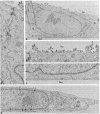
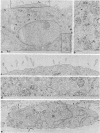
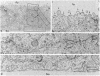
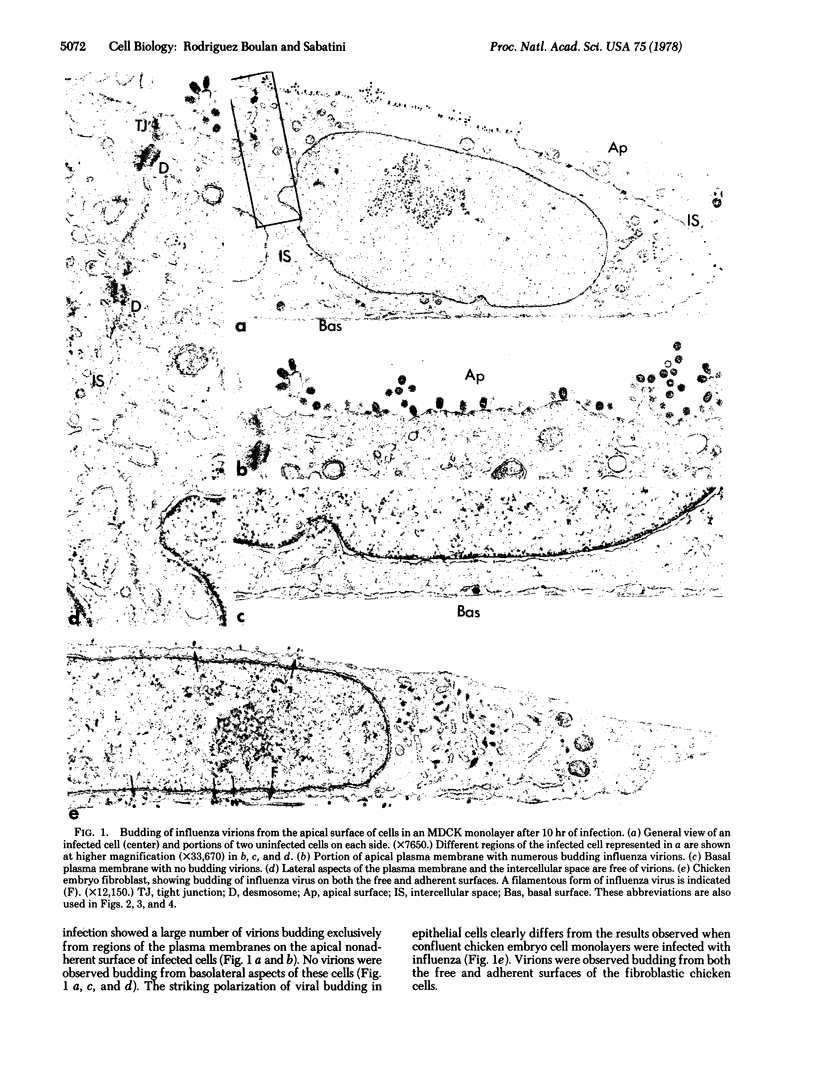
Abstract
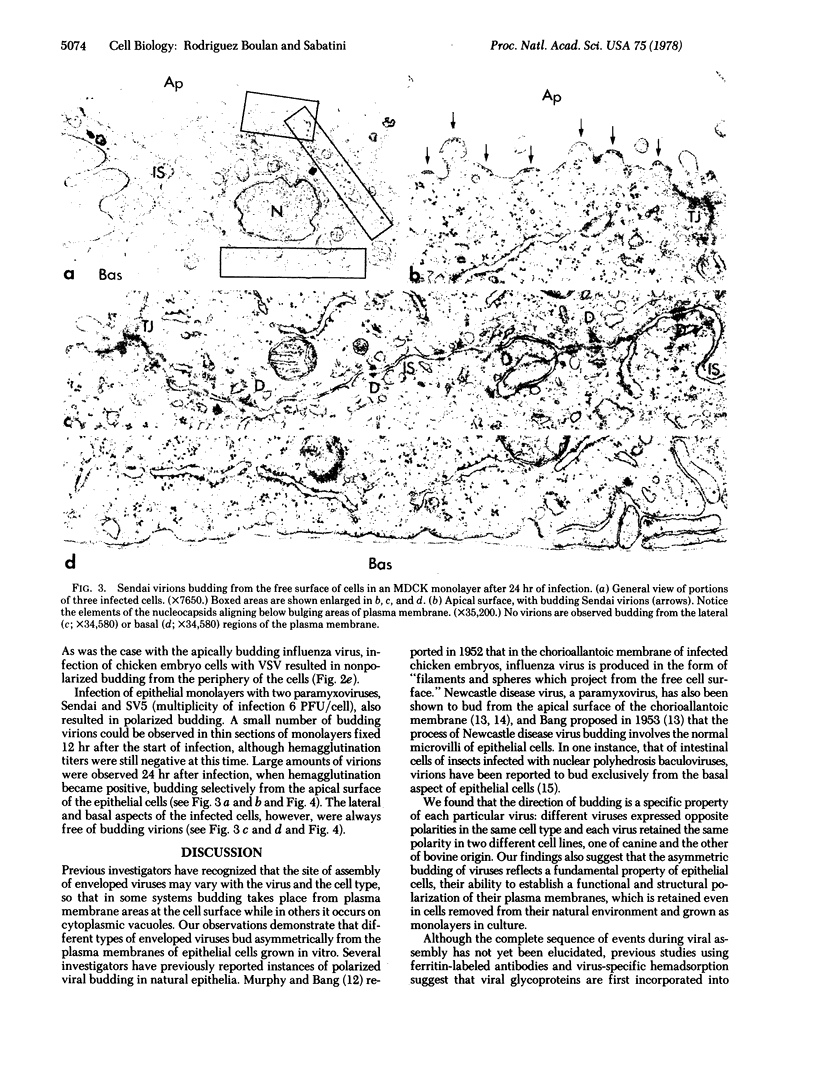
Infection of two different lines of polarized epithelial cells grown as monolayers with several types of enveloped viruses results, for each virus type, in a characteristic asymmetric budding of virions. Influenza virus (WSN strain), simian virus 5, and Sendai virus bud exclusively from the free (apical) surface of the cells, while vesicular stomatitis virus acquires its envelope only from the basolateral plasma membrane. Because different viruses select specific domains of plasma membrane in the same cell type, virus-infected epithelial monolayers can provide an excellent model system for studies of the mechanisms that generate regional differences in the distribution of plasma membrane components of epithelial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANG F. B. The development of Newcastle disease virus in cells of the chorio-allantoic membrane as studied by thin section. Bull Johns Hopkins Hosp. 1953 Apr;92(4):309–329. [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst S. A., Mills J. W. Basolateral plasma membrane localiztion of ouabain-sensitive sodium transport sites in the secretory epithelium of the avian salt gland. J Cell Biol. 1977 Oct;75(1):74–94. doi: 10.1083/jcb.75.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U., Dougherty R. M., Di Stefano H. S. Morphogenesis of Newcastle disease virus in chorioallantoic membrane. J Virol. 