Abstract
Approximately 15–30% of breast cancers over-express the HER2/neu receptor. Historically, over-expression of HER2/neu has been identified using IHC or FISH, both of which are invasive approaches requiring tissue samples. Recent evidence has shown that some tumors identified as “negative” using these methods can respond to HER2/neu targeted therapy. Shedding of the extracellular domain (ECD) of the receptor into the circulation has led to the development of serum test of HER2 ECD as an additional approach to probe HER2/neu overexpression. The serum test will be able to monitor the dynamic changes of HER2 status over the course of disease progression. Some studies further suggest that the serum HER2 ECD level and its change may serve as a biomarker to reflect patients’ response to therapy. Yet more than 10 years after the first serum HER2 ECD test was approved by the FDA, serum HER2 testing has yet to be widely used in clinical practice. In this article we will review the progress of the serum HER2 ECD test and discuss some obstacles impeding its incorporation into broad clinical practice. We will also discuss recent improvements in the sensitivity and specificity of the assay that offer some hope for the future of serum HER2 test.
Keywords: HER2, extracellular domain (ECD), targeted therapy, serum biomarker, detection
1. Introduction
The HER2/neu gene (also known as c-erbB2) expresses a 185 kDa transmembrane glycoprotein (HER2/neu, also known as p185her2/neu) that is overexpressed in 15–30% of breast cancers [6] and other cancers [7, 8]. Her2/neu belongs to the ErbB family of receptor tyrosine kinases, which consists of four members: EGFR, HER2/neu, HER3, and HER4. Like the other members, the HER2/neu protein has three domains: a 105 kDa extracellular domain (ECD), a short transmembrane region, and an intracellular domain with tyrosine kinase activity.
The importance of HER2/neu to tumorigenesis has been clearly recognized since the discovery of neu, the rat homologue of HER2/neu, as the oncogene that led to neuroblastoma and transformation of NIH3T3 fibroblast cells [10, 11]. In humans, breast cancers associated with HER2/neu amplification or receptor overexpression have an accelerated growth and recurrance-rate, and are associated with decreased overall patient survival [15].
1.1 HER2/neu as targets for therapies
Since HER2/neu is often amplified/overexpressed in cancer cells, it has been regarded as a tumor-specific target in molecular therapies. The original work of Drebin et al. using monoclonal antibodies targeting the onco-protein on tumor cells, has laid out the foundation for antibody therapies to HER2/neu as well as other tumor associated-antigens. Trastuzumab (Herceptin), the humanized anti-HER2/neu monoclonal antibody, was approved by the FDA in 1998 to treat metastatic breast cancer. The use of Trastuzumab in the treatment of operable HER2/neu positive breast cancer in the adjuvant setting began after the landmark study in 2005 {Romond, 2005 #437}. Later in 2010, trastuzumab was approved for metastatic gastric or gastroesophageal (GE) junction adenocarcinoma. Another anti-HER2/neu antibody, Pertuzumab, has completed clinical trials in combination with trastuzumab plus docetaxel on metastatic breast cancer and is currently pending FDA-approval. Lapatinib, a tyrosine kinase inhibitor with specificity for both EGFR and HER2/neu was also approved in 2010 to treat hormone receptor positive metastatic breast cancer. In addition, chimeric antigen receptor (CAR)-modified T cells was also developed as a cancer immunotherapy to target HER2/neu positive tumors [17], despite early indication of over-reactivity in clinical trials.
1.2 Diagnostic and screening tests for HER2/neu targeted therapies
For HER2/neu- targeted therapies, such as the trastuzumab-based therapy, a screening test for HER2/neu is required before treatment can be administrated. Currently, HER2/Neu status is mainly evaluated using immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) (when IHC results are equivocal) in the clinical setting [21]. According to the most recent guidelines published by the American Society of Clinical Oncology and the College of American Pathologists, only patients with a uniform intense membrane staining of more than 30% of invasive tumor cells on IHC, and a HER2/neu-to-chromosome 17 centromere (CEP17) ratio of greater than 2.2 on FISH are considered HER2/neu-positive and are eligible for Trastuzumab treatment [22].
However, both IHC and FISH have been shown to have numerous limitations. One such limitation is the lack of ‘real-time” follow-up due to the dependency of both assays on tumor biopsies. When patients diagnosed with HER2/neu positive breast cancer undergoing treatment return for follow-up, the Her2-neu receptor status of the treated breast cancer is not routinely re-tested. However, at times, the clinician may need to learn whether the HER2/neu status of the tumor has changed and to monitor treatment response. Neither IHC nor FISH is a practical assay. Inconsistent reporting of IHC or FISH may pose another problem which partly stems from variation in procedures by different labs and partly due to inherent heterogeneity in the distribution of HER2-neu positive tumor cells within the tumor. As many as 12% to 20% of the HER2/neu assays performed in the field may yield erroneous results due to intra- and interobserver variability, and non-standardized IHC assays and scoring systems [22–26]. In many cases there is also a significant discordance between the IHC and the FISH results. Loss of protein antigens in fixed tissue slides and subjective observations during the IHC procedure (IHC−, FISH+), as well as protein overexpression without gene amplification (IHC+, FISH−) account for such discrepancies. This raises many concerns as to whether IHC and FISH suffice for HER2/neu testing.
Serum extracellular domain fragment of HER2 (HER2 ECD) represents a noninvasive and quantifiable biomarker that could supplement existing HER2/neu testing. This extracellular fragment of the receptor is released from the surface of tumor cells into the circulation by the ADAM proteases (A Disintegrin And Metalloproteinase domain-containing protein) [30, 31]. Studies have found elevated levels of HER2 ECD in the serum of many breast cancer patients, and in most cases, high levels of serum HER2 ECD are associated with higher relapse rates and worse prognosis [29, 32]. However, the clinical usefulness of serum HER2 ECD has not been validated as a result of conflicting data. This review will focus mainly on the value of serum HER2 ECD as a biomarker for both diagnosis and prognosis, and recent developments in the field that could break through limitations of current serum HER2 ECD tests.
2. Prevalence of Her2 ECD in breast cancers
Many studies have measured the concentrations of serum HER2 ECD in breast cancer patients. We have reviewed 67 published studies (1991–2011) with prevalence of increased serum HER2 ECD in breast cancer patients (Fig. 1; Table S1). Of the 67 published studies, at least 5 different assays and 10 different cut off values were used. Some cutoff values were arbitrarily chosen based on serum values collected from healthy controls, whereas others followed the manufacturers’ recommended cutoff value. Moreover, some of these studies used customized anti- HER2/neu antibodies for the assay.
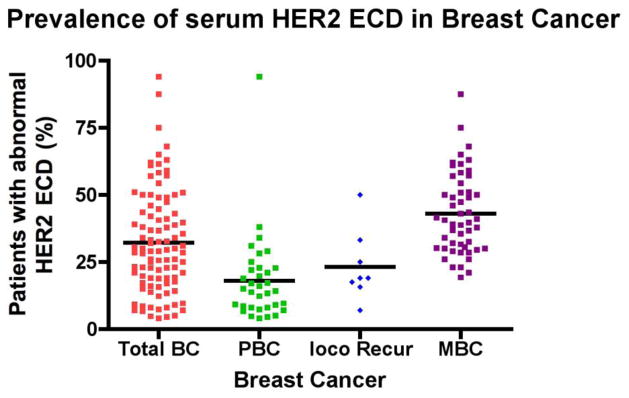
Figure 1. Prevalence of serum HER2 ECD in breast cancer.
This a summary of 70 studies published between 1991–2011 (see Table S1 for detail). In average, abnormal levels of HER2 ECD were found in 33% of all breast cancers (Total BC), 18% of primary breast cancer (PBC), 23% of breast cancers with locoregional recurrence (loco Recur), and 44% of metastatic breast cancer (MBC).
Although most assays used the Immuno One (Bayer) selected cutoff value at 15 ng/ml it will be impossible to generalize a cutoff value as a cross-reference for all studies due to the variations between assays of these studies. In one study, abnormal HER2 ECD levels (≥ 2000U/ml) were found in 94% breast cancer serum samples but the selectivity/specificity was only 60%, indicating an unusual large percentage of healthy controls with “abnormal” levels above this cutoff [34]. Therefore, the cutoff level should be carefully defined to obtain the best sensitivity and specificity. In fact, Kong et al suggested that different ethnicities have different cut off values and it may be beneficial to establish cut off values for each ethnic population [35].
Nevertheless, the body of these studies has demonstrated that a great percentage (32% in average) of breast cancer patients have elevated serum HER2 ECD. Abnormal HER2 ECD is more frequently detected in patients after the disease progresses. Metastatic breast cancer (MBC) patients appear to have a higher percentage of positive serum HER2 ECD than primary breast cancer (PBC) (43% v.s. 18%) (Figure 1) [32, 37, 38]. Serum HER2 ECD prevalence rate from our analysis is in general consistent with previous report in 2003 [42].
