Abstract
Effective public health responses to an influenza pandemic require an effective vaccine that can be manufactured and administered to large populations in the shortest possible time. In this study, we evaluated a method for vaccination against avian influenza virus that uses a DNA vaccine for rapid manufacturing and delivered by a microneedle skin patch for simplified administration and increased immunogenicity. We prepared patches containing 700 µm-long microneedles coated with an avian H5 influenza hemagglutinin DNA vaccine from A/Viet Nam/1203/04 influenza virus. The coating DNA dose increased with DNA concentration in the coating solution and the number of dip coating cycles. Coated DNA was released into the skin tissue by dissolution within minutes. Vaccination of mice using microneedles induced higher levels of antibody responses and hemagglutination inhibition titers, and improved protection against lethal infection with avian influenza as compared to conventional intramuscular delivery of the same dose of the DNA vaccine. Additional analysis showed that the microneedle coating solution containing carboxymethylcellulose and a surfactant may have negatively affected the immunogenicity of the DNA vaccine. Overall, this study shows that DNA vaccine delivery by microneedles can be a promising approach for improved vaccination to mitigate an influenza pandemic.
Keywords: avian influenza virus, microneedle, DNA vaccine, coating, DNA stability
1. Introduction
Global concern over the possibility of an influenza pandemic has been heightened by the spread of highly pathogenic avian influenza virus of the H5N1 subtype from birds to humans and the 2009 H1N1 pandemic [1, 2]. The most effective way to prevent influenza infection is immunization, which is currently carried out with trivalent inactivated or live-attenuated virus vaccines to reduce morbidity and mortality due to the annual epidemics of seasonal influenza [3]. Because vaccines are most effective only if the match to the circulating virus is optimized, periodic updates of the antigen are needed to minimize the impact of antigenic drift due to mutation in the hemagglutinin (HA) gene. The emergence of a novel pandemic influenza strain will require a strain-specific vaccine. The recent 2009 influenza pandemic demonstrated that manufacturing and administering of influenza vaccine to the public by current methods requires up to 6–7 months [4] after identification and isolation of a novel virus. Expedited methods of pandemic vaccine manufacturing and administration are therefore needed.
In part to address this concern, DNA vaccines have been developed as inexpensive, rapidly produced, stable and safe vaccines [5]. Manufacturing of recombinant DNA vaccines has the potential to be relatively easy and rapid in mass production, since it is produced by bacterial culture techniques [6]. In this approach, an antigen-encoding plasmid DNA is administered to induce cellular and humoral immune responses [7]. Efficacy of DNA vaccines against influenza viruses have been studied in animal models [8, 9] and in humans [10]. Additionally, experimental DNA vaccines encoding hemagglutinin (HA) [11–14] or nucleoprotein (NP) [12] of avian influenza viruses have been designed and evaluated [11]. However, it has been shown that DNA vaccines are often poorly immunogenic [15–17]. To improve delivery and immunogenicity of DNA vaccines, various methods have been used such as jet injection [18], electroporation with injection [19, 20], gene-gun [21], transcutaneous DNA vaccination [22] and microneedles [23].
Immunogenicity might be further enhanced by vaccination in the skin, as shown previously for a number of vaccines [24, 25]. More specifically, intradermal injection of conventional seasonal influenza vaccine has been shown to increase immunogenicity in the elderly and is licensed for clinical use in many countries [26]. This increased immunogenicity is believed to be caused by targeted delivery of the vaccine to the skin’s potent antigen-presenting cells, such as Langerhans cells and dermal dendritic cells, although the role of Langerhans cells as antigen-presenting cells is still in debate [24, 25]. Direct lymphatic drainage of antigen to lymph nodes may also play a role [27].
However, vaccination in the skin can be difficult to achieve. Conventional intradermal injection using a hypodermic needle requires specialized training and can be unreliable [28]. The new intradermal injection device recently introduced for influenza vaccination improves reliability, but still requires administration by medical personnel [29], which provides a bottleneck to rapid vaccination of large populations. The gene gun has received attention specifically for DNA vaccination in the skin, but has been limited by its high cost and Th2-biased immune response [21, 30].
In this study, we propose the use of a microneedle patch to administer influenza DNA vaccine as an approach that is rapid to manufacture and administer with increased immunogenicity. In this way, a patch of solid metal microneedles measuring hundreds of microns in length were coated with DNA using inexpensive manufacturing processes [31]. The resulting microneedle patch can be simply and painlessly applied to the skin without the need for medical expertise [4], and the DNA coating dissolves off the microneedles in the skin within minutes [32]. Previous studies have demonstrated the efficacy of microneedles for influenza vaccination using whole-inactivated virus, virus-like particle and protein subunit vaccines [33–38], as well as vaccination against other diseases [24]. DNA vaccination with microneedles has been studied previously in the context of a few other vaccines [23, 26, 39], but not influenza.
This study tested the hypothesis that an avian H5 influenza DNA vaccine administered to the skin using microneedles provides stronger protective immunity compared to intramuscular vaccination using the same vaccine at the same dose in a mouse model, because DNA vaccine can be delivered into antigen-presenting cells in epidermis and dermis by microneedle vaccination. We assessed this hypothesis by first developing a method to coat microneedles with the DNA vaccine and rapidly deliver it to the skin. Immunogenicity was then assessed by measuring antibody responses after vaccination, as well as protective responses after lethal challenge with influenza virus.
