Abstract
Background
Liver fluke infection of livestock causes economic losses of over US$ 3 billion worldwide per annum. The disease is increasing in livestock worldwide and is a re-emerging human disease. There are currently no commercial vaccines, and only one drug with significant efficacy against adult worms and juveniles. A liver fluke vaccine is deemed essential as short-lived chemotherapy, which is prone to resistance, is an unsustainable option in both developed and developing countries. Protein superfamilies have provided a number of leading liver fluke vaccine candidates. A new form of glutathione transferase (GST) family, Sigma class GST, closely related to a leading Schistosome vaccine candidate (Sm28), has previously been revealed by proteomics in the liver fluke but not functionally characterised.
Methodology/Principal Findings
In this manuscript we show that a purified recombinant form of the F. hepatica Sigma class GST possesses prostaglandin synthase activity and influences activity of host immune cells. Immunocytochemistry and western blotting have shown the protein is present near the surface of the fluke and expressed in eggs and newly excysted juveniles, and present in the excretory/secretory fraction of adults. We have assessed the potential to use F. hepatica Sigma class GST as a vaccine in a goat-based vaccine trial. No significant reduction of worm burden was found but we show significant reduction in the pathology normally associated with liver fluke infection.
Conclusions/Significance
We have shown that F. hepatica Sigma class GST has likely multi-functional roles in the host-parasite interaction from general detoxification and bile acid sequestration to PGD synthase activity.
Author Summary
Combating neglected parasitic diseases is of paramount importance to improve the health of human populations and/or their domestic animals. Uncovering key roles in host-parasite interactions may support the vaccine potential portfolio of a parasite protein. Fasciola hepatica causes global disease in humans and their livestock but no commercial vaccines are available. Members of the Sigma class glutathione transferase (GST) family have long been highlighted as vaccine candidates towards parasitic flatworms. To this end, a Sigma class GST is currently undergoing phase II clinical trials to protect against infection from the schistosomes. In this study we characterise the protein from F. hepatica following four work pathways that 1) confirm its designation as a Sigma class GST using substrate profiling, 2) assess prostaglandin synthase activity and its effect on host immune cells, 3) localise the Sigma GST within adult fluke and between ontogenic stages and 4) measure its potential as a vaccine candidate. The work presented here shows F. hepatica Sigma class GST to have key host-parasite roles and we suggest, warrants further investigation for inclusion into vaccine formulations.
Introduction
The liver flukes, Fasciola hepatica and Fasciola gigantica are the causative agents of fasciolosis, a foodborne zoonotic disease affecting grazing animals and humans worldwide [1]. Liver fluke causes economic losses of over US$ 3 billion worldwide per annum to livestock via mortality, reduction in host fecundity, susceptibility to other infections, decrease in meat, milk and wool production and condemnation of livers [1]. The disease is increasing in livestock worldwide with contributing factors such as climate change (warmer winters and wetter summers supporting larger intermediate mud snail host populations); fragmented disease management (only treating sheep not cattle and limiting veterinary interaction); encouragement of wet-lands; livestock movement; and/or failure/resistance of chemical control treatments in the absence of commercial vaccines [1], [2]. Fasciolosis is also a re-emerging human disease with estimates of between 2.4 and 17 million people infected worldwide [3]. In response, the World Health Organisation have added fasciolosis to the preventative chemotherapy concept [4].
There are currently no commercial vaccines and triclabendazole (TCBZ) is the most important fasciolicide, as the only drug with significant efficacy against adult worms and juveniles [5]. Evidence from developed countries where TCBZ has been used widely exposes the reliance on this drug as an Achilles heel of liver fluke chemotherapeutic control, with well-established evidence of drug-resistance [5]. Therefore, TCBZ does not offer a long-term sustainable option for livestock farmers worldwide. The need for a liver fluke vaccine is further underscored by the fact that the costs associated with anthelmintic intervention for fluke control make short-lived chemotherapy an unsustainable option in developing countries. Protein superfamily studies in liver fluke have provided a number of leading vaccine candidates. High quality one-gene based vaccine discovery research has identified several vaccine candidates from protein superfamilies that provide significant, but often variable protection rates in challenge animal trials against liver fluke.
For example, Mu class Glutathione transferase (GSTs) have been widely investigated as vaccine candidates for fasciolosis [6]–[9]. The Mu class GSTs have established roles in general Phase II detoxification of xenobiotic and endogenously derived toxins in F. hepatica within the host bile environment [10]. The general detoxification role is supported by GSTs contributing to 4% of the total soluble protein in F. hepatica, with a widespread tissue distribution. Proteomics and EST sequencing approaches have now delineated what members of the GST family are expressed in F. hepatica and two new classes of GST, Sigma and Omega, have been uncovered [11]. In the related trematode, Schistosoma mansoni, the Sigma class GST (Sm28) has generally shown more robust protection in vaccine trials against schistosome infection [12], than the F. hepatica Mu GSTs against F. hepatica infection.
Sigma class GSTs, unlike Mu Class GSTs, have been characterized as GSH-dependent hematopoietic prostaglandin synthases responsible for the production of prostaglandins in both mammals and parasitic worms [13]–[18]. Prostaglandins have been extensively studied in mammals and are shown to be involved in a range of physiological and pathological responses [19]–[23]. Parasite-produced prostaglandins may be involved in parasite development and reproduction as well as the modulation of host immunity, allergy and inflammation during establishment and maintenance of a host infection [16], [24]–[28]. The host protection success of Sigma GST based vaccinations in schistosomiasis may therefore be related to neutralising specific functions in host-parasite interplay, such as prostaglandin synthase activity.
In this manuscript we follow four work pathways to functionally characterise the newly identified Sigma GST from F. hepatica. 1) We confirm its designation as a Sigma class GST using substrate profiling, 2) we assess prostaglandin synthase activity and its effect on host immune cells, 3) we localise the Sigma GST within adult fluke and between ontogenic stages and 4) assess its potential as a vaccine candidate.
Materials and Methods
Sequence analysis
GST proteins representative of recognised GST superfamily classes were obtained from European Bioinformatics Institute Interpro database (http://www.ebi.ac.uk/interpro/), and from non-redundant databases at NCBI (http://www.ncbi.nlm.nih.gov/). A mammalian and a helminth or invertebrate GST sequence were selected for each GST class where available. Sequences were aligned via ClustalW program [29] in BioEdit Sequence Alignment Editor Version 7.0.5.2. [30] and sequence identity matrices produced from multiple alignments. Phylogenetic bootstrap neighbour-joining trees were produced as PHYLIP output files in ClustalX Version 1.83 [31] according to the neighbour-joining method of Saitou and Nei [32]. ClustalX default settings for alignments were accepted using the GONNET protein weight matrices with PHYLIP tree format files viewed within TREEVIEW [33].
Recombinant Fasciola hepatica glutathione transferase Sigma class (rFhGST-S1) production
Full-length cDNA for FhGST-S1 was available in the form of an expressed sequence tag (EST) clone Fhep24h03, details of which can be obtained from the previously published Sigma class GST [11] and is identical to the submitted GenBank accession No. DQ974116.1 (NCBI http://www.ncbi.nlm.nih.gov/).
FhGST-S1 was amplified via PCR using the following primer pair: rFhGST-S1 forward primer, 5′ GGAATTCCATATGGACAAACAGCATTTCAAGTT 3′;rFhGST-S1 reverse primer, 5′ ATAAGAATGCGGCCGCCTAGAATGGAGTTTTTGCACGTTTTTT 3′. Restriction enzyme sites (in bold type and underlined) for NdeI (forward primer) and NotI (reverse primer) were included so that the entire ORF could be directionally cloned into the pET23a (Novagen) vector. Recombinant protein was produced in Escherichia coli BL21(DE3) cells (Novagen).
Protein purification of rFhGST-S1 and native F. hepatica GSTs
rFhGST-S1 protein was purified according to the glutathione affinity chromatography method of Simons and Vander Jagt [34] from transformed E. coli cytosol following protein expression. Native GSTs were purified from F. hepatica soluble cytosolic supernatants as previously described [11]. Purity of rFhGST-S1 was assessed by electrospray ionisation (ESI) mass spectrometry, sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and 2DE according to LaCourse et al. [35].
Substrate profiling of Sigma GST
A range of model and natural substrates (see Table 1 for details) were used to profile the Sigma GST. A number of ligands were also assessed for their ability to inhibit GST activity with 1-chloro-2, 4-dinitrobenzene (CDNB) as the second substrate [36]. Values were reported as the concentration of inhibitor required to bring GST specific activity to 50% of its original activity (IC50). At least six different inhibitor concentrations were used in each IC50 determination in triplicate. Inhibitors were pre-incubated for 5 minutes prior to starting reactions. IC50 values were estimated graphically [37].