3. Serum HER2 ECD and tumor tissue HER2/neu status
Determining the HER2/neu status is an integral part of the diagnostic workup for breast cancer patients. The precise status of HER2 is clinically important because it directs the course of therapeutic treatments for breast cancer patients. One original goal of serum HER2 ECD is to determine if this type of assay can be used to match the established FISH/IHC tests for HER2 status.
Many studies have evaluated the correlation between abnormal HER2 ECD in serum and HER2/neu positivity in tissue. Some of these studies have indicated a significant relationship while others have found no or poor correlations. Table 1 summarizes these studies.
Table 1.
Summary of studies for the relationship between abnormal HER2 ECD in serum and tissue expression positivity.
| Reference | Patient population | Cutoff | Serum Assay | Correlation? |
|---|---|---|---|---|
| Cheung et al [3] | 20 MBC | 20 ng/ml | Bayer Immuno1 | Yes. |
| Anderson et al [9] | 168 PBC (*) | 1600 HNU/ml | Oncogene Science | Yes. |
| Garoufali et al [12] | 116 MBC | 12.7 ng/ml | Bayer Immuno1 | Yes. |
| Colomer et al [13] | 40 MBC | 450 fmol.ml | Calbiochem | Yes. |
| Sugano et al [14] | 158 PBC | 5.4 ng/ml | Nichirei | Yes. |
| Krainer et al [16] | 47 PBC | 20 U/ml | Triton Diagnositic | Yes. |
| Pallud et al [18] | 157 PBC | 15 ng/ml | Bayer Immuno1 | Yes for invasive |
| Ludovini et al [19] | 256 PBC | 15 ng/ml | Oncogene Science & ADVIA Centaur | Yes. |
| Farzadnia [20] | 74 PBC | 18.4 ng/ml | Bender MedSystem | Yes |
| Kong et al [27] | 195 MBC | 37 ng/ml | ADVIA Centaur System | Yes |
| Fornier et al [28] | 55 MBC | 15ng/ml | Bayer Immuno1 | Yes. |
| Muller et al [29] | 29 MBC | 15 ng/ml | Oncogene Science | Yes. |
| Molina et al [33] | 77 MBC, 84 locoregional | 15 U/ml | Ciba Corning | Yes |
| James et al [36] | 100 MBC | 15 ng/ml | Bender MedSystem | Yes |
| Narita et al [39] | 55 PBC | 20 U/ml | Triton Diagnositic | Yes for MBC, no for PBC. |
| Harris et al [40] | 355 MBC | 20 U/ml | Chiron Diagnostics | Yes |
| Witzel et al [41] | 167 PBC | 15 ng/ml | Siemens | Yes |
| Kandl et al [43] | 24 MBC | 10 U/ml | Triton Diagnositic | No. |
| Willsher et al [2] | 57 PBC & MBC | 20 ng/ml | Bender MedSystem | No. |
| Quaranta et al [1] | 108 PBC | 1368 HNU/ml | Oncogene Science | No. |
| Fontana et al [44] | 62 PBC | 8 U/ml | Triton Diagnositic | No. |
| Kong et al [35] | 86 PBC | 10.2 ug/L | ADVIA Centaur System | No. |
| Sorensen et al [46] | 826 MBC | 15 ng/ml | ADVIA Centaur System | No. |
In a prospective trial, Ludovini et al. found a concordance of 87.1% between elevated serum HER2 ECD levels (≥15 ng/ml) (23 patients, 9.0%) and HER2/neu-positive status in the tumor tissue (42 patients, 16.4%) of stage I–III breast cancer patients [19]. Correlation between serum and tissue HER2/neu status was also observed in some other studies [3, 9, 14, 16, 20, 41]. Muller et al. reported a correlation between abnormal HER2 ECD and the tissue HER2/neu status in primary tumors of metastatic breast cancer patients (p = 0.018)[29].
Garoufali et al reported significant difference in serum HER2 ECD between early and advanced breast cancer (p<0.001). However, a positive correlation of serum HER2 ECD with tissue HER2/neu status by IHC was only observed in advanced-disease patients (p=0.002) [12]. In patients with distant metastases, a close correlation between expression of HER2/neu protein in the primary tumor and the serum HER2 ECD level was also reported by Narita et al. [39].
Correlation between serum HER2 ECD and tissue expression also extends to other tumors. In uterine serous carcinoma, patients with positive tissue HER2/neu were found to have higher serum HER2 ECD than patients with negative tissue HER2/neu or healthy female subjects [45].
However, several studies did not find a correlation between serum HER2 ECD and tissue HER2/neu status [1, 2, 40], especially in primary breast cancer without distant metastasis [44]. Sorensen et al. studied serum HER2 ECD in 1348 patients with breast cancer and found, in 206 HER2/neu positive patients, only 35 had abnormal serum HER2 ECD (16.9%)[46]. Of the 437 HER2/neu tissue negative patients, 69 were serum HER2 ECD positive (15.7%). Overall, these data demonstrate a moderate discordance between the tissue status and serum HER2 ECD levels in this assay.
It is important to note that the two HER2/neu tissue assays, IHC and FISH, are designed to examine HER2/neu at different levels: protein overexpression and gene amplification, respectively. As reported by Harris et al., serum HER2 ECD correlated reliably with tissue HER2/neu status by IHC but poorly with that by FISH [40]. However, current clinical practice favors FISH over IHC, as it is well perceived that FISH is more strongly correlated with responsiveness to either trastuzumab or lapatinib treatment [25].
The discrepancy between serum HER2 ECD levels and tissue status was somehow unexpected to researchers in the field and has become an obstacle for the clinical utility of the baseline HER2 ECD. There are several explanations accounting for this discrepancy:
Imperfection in the definition of tissue positivity for HER2/neu
One major source of discordance is the classification system for HER2/neu positivity. As mentioned before, to be qualified as HER2/neu positive, tumors have to contain more than 30% of positively stained tumor cells in IHC or show a HER2/CEP17 ratio of greater than 2.2 in FISH. It is argued that a population of patients may actually be incorrectly classified as HER2/neu negative because they had less than the recommended percentage of HER2/neu positive cells in the primary tumor and, therefore, did not fall under the category of HER2/neu positive [46, 47]. These HER2/neu positive cells in the primary tumor may have growth advantage and be sufficient to produce HER2/neu positive metastatic lesions. This may help explain why some HER2/neu “negative” patients can respond to trastuzumab. Likewise, the HER2/neu positive cells in the HER2 “negative” tumor can produce small amount of HER2/neu protein in the serum and become detectable with increased tumor volume.
Heterogeneity of breast cancers
There is some evidence that the origin and size of tumors can have a great influence on serum HER2 ECD levels. Pallud et al. found elevated serum HER2 ECD in 21 of the 157 primary breast cancers with paired serum and tissue samples [18]. HER2/neu tissue positive patients as determined by the Dako (3+) or CB11 (2+ and 3+) IHC had serum positive rate of 48% and 60% respectively. Most interestingly, abnormal HER2 ECD was observed in tissue positive patients only when the tumor histological size (pT) exceeded 28–30 mm. In all tissue positive patients with pure intraductal tumors (Dako+3), serum HER2 ECD was normal (<15ng/ml).
Conversion of HER2/neu status and the kinetic nature of serum HER2 ECD levels
Conventionally, IHC or FISH determines the classification of patients with HER2/neu positive or negative on the primary tumor that is generally removed a long time before therapuetic interventions. However, cancer is a progressive disease. Several publications have confirmed that the HER2/neu status of the primary and metastatic tumor can be discordant. Zidan et al. reported a 14% discordance in HER2/neu status between primary and its corresponding metastases from 58 patients [48]. The HER2/neu status changed from positive in the primary tumor to negative in the metastatic tumor in one patient, but 7 patients developed HER2/neu positive metastases from HER2/neu negative primary breast cancer. Hoefnagel et al also reported change in HER2/neu status in 5.2% of patients, with about half of them from negative to positive and the other half from positive to negative [49].
It is proposed that a small percentage of cancer cells with HER2/neu overexpression can exist in HER2/neu “negative” tumors. These HER2 shedding cells have growth advantage and their percentage could increase during the course of disease progression, leading to a transition of HER2 negative to positive tumor. [50]. Due to the progression of the disease state and the kinetic nature of serum HER2 ECD levels, it is highly possible that a patient could have both abnormal and normal serum HER2 ECD levels depending on the time of serum collection.
Asgeirsson et al studied paired serum samples collected at the time of primary and metastatic diagnoses from 57 patients [5]. Abnormal serum HER2 ECD (>15 ng/ml) was observed in 16 out of 57 patients (28%) at primary breast cancer diagnosis and in 31 out of 57 (54%) at metastasis. Serum levels changed in 18 patients from normal at the primary to abnormal at metastasis. More interestingly, serum levels changed in the opposite direction in 5 patients. In another study, the percentage of abnormal levels of serum HER2 ECD increased from 9.6% to 30.7% during the disease course. Only 9% of patients without recurrent disease had abnormal levels of serum HER2 ECD [51].
