux from the SR, and (D) Ca2+ in the sub membrane space.
(TIF)
(DOC)
(DOC)
(DOC)