Table 1. Substrate specificities of rFhGST-S1.
| SUBSTRATE CLASS | SUBSTRATE | [Substrate] (mM) | [GSH] (mM) | 100 mM KHPO4 (pH) | Temp. (°C) | l Max (nm) | e (mM−1cm−1) | rFhGST-S1 | Sm28GST** | Assay Ref. |
| Specific Activity (nmol min−1mg−1) | Specific Activity (nmol min−1mg−1) | |||||||||
| MODEL SUBSTRATES | 1-Chloro-2,4-dinitrobenzene (CDNB) | 1 | 1 | 6.5 | 25 | 340 | 9.6 | 4736±292 | 7269±218 | [36] |
| 1,2-Dichloro-4-nitrobenzene (DCNB) | 1 | 5 | 7.5 | 25 | 345 | 9.6 | ND<5 | ND<5 | [36] | |
| Ethacrynic Acid | 0.08 | 1 | 6.5 | 25 | 270 | 5 | 898±204 | 1580±97 | [70] | |
| REACTIVE ALDEHYDES | 4-hydroxynonenal | 0.1 | 0.5 | 6.5 | 30 | 224 | 13.75 | 645±129 | 287±17 | [68] |
| Trans-2-nonenal | 0.023 | 1 | 6.5 | 25 | 225 | −19.2 | 333±43 | 447±6 | [36] | |
| Trans, trans-2,4-decadienal | 0.023 | 1 | 6.5 | 25 | 280 | −29.7 | 51±0.3 | 221 | [36] | |
| LIPID PEROXIDES | Cumene hydroperoxide | 1.2 | 1 | 7 | 25 | 340 | 6.22 | 7081±1009 | 162±7 | [71] |
| 0.25 | 1 | 7 | 25 | 340 | 6.22 | 2209±122 | - | [71] | ||
| t-butyl hydroperoxide | 0.25 | 1 | 7 | 25 | 340 | 6.22 | 193±1.8 | ND<10 | [69] | |
| 2.5 | 1 | 7 | 25 | 340 | 6.22 | 1827±198 | - | [69] | ||
| Linoleic Acid | 0.25 | 1 | 7 | 25 | 340 | 6.22 | 430±69 | - | [51] | |
| 0.05 | 1 | 7 | 30 | 340 | 6.22 | - | ND<10 | [51] |
Recombinant FhGST-S1 shows activity towards a broad range of model and natural GST substrates with a similar enzymatic profile to the Schistosomiasis vaccine trialist (Sm28GST - P09792). rFhGST-S1 also displays high glutathione-dependent lipid peroxidase activity compared to both Sm28GST and Sj26GST (Q26513) [47]. Reasonably high GSH-dependent lipid peroxidase activity has also been seen in a ‘weak affinity’ fraction following chromatofocusing of GSH transferase activity that failed to bind GSH-sepharose [10]. ND – Not determined.
**: Data taken from Walker et al. [47].
Prostaglandin synthase activity was assessed via an adapted method based upon those of Sommer et al. [26] and Meyer et al. [16], [38], with extraction modifications based upon Schmidt et al. [39]. In brief, reactions were performed in glass vials in 2 mM sodium phosphate buffer, pH 7.4, containing 10 mM glutathione, 50 mM NaCl, 0.5 mM tryptophan, 1 µM hematin, 1 U COX-1 enzyme, 100 µM arachidonic acid (All Sigma, UK. COX-1 [C0733]) and rFhGST-S1 at final concentration ranges of 0.1–100.0 µg/ml. Negative control reactions lacking either GST or COX-1 were also prepared. Reactions were incubated for 5–10 min in a water bath at 37°C. This was followed by 4 minutes incubation at 25°C in a shaking water bath. Prostaglandins were extracted by adding 860 µL of ice-cold ethyl acetate. Reactions were vortexed for 30 s then centrifuged briefly at 10,000× g at 4°C for 2 min. The upper ethyl acetate layer was retained and solvent was evaporated under a nitrogen stream at 45°C. The remaining residue was reconstituted in 50 µl of methanol/water/fomic acid (25∶75∶0,1) mix at pH 2.8 and stored at −80°C until ready for mass spectrometry analysis. Standards of prostaglandins D2, E2 and F2α (Cayman, Ltd) were also prepared in methanol/water/formic acid mix for analysis.
Prostaglandin detection
The nano LC-MS analyses were performed using a Waters Q-Tof micro mass spectrometer (Waters) coupled to a LC-Packings Ultimate nano LC system (Dionex). The pre-column used was a LC Packings C18 PepMap 100 and the nano LC column used was a LC Packings 15 cm PepMap 100 C18 (both Dionex). Samples were loaded on the pre-column with mobile phase A (25% methanol with 0.1% formic acid added). Loading flow rate was 0.03 ml/min for 6 min. The samples were eluted on to the nano LC column using mobile phases B (60% acetonitrile) and C (100% methanol). A typical gradient profile was 100% B to 100% C in 10 min (flow rate of 0.2 µl/min) with the column held at 100% C for 1 hour. The mass spectrometer was operated in the negative ion nano electrospray mode with a source temperature of 80°C and capillary voltage 2.8 kV. The scan range was 40 to 400 Da for 1.5 s.
Liver fluke extract and excretory/secretory (ES) product preparation
F. hepatica adults were collected, cultured in vitro for 4 h and the ES products collected and prepared as previously described [40]. Newly excysted juveniles (NEJ) were excysted from metacercariea in vitro and cultured in Fasciola saline for 4 h post excystment as previously described [41]. F. hepatica (adult and NEJ) soluble fractions were obtained by homogenisation of frozen fluke at 4°C in a glass grinder in lysis buffer (20 mM KHPO4, pH 7.0, 0.1% Triton-X100 and a cocktail of protease inhibitors [Roche, Complete-Mini, EDTA-free]). Homogenates were centrifuged at 100,000× g for 1 h at 4°C. Supernatants were considered as the soluble cytosolic fraction. Cytosolic protein extracts were treated and resolved by 2DE as described previously [11]. F. hepatica eggs were isolated, cultured and protein extracted as previously described [42].
Western blotting
Recombinant F. hepatica Sigma GST (rFhGST-S1), and native F. hepatica S-hexylGSH-affinity purified GST samples (and human/rat recombinant PGD-synthase) were subjected to standard SDS-PAGE and 2DE, electro-transferred to membranes [43], [44] and western blotted with a polyclonal antibody (1∶20,000 dilution) raised in rabbits to the recombinant F. hepatica Sigma GST by Lampire Biological Laboratories, USA. Membranes were also probed with Mu class GST antibody (represented by the anti-Schistosoma japonicum GST26 Mu class antibody [1∶1,000 dilution] and an anti-rat PGD-synthase antibody [1∶1,000 dilution], Pharmacia-Biotech 27-4577). F. hepatica eggs, NEJs (somatic and ES preparations) and adults (somatic and ES preparations) were subjected to SDS-PAGE and also electro-transferred as described above and probed with the polyclonal antibody raised in rabbits to the recombinant F. hepatica Sigma GST. All western blots were developed as described previously [11].
Immunolocalisation studies
F. hepatica Sigma class GST (FhGST-S1) was detected by immunohistology in tissue sections of whole adult F. hepatica extracted from bile ducts of sheep liver and also in situ from sections of liver.
Staining for FhGST-S1 was performed on formalin-fixed and paraffin-embedded tissue sections according to the method described previously [45]. Sections were washed in Tris-buffered saline (TBS; 0.1 M Tris-HCl with 0.9% NaCl [pH 7.2]), treated with 0.05% (w/v) protease (type XXIV, bacterial: Sigma) in TBS for 5 min at 37°C for antigen retrieval, before three further 5 min washes in ice-cold TBS. Following TBS washes, sections were incubated for 10 min in 50% (v/v) swine serum in TBS followed by incubation for 15–18 h at 4°C in rFhGST-S1 polyclonal antibody (diluted at 1∶500 in 20% swine serum in TBS). Sections were again washed in TBS before further incubation at ambient temperature (approximately 20°C+/−3°C) with anti-rabbit peroxidise anti-peroxidase (PAP; diluted at 1∶100 in 20% swine serum in TBS). Following washes with TBS, sections were incubated, with stirring, for 10 min, with 3,3-diaminobenzidine tetrahydrochloride (DAB; Fluka, Buchs, Switzerland) with 0.01% v/v hydrogen peroxide in 0.1 M imidazole buffer pH 7.1, before counterstaining with Papanicolaou's hematoxylin for 30 s. Sections were then rinsed, dehydrated in alcohol, cleared in xylene, and mounted. Consecutive sections from each tissue were used as negative controls in which the rFhGST-S1 polyclonal antibody was replaced by TBS.
Induction of prostaglandin from dendritic cells
Animals
C57BL/6 mice were purchased from Harlan Ltd (UK) and TLR4KO (on a C57BL/6 background) bone marrow cells were a gift from Professor Padraic Fallon (Trinity College Dublin, Ireland). All mice were maintained according to the Irish Department of Children and Health.
Cell culturing and cytokine analysis
Bone marrow-derived immature dendritic cells (DCs) were prepared by culturing bone marrow cells isolated from the femurs and tibia of C57BL/6j and TLR4−/− mice in complete RPMI 1640 (cRPMI; 5% [v/v] heat inactivated Fetal Calf Serum [FCS] [30 mins at 60°C], 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine and 50 µM 2-mercaptoethanol) with recombinant mouse GM-CSF (20 ng/ml; R&D Systems), at 37°C. On days 3 and 6 of culture, fresh medium with GM-CSF (20 ng/ml) was added to the cells. On day 8, cells were harvested, counted and stained with CD11c (Caltag Laboratories) for analysis by flow cytometry to determine purity (>90%). J774 cells and RAW264.7, murine macrophage cell lines were cultured in cRPMI 1640 medium containing 10% (v/v) FCS. All cells were used to conduct experiments when they reached ∼90% confluence.
For all experiments, cells were seeded into 24-well plates (Nunc) at 106/ml in complete RPMI 1640 except for DCs where GM-CSF (5 ng/ml) was also added. Cells were treated with medium only, rFhGST-S1 (10 µg) or LPS (Alexa; 100 ng/ml) for 18 h. Levels of total prostaglandin (PG), prostaglandin E2 (PGE2) and prostaglandin D2 (PGD2) were measured using the Cayman competitive EIA. The values were calculated using free data analysis software available at www.caymanchem.com/analysis/eia. Data are presented as the mean ± SEM following subtraction of medium controls and are representative of two separate experiments. Prior to experimentation, rFhGST-S1 was assessed for endotoxin (i.e. LPS) contamination using the Pyrogene endotoxin detection system (Cambrex).