Surgery and treatment can also influence HER2 ECD serum levels. Fehm et al. retrospectively studied the kinetics of serum HER2 ECD levels for 52 primary breast cancer patients who had developed metastatic disease during follow-up [52]. Elevated baseline serum HER2 ECD levels were observed in 31% (16/52) of these patients. After the removal of the primary tumor, elevated serum HER2 ECD levels were still observed in 13% and 19% of patients at 3 months and 6 months, respectively. Nine out of 16 patients with abnormal serum HER2 ECD became normal, while 7 remained abnormal. Meanwhile, 3 out of the 36 patients with normal serum HER2 ECD developed abnormal levels by 6 months after surgery. At 6 months and 3 months prior to clinical diagnosis of metastases, elevated serum HER2 ECD levels were found in 27% and 50% of the patients, respectively. At metastases, 62% patients had elevated HER2 ECD levels. Twenty-four out of 45 metastatic patients had serum HER2 ECD changes inconsistent with response to first line treatment (hormonal or chemotherapy).
Definition of cutoff levels
Sorensen et al reported no correlation between serum HER2 ECD levels and tissue expression when using a cutoff value of 15 ng/ml. It is noted that the analysis included samples from patients who might have already been treated for breast cancer thus, HER2 ECD in those sample might already be reduced. Nevertheless, when compared to the actual ECD levels in tissue negative patients vs that in tissue positive patients, a significant difference can be detected (p<0.000001) [46]. This raises the question if the cutoff value in this study was optimal.
In fact, Kong et al reported that a higher cutoff was required for serum HER2 ECD to correlate with tissue status [27]. In 195 patients with metastatic breast cancer, 76 (39%) were IHC 3+ and 19 were IHC 2+ but FISH positive. These patients have higher average serum HER2 ECD levels than HER2/neu tissue negative patients. ROC curve analysis was used to identify a cut-off level of 37 ng/ml with a specificity and sensitivity of 95% and 62%, respectively, in predicting tissue HER2/neu positivity.
In another study, Witzel et al found a correlation between serum HER2 ECD and tissue expression [41]. However, using 15 ng/ml as the cutoff only led to a sensitivity of 49%. ROC analysis suggested that better sensitivity (72%) could be obtained while good specificity (85%) retained when the cutoff was changed to 10 ng/ml.
Serum interference
A higher cutoff value means less impact of “noise” or false positive results in serum samples from some healthy controls and HER2/neu tissue negative patients. As we will discuss later in this review, serum interference might be a culprit for the unexpected elevated levels of serum HER2 ECD. Reported rates of elevated serum HER2 ECD among healthy controls vary significantly across studies, ranging from 4% to as high as 22.2% (Table 2). By reducing the serum interference, it is possible that HER2 ECD can better correlate with tissue HER2 status.
Table 2.
Abnormal serum HER2 ECD in healthy controls.
With the consideration of all these factors, it is unlikely that a strong consensus will be reached on the correlation between abnormal serum HER2 and tissue positivity, especially for early stage primary tumors. This lack of consensus stands as a hurdle for clinical adoption of serum HER2 ECD assays. However, increasing concerns have been raised surrounding the validity of tissue HER2/neu tests as the gold standard. It must be remembered that when using tissue HER2/neu status to select patients for treatment, only about 30% of patients respond to trastuzumab monotherapy [53].
The unexpected low response rate is not a clinical issue as the objective response rate is greatly improved when trastuzumab is combined with chemotherapies, which render FISH/IHC tests acceptable for the purpose of screening patients for targeted therapies. Nevertheless, recent discovery about benefit of trastuzumab based treatment for HER2/neu negative patients indicates that a better test is needed. With recent guidelines from the ASCO to increase the cutoff of tumor membrane staining from 10% to 30% [22], a larger subset of breast cancer patients may potentially be denied of early treatments by HER2 targeted therapies. Serum HER2 ECD has been studied to understand if it can have clinical utility as a helpful biomarker for therapies.
4. Clinical Utility of Serum HER2 ECD Measurement
Since the FDA approved the first HER2 ECD ELISA test in 2000, many studies have sought to define its clinical utility in reference to therapeutic response and other outcomes. These studies were first directed towards women with metastatic disease undergoing additional therapy, but have progressed to include women with more limited disease burden. This section will provide an overview of the various clinical applications proposed for HER2 ECD assay.
In general, elevated serum HER2 ECD in metastatic breast cancer patients was found to link to much higher risk of disease progression [54], decreased disease-free survival and overall survival [55], and decreased survival-after-relapse [56]. Breast cancer patients with elevated HER2 ECD serum levels had a limited response to hormone or chemotherapy [13, 38, 40, 54] and a reduced response rate to tamoxifen (an antiestrogen) or letrozole (an aromatase inhibitor) [57].
A number of studies have monitored the circulating levels of ECD in response to HER2/neu targeted therapies, such as trastuzumab and lapatinib. Although some studies utilize these treatments as monotherapy, most include other chemotherapeutic regimens as well. In general these studies are designed to answer two questions: 1.) Is the baseline HER2 ECD level predictive of response to treatment? and 2.) Is the change of HER2 ECD levels from the baseline a better predictive biomarker of treatment response or disease progression?
4.1. Serum HER2 ECD Levels as a prognostic biomarker
A review by Carney et al based on 20 studies involving 4430 breast cancer patients indicates a strong correlation between abnormal HER2 ECD levels and worse prognosis (e.g. shorter time to progression (TTP), decreased overall survival (OS), and decreased progression-free survival (PFS) [42]. Metastatic breast cancer patients with abnormal HER2 ECD had a significantly worse OS and PFS than patients with normal ECD following high-dose chemotherapy and autologous stem cell transplantation [40, 58]. Pre-surgical high HER2 ECD in both node-negative and node-positive patients predicted a high possibility of metastases (mainly liver metastases) and poorer clinical outcome [33]. In one study, reduce OS (median: 17.1 months) was reported in metastatic breast cancer patients with abnormal ECD than those with ECD in the normal range [59].
In several studies, serum HER2 ECD was a better prognostic biomarker than tissue HER2/neu for metastasis and recurrence [40, 60]. Sorensen et al found 25 out of 35 tissue-positive and serum abnormal patients had recurrence in the form of metastases [46], suggesting a combination of serum and tissue HER2/neu status could be much more effective for prognosis.
4.2. Baseline serum HER2 ECD levels to predict response to HER2/neu Targeted Therapy
Several studies have reported no significant relationship between baseline serum HER2 ECD levels and clinical outcome in response to HER2/neu-targeted therapies [61, 62]. However, the combination of serum HER2 ECD testing with IHC and FISH can potentially identify, with more accuracy, patients who will more likely to benefit from trastuzumab treatment, and may provide useful information that is lacking in IHC and FISH testing, such as early diagnosis of recurrence and real-time biomarker for longitudinal assessments. Recently in a neoadjuvant therapy trial with breast cancer patients, Witzel et al found significantly higher pathological complete response (pCR) rate associated with abnormal HER2 ECD in HER2 tissue positive patients who received chemotherapies plus trastuzumab [41].
For metastatic breast cancer patients receiving trastuzumab (with or without other chemotherapy agents), Ali et al. showed there was a significantly better overall survival (OS) (p = 0.017) and time to progression (TTP) (p = 0.007) for patients who had normal baseline serum HER2 ECD levels (<15 ng/ml) than for patients with abnormal serum HER2 ECD [63]. This finding of reduced trastuzumab benefit in patients with abnormal HER2 ECD was unexpected.
One possible explanation for the reduced trastuzumab benefit may be linked to pharmacokinetic interferences with trastuzumab by HER2 ECD. In the study by Ali et al, the highest HER2 ECD baseline levels reached 4000–6000 ng/ml. Although the pharmacokinetic trough level of the therapeutic antibody is expected to be more than 10 μg/ml, the presence of extremely high concentrations of HER2 ECD (> 500 ng/ml) has been shown to greatly reduce the serum half life of trastuzumab from 9.1 days to 1.8 days, resulting in a very low trough trastuzumab level (<0.2 μg/ml) [64]. It has also been suggested that the presence of a large amount of circulating HER2 ECD might neutralize the therapeutic effect of trastuzumab. In cytotoxicity experiments, trastuzumab-mediated ADCC activity was significantly decreased by the addition of Her2 ECD [45]. These studies suggest that the pharmacokinetic interactions between trastuzumab and circulating levels of serum HER2 ECD must be considered and carefully evaluated in order to achieve optimal therapeutic effect on breast cancer patients.
In a study of the utility of HER2 ECD to predict response to lapatinib, 79% of tissue positive metastatic breast cancer patients had elevated HER2 ECD levels [65]. Out of these 138 subjects treated with lapatinib monotherapy, 33 patients had overall response (either complete or partial response). Patients with response to lapatinib monotherapy had higher baseline HER2 ECD levels than patients without response (mean 159.2 vs 94.1 ng/ml, p =0.043)[65], but the baseline levels were not associated with progression free survival.