1969 Nov;4(5):753–762. doi: 10.1128/jvi.4.5.753-762.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycolipid content of vesicular stomatitis virus grown in baby hamster kidney cells. J Virol. 1971 Mar;7(3):416–417. doi: 10.1128/jvi.7.3.416-417.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Compans R. W., Choppin W. P. An electron microscopic study of the presence or absence of neuraminic acid in enveloped viruses. Virology. 1970 Dec;42(4):1158–1162. doi: 10.1016/0042-6822(70)90368-5. [DOI] [PubMed] [Google Scholar]
- Kyte J. Properties of the two polypeptides of sodium- and potassium-dependent adenosine triphosphatase. J Biol Chem. 1972 Dec 10;247(23):7642–7649. [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lojda Z. Cytochemistry of enterocytes and of other cells in the mucous membrane of the small intestine. Biomembranes. 1974;4A(0):43–122. [PubMed] [Google Scholar]
- MURPHY J. S., BANG F. B. Observations with the electron microscope on cells of the chick chorio-allantoic membrane infected with influenza virus. J Exp Med. 1952 Mar;95(3):259–268. doi: 10.1084/jem.95.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Carbohydrate composition of vesicular stomatitis virus. J Virol. 1971 Mar;7(3):412–415. doi: 10.1128/jvi.7.3.412-415.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Wolken K. Electronmicroscope investigations of the underside of cells in culture. Exp Cell Res. 1973 Mar 30;78(1):1–14. doi: 10.1016/0014-4827(73)90031-1. [DOI] [PubMed] [Google Scholar]