Vaccination Strategy
Experimental design
Nineteen 5-month old, Malagueña breed goats were used for a vaccine trial. The animals were free of parasitic and infectious diseases as indicated by fecal analysis and absence of clinical signs. Group 1 (n = 10) were immunized with two subcutaneous injections of 100 µg of rFhGST-S1 in 1 ml of Quil A in 1 ml of PBS each injection separated by 4 weeks. Group 2 (n = 9) served as an infected control and was immunized at the same time with 1 ml of Quil A in 1 ml of PBS. Twelve weeks after the first immunization, animals were orally infected with 100 F. hepatica metacercariae of bovine origin. Three animals from each group were killed at 7, 8 and 9 days post-infection to study hepatic changes and host response during the early stages post-infection; the remaining animals (7 and 6 goats per group) were killed 15 weeks after infection to study fluke burdens, fecal egg counts and hepatic lesions. All goats were sacrificed by intravenous injection of thiobarbital. The experiment was approved by the Bioethical Committee of the University of Cordoba (N. 7119) and it was carried out according to European (86/609/CEE) and Spanish (RD 223/1988) directives for animal experimentation.
Fluke burdens and morphometrics
At necropsy, gallbladders and bile ducts were opened and flukes recovered. Llivers were cut (∼1 cm pieces) and washed in hot water to collect the remaining flukes. Flukes were counted, measured and weighed.
Fecal egg counts
Sedimentation techniques at 12 and 13 weeks after infection using four grams of feces were conducted to give eggs per gram (EPG).
Pathological assessment
At necropsy livers were photographed by the visceral and diaphragmatic aspects for gross pathology evaluation as described previously [46]. Gross hepatic lesions were scored as: absent [−]; mild [+] (less than 10% of hepatic surface affected); moderate [++] (10–25% of hepatic surface affected); severe [+++] (25–50% of hepatic surface affected) and very severe [++++] (more than 50% of hepatic surface affected). Tissue samples were collected from the left (6 samples) and right (2 samples) hepatic lobes, fixed in 10% buffered formalin and embedded in paraffin wax. Tissue sections (4 µm) were stained with haematoxylin and eosin (HE) for the histopathological study.
Specific IgG response
Specific IgG anti-rFhGST-S1were measured using the ELISA method as described previously [46]. A total of 10 µg/ml of rFhGST-S1 was used to coat microtitre plates, 100 µl/well of goat sera diluted in blocking buffer and rabbit anti-goat IgG peroxidase conjugated (whole molecule - Sigma) diluted in blocking buffer at 1∶10000. Serum pools were from ten experimentally infected goats and ten uninfected goats as positive and negative controls, respectively. All samples were analysed in duplicate. Results were expressed as antibody titre (Log10).
Results
Expression, purification and characterisation of rFhGST-S1
Aligning Sigma class GSTs of trematodes shows the extent of identity and similarity across this class of GSTs (Figure S1). An amino acid sequence comparison of FhGST-S1 with other trematode GSTs places FhGST-S1 into the Sigma class of GSTs, with identities averaging approximately 45%. Comparison with the most closely matching mammalian GSTs shows sequence identities averaging only approximately 28% (Table S1). Despite phylogenetic neighbour-joining trees place mammalian and trematode GSTs within the same broad Sigma class (Figure S1) there remains a distinct separation of the trematode and mammalian clusters.
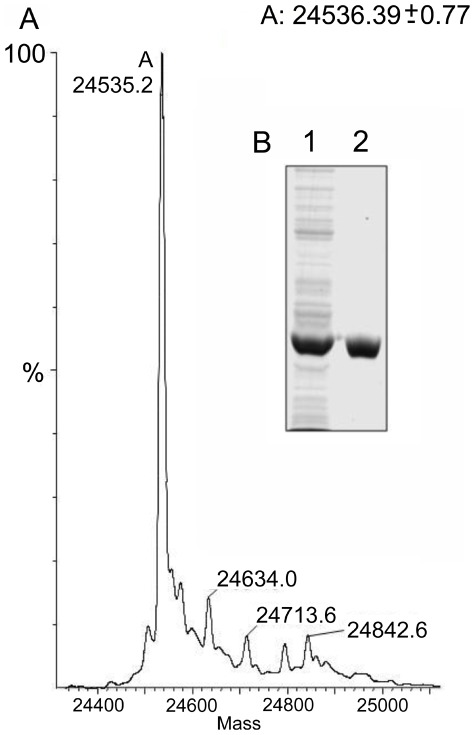
Full sequence length recombinant F. hepatica Sigma Class GST (rFhGST-S1) was shown to be purified to a high level from transformed E. coli cytosol following expression yielding 57.3 mg of rFhGST-S1 from a 1 litre culture of BL21 (DE3) cells. Purity was judged by the presence of a single band upon SDS-PAGE at the estimated size and a dominating single peak via ESI MS at the precise calculated theoretical mass for the complete protein sequence (Figure 1). Analysing this fraction by 2D SDS-PAGE revealed a single protein resolving into 3 protein spots. Western blotting of the 2DE profile with anti-rFhGST-S1 antibody confirmed all 3 resolved protein spots as rFhGST-S1 (2DE and western blot data not shown). No recognition was seen probing the 3 spots with an anti-Mu class antibody.
Figure 1. Expression and purification of recombinant FhGST-S1.
A) ESI mass spectrum of the GSH-affinity purified rFhGST-S1 showing the MW of rFhGST-S1 at 24536.39±0.77 Da. B) SDS-PAGE gel of the expression and purification of rFhGST-S1. Lane 1. E. coli total cytosolic protein. Lane 2. GSH-affinity purified recombinant rFhGST-S1 protein. Ran on 12.5% SDS PAGE and coomassie blue stained.
rFhGST-S1 was produced as an active protein, displaying significant enzymic activity towards the model GST substrate 1-chloro-2,4-dinitrobenzene (CDNB) and a range of substrates commonly used to characterise GSTs (Table 1). F. hepatica GST is very similar in terms of its enzymatic profile to the GST of S. japonicum currently undergoing clinical vaccine trials. FhGST-S1 also displays higher glutathione-dependent lipid peroxidase activity compared to both Sm28GST and Sj26GST [47]. Interestingly, ligand inhibition studies on rFhGST-S1 showed the enzymic activity of rFhGST-S1 with CDNB was inhibited by the major pro-active form of the main liver fluke drug Triclabendazole. The sulphoxide derivative (TCBZ SO) gave an IC50 (50% enzyme inhibition) of 57±5 µM (5 replicates). Bile acids, potentially natural ligands for liver fluke tegumental associated proteins in the host bile environment, were also assessed for activity inhibition. The rFhGST-S1 interacted with all three bile acids tested using five replicate assays: Cholic acid (IC50 302±73 µM); Deoxycholic acid (IC50 223±21 µM) and Chenodeoxycholic acid (IC50 64±9 µM).
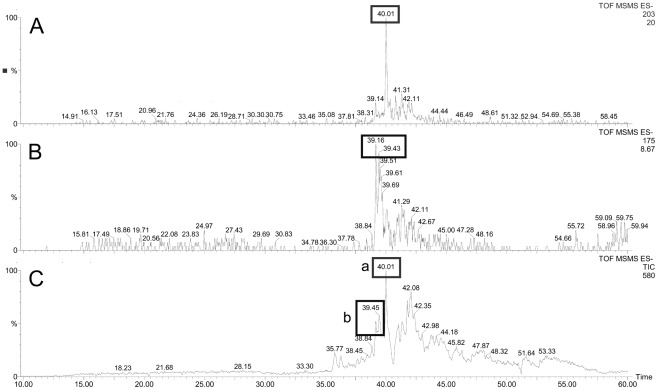
Previous studies on the Sigma class GSTs from both mammals and helminth parasites have revealed a capacity to synthesise Prostaglandin D2 (PGD2) and PGE2. Since prostaglandin synthase activity may be a conserved role of Sigma class GSTs, we also tested the ability of rFhGST-S1 to synthesise prostaglandin eicosanoids using a coupled assay with COX-1. COX-1 catalyses the conversion of arachidonic acid to the H2 form before the prostaglandin isomer is converted to either the D or E form. Nano-LC/MS analysis enabled us to detect the presence of both PGD2 and PGE2 in the assay mixture with the PGD2 form being the more abundant of the two prostanoids (Figure 2). While some PGE2 in the mixture could have arisen from rapid degradation of the unstable PGH2, nano-LC-MS was unable to detect either PGD2 or PGE2 in negative control reactions lacking either COX-1 or GST. The rFhGST-S1 catalyses PGD2 formation in a concentration-dependent manner as previously described for rOvGST-1 [26]. PGD2 was also detected in coupled assays with rFhGST-S1 and COX-1 using an Enzyme Immno Assay (EIA) detection kit (Cayman) and showed similar results (results not shown).
Figure 2. Detection of prostaglandin synthase activity of rFhGST-S1 via a mass spectrometry approach.
A coupled assay with rFhGST-S1 and COX-1 catalyses the conversion of arachidonic acid to the H2 form before the prostaglandin isomer is converted to either the D or E form. Nano-LC/MS analysis allowed detection of both PGD2 (A) and PGE2 (B) in the assay mixture with the PGD2 form being the more abundant of the two prostanoids (C). Boxed figures above peaks show the fragmentation ions specific to detection of PGD2 (a) and PGE2 (b) according to the method of Schmidt et al. [39].