4.3. Change of serum HER2 ECD Levels for Monitoring HER2/neu Targeted Therapy
It turns out that the changes of HER2 ECD levels after HER2/neu-targeted therapy might be a better biomarker than the baseline itself. In one study, investigators found that a 9% drop in serum HER2 ECD between weeks 3 and 6 followed initial chemotherapy significantly predicted a pathological complete response (pCR) (p = 0.04) in 39 patients with local/locally advanced disease (stage II–III) who were treated with paclitaxel, flurouracil, epirubicin and cyclophosphamide [61]. The pCR rate was also higher in the group receiving trastuzumab.
In another study, patients with a 20% or greater decrease in concentrations of serum HER2 ECD after receiving trastuzumab (with or without adjuvant chemotherapy) were significantly more likely to have a higher response rate, longer disease free interval (320 vs. 180 days) and increased overall survival (1023 vs. 503 days) than did patients who had a decrease in serum concentrations of 20% of lower [63]. Most importantly, in a subset of patients with HER2/neu tissue positive tumor, patients who achieved a significant decline in serum HER2 ECD had a much better clinical response than did patients without such decline: overall response rate: 58.3% vs 25%; median time to progression: 334 days vs 173 days; overall survival: 1023 days vs 519 days [63].
Lipton and colleagues also found similar benefits of this ≥20% reduction in serum HER2 ECD from the baseline [65]. This study looked at 138 patients with metastatic disease who were taking lapatinib monotherapy as first line HER2/neu directed treatment (i.e. these patients were trastuzumab naïve). Patients were randomized to either lapatinib at the dose of 1500 mg daily or 500 mg BID. Serum was collected every 4 weeks for a total of 16 weeks. The investigators found that a 20% decrease from baseline conferred a significantly greater overall response rates as well as increased progression free survival. Additionally, they found that the overall response rate was significantly worse in patients who had a 20% increase of serum HER2 ECD from the baseline.
In contrast, one study found that a reduction of over 55% from baseline serum HER2 ECD levels is necessary to predict a significant therapeutic response in patients [28]. Using a ROC curve with therapeutic response as the outcome, the investigators determined that a 55% reduction was the minimal number to produce an odds ratio significantly different from 1.0. In addition, the authors also claimed that patients with baseline serum levels over 15 ng/ml who achieved a reduction of serum HER2 ECD levels to under 15 mg/ml at week 12 of treatment had a greater likelihood of therapeutic response (p = 0.005) [28].
Other studies also reported a decline in serum HER2 ECD in responding patients [37, 41, 66]. Decline of serum HER2 ECD levels could be observed as early as 8 days after treatment started, and disease progression could be predicted by the decline of serum HER2 ECD at day 15 of treatment [66].
There have been reports, however, of responsive patients who experienced no reduction in serum HER2 ECD. Ali et al observed 28% of patients who responded to trastuzumab-based therapy did not achieve a 20% decline in HER2 ECD [63]. This indicates that patients should not be taken off trastuzumab therapies only because no significant reduction of serum HER2 ECD levels is observed. Instead, these patients should be considered as unlikely to be very responsive and can have other treatment options if available.
4.4. Rising of serum HER2 ECD before clinical evidence of recurrence
While decline of serum HER2 ECD may indicate response to therapies, rising levels may also foretell disease progression. As shown in Fig 1, up trend in the percentage of HER2 ECD abnormal patients can be seen from primary breast cancer to recurrent disease and to metastatic breast cancer. In a study by Fehm et al, abnormal ECD was found in 27% patients at 6 months before metastasis, 50% at 3 months before metastasis, and 62% at the time of clinically diagnosed metastasis [52].
In a study of 250 primary breast cancers with no evidence of residual disease (NED) after radical treatment (radical mastectomy or simple mastectomy and radiotherapy), 95 patients developed metastases during follow-up (up to 4 years). Rising serum HER2 ECD to abnormal levels were recorded in 28.4% of these patients before diagnosis of metastasis, with a lead time of 4.2 +/− 2.4 months [67]. In patients with HER2/neu tissue positive primary tumors, rising HER2 ECD levels predicted 83.3% metastasis (10 out of 12 patients). Similarly, rising serum ECD levels was detected 3–24 months before recurrence was clinically confirmed in 10 HER2/neu tissue positive patients [46].
Schondorf et al monitored serum HER2 ECD changes in 23 patients with HER2/neu tissue positive tumor on trastuzuma therapy. All patients were treated by mastectomy and axillary lymph node dissection and all tissue histologically displayed the invasive ductal subtype [68]. Serum HER2 ECD were collected and measured periodically from these patients for circulating levels of HER2 ECD. To study the change of HER2 ECD levels, a total of 19 patients with HER2 ECD over 20 ng/ml were analyzed, and rising HER2 ECD levels were observed in 12 of these patients (63%). Within this group, 35 clinical remissions or progressions were documented, and changes of HER2 ECD levels correlated with 26 of these events (74%). Patients with very high HER2 ECD levels appeared to be associated with liver metastases.
Pectasides et al studied metastases after trastuzumab therapy [69] and found 37% of metastases were HER2/neu tissue negative, despite the primary tumors were positive. Five out of 16 patients had elevated serum HER2 ECD levels at baseline, and the levels returned into the normal range during treatment. However, 2 patients regained abnormal serum HER2 ECD levels and the metastasis tissue confirmed HER2/neu positive. Serum HER2 ECD levels in the other three patients remained in the normal range and the metastasis tissue were HER2/neu negative. This study indicates that serum ECD might serve as a way to predict the status of metastasis. With more targeted therapy becoming clinically available for both HRE2 positive and negative tumors, the patients may be able to choose other treatment options without waiting for test from metastatic tissues.
4.5. Issues in monitoring HER2 ECD change
However, not all studies consistently confirm the correlation of reduction of serum HER2 ECD levels with response to treatments [70]. Lennon et al retrospectively analyzed data from four different clinical trials involving trastuzumab treatment on both metastatic and locally advanced breast cancer [62]. Serum HER2 ECD levels was determined using the commercial ELISA from Oncogene Science (Cambridge, MA). Although post-treatment reduction of serum HER2 ECD levels was observed in some patients with significantly elevated levels at baseline, there was no indication that the baseline level or the change of serum HER2 ECD could be used to predict treatment outcome.
Of note, the authors did not count changes within the normal range of serum HER levels (under 15 ng/ml) as significant. It is suggested that a defined percentage of changes should be used to analyze whether the response to treatment is linked to only significant changes of the serum HER2 ECD levels [71]. In a study by Lennon et al., a clear relationship between reduction of HER2 ECD and response was only noticed when trastuzumab was used as monotherapy [62]. It is not clear if chemotherapeutic agents that induce death of tumor cells can also release certain levels of the tumor receptor into blood, thus complicating the analysis of the change of HER2 ECD levels. It is also recommended that a prospective, not retrospective, study should be conducted to clarify the controversies in the field [72].
It has also been reported that HER2/neu targeted therapies can interfere with the release of HER2 ECD from tumor cells. Trastuzumab was shown to prevent the cleavage of ECD as it binds around the juxtamembrane cleavage site [73]. On the other hand, lapatinib was reported to increase the release of ECD [74].
4.6. Elevated serum HER2 ECD in patients with HER2/neu tissue negative breast cancer may predict response to trastuzumab based treatment
It has been documented that a certain percentage of primary breast cancers with negative tissue HER2/neu status develop abnormal HER2 ECD levels in serum [9, 16, 43]. Initially this observation was just considered as discrepancy between different methods assessing HER2 levels. It is now realized that this population might be responsive to HER2/neu targeted therapies. Thus, a potential utility of HER2 ECD is the identification of patients who miss the opportunity of being treated with HER2/neu targeted therapies due to lack of overexpression HER2/neu in tumor tissues.
Ardavanis et al. focused on women with metastatic disease who had negative tissue statue but abnormal ECD levels in serum [75]. These patients had all failed at least two regimens of chemotherapy, but none of them had received trastuzumab previously as they were FISH negative. Five out of 22 women who later received trastuzumab-based chemotherapy had a reduction in tumor mass and 11 other had stable disease after trastuzumab treatment. In this study, patients with the highest serum HER2 ECD levels (~ 250 ng/ml) at baseline were found to have reduced levels after 3 courses of treatment, accompanying tumor reduction in 4 patients and stable disease in 3. Considering the advanced disease stages and the resistance to previous therapies, the 73% beneficial effect (16/22) in this study strongly suggests that the HER2/neu tissue negative but serum positive patients should be investigated at a larger scale for trastuzumab benefit in this population.
4.7. Serum HER2 ECD Levels for Monitoring other Therapies
Although the previously mentioned studies have focused on monitoring serum HER2 ECD levels after HER2/neu-therapy, this biomarker is also useful in other non-targeted treatment. Decline of serum HER2 ECD levels from abnormal to normal was found in 9 out of 16 patients underwent surgery to remove tumors [52]. Reduction of serum HER2 ECD levels was also noticed in the control arm of patients receiving no trastuzumab but only chemotherapy treatment [62]. Breast conserving therapy offered after neoadjuvant therapy was reported to link to decline of HER2 ECD levels in HER2/neu tissue positive patients but not in tissue negative patients [41].
Muller et al. studied 103 women with metastatic breast cancer who were randomized to either epirubicin and paclitaxel or epirubicin and cyclophosphamide [29]. Of these patients, only 46 follow-up serum samples were taken. An increased serum HER2 ECD baseline (above 15 ng/mL) was associated with decreased overall survival, but not with shorter progression free survival or response to treatment. However, patients in the epirubicin and paclitaxel arm with increased initial serum HER2 ECD level had increased progression free survival compared to the other arm (p = 0.0341). In patients showing a complete response, their average serum HER2 ECD levels dropped from 17.1 to 11.4 ng/mL (or a 33% decrease) after 3 courses of therapy. This was not seen in patients with no change in clinical course or in patients with progressive disease.
Serum HER2 ECD was also studied to predict response to metronomic chemotherapy in patients with advanced breast cancer [76]. Abnormal serum HER2 ECD at 2 months after treatment, as well as an increase of HER2 ECD levels from baseline, was significantly associated with reduced long-term clinical benefit (24 weeks) (P < .001).
For patients with HER2/neu tissue negative primary tumors, monitoring serum HER2 ECD levels may identify patients with HER2/neu status conversion so they can benefit from HER2/neu targeted therapies. In one such study, Zidan et al. identified 7 HER2 ECD positive metastases in patients with negative primary tumors, four of which were offered trastuzumab-based therapy after chemotherapy failure, and three of them responded to transtuzumab [48]. In another study with HER2/neu negative primary breast cancer patients who received tamoxifen or letrozole, 26% converted from serum HER2 ECD negative to positive at the time of disease progression and showed significantly shorter survival compared to patients who remained negative for serum HER2 ECD [77].
5. Developments for future serum HER2 ECD test
Serum HER2 ECD concentrations are mostly assessed by ELISA based assays. Although ELISA is a relatively straightforward assay that is conventional in many labs, there are reports on incompatibility issues for some antibodies if the serum samples are from patients who are treated with trastuzumab. Hayashi et al compared an EIA assay (Nichieri Biosciences) with CLIA assay (Bayer) and reported greatly reduced readout with the EIA assay in patients receiving trastuzumab treatment [70]. It was suspected that trastuzumab from treatment remained in the circulation and might compete with the two antibodies used in the EIA assay (6G10 and SV-2-61). In some other assays, such as the Siemens Immuno-1 automated assay, trastuzumab was tested not to interfere with the assay [78].
Even with optimized antibody pairs for ELISA, testing HER2 ECD from serum or plasma still faces some issues that are introduced by clinical samples. Emerging technologies over the past two decades have enhanced or completely revolutionized existing immunological tests. We have seen some efforts in the field to improve the sensitivity and specificity of the assay, which may help to develop the next generation of serum tests to help HER2/neu targeted therapies and other treatments.
5.1. Emerging Assays with higher sensitivity
While conventional methodologies such as ELISA and EIA are still widely used, they are limited by the concentration of the antigens and their sensitivity is generally in the ng/ml range (24, 101). Since the normal serum HER2 ECD level (< 15 ng/ml) is very close to the limit of ELISA assay, the conventional HER2 ECD ELISA assays are susceptible to assay interference that produces noise and false results. The development of chemiluminescence immunoassays (CLIA) has improved the sensitivity and its usefulness has been confirmed in serum HER2 ECD of breast cancer patients [37, 70]. Detection limit, however, is still an issue and highlights the need for ultrasensitive immunological tests. Loo et al developed a piezoelectric microcantilever sensors (PEMS) with 5 fold higher sensitivity than ELISA and was able to detect HER2 ECD in serum at concentrations below the low end of normal range (2 ng/ml)[79]. A new assay based on linear RNA amplification termed Fluorescent Amplification Catalyzed by T7 RNA polymerase Technology (FACTT) reported even higher sensitivities [80].
In FACTT, the antigen of interest is captured by the plate-associated antibody and detected by antibodies coupled to a double stranded DNA template that accommodates the attachment of the T7 RNA polymerase enzyme. The interaction of T7 leads to the production of RNA species that is monitored with a fluorescent RNA intercalating dye (RiboGreen, InVitrogen). We have applied FACTT to multiple protein targets, including HER2 ECD, TNFα, G-CSF, and the prion protein, and demonstrated at least 1000-fold higher sensitivity than ELISA [81]. In addition, FACTT is not subjected to background noise that is associated with substrate in ELISA assays. In a study of ten women with FISH+ tumors, FACTT detected elevated HER2 ECD in eight, while ELISA only detected elevated levels in two [80].
5.2. Approaches to enhance selectivity in serum test
One persistent problem that plagues serum biomarker tests by ELISA is the false results caused by the heterophilic human anti-animal immunoglobulin antibodies (HAIA), including human auto-antibodies that bind to animal antibodies by cross reactivity [82, 83]. An example of HAIA is the human anti-mouse antibodies (HAMA), which are generated in people who have come in contact with mouse immunoglobulins or present as human auto-antibodies but with cross reactivity.
HAIA are easily acquired through blood transfusions, vaccinations, treatment with mouse monoclonal antibodies, or from handling animals [16]. The prevalence of HAIA is varied in different reports, ranging from <1% to 99% of the population and from μg/mL to mg/mL concentrations in serum [16]. In one study, human anti-bovine antibodies were detected in 99% of the donor serum samples [84].
HAIA or HAMA is known to interfere with a number of immunological assays used for detecting cancer biomarkers, including serum HER2 ECD. It has also been reported to interfere with ELISA tests used for measuring IL-8, prostate specific antigen, and serum thyroglobulin levels in melanoma, prostate, and thyroid cancers, respectively [85–87]. In ELISA assays, HAMA can crosslink the capture and signal antibodies giving a false positive result, or it can bind to the capture antibody, sterically blocking binding of the signal antibody, resulting in a false negative reading [85, 88, 89].
Misleading results often result in inappropriate or unnecessary therapies, evident from the nationally publicized Rufer case. Rufer was diagnosed to have a form of cancer in her uterus by a human chorionic gonadotropin (hCG) blood test. The test stayed positive after she underwent chemotherapy and later hysterectomy. In fact, the test was repeated 44 times with the assay made by Abbott Laboratory, all showing abnormal levels of hCG. More chemotherapy was administered and a full-body CT-scan identified a suspicious nodule in her lung. As a result, a portion of her lung was removed to prevent further “metastasis”. Eventually, an hCG test manufactured by a different company was performed and the result was negative. It turned out that the previous results were all false positives.
To avoid HAIA-related problems in ELISA assays, many approaches have been reported to reduce interference, including removing immunoglobulin from serum samples [90], using mouse IgG-derived blocking reagents [91], and replacing antibody with non-IgG detection agents [92]. A unique approach was developed to resolve this issue using a universal blocking buffer to dilute serum samples [86]. In the ELISA assay, non-specific signals caused by HAIA mediated serum interference can be noticed when a positive reading is recorded with the use of non-matching pair of antibodies that recognize different unrelated proteins. A specially designed MBB buffer was shown to eliminate such an interaction [86].
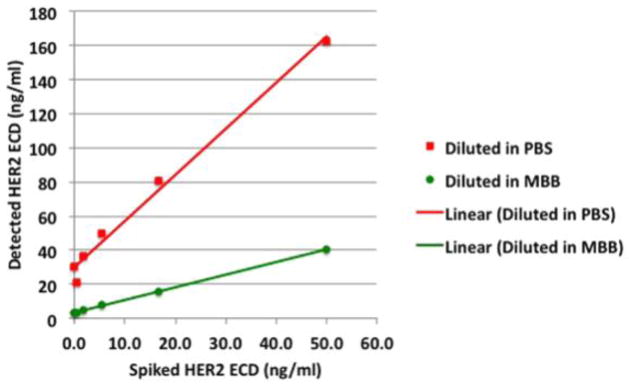
To visualize the potential for serum interference in a serum HER2 ECD test, we performed an ELISA with recombinant HER2 ECD serially diluted in PBS, a standard buffer used in ELISA assays,as the standard curve. In parallel, the same dilution was performed but each sample was spiked with a HAIA serum sample (at 1:20 dilution). As shown in Fig 2, the calculated concentrations of HER2 ECD in the spiked samples were statistically different from the expected concentrations (paired t test, p = 0.012). A background reading (20–30 ng/ml) appeared when there was no or very low concentration of recombinant HER2 ECD. On the other hand, a very high reading of 162.9 ng/ml was detected when the sample only contained 50 ng/ml recombinant protein. In contrast, when the ELISA was performed with the MBB buffer, a close correlation between the detected and expected concentration in samples containing HAIA was observed (p = 0.975).
Figure 2. Detection of recombinant HER2 ECD in samples containing HAIA serum.
Recombinant HER2 ECD (eBioscience, Inc.) at different concentrations but with consistent levels of HAIA serum (5%) was detected using anti HER2/neu monoclonal antibodies 6E2 and biotin-A21. The presence of MBB buffer corrected the influence of the HAIA serum and showed good correlation between detected and expected HER2 ECD levels.