Tissue localisation of Sigma GST
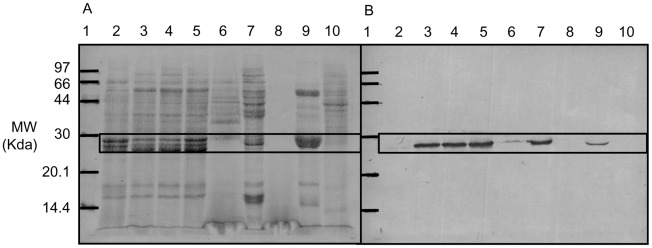
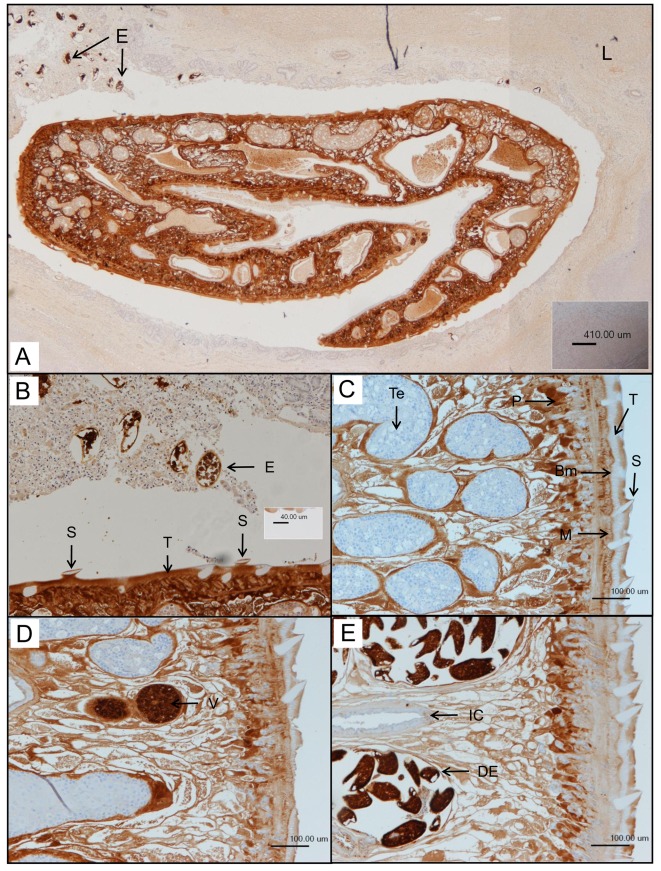
FhGST-S1 was first identified in adult liver fluke in S-hexyl-GSH affinity isolated fractions of cytosol [11]. Western blots confirmed the presence of FhGST-S1 in NEJs and adult flukes and further enabled us to identify the Sigma GST in relative abundance in egg extracts, suggesting that it may play a metabolic role in embryogenesis/reproduction (Figure 3). Western blot analyses demonstrate that FhGST-S1 is consistently expressed during the course of in vitro parasite embryonation (days 1–9, only data for days 2, 7 and 9 shown in Figure 3). In contrast, immunoblot analysis of freshly voided (day 0) eggs reveals that expression of the Sigma class GST is greatly reduced at the time of voiding from the host (Figure 3). However, immunolocalisation studies of adult parasites revealed an abundance of FhGST-S1 in the vitelline cells and eggs, emphasising the likely importance of this enzyme in egg formation and development. Some staining was also found in the parasite parenchyma and tegument, also suggesting a role at the host-parasite interface (Figure 4). Indeed, FhGST-S1 was detected in ES products of adult fluke cultured in vitro (Figure 3) suggesting that the protein could, in principle, come into contact with the host immune system as it is released from the tegument during tegumental turnover and sloughing of the fluke body surface.
Figure 3. Western Blotting localising FhGST-S1 in embryonating eggs, NEJs, adults and adult ES products.
10 µg of each protein sample was resolved through 14% SDS PAGE and electrophoretically transferred to Hybond C nitrocellulose membrane. Membranes were amido black stained membrane to assess protein transfer (A) Membranes were incubated with anti-FhGST-S1 antibody diluted 1∶30,000 and developed using the BCIP/NBT liquid substrate system according to manufacturer's instructions (B). 1: Low Molecular Weight Marker (GE Biosciences); 2: Day 0 Egg; 3: Day 2 Egg; 4: Day 7 Egg; 5: Day 9 Egg; 6: NEJ Somatic Sample; 7: Adult Somatic Sample; 8: NEJ ES Products; 9: Adult ES Products; 10: Uninfected Galba truncatula –ve Control.
Figure 4. Images of FhGST-S1 localisation within F. hepatica tissue.
A) Anti-F. hepatica FhGST-S1 immunohistochemical stain of a fluke in cross section within the host sheep liver bile duct. Heavily stained eggs (E) are shown released from the fluke into the bile duct in the top left-hand corner. Brown stained areas show the presence of FhGST-S1 proteins. The lack of staining in the host liver (L) highlights the specificity of the antibody. Composite picture. B) Enlarged region of A showing the intense anti-F. hepatica FhGST-S1staining in the voided eggs (E). The spines (S) present in the tegument (T) can be clearly distinguished by their lack of FhGST-S1 presence. C–E) Cross sections of a F. hepatica adult highlighting staining of FhGST-S1 in the parenchyma (P), musculature (M),the tegument (T), basal membrane (Bm) and most intensely in the vitelline cells (V) and developing eggs (DE). No staining can be seen in the tegumental spines (S), testes (T) or the intestinal caecum (IC).
Influence of rFhGST-S1 on prostaglandin synthesis in host immune cells
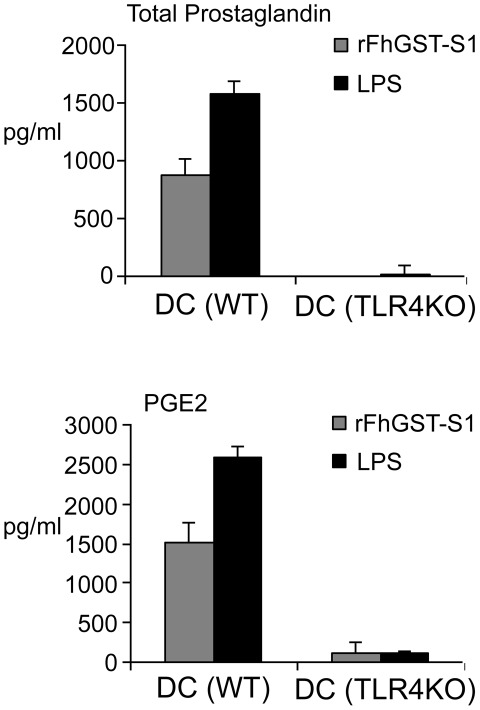
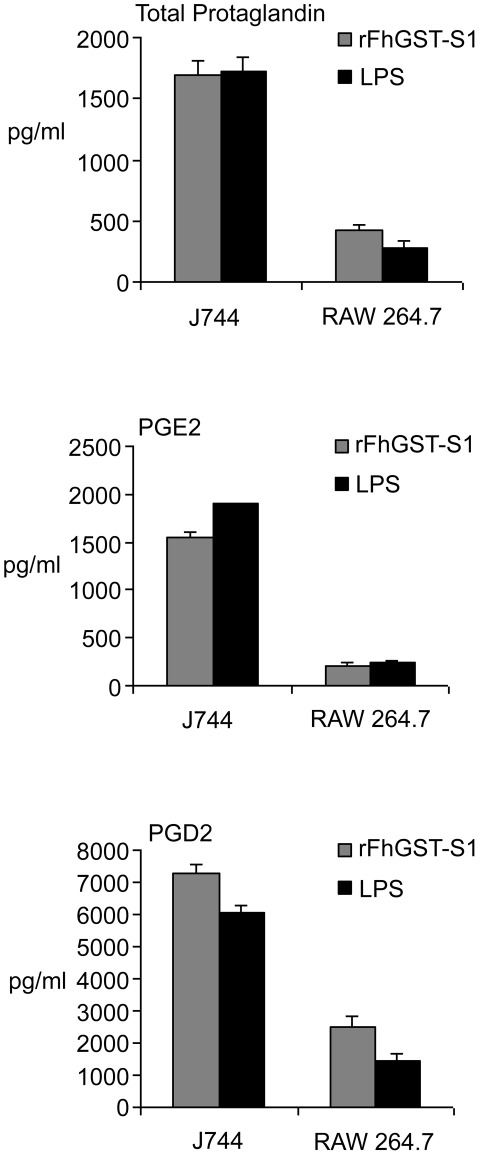
rFhGST-S1 exhibited prostaglandin synthase activity producing PGE2 and PGD2. In addition, it has been shown previously that rFhGST-S1 activates DCs in vitro [48]. Therefore, an attempt to determine if rFhGST-S1 could induce the secretion of total prostaglandin, PGE2 and PGD2 from DCs was performed. Prior to experimentation, endotoxin levels in rFhGST-S1 were assessed and were similar to that of the media alone. Both of which were below the lower limit of detection (<0.01 EU/ml). When examining prostaglandin induction DCs stimulated with rFhGST-S1 secreted total prostaglandin and PGE2 (DC (WT); Figure 5) but not PGD2 (data not shown). Since it has been previously determined that the activation of DCs by rFhGST-S1 was dependent upon TLR4 [48] we repeated the experiment in DCs from TLR4KO mice and in keeping with previous findings demonstrated that the secretion of total prostaglandin and PGE2 by rFhGST-S1 was significantly reduced in the absence of the TLR4 receptor (DC (TLR4KO); Figure 5). rFhGST-S1 was then further assessed for its potential to induce prostaglandin secretion from macrophages by exposing two macrophage cell lines with rFhGST-S1. After 18 hours the levels of total prostaglandin, PGE2 and PGD2 were measured. In this assay, both macrophage cells lines stimulated with rFhGST-S1 secreted total prostaglandin, PGE2 and PGD2 (Figure 6). However, the levels secreted by J744 cell line were higher when compared to the amount secreted by RAW264.7 cell line. In these experiments we included medium only as a negative control and LPS as a positive control. In all experiments the levels of prostaglandin in response to rFhGST-S1 was comparable to the levels secreted in responses to LPS.
Figure 5. rFhGST-SI stimulates the production total prostaglandin and PGE2 from dendritic cells (DCs) in a TLR4 dependent manner.
DCs derived from the bone marrow from C57BL/6j mice were cultured in vitro with medium, rFhGST-S1 (10 µg/ml) or LPS (100 ng/ml) for 18 hours, and the production of total prostaglandin, PGE2 and PGD2 (data for PGD2 not shown) released into supernatants determined by competitive EIA. Data are presented as the mean ± SEM following subtraction of medium controls and are representative of two experiments. WT – wild type; TLR4KO – Toll like receptor 4 knock out.
Figure 6. rFhGST-SI stimulates the production PGE2 and PGD2 from the macrophage cell lines J774 and RAW264.7.