Considering the lack of information on the serum interference issue in a considerable numbers of previous studies, it is highly possible that some HER2 ECD levels in the literature might be misleading. The issue of HAIA could be significant if results of these studies were based on a single pair of antibodies and only a very small number of patients were surveyed. However, as most of these studies involved a sufficient number of subjects, the impact of serum influence is minimized. Nevertheless, it is highly recommended that future HER2 ECD tests should include HAIA-preventing mechanism such as the MBB buffer.
Summary
Prevalence of elevated serum HER2 ECD in breast cancer patients has been documented in many studies. The abnormal serum HER2 ECD levels are more frequently observed in metastatic breast cancers. The concentration of HER2 ECD in serum is dynamic during disease progression and treatment. Baseline levels may not correlate with tissue status, especially in early stage breast cancer patients, while serum HER2 ECD in metastatic breast cancer is more closely correlated with tissue expression of HER2/neu proteins. Monitoring of HER2 ECD will not replace FISH/IHC assays but definitely will complement the tissue assay to offer a real time picture of HER2/neu status in patients. Decline of serum HER2 ECD levels after treatment (>20%) might indicate response to treatments. While other assays exist that may offer increased sensitivity over ELISA in measuring serum HER2 ECD, there are also methods (MBB buffer) that may further refine the specificity of ELISA testing.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (M.I.G. R01 CA055306, 5RO1CA089481-09A2), the Breast Cancer Research Foundation (M.I.G.), and the Abramson Family Cancer Research Institute (M.I.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quaranta M, Daniele A, Coviello M, Savonarola A, Abbate I, Venneri MT, Paradiso A, Stea B, Zito A, Labriola A, Schittulli F. c-erbB-2 protein level in tissue and sera of breast cancer patients: a possibly useful clinical correlation. Tumori. 2006;92:311–317. doi: 10.1177/030089160609200409. [DOI] [PubMed] [Google Scholar]
- 2.Willsher PC, Beaver J, Pinder S, Bell JA, Ellis IO, Blamey RW, Robertson JF. Prognostic significance of serum c-erbB-2 protein in breast cancer patients. Breast cancer research and treatment. 1996;40:251–255. doi: 10.1007/BF01806813. [DOI] [PubMed] [Google Scholar]
- 3.Cheung KL, Pinder SE, Paish C, Sadozye AH, Chan SY, Evans AJ, Blamey RW, Robertson JF. The role of blood tumor marker measurement (using a biochemical index score and c-erbB2) in directing chemotherapy in metastatic breast cancer. Int J Biol Markers. 2000;15:203–209. doi: 10.1177/172460080001500310. [DOI] [PubMed] [Google Scholar]
- 4.Breuer B, De Vivo I, Luo JC, Smith S, Pincus MR, Tatum AH, Daucher J, Minick CR, Miller DG, Nowak EJ, et al. erbB-2 and myc oncoproteins in sera and tumors of breast cancer patients. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1994;3:63–66. [PubMed] [Google Scholar]
- 5.Asgeirsson KS, Agrawal A, Allen C, Hitch A, Ellis IO, Chapman C, Cheung KL, Robertson JF. Serum epidermal growth factor receptor and HER2 expression in primary and metastatic breast cancer patients. Breast cancer research: BCR. 2007;9:R75. doi: 10.1186/bcr1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JA, Weiner DB, More KF, Kokai Y, Williams WV, Maguire HC, Jr, LiVolsi VA, Greene MI. Expression pattern of the neu (NGL) gene-encoded growth factor receptor protein (p185neu) in normal and transformed epithelial tissues of the digestive tract. Oncogene. 1989;4:81–88. [PubMed] [Google Scholar]
- 8.Williams TM, Weiner DB, Greene MI, Maguire HC., Jr Expression of c-erbB-2 in human pancreatic adenocarcinomas. Pathobiology. 1991;59:46–52. doi: 10.1159/000163614. [DOI] [PubMed] [Google Scholar]
- 9.Andersen TI, Paus E, Nesland JM, McKenzie SJ, Borresen AL. Detection of c-erbB-2 related protein in sera from breast cancer patients. Relationship to ERBB2 gene amplification and c-erbB-2 protein overexpression in tumour. Acta Oncol. 1995;34:499–504. doi: 10.3109/02841869509094014. [DOI] [PubMed] [Google Scholar]
- 10.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, Weinberg RA. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 11.Shih C, Shilo BZ, Goldfarb MP, Dannenberg A, Weinberg RA. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979;76:5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garoufali A, Kyriakou F, Kountourakis P, Yioti I, Malliou S, Nikaki A, Kardara E, Frangos I, Koumna S, Baziotis N, Scorilas A, Ardavanis A. Extracellular domain of HER2: a useful marker for the initial workup and follow-up of HER2-positive breast cancer. J Buon. 2008;13:409–413. [PubMed] [Google Scholar]
- 13.Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, Massuti B, Cortes-Funes H, Lloveras B. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6:2356–2362. [PubMed] [Google Scholar]
- 14.Sugano K, Ushiama M, Fukutomi T, Tsuda H, Kitoh T, Ohkura H. Combined measurement of the c-erbB-2 protein in breast carcinoma tissues and sera is useful as a sensitive tumor marker for monitoring tumor relapse. Int J Cancer. 2000;89:329–336. doi: 10.1002/1097-0215(20000720)89:4<329::aid-ijc3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 16.Krainer M, Brodowicz T, Zeillinger R, Wiltschke C, Scholten C, Seifert M, Kubista E, Zielinski CC. Tissue expression and serum levels of HER-2/neu in patients with breast cancer. Oncology. 1997;54:475–481. doi: 10.1159/000227606. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, Grossman RG, Heslop HE, Gottschalk S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallud C, Guinebretiere JM, Guepratte S, Hacene K, Neumann R, Carney W, Pichon MF. Tissue expression and serum levels of the oncoprotein HER-2/neu in 157 primary breast tumours. Anticancer research. 2005;25:1433–1440. [PubMed] [Google Scholar]
- 19.Ludovini V, Gori S, Colozza M, Pistola L, Rulli E, Floriani I, Pacifico E, Tofanetti FR, Sidoni A, Basurto C, Rulli A, Crino L. Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008;19:883–890. doi: 10.1093/annonc/mdm585. [DOI] [PubMed] [Google Scholar]
- 20.Farzadnia M, Meibodi NT, Shandiz FH, Mahmoudi M, Bahar MM, Memar B, Amoian S, Maroozi F, Moheghi N. Evaluation of HER2/neu oncoprotein in serum and tissue samples of women with breast cancer: correlation with clinicopathological parameters. Breast. 2010;19:489–492. doi: 10.1016/j.breast.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Allison M. The HER2 testing conundrum. Nature biotechnology. 2010;28:117–119. doi: 10.1038/nbt0210-117. [DOI] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 23.Paik S, Bryant J, Tan-Chiu E, Romond E, Hiller W, Park K, Brown A, Yothers G, Anderson S, Smith R, Wickerham DL, Wolmark N. Real-world performance of HER2 testing--National Surgical Adjuvant Breast and Bowel Project experience. Journal of the National Cancer Institute. 2002;94:852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 24.Sui W, Ou M, Chen J, Wan Y, Peng H, Qi M, Huang H, Dai Y. Comparison of immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) assessment for Her-2 status in breast cancer. World J Surg Oncol. 2009;7:83. doi: 10.1186/1477-7819-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 26.Perez EA, Suman VJ, Davidson NE, Martino S, Kaufman PA, Lingle WL, Flynn PJ, Ingle JN, Visscher D, Jenkins RB. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 27.Kong SY, Nam BH, Lee KS, Kwon Y, Lee ES, Seong MW, Lee do H, Ro J. Predicting tissue HER2 status using serum HER2 levels in patients with metastatic breast cancer. Clinical chemistry. 2006;52:1510–1515. doi: 10.1373/clinchem.2006.067512. [DOI] [PubMed] [Google Scholar]
- 28.Fornier MN, Seidman AD, Schwartz MK, Ghani F, Thiel R, Norton L, Hudis C. Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2005;16:234–239. doi: 10.1093/annonc/mdi059. [DOI] [PubMed] [Google Scholar]
- 29.Muller V, Witzel I, Luck HJ, Kohler G, von Minckwitz G, Mobus V, Sattler D, Wilczak W, Loning T, Janicke F, Pantel K, Thomssen C. Prognostic and predictive impact of the HER-2/neu extracellular domain (ECD) in the serum of patients treated with chemotherapy for metastatic breast cancer. Breast cancer research and treatment. 2004;86:9–18. doi: 10.1023/B:BREA.0000032919.83803.48. [DOI] [PubMed] [Google Scholar]