J744 and RAW264.7 macrophage cell lines were cultured in vitro with medium, rFhGST-S1 (10 µg/ml) or LPS (100 ng/ml) for 18 hours, and the production of total prostaglandin, PGE2 and PGD2 released into supernatants determined by competitive EIA. Data are presented as the mean ± SEM following subtraction of medium controls and are representative of two experiments.
Assessment of goat vaccinations with rFhGST-S1 challenged with F. hepatica
Following the completion of the vaccine trial, liver fluke were recovered and the livers scored. The resulting data is summarised in Table 2. When assessing fluke burdens, length, weight and fecal egg counts, no significant differences between rFhGST-S1 immunised and Quil A immunised groups were observed. Despite this lack of significance, at 7–9 days post-infection (dpi) the number of gross hepatic lesions appeared reduced in rFhGST-S1 immunised groups compared to the Quil A control group. At 15 weeks post-infection (wpi), a similar outcome is observed. Liver hepatic lesion scoring appeared to show reductions in the severity of damage occurred in the rFhGST-S1 immunised group compared to the Quil A only group, despite no significant differences in the aforementioned morphometric data.
Table 2. Results of parasitological and hepatic gross morphometric studies from vaccination.
| Group | Parasitological Study | FEC (epg) | Gross Hepatic Leisons | Liver Scores (Number of Animals) | |||||||||
| Fluke Burdens | Fluke Length (mm) | Fluke Weight (g) | 12 WPI | 13 WPI | 7 DPI | 8 DPI | 9 DPI | − (0) | + (<10) | ++ (10–25) | +++ (25–50) | ++++ (>50) | |
| 1 | 59±32.5 | 17.3±3 | 4.4±2.4 | 82.1 | 96.4 | 26 | 48 | 89 | 0 | 2 | 3 | 2 | 0 |
| 2 | 55.2±12.4 | 16.8±3 | 4.6±3.1 | 100 | 110.7 | 85 | 165 | 172 | 0 | 1 | 2 | 3 | 0 |
Group 1 (goats immunised with recombinant FhGST-S1) and Group 2 (infected control group immunised with Quil A only). Liver scores were recorded at necropsy 15 wpi. WPI – Weeks post infection. DPI – Days post infection.
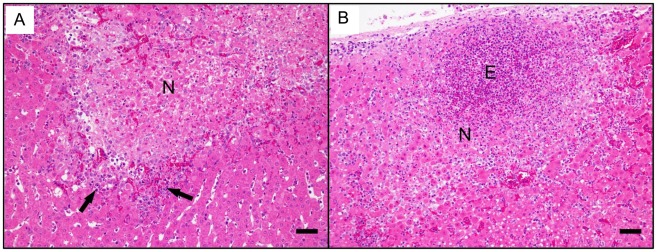
Microscopically, at 7–9 dpi animals from the Quil A group showed tortuous necrotic tracts surrounded by a scarce inflammatory infiltration with occasional eosinophils (Figure 7A). Older necrotic areas were surrounded by macrophages, epithelioid cells and multinucleate giant cells and lymphocytes. Some migrating larvae were found in the liver parenchyma without inflammatory infiltrate associated to them. In goats immunised with rFhGST-S1 smaller necrotic areas associated to a heavy infiltration of eosinophils (Figure 7B) were seen. Unlike the Quil A immunised group, all migrating larvae found were surrounded by a heavy infiltration of eosinophils.
Figure 7. The effects of vaccination with rFhGST-S1 or Quil A.
A) Photomicrograph of the liver from the Quil A immunised group showing an area of coagulative necrosis (N) surrounded by scarce inflammatory infiltration (arrows) with occasional eosinophils. B) Photomicrograph of the liver from the rFhGST-S1 immunised group showing a coagulative necrotic area (N) associated to numerous eosinophils (E). Both images haematoxylin and eosin stained. Both bar represent 100 µm.
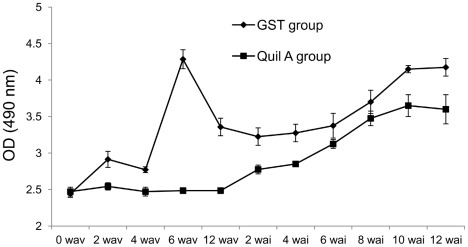
A significant increase of IgG anti-rFhGST-S1 was observed two weeks after vaccination with a strong increase after the second injection at week 4 in immunised animals (Figure 8). The Quil A control group did not show any specific IgG response until 2 weeks after infection. Specific IgG titres increased during infection in both groups, but they were consistently higher in the immunised group throughout the duration of the the experiment.
Figure 8. Specific IgG response.
Serum titres of IgG anti-r-FhGST-S1 at 0, 2, 4 and 6 weeks after vaccination (wav) and at 2, 4, 6, 8, 10 and 12 weeks after infection (wai). Results expressed in log10.
Discussion
Previous studies have highlighted the importance of parasite GSTs, including Sigma class GSTs, in host-parasite interactions and as potential vaccination candidates. With this in mind, we have studied the relatively newly identified Sigma class GST from F. hepatica to both enhance our understanding of this important enzyme in Fasciola and the Sigma class of GSTs as a whole.
Alignments and phylogenetics classified FhGST-S1 alongside trematode and mammalian Sigma class GSTs, yet there remains a distinct divide between the parasites and their hosts, a phenomenon also observed for the recently reclassified ‘Nu’ class of GSTs from nematodes [49]. Therefore, it may be that trematode GSTs are sufficiently distinct to support a sub-classification within the broad Sigma class. The distinction of FhGST-S1 from fasciolosis host Sigma class GSTs enhances its potential as a therapeutic target.
Substrate activity profiling of rFhGST-S1 using model substrates showed the enzyme to have comparable activity to other trematode Sigma class GSTs such as Sm28GST [47]. However, rFhGST-S1 exhibits relatively high GSH-conjugating activity towards the potentially natural reactive aldehyde, 4-hydroxy-nonenal (4-HNE) toxin and high GSH-dependent peroxidase activity towards the tested lipid peroxides which includes the endogenous substrate linoleic acid hydroperoxide. 4-HNE is the major aldehydic end-product of lipid peroxidation that is involved in signalling of host immune cells leading to apoptosis of T- and B-cells [50].
Assessing the inhibition of rFhGST-S1 activity with CDNB revealed that both bile acids and the flukicide TCBZ appear to bind to the enzyme. In particular, the interaction of the bile acid cholate with rFhGST-S1 is approximately ten fold higher than GSTs from the sheep intestinal cestode Moniezia expansa [51]. Host bile acids are known as triggers of physiological processes in trematodes including Fasciola sp. [52], [53]. Therefore, molecular interaction of bile acids with FhGST-S1 warrants further investigation especially, given that FhGST-S1 is localised to near the body surface of the fluke, where it could potentially bind cholate and other free bile acids found in abundance in host bile (cholate is found at approximately 100 mM in sheep bile) [54]. The hydroxy-TCBZ SO levels in the bile have been shown to be in excess of 100 µM [55] thus, the IC50 of 57±5 µM for TCBZ SO suggests the abundant FhGST-S1 could be involved in TCBZ response in phase III sequestration based detoxification. This finding warrants further investigation to understand the role of FhGST-S1 in TCBZ action or detoxification.
Sigma class GSTs from both parasites and mammals have been known to exhibit prostaglandin synthase activity. To this end, the Sigma GST from F. hepatica shares a high sequence identity with recognised Sigma class GSTs with prostaglandin synthase activity, including rOvGST-1 from the filarial parasite, Onchocerca volvulus. Using a coupled assay with COX-1 we have shown that rFhGST-S1 is capable of synthesizing both PGD2 and PGE2, with PGD2 being the predominant prostanoid. Parasite-derived eicosanoids, including prostaglandins, are known to be important in the establishment of parasitic infection and the survival and proliferation within the host. Therefore, eicosanoids produced by parasitic helminths may play a role in pathophysiological changes during helminth infections. For example, chronic fasciolosis is associated with fever and changes in liver biochemistry, both of which could be associated with parasite-derived eicosanoids thromboxane B2 (TXB2), PGI2, PGE2 and leukotriene B4 (LTB4), detected in the ES products and homogenates of adult F. hepatica worms [56]. In addition, the migration of host epidermal Langerhans cells, which play a key part in immune defence mechanisms, has been shown to be inhibited by parasite-derived PGD2 in the Schistosoma mansoni-mouse model of human infection, thus allowing schistosomes to manipulate the host immune system [57]. Earlier studies have revealed the presence of eicosanoids produced by S. mansoni cercariae which could also play a role in establishment of infections through loss of the cercarial tail following penetration of the skin [58]. It therefore seems likely that prostaglandins synthesised via FhGST-S1 will have a role in establishing the infection within the host.
In general, prostaglandins and eicosanoids have potent biological activities in reproduction. For example in the zebrafish egg, high levels of PGE2 were seen post fertilisation coupled with high PGD2 synthase transcript levels during the early stages of egg development concomitant with an exponential decrease of PGD2 levels over the next 120 h post fertilisation [59]. However, in F. hepatica, eggs in gravid adults are released in an immature state in the bile duct, where they pass to the external environment via the host's excretory system and complete embryogenesis ex-host. Therefore, FhGST-S1 may have a secondary, or indeed primary, function in egg development and embyrogenesis. A role in egg development is further supported by proteomic studies of F. hepatica ontogenic stages which reveal the presence of FhGST-S1 in eggs ([42] and the current study).
FhGST-S1 appears to be highly abundant in eggs with western blotting showing FhGST-S1 to be constitutively expressed, despite its association with a large spot consisting of multiple co-migrating proteins unresolved via 2DE (for association see [42]). Immunolocalisation studies revealed that FhGST-S1 is closely associated with vitelline cells of mature adult worms. Given the importance of PGs in reproduction, we hypothesize that PG synthase activity exhibited by rFhGST-S1 contributes to developmental cues during egg formation. Interestingly, no FhGST-S1 was seen in day 0, un-embryonated, eggs by western blotting yet in situ immunlocalisation showed freshly voided eggs, equivalent to day 0 eggs, to contain copious amounts of FhGST-S1. While it is most likely that FhGST-S1 is present in day 0 eggs, albeit at a reduced expression, the discrepancy seen between the two techniques is probably related to the antibody dilutions used for each method; in total a 40-fold difference in favour of immunolocalisation.