- 30.Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer research. 1999;59:1196–1201. [PubMed] [Google Scholar]
- 31.Liu PC, Liu X, Li Y, Covington M, Wynn R, Huber R, Hillman M, Yang G, Ellis D, Marando C, Katiyar K, Bradley J, Abremski K, Stow M, Rupar M, Zhuo J, Li YL, Lin Q, Burns D, Xu M, Zhang C, Qian DQ, He C, Sharief V, Weng L, Agrios C, Shi E, Metcalf B, Newton R, Friedman S, Yao W, Scherle P, Hollis G, Burn TC. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer biology & therapy. 2006;5:657–664. doi: 10.4161/cbt.5.6.2708. [DOI] [PubMed] [Google Scholar]
- 32.Hayes DF, Yamauchi H, Broadwater G, Cirrincione CT, Rodrigue SP, Berry DA, Younger J, Panasci LL, Millard F, Duggan DB, Norton L, Henderson IC. Circulating HER-2/erbB-2/c-neu (HER-2) extracellular domain as a prognostic factor in patients with metastatic breast cancer: Cancer and Leukemia Group B Study 8662. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7:2703–2711. [PubMed] [Google Scholar]
- 33.Molina R, Jo J, Filella X, Zanon G, Pahisa J, Munoz M, Farrus B, Latre ML, Gimenez N, Hage M, Estape J, Ballesta AM. C-erbB-2 oncoprotein in the sera and tissue of patients with breast cancer. Utility in prognosis. Anticancer research. 1996;16:2295–2300. [PubMed] [Google Scholar]
- 34.Streckfus C, Bigler L, Dellinger T, Dai X, Kingman A, Thigpen JT. The presence of soluble c-erbB-2 in saliva and serum among women with breast carcinoma: a preliminary study. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6:2363–2370. [PubMed] [Google Scholar]
- 35.Kong SY, Kang JH, Kwon Y, Kang HS, Chung KW, Kang SH, Lee DH, Ro J, Lee ES. Serum HER-2 concentration in patients with primary breast cancer. J Clin Pathol. 2006;59:373–376. doi: 10.1136/jcp.2005.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James R, Thriveni K, Ramaswamy G, Krishnamoorthy L, Mukherjee G, Deshmane PV, Bapsy P. EVALUATION OF IMMUNOHISTOCHEMISTRY AND ENZYME LINKED IMMUNOSORBENT ASSAY FOR HER-2/NEU EXPRESSION IN BREAST CARCINOMA. Indian Journal of Clinical Biochemistry. 2008;23:345–351. doi: 10.1007/s12291-008-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroda N, Kontani K, Kajikawa T, Taminato T. Study of the measurement of serum extracellular domain of HER-2/neu protein with CLIA method. Rinsho Byori. 2010;58:541–552. [PubMed] [Google Scholar]
- 38.Classen S, Kopp R, Possinger K, Weidenhagen R, Eiermann W, Wilmanns W. Clinical relevance of soluble c-erbB-2 for patients with metastatic breast cancer predicting the response to second-line hormone or chemotherapy. Tumour Biol. 2002;23:70–75. doi: 10.1159/000059706. [DOI] [PubMed] [Google Scholar]
- 39.Narita T, Funahashi H, Satoh Y, Takagi H. C-erbB-2 protein in the sera of breast cancer patients. Breast cancer research and treatment. 1992;24:97–102. doi: 10.1007/BF01961242. [DOI] [PubMed] [Google Scholar]
- 40.Harris LN, Liotcheva V, Broadwater G, Ramirez MJ, Maimonis P, Anderson S, Everett T, Harpole D, Moore MB, Berry DA, Rizzeri D, Vredenburgh JJ, Bentley RC. Comparison of methods of measuring HER-2 in metastatic breast cancer patients treated with high-dose chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:1698–1706. doi: 10.1200/JCO.2001.19.6.1698. [DOI] [PubMed] [Google Scholar]
- 41.Witzel I, Loibl S, von Minckwitz G, Mundhenke C, Huober J, Hanusch C, Henschen S, Hauschild M, Lantzsch T, Tesch H, Latos K, Just M, Hilfrich J, Barinoff J, Eulenburg CZ, Roller M, Untch M, Muller V. Monitoring serum HER2 levels during neoadjuvant trastuzumab treatment within the GeparQuattro trial. Breast cancer research and treatment. 2010;123:437–445. doi: 10.1007/s10549-010-1030-9. [DOI] [PubMed] [Google Scholar]
- 42.Carney WP, Neumann R, Lipton A, Leitzel K, Ali S, Price CP. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clinical chemistry. 2003;49:1579–1598. doi: 10.1373/49.10.1579. [DOI] [PubMed] [Google Scholar]
- 43.Kandl H, Seymour L, Bezwoda WR. Soluble c-erbB-2 fragment in serum correlates with disease stage and predicts for shortened survival in patients with early-stage and advanced breast cancer. British journal of cancer. 1994;70:739–742. doi: 10.1038/bjc.1994.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontana X, Ferrari P, Namer M, Peysson R, Salanon C, Bussiere F. C-erb-B2 gene amplification and serum level of c-erb-B2 oncoprotein at primary breast cancer diagnosis. Anticancer research. 1994;14:2099–2104. [PubMed] [Google Scholar]
- 45.Todeschini P, Cocco E, Bellone S, Varughese J, Lin K, Carrara L, Guzzo F, Buza N, Hui P, Silasi DA, Ratner E, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. Her2/neu extracellular domain shedding in uterine serous carcinoma: implications for immunotherapy with trastuzumab. British journal of cancer. 2011;105:1176–1182. doi: 10.1038/bjc.2011.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen PD, Jakobsen EH, Langkjer ST, Bokmand S, Ostergaard B, Olsen DA, Madsen JS, Brandslund I. Serum HER-2 concentrations for monitoring women with breast cancer in a routine oncology setting. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2009;47:1117–1123. doi: 10.1515/CCLM.2009.241. [DOI] [PubMed] [Google Scholar]
- 47.Carney WP. Hidden HER-2/neu-positive breast cancer: how to maximize detection. IDrugs. 2009;12:238–242. [PubMed] [Google Scholar]
- 48.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. British journal of cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, van der Valk P, van der Groep P, de Vries EG, van der Wall E, van Diest PJ. Receptor conversion in distant breast cancer metastases. Breast cancer research: BCR. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fehm T, Maimonis P, Weitz S, Teramoto Y, Katalinic A, Jager W. Influence of circulating c-erbB-2 serum protein on response to adjuvant chemotherapy in node-positive breast cancer patients. Breast cancer research and treatment. 1997;43:87–95. doi: 10.1023/a:1005700812422. [DOI] [PubMed] [Google Scholar]
- 51.Isola JJ, Holli K, Oksa H, Teramoto Y, Kallioniemi OP. Elevated erbB-2 oncoprotein levels in preoperative and follow-up serum samples define an aggressive disease course in patients with breast cancer. Cancer. 1994;73:652–658. doi: 10.1002/1097-0142(19940201)73:3<652::aid-cncr2820730324>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 52.Fehm T, Gebauer G, Jager W. Clinical utility of serial serum c-erbB-2 determinations in the follow-up of breast cancer patients. Breast Cancer Res Treat. 2002;75:97–106. doi: 10.1023/a:1019601022456. [DOI] [PubMed] [Google Scholar]
- 53.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 54.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:4423–4434. [PubMed] [Google Scholar]
- 55.Imoto S, Wada N, Hasebe T, Ochiai A, Kitoh T. Serum c-erbB-2 protein is a useful marker for monitoring tumor recurrence of the breast. International journal of cancer. Journal international du cancer. 2007;120:357–361. doi: 10.1002/ijc.22166. [DOI] [PubMed] [Google Scholar]
- 56.Fehm T, Jager W, Kramer S, Sohn C, Solomayer E, Wallwiener D, Gebauer G. Prognostic significance of serum HER2 and CA 15-3 at the time of diagnosis of metastatic breast cancer. Anticancer research. 2004;24:1987–1992. [PubMed] [Google Scholar]
- 57.Lipton A, Ali SM, Leitzel K, Demers L, Harvey HA, Chaudri-Ross HA, Brady C, Wyld P, Carney W. Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21:1967–1972. doi: 10.1200/JCO.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 58.Bewick M, Conlon M, Gerard S, Lee H, Parissenti AM, Zhang L, Gluck S, Lafrenie RM. HER-2 expression is a prognostic factor in patients with metastatic breast cancer treated with a combination of high-dose cyclophosphamide, mitoxantrone, paclitaxel and autologous blood stem cell support. Bone Marrow Transplant. 2001;27:847–853. doi: 10.1038/sj.bmt.1703005. [DOI] [PubMed] [Google Scholar]
- 59.Ali SM, Leitzel K, Chinchilli VM, Engle L, Demers L, Harvey HA, Carney W, Allard JW, Lipton A. Relationship of serum HER-2/neu and serum CA 15-3 in patients with metastatic breast cancer. Clinical chemistry. 2002;48:1314–1320. [PubMed] [Google Scholar]
- 60.Mansour OA, Zekri AR, Harvey J, Teramoto Y, el-Ahmady O. Tissue and serum c-erbB-2 and tissue EGFR in breast carcinoma: three years follow-up. Anticancer research. 1997;17:3101–3106. [PubMed] [Google Scholar]