FhGST-S1 was also identified in both NEJs and adult worms using western blotting. This finding emphasises the multi-functionality of FhGST-S1, where in NEJs egg productions is not yet in process, suggesting its main function is in PG synthesis for host modulation or as a detoxification enzyme. In the adult worm, FhGST-S1 could also be localised, to a smaller extent, in the parenchyma and tegument. Given the high activity of FhGST-S1 towards the toxic 4-HNE and to lipid hydroperoxides this suggests a detoxification role at the host-parasite interface.
With near surface expression of FhGST-S1, in the parenchyma and tegument, there is the potential for this enzyme to be readily released into the host environment. Indeed, we have identified FhGST-S1 in the ES products of adult worms. With this in mind, previous studies have highlighted the importance of parasite Sigma class GSTs in immunomodulation of the host immune response. This includes our recent study implicating rFhGST-S1 in chronic inflammation through the activation of dendritic cells (DCs) [48]. While active rFhGST-S1 was able to induce levels of IL-12p40 and IL-6 cytokines in DCs in a dose-dependent manner, the previously described F. hepatica Mu-class GSTs failed to induce any cytokine secretion. Since denatured rFhGST-S1 also failed to induce any cytokines in DCs, activation of DCs is likely related to the structure and activity of the enzyme. However, inhibition of nitric oxide production, involved in driving a Th2 immune response, may also be a contributing factor in skewing the host response to fasciolosis [60].
F. hepatica infections are associated with a T-helper-cell type 2 (Th2) immune response dominating during the chronic phases of infection [61], but pro-inflammatory responses are suppressed [62]. Suppression of allergic responses during chronic parasitic worm infections has a mutually beneficial effect on the parasites' proliferation and the hosts' survival. Prostanoids, including PGD2, are important in mediating these allergic inflammatory responses. While generally regarded as pro-inflammatory molecules, these important lipid molecules are also involved in mediating anti-inflammatory responses [63]. Helminth-derived molecules are thought to be involved in driving the Th2 response stereotypical of parasitic worm infections. DC and macrophage cell cultures exposed to rFhGST-S1 showed elevated levels of Th2 cytokines after 24 h [48]. In this study, the effects of rFhGST-S1 exposure onprostanoid synthesis in host immune cells was investigated. The results of which show the stimulation of PGD2 and PGE2 in both DCs and macrophage cell lines suggesting FhGST-S1 is one such helminth derived molecule capable of driving the Th2 response.
As we have shown FhGST-S1 to have key roles in F. hepatica, both in NEJs and adult worms, coupled with the near surface expression and release of the enzyme via the ES products, we assessed the potential of FhGST-S1 to be used as a vaccine candidate. This was especially poignant given that the S. mansoni Sigma GST homologue (Sm28) is in phase II clinical trials [12]. Unfortunately, the current goat based vaccine trial did not show any significant differences in fluke burdens between the rFhGST-S1 immunised and Quil A control group. However, a high individual variability was recorded, particularly in the vaccinated group also reported in previous trials using goats vaccinated with alternative candidates such as cathepsin L1 [64] and Sm14 [65]. The vaccine trial shown here using a target species with an acceptable adjuvant may have been adversely affected by the strain of F. hepatica used to challenge goats. Here we have shown an unusually high infectivity rate with the strain of F. hepatica used; which we have reported in a previous trial using goats [64]. Using an alternative strain of F. hepatica for experimental infections in this species has given normal infectivity rates ranging from 14% to 26.5% [65].
In the present trial it appeared that goats immunised with rFhGST-S1, despite no variations in fluke burdens or morphometrics, showed reduced gross hepatic lesions during early infection, up to day 9 post infection, which continued to week 15 post infection where liver scores for hepatic lesions appeared reduced for rFhGST-S1 immunised animals. These results suggest that animals from the immunised group produced an early response to migrating larvae that has induced some partial protection from liver damage. The early and consistent specific IgG response found in the present work also agrees with the results obtained in a previous trial using naïve FhGST [46]. However, in both studies high levels of specific IgG did not induced a protective response reducing worm burdens.
A promising aspect of producing anti-helminth vaccines is developing multivalent vaccines. In many cases the greatest protection from challenge is by vaccinating with a combination of Fasciola antigens [66], [67]. Therefore, based on the immunisation with FhGST-S1 showing an early response reducing hepatica damage, could be considered for inclusion into a multivalent vaccine against Fasciolosis. In addition, in light of our findings showing FhGST-S1 to be highly prominent in egg production and the egg itself, as with previous vaccination trials [67], it will be important to investigate the ability of eggs voided from vaccinated animals to embryonate. The potential to reduce pasture contamination by inhibiting egg embryonation, combined with the demonstrated reduction in liver damage, warrants further exploration using rFhGST-S1 as a vaccine candidate.
In summary, we have further promoted the concept that FhGST-S1 clearly demonstrates key host-parasite roles in synthesising PGs and stimulating PG release from host innate immune cells. In addition we have shown FhGST-S1 to be a key protein for detoxification, which may well be involved in TCBZ response. In line with current vaccine development theory we have shown FhGST-S1 to have multi-functional roles in the liver fluke physiology. Furthermore, we have shown FhGST-S1 to be expressed across ontogenic stages, localised to the fluke surface, and to the egg, both characteristics vital for vaccine development and success. Whilst no protection from fluke burden was seen in trials, the inclusion of rFhGST-S1 as a multivalent vaccine component should be investigated. However, it is important to fully characterise the host immune response during the early stages post-infection to better understand the mechanism mediating an effective host response. This will be essential to improve any future vaccine formulation.
[36,51,68–71] Table 2 Refs.
Supporting Information
Multiple sequence alignment and neighbour-joining phylogenetic tree across seven species-independent classes of GSTs. A) Alignment of the sigma class GSTs of trematodes shows the extent of identity and similarity across this class of GSTs. Boxed residues indicate complete identity between all sequences. Residues shaded in grey indicate conserved residues. B) Neighbour-joining tree placing mammalian and trematode GSTs within the same broad Sigma class. A distinct separation of clusters within this Sigma class is observed as with the recently reclassified ‘Nu’ class of GSTs from nematodes [49]. Sequences were aligned via the ClustalW program [29] in BioEdit Sequence Alignment Editor version 7.0.5.2. [30]. Phylogenetic neighbour-joining bootstrap trees were produced and viewed within TREEVIEW [33]. Key to sequences in 1a and 1b. Xenopus laevis; Fhep49c06_omega Fasciola hepatica; Fhep54b04_omega Fasciola hepatica; O09131_GSTO1_MUS Mus musculus; O35543_PTGD2lowbar;RAT Rattus norvegicus; O60760_PTGD2_HOMO Homo sapiens; O73888_PTGD2_GGAL Gallus gallus; O97096_GST_CLOSI Clonorchis sinensis; P08263_GSTA1_HOMO Homo sapiens; P08515_GSTM_SCHJA Schistosoma japonicum; P09488_GSTM1.1_HOMO Homo sapiens; P09792_GST28_SCHMA Schistosoma mansoni; P10299_GSTP1_CE Caenorhabditis elegans; P19157_GSTP1_MUS Mus musculus; P20432_GSTT1_DROME Drosophila melanogaster; P28801_GSTP1_BOVIN Bos taurus; P30113_GST28_SCHBO Schistosoma bovis; P30114_GST28_SCHHA Schistosoma haematobium; P34345_GSTO_CE Caenorhabditis elegans; P35661_GSTM_SCHMA Schistosoma mansoni; P41043_GSTS1_DROME Drosophila melanogaster; P46436_GSTS1_ASCSU Ascaris suum; P51781_GSTA1_PIG Sus scrofa; P78417_GSTO1_HOMO Homo sapiens; P80031_GSTP1_PIG Sus scrofa; P91253_GSTS7_CE Caenorhabditis elegans; Q000H8_GSTM2_PIG Sus scrofa; Q06A71_FhGST-S1 Fasciola hepatica; Q26200_GST_PARWE Paragonimus westermani; Q26513_GST_SCHJA Schistosoma japonicum; Q28035_GSTA1_BOVIN Bos taurus; Q30B87_GSTM3_SHEEP Ovis aries; Q58ET5_GSTM1_MUS Mus musculus; Q5TZY2_GSTT1_HOMO Homo sapiens; Q5TZY3_GSTP1_HOMO Homo sapiens; Q6IB17_GSTZ1.1_HOMO Homo sapiens; Q6P8Q0_GSTA1_MUS Mus musculus; Q86LC0_GSTO_SCHMA Schistosoma mansoni; Q8ISK1_GST_OPIVI Opisthorchis viverrini; Q91X50_GSTT1_MUS Mus musculus; Q9JHF7_PTGD2_MUS Mus musculus; Q9N0V4_GSTM1_BOVIN Bos taurus; Q9N2J6_GSTMic_SHEEP Ovis aries; Q9N4H6_GSTZ43_CE Caenorhabditis elegans; Q9NAW7_GSTS_HCON Haemonchus contortus; Q9TTY8_GSTP1_CAPHI Capra hircus; Q9WVL0_GSTZ1_MUS Mus musculus; Q9XS30_GST_SHEEP Ovis aries; XP_535659.1_PGDS_CFAM Canis familiaris. A1BNE5_GST_CLOSI Clonorchis sinensis; AAH53774.1_GSTS1-1_XLAE
(TIF)
Amino acid identity comparisons of FhGST-S1 with GSTs from cytosolic classes across a variety of taxa. Amino acid sequence comparison of FhGST-S1 with other trematode GSTs clearly places FhGST-S1 into the Sigma class of GSTs, with identities averaging approximately 45%. Comparison with the most closely matching mammalian GSTs shows sequence identities averaging only approximately 28%. PTGD – Prostaglandin D synthase; Mic -Microsomal.