- 61.Mazouni C, Hall A, Broglio K, Fritsche H, Andre F, Esteva FJ, Hortobagyi GN, Buzdar AU, Pusztai L, Cristofanilli M. Kinetics of serum HER-2/neu changes in patients with HER-2-positive primary breast cancer after initiation of primary chemotherapy. Cancer. 2007;109:496–501. doi: 10.1002/cncr.22418. [DOI] [PubMed] [Google Scholar]
- 62.Lennon S, Barton C, Banken L, Gianni L, Marty M, Baselga J, Leyland-Jones B. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:1685–1693. doi: 10.1200/JCO.2008.16.8351. [DOI] [PubMed] [Google Scholar]
- 63.Ali SM, Carney WP, Esteva FJ, Fornier M, Harris L, Kostler WJ, Lotz JP, Luftner D, Pichon MF, Lipton A. Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer. 2008;113:1294–1301. doi: 10.1002/cncr.23689. [DOI] [PubMed] [Google Scholar]
- 64.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 65.Lipton A, Leitzel K, Ali SM, Carney W, Platek G, Steplewski K, Westlund R, Gagnon R, Martin AM, Maltzman J. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer. 2011;117:5013–5020. doi: 10.1002/cncr.26101. [DOI] [PubMed] [Google Scholar]
- 66.Kostler WJ, Schwab B, Singer CF, Neumann R, Rucklinger E, Brodowicz T, Tomek S, Niedermayr M, Hejna M, Steger GG, Krainer M, Wiltschke C, Zielinski CC. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:1618–1624. doi: 10.1158/1078-0432.ccr-0385-3. [DOI] [PubMed] [Google Scholar]
- 67.Molina R, Jo J, Filella X, Zanon G, Farrus B, Munoz M, Latre ML, Pahisa J, Velasco M, Fernandez P, Estape J, Ballesta AM. C-erbB-2, CEA and CA 15.3 serum levels in the early diagnosis of recurrence of breast cancer patients. Anticancer research. 1999;19:2551–2555. [PubMed] [Google Scholar]
- 68.Schondorf T, Hoopmann M, Warm M, Neumann R, Thomas A, Gohring UJ, Eisberg C, Mallmann P. Serologic concentrations of HER-2/neu in breast cancer patients with visceral metastases receiving trastuzumab therapy predict the clinical course. Clinical chemistry. 2002;48:1360–1362. [PubMed] [Google Scholar]
- 69.Pectasides D, Gaglia A, Arapantoni-Dadioti P, Bobota A, Valavanis C, Kostopoulou V, Mylonakis N, Karabelis A, Pectasides M, Economopoulos T. HER-2/neu status of primary breast cancer and corresponding metastatic sites in patients with advanced breast cancer treated with trastuzumab-based therapy. Anticancer research. 2006;26:647–653. [PubMed] [Google Scholar]
- 70.Hayashi N, Nakamura S, Tokuda Y, Yagata H, Yoshida A, Ota H, Hortobagyi GN, Cristofanilli M, Ueno NT. Serum HER2 levels determined by two methods in patients with metastatic breast cancer. International journal of clinical oncology/Japan Society of Clinical Oncology. 2011 doi: 10.1007/s10147-011-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali SM, Leitzel K, Lipton A, Carney WP, Kostler WJ. Value of serum human epidermal growth factor receptor 2 (HER2)/neu testing for early prediction of response to HER2/neu-directed therapies is still an open one and deserves further study in large prospective trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:e273. doi: 10.1200/JCO.2009.23.4674. author reply e274–275. [DOI] [PubMed] [Google Scholar]
- 72.Tse C, Lamy PJ. Clinical utility of serum human epidermal growth factor receptor 2 extracellular domain levels: stop the shilly-shally--it is time for a well-designed, large-scale prospective study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:e286–287. doi: 10.1200/JCO.2009.24.5100. [DOI] [PubMed] [Google Scholar]
- 73.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer research. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 74.Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Del Barco S, Martin-Castillo B, Menendez JA. Lapatinib, a dual HER1/HER2 tyrosine kinase inhibitor, augments basal cleavage of HER2 extracellular domain (ECD) to inhibit HER2-driven cancer cell growth. J Cell Physiol. 2011;226:52–57. doi: 10.1002/jcp.22333. [DOI] [PubMed] [Google Scholar]
- 75.Ardavanis A, Kountourakis P, Kyriakou F, Malliou S, Mantzaris I, Garoufali A, Yiotis I, Scorilas A, Baziotis N, Rigatos G. Trastuzumab plus paclitaxel or docetaxel in HER-2-negative/HER-2 ECD-positive anthracycline- and taxane-refractory advanced breast cancer. Oncologist. 2008;13:361–369. doi: 10.1634/theoncologist.2007-0207. [DOI] [PubMed] [Google Scholar]
- 76.Sandri MT, Johansson HA, Zorzino L, Salvatici M, Passerini R, Maisonneuve P, Rocca A, Peruzzotti G, Colleoni M. Serum EGFR and serum HER-2/neu are useful predictive and prognostic markers in metastatic breast cancer patients treated with metronomic chemotherapy. Cancer. 2007;110:509–517. doi: 10.1002/cncr.22825. [DOI] [PubMed] [Google Scholar]
- 77.Lipton A, Leitzel K, Ali SM, Demers L, Harvey HA, Chaudri-Ross HA, Evans D, Lang R, Hackl W, Hamer P, Carney W. Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer. 2005;104:257–263. doi: 10.1002/cncr.21202. [DOI] [PubMed] [Google Scholar]
- 78.Payne RC, Allard JW, Anderson-Mauser L, Humphreys JD, Tenney DY, Morris DL. Automated assay for HER-2/neu in serum. Clin Chem. 2000;46:175–182. [PubMed] [Google Scholar]
- 79.Loo L, Capobianco JA, Wu W, Gao X, Shih WY, Shih WH, Pourrezaei K, Robinson MK, Adams GP. Highly sensitive detection of HER2 extracellular domain in the serum of breast cancer patients by piezoelectric microcantilevers. Anal Chem. 2011;83:3392–3397. doi: 10.1021/ac103301r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Cheng X, Richter M, Greene MI. A sensitive and high-throughput assay to detect low-abundance proteins in serum. Nat Med. 2006;12:473–477. doi: 10.1038/nm1378. [DOI] [PubMed] [Google Scholar]
- 81.Chang B, Cheng X, Yin S, Pan T, Zhang H, Wong P, Kang SC, Xiao F, Yan H, Li C, Wolfe LL, Miller MW, Wisniewski T, Greene MI, Sy MS. Test for detection of disease-associated prion aggregate in the blood of infected but asymptomatic animals. Clin Vaccine Immunol. 2007;14:36–43. doi: 10.1128/CVI.00341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clinical chemistry. 1999;45:942–956. [PubMed] [Google Scholar]
- 83.Leach MF, Aubuchon JP. False reactivity in GTI Pak Plus ELISA kits due to the presence of anti-mouse antibody in patients’ samples. Immunohematology. 2003;19:112–116. [PubMed] [Google Scholar]
- 84.Andersen DC, Koch C, Jensen CH, Skjodt K, Brandt J, Teisner B. High prevalence of human anti-bovine IgG antibodies as the major cause of false positive reactions in two-site immunoassays based on monoclonal antibodies. J Immunoassay Immunochem. 2004;25:17–30. doi: 10.1081/ias-120027223. [DOI] [PubMed] [Google Scholar]
- 85.Preissner CM, O’Kane DJ, Singh RJ, Morris JC, Grebe SK. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab. 2003;88:3069–3074. doi: 10.1210/jc.2003-030122. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H, Fu T, McGettigan S, Kumar S, Liu S, Speicher D, Schuchter L, Xu X. IL8 and Cathepsin B as Melanoma Serum Biomarkers. International journal of molecular sciences. 2011;12:1505–1518. doi: 10.3390/ijms12031505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan BR, Tarter TH. Serum heterophile antibodies interfere with prostate specific antigen test and result in over treatment in a patient with prostate cancer. J Urol. 2001;166:2311–2312. [PubMed] [Google Scholar]
- 88.Levinson SS, Miller JJ. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin Chim Acta. 2002;325:1–15. doi: 10.1016/s0009-8981(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 89.Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000;124:921–923. doi: 10.5858/2000-124-0921-HAMA. [DOI] [PubMed] [Google Scholar]
- 90.Ismail AA. A radical approach is needed to eliminate interference from endogenous antibodies in immunoassays. Clinical chemistry. 2005;51:25–26. doi: 10.1373/clinchem.2004.042523. [DOI] [PubMed] [Google Scholar]
- 91.Reinsberg J. Different efficacy of various blocking reagents to eliminate interferences by human antimouse antibodies with a two-site immunoassay. Clinical Biochemistry. 1996;29:145–148. doi: 10.1016/0009-9120(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 92.Andersson M, Ronnmark J, Arestrom I, Nygren PA, Ahlborg N. Inclusion of a non-immunoglobulin binding protein in two-site ELISA for quantification of human serum proteins without interference by heterophilic serum antibodies. Journal of immunological methods. 2003;283:225–234. doi: 10.1016/j.jim.2003.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.