(XLS)
Acknowledgments
The authors would like to thank following: Dr. Deborah Ward at the University of Liverpool, School of Biological Sciences, for technical assistance with 2DE, and Dr. Dianna Williams, Sean Williams and Marie O'Brien for immunohistochemical technical advice and preparation.
Footnotes
The authors have declared that no competing interests exist.
This work was funded by the European Union (DELIVER: Grant FOOD-CT-2005-023025) and the BBSRC (grants BBH0092561 and BB/C503638/2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boray JC. Chemotherapy of infections with fasciolidae. In: Boray JC, editor. Immunology, pathobiology and control of Fasciolosis. New Jersey: MSD AGVET Rahway; 1997. pp. 83–97. [Google Scholar]
- 2.Rim HJ, Farag HF, Sornmani S, Cross JH. Food-borne trematodes: Ignored or emerging. Parasitology Today. 1994;10:207–209. [Google Scholar]
- 3.Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bulletin of the World Health Organization. 1999;77:340–346. [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Report of the World Health Organisation Informal Meeting on use of triclabendazole in fascioliasis control. 2006. Geneva, Switzerland: WHO headquarters. Available online: http://www.who.int/neglected_diseases/preventive_chemotherapy/WHO_CDS_NTD_PCT_2007.1.pdf.
- 5.Brennan GP, Fairweather I, Trudgett A, Hoey E, McCoy, et al. Understanding triclabendazole resistance. Experimental and Molecular Pathology. 2007;82:104–109. doi: 10.1016/j.yexmp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Panaccio M, Wilson LR, Crameri SL, Wijffels GL, Spithill TW. Molecular characterization of cDNA sequences encoding glutathione S-transferases of Fasciola hepatica. Experimental Parasitology. 1992;74:232–237. doi: 10.1016/0014-4894(92)90051-b. [DOI] [PubMed] [Google Scholar]
- 7.Wijffels GL, Sexton JL, Salvatore L, Pettitt JM, Humphris DC, et al. Primary sequence heterogeneity and tissue expression of glutathione S-transferases of Fasciola hepatica. Experimental Parasitology. 1992;74:87–99. doi: 10.1016/0014-4894(92)90142-w. [DOI] [PubMed] [Google Scholar]
- 8.Salvatore L, Wijffels G, Sexton JL, Panaccio M, Mailer S, et al. Biochemical analysis of recombinant glutathione S-transferase of Fasciola hepatica. Molecular and Biochemical Parasitology. 1995;69:281–288. doi: 10.1016/0166-6851(94)00205-2. [DOI] [PubMed] [Google Scholar]
- 9.Rossjohn J, Feil SC, Wilce MCJ, Sexton JL, Spithill TW, et al. Crystallization, structural determination and analysis of a novel parasite vaccine candidate: Fasciola hepatica glutathione S-transferase. Journal of Molecular Biology. 1997;273:857–872. doi: 10.1006/jmbi.1997.1338. [DOI] [PubMed] [Google Scholar]
- 10.Brophy PM, Crowley P, Barrett J. Detoxification reactions of Fasciola hepatica cytosolic glutathione transferases. Molecular and Biochemical Parasitology. 1990;39:155–162. doi: 10.1016/0166-6851(90)90054-p. [DOI] [PubMed] [Google Scholar]
- 11.Chemale G, Morphew R, Moxon JV, Morassuti AL, LaCourse EJ, et al. Proteomic analysis of glutathione transferases from the liver fluke parasite, Fasciola hepatica. Proteomics. 2006;6:6263–6273. doi: 10.1002/pmic.200600499. [DOI] [PubMed] [Google Scholar]
- 12.Capron A, Riveau G, Capron M, Trottein F. Schistosomes: the road from host-parasite interactions to vaccines in clinical trials. Trends in Parasitology. 2005;21:143–149. doi: 10.1016/j.pt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Hervé M, Angeli V, Pinzar E, Wintjens R, Faveeuw C, et al. Pivotal roles of the parasite PGD2 synthase and of the host D prostanoid receptor 1 in schistosome immune evasion. European Journal of Immunology. 2003;33:2764–2772. doi: 10.1002/eji.200324143. [DOI] [PubMed] [Google Scholar]
- 14.Kanaoka Y, Ago H, Inagaki E, Nanayama T, Miyano M, et al. Cloning and crystal structure of hematopoietic prostaglandin D synthase. Cell. 1997;90:1085–1095. doi: 10.1016/s0092-8674(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 15.Kanaoka Y, Urade Y. Hematopoietic prostaglandin D synthase. Prostaglandins Leukotrienes and Essential Fatty Acids. 2003;69:163–167. doi: 10.1016/s0952-3278(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 16.Meyer DJ, Muimo R, Thomas M, Coates D, Isaac RE. Purification and characterization of prostaglandin-H E-isomerase, a sigma-class glutathione S-transferase, from Ascaridia galli. Biochemical Journal. 1996;313:223–227. doi: 10.1042/bj3130223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehan D, Meade G, Foley VM, Dowed CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochemical Journal. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson AM, Meyer DJ, Hayes JD. Sequence, catalytic properties and expression of chicken glutathione dependent prostaglandin D2 synthase, a novel class Sigma glutathione S- transferase. Biochemical Journal. 1998;333:317–325. doi: 10.1042/bj3330317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayaishi O, Urade Y. Prostaglandin D2 in sleep-wake regulation: recent progress and perspectives. Neuroscientist. 2002;8:12–15. doi: 10.1177/107385840200800105. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Ogawa K, Sugamura K, Nakamura M, Takano S, et al. Cutting edge: differential production of prostaglandin D2 by human helper T cell subsets. Journal of Immunology. 2000;164:2277–2280. doi: 10.4049/jimmunol.164.5.2277. [DOI] [PubMed] [Google Scholar]
- 22.Hart PH. Regulation of the inflammatory response in asthma by mast cell products. Immunology and Cell Biology. 2001;79:149–153. doi: 10.1046/j.1440-1711.2001.00983.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller SB. Prostaglandins in health and disease: an overview. Seminars in Arthritis and Rheumatism. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Liu LX, Serhan CN, Weller PF. Intravascular filarial parasites elaborate cyclooxygenase-derived eicosanoids. Journal of Experimental Medicine. 1990;172:993–996. doi: 10.1084/jem.172.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdel Baset H, O'Neill GP, Ford-Hutchinson AW. Characterization of arachidonic-acid-metabolizing enzymes in adult Schistisoma mansoni. Molecular and Biochemical Parasitology. 1995;73:31–41. doi: 10.1016/0166-6851(95)00085-f. [DOI] [PubMed] [Google Scholar]
- 26.Sommer A, Rickert R, Fischer P, Steinhart H, Walter RD, et al. A dominant role for extracellular glutathione S-transferase from Onchocerca volvulus is the production of prostaglandin D2. Infection and Immunity. 2003;71:3603–3606. doi: 10.1128/IAI.71.6.3603-3606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubata BK, Duszenko M, Martin KS, Urade Y. Molecular basis for prostaglandin production in hosts and parasites. Trends in Parasitology. 2007;23:325–331. doi: 10.1016/j.pt.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Maizels RM, Bundy DAP, Selkirk ME, Smith DF, Anderson RM. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- 31.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 33.Page RD. TreeView: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 34.Simons PC, Vander Jagt DL. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Analytical Biochemistry. 1977;82:334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- 35.LaCourse EJ, Hernandez-Viadel M, Svendsen C, Spurgeon D, Jefferies JR, et al. Glutathione transferase (GST) as a candidate molecular based biomarker for soil toxin exposure in the earthworm Lumbricus rubellus. Environmental Pollution. 2009;157:2459–2469. doi: 10.1016/j.envpol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 37.Hayes JD, Mantle TJ. Inhibition of hepatic and extrahepatic glutathione S- transferases by primary and secondary bile acids. Biochemical Journal. 1986;233:407–415. doi: 10.1042/bj2330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer DJ, Thomas M. Characterization of a rat spleen prostaglandin-H D-isomerase as a Sigma class glutathione S-transferase. Biochemical Journal. 1995;311:739–742. doi: 10.1042/bj3110739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt R, Coste O, Geisslinger G. LC-MS/MS-analysis of prostaglandin E2 and D2 in microdialysis samples of rats. Journal of Chromatography B. 2005;826:188–197. doi: 10.1016/j.jchromb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Morphew RM, Wright HA, LaCourse EJ, Porter J, Barrett J, et al. Towards Delineating Functions within the Fasciola Secreted Cathepsin L Protease Family by Integrating In Vivo Based Sub-Proteomics and Phylogenetics. Plos Neglected Tropical Diseases. 2011;5:e937. doi: 10.1371/journal.pntd.0000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGonigle L, Mousley A, Marks NJ, Brennan GP, Dalton JP, et al. The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. International Journal for Parasitology. 2008;38:149–155. doi: 10.1016/j.ijpara.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Moxon JV, LaCourse EJ, Wright HA, Perally S, Prescott MC, et al. Proteomic analysis of embryonic Fasciola hepatica: Characterization and antigenic potential of a developmentally regulated heat shock protein. Veterinary Parasitology. 2010;169:62–75. doi: 10.1016/j.vetpar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Harlow D, Lane D. Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory. NY: Cold Spring Harbor; 1988. [Google Scholar]
- 44.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kipar A, Bellmann S, Kremendahl J, KoÈhler K, Reinacher M. Cellular composition, coronavirus antigen expression and production of specific antibodies in lesions in feline infectious peritonitis. Veterinary Immunology and Immunopathology. 1998;65:243–257. doi: 10.1016/S0165-2427(98)00158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buffoni L, Zafra R, Perez-Ecija A, Martinez-Moreno FJ, Martinez-Galisteo E, et al. Immune response of goats immunised with glutathione S-transferase and experimentally challenged with Fasciola hepatica. Parasitology International. 2010;59:147–153. doi: 10.1016/j.parint.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Walker J, Crowley P, Moreman AD, Barrett J. Biochemical properties of cloned glutathione S-transferases from Schistosoma mansoni and Schistosoma japonicum. Molecular and Biochemical Parasitology. 1993;61:255–264. doi: 10.1016/0166-6851(93)90071-5. [DOI] [PubMed] [Google Scholar]
- 48.Dowling DJ, Hamilton CM, Donnelly S, La Course J, Brophy PM, et al. Major Secretory Antigens of the Helminth Fasciola hepatica Activate a Suppressive Dendritic Cell Phenotype That Attenuates Th17 Cells but Fails To Activate Th2 Immune Responses. Infection and Immunity. 2010;78:793–801. doi: 10.1128/IAI.00573-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schüller DJ, Liu Q, Kriksunov IA, Campbell AM, Barrett J, et al. Crystal structure of a new class of glutathione transferase from the model human hookworm nematode Heligmosomoides polygyrus. . Proteins. 2005;61:1024–1031. doi: 10.1002/prot.20649. [DOI] [PubMed] [Google Scholar]
- 50.Kalinich JF, Ramakrishnan R, McClain DE, Ramakrishnan N. 4-Hydroxynonenal, an end-product of lipid peroxidation, induces apoptosis in human leukemic T- and B-cell lines. Free Radical Research. 2000;33:349–358. doi: 10.1080/10715760000300891. [DOI] [PubMed] [Google Scholar]
- 51.Brophy PM, Southan C, Barrett J. Glutathione transferase in the tapeworm Moniezia expansa. Biochemical Journal. 1989;262:939–946. doi: 10.1042/bj2620939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixon KE. The physiology of excystment of metacercaria of Fasciola hepatica L. Parasitology. 1966;56:431–456. doi: 10.1017/s0031182000068931. [DOI] [PubMed] [Google Scholar]
- 53.Sukhdeo MVK, Keith S, Mettrick DF. The effects of bile on the locomotory cycle of Fasciola hepatica. Journal of Parasitology. 1988;74:493–495. [PubMed] [Google Scholar]
- 54.Peric-Golia L, Socic H. Free bile acids in sheep. Comparative Biochemistry and Physiology. 1968;26:741–744. doi: 10.1016/0010-406x(68)90667-1. [DOI] [PubMed] [Google Scholar]
- 55.Hennessy DR, Lacey E, Steel JW, Prichard RK. The Kinetics of Triclabendazole Disposition in Sheep. Journal of Veterinary Pharmacology and Therapeutics. 1987;10:64–72. doi: 10.1111/j.1365-2885.1987.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 56.Ali SF, Joachim A, Daugschies A. Eicosanoid production by adult Fasciola hepatica and plasma eicosanoid patterns during fasciolosis in sheep. International Journal for Parasitology. 1999;29:743–748. doi: 10.1016/s0020-7519(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 57.Angeli V, Faveeuw C, Roye O, Fontaine J, Teissier E, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. Journal of Experimental Medicine. 2001 doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fusco AC, Salafsky B, Kevin MB. Schistosoma mansoni: eicosanoid production by cercariae. Experimental Parasitology. 1985;59:44–50. doi: 10.1016/0014-4894(85)90055-4. [DOI] [PubMed] [Google Scholar]
- 59.Yeh HC, Wang LH. Profiling of prostanoids in zebrafish embryonic development. Prostaglandins Leukotrienes and Essential Fatty Acids. 2006;75:397–402. doi: 10.1016/j.plefa.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Else KJ. Have gastrointestinal nematodes outwitted the immune system? Parasite Immunology. 2005;27:407–415. doi: 10.1111/j.1365-3024.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 61.O'Neill SM, Brady MT, Callanan JJ, Mulcahy G, Joyce P, et al. Fasciola hepatica Infection Down Regulates Th1 Responses in Mice. Parasite Immunology. 2000;22:147–155. doi: 10.1046/j.1365-3024.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 62.O'Neill SM, Mills KHG, Dalton JP. Fasciola hepatica cathepsin L cysteine proteinase suppresses Bordetella pertussis-specific interferon-gamma production in vivo. Parasite Immunology. 2001;23:541–547. doi: 10.1046/j.1365-3024.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 63.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. Journal of Invetigative Medicine. 2009;57:703–708. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 64.Pérez-Écija RA, Mendes RE, Zafra R, Buffonni L, Martínez-Moreno A, et al. Pathological and parasitological protection in goats immunised with recombinant cathepsin L1 and challenged with Fasciola hepatica. Veterinary Journal. 2010;185:351–353. doi: 10.1016/j.tvjl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Zafra R, Buffoni L, Martínez-Moreno A, Pérez-Ecija A, Martinez-Moreno FJ, et al. A study of the liver of goats immunized with a synthetic peptide of the Sm14 antigen and challenged with Fasciola hepatica. Journal of Comparative Pathology. 2008;139:169–176. doi: 10.1016/j.jcpa.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Jayaraj R, Piedrafita D, Dynon K, Grams R, Spithill TW, et al. Vaccination against fasciolosis by a multivalent vaccine of stage-specific antigens. Veterinary Parasitology. 2009;160:230–236. doi: 10.1016/j.vetpar.2008.10.099. [DOI] [PubMed] [Google Scholar]
- 67.Dalton JP, McGonigle S, Rolph TP, Andrews SJ. Induction of protective immunity in cattle against infection with Fasciola hepatica by vaccination with cathepsin L proteinases and with hemoglobin. Infection and Immunity. 1996;64:5066–5074. doi: 10.1128/iai.64.12.5066-5074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Letters. 1985;179:267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- 69.Flohé L, Günzler WA. Assays of glutathione peroxidise. Methods in Enzymology. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 70.Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods in Enzymology. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 71.Jaffe JJ, Lambert RA. Glutathione S-transferase in adult Dirofilaria immitis and Brugia pahangi. Molecular and Biochemical Parasitology. 1986;20:199–206. doi: 10.1016/0166-6851(86)90032-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple sequence alignment and neighbour-joining phylogenetic tree across seven species-independent classes of GSTs. A) Alignment of the sigma class GSTs of trematodes shows the extent of identity and similarity across this class of GSTs. Boxed residues indicate complete identity between all sequences. Residues shaded in grey indicate conserved residues. B) Neighbour-joining tree placing mammalian and trematode GSTs within the same broad Sigma class. A distinct separation of clusters within this Sigma class is observed as with the recently reclassified ‘Nu’ class of GSTs from nematodes [49]. Sequences were aligned via the ClustalW program [29] in BioEdit Sequence Alignment Editor version 7.0.5.2. [30]. Phylogenetic neighbour-joining bootstrap trees were produced and viewed within TREEVIEW [33]. Key to sequences in 1a and 1b. Xenopus laevis; Fhep49c06_omega Fasciola hepatica; Fhep54b04_omega Fasciola hepatica; O09131_GSTO1_MUS Mus musculus; O35543_PTGD2lowbar;RAT Rattus norvegicus; O60760_PTGD2_HOMO Homo sapiens; O73888_PTGD2_GGAL Gallus gallus; O97096_GST_CLOSI Clonorchis sinensis; P08263_GSTA1_HOMO Homo sapiens; P08515_GSTM_SCHJA Schistosoma japonicum; P09488_GSTM1.1_HOMO Homo sapiens; P09792_GST28_SCHMA Schistosoma mansoni; P10299_GSTP1_CE Caenorhabditis elegans; P19157_GSTP1_MUS Mus musculus; P20432_GSTT1_DROME Drosophila melanogaster; P28801_GSTP1_BOVIN Bos taurus; P30113_GST28_SCHBO Schistosoma bovis; P30114_GST28_SCHHA Schistosoma haematobium; P34345_GSTO_CE Caenorhabditis elegans; P35661_GSTM_SCHMA Schistosoma mansoni; P41043_GSTS1_DROME Drosophila melanogaster; P46436_GSTS1_ASCSU Ascaris suum; P51781_GSTA1_PIG Sus scrofa; P78417_GSTO1_HOMO Homo sapiens; P80031_GSTP1_PIG Sus scrofa; P91253_GSTS7_CE Caenorhabditis elegans; Q000H8_GSTM2_PIG Sus scrofa; Q06A71_FhGST-S1 Fasciola hepatica; Q26200_GST_PARWE Paragonimus westermani; Q26513_GST_SCHJA Schistosoma japonicum; Q28035_GSTA1_BOVIN Bos taurus; Q30B87_GSTM3_SHEEP Ovis aries; Q58ET5_GSTM1_MUS Mus musculus; Q5TZY2_GSTT1_HOMO Homo sapiens; Q5TZY3_GSTP1_HOMO Homo sapiens; Q6IB17_GSTZ1.1_HOMO Homo sapiens; Q6P8Q0_GSTA1_MUS Mus musculus; Q86LC0_GSTO_SCHMA Schistosoma mansoni; Q8ISK1_GST_OPIVI Opisthorchis viverrini; Q91X50_GSTT1_MUS Mus musculus; Q9JHF7_PTGD2_MUS Mus musculus; Q9N0V4_GSTM1_BOVIN Bos taurus; Q9N2J6_GSTMic_SHEEP Ovis aries; Q9N4H6_GSTZ43_CE Caenorhabditis elegans; Q9NAW7_GSTS_HCON Haemonchus contortus; Q9TTY8_GSTP1_CAPHI Capra hircus; Q9WVL0_GSTZ1_MUS Mus musculus; Q9XS30_GST_SHEEP Ovis aries; XP_535659.1_PGDS_CFAM Canis familiaris. A1BNE5_GST_CLOSI Clonorchis sinensis; AAH53774.1_GSTS1-1_XLAE
(TIF)
Amino acid identity comparisons of FhGST-S1 with GSTs from cytosolic classes across a variety of taxa. Amino acid sequence comparison of FhGST-S1 with other trematode GSTs clearly places FhGST-S1 into the Sigma class of GSTs, with identities averaging approximately 45%. Comparison with the most closely matching mammalian GSTs shows sequence identities averaging only approximately 28%. PTGD – Prostaglandin D synthase; Mic -Microsomal.
(XLS)