2. Materials and Methods
2.1. Fabrication of metal microneedles
Arrays of solid metal microneedles were cut into stainless steel sheets (SS304, 75 µm thick, McMaster-Carr, Atlanta, GA) with an infrared laser (Resonetics Maestro, Nashua, NH). The microneedles used in this study measured 700 µm in length and 160 µm in width at the base, and were aligned in a row of five needles per device
2.2. Preparation of H5 influenza DNA vaccine plasmid
The DNA encoding the HA (subtype H5) from influenza A/Viet Nam/1203/04 (H5N1) virus (designated VN/04) engineered to lack multi-basic amino acids at the HA cleavage site was chemically synthesized (Piscataway, Genscript, NJ). The synthetic HA gene DNA was cloned into the expression plasmid vector pCAGGS under the control of the chicken -actin promoter (Fig. 1A). Vaccine plasmid was prepared from E.coli DH5 strain and purified using a Giga Quagen kit (Valencia, CA) according to the manufacturer’s instructions. The expression of the HA protein was confirmed by transfection and Western blotting (Fig. 1B). Briefly, for transient expression of HA protein, 2×106 CV-1 cells at 70% confluence in a 60-mm dish were transfected with a 100 µg of plasmid DNA and harvested 30 h and 70 h later. Equal amounts (10 µg) of total protein from HA protein expressing cell were loaded for SDS-PAGE using 12% polyacrylamide gels and then transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). After being blocked overnight at 4°C in blocking buffer (2% skim-milk, 0.1% Tween 20 in PBS), the membranes were incubated with a 1:2000 dilution of rabbit anti-HA polyclonal antibody (ProSciinc., Poway, CA) for 1 h followed by washes. Then the membranes were incubated with Horseradish peroxidase (HRP) conjugated goat anti-rabbit immunoglobulin G at a 1:5,000 dilution for 30 min. Following washes, the signals were detected by using an Amersham ECL Plus reagent (GE Healthcare, Piscataway, NJ). Purified recombinant H5HA protein was obtained from the NIH Biodefense and Emerging Infections Research Resources Repository (NIAID, NIH).
Figure 1.
H5 influenza HA DNA vaccine. (A) Schematic diagram of H5 HA in the pCAGGS protein expression vector. The synthesized HA gene from influenza A/Vietnam/1203/04 (H5N1) virus was cloned into the pCAGGS vector between chicken beta actin promoter and rabbit beta globin polyA site. Multi-basic amino acids (RRRK) in the HA1/HA2 cleavage site were deleted and codon usage was optimized for the sf9 insect cell. (B) Western blotting analysis of H5 HA expression. HA expression was confirmed by Western blotting of culture supernatants from CV1 cells transfected with pCAGGS/H5 HA vaccine plasmid at 30 h (Lane 2) or 60 h (Lane 3) after transient transfection. Culture supernatant from non-transfected cells (Lane 1) and recombinant HA proteins (Lane 4) were used as negative and positive control, respectively.
2.3. Labeling DNA vaccine and coating on microneedles
To label the DNA vaccine, a Label IT Tracker Cy3 kit was used (Mirus Bio, Madison, WI). We first mixed 37.5 µl sterile water (DNase and RNase free), 5 µl 10× labeling buffer A, 5 µl DNA vaccine (1 mg/ml), and Label IT Tracker reagent, and then incubated at 37 °C for 1 h. Unreacted reagents were removed by ethanol precipitation. The labeled DNA pellet was obtained by centrifugation for 10 min at 28,000 × g and washed with 500 µl of 70% ethanol. Finally, the labeled DNA vaccine was re-suspended in sterile water.
The microneedle coating solution was composed of 1% (w/v) carboxymethylcellulose (CMC) sodium salt (USP grade, Carbo-Mer, San Diego, CA), 0.5% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, NJ), and DNA vaccine (1 – 5 mg/ml) in deionized water. An individual row of microneedles was dip-coated by horizontally dipping the microneedles into the coating solution held in a dip-coating device, as previously described [40]. After vaccine coating, microneedles were air dried at room temperature overnight.
The amount of DNA vaccine coated onto the microneedles was determined by incubating microneedles in deionized water for 12 h at 4 °C and then measuring the amount of DNA dissolved off by spectroscopy (NanoDrop, Thermo Scientific, Wilmington, DE).
2.4. Immunization and ELISA assay for IgG
Female, 6-to-8-weeks-old BALB/c mice (Charles River, Wilmington, MA) were anesthetized by intramuscular injection of 110 mg/kg ketamine (Abbott Laboratories, Chicago, IL) mixed with 11 mg/kg xylaxine (Phoenix Scientific, St. Joseph, MO). The skin on the back of the mouse was exposed by removing hair with depilatory cream (Nair, Princeton, NJ), washed with 70% ethanol, and dried with a hair dryer. A prime and two boost vaccinations were performed using (i) DNA-coated microneedles, (ii) intramuscular injection of the DNA vaccine or (iii) intramuscular injection of phosphate-buffered saline (n=9 mice per group) at weeks 0, 5, and 10. For microneedle-based vaccination, a five-needle array of microneedles coated with 3 µg of DNA vaccine was manually inserted into the skin and left for 20 min to dissolve the coated DNA vaccine into the skin. As comparative controls, groups of mice were intramuscularly immunized with DNA vaccine (3 µg) previously dissolved from coated microneedles (designated IM). Thus, the IM group was immunized with the same amount of DNA vaccine that was subjected to the same coating procedures as the microneedle group, which allowed a head-to-head comparison between the different routes of delivery. There was also a negative control “mock” vaccination group that was inserted with microneedles coated with coating solution but no vaccine. All animal studies were approved by the Emory University and Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Committees (IACUC).
Influenza virus-specific total antibody (IgG) and subtypes (IgG1, IgG2a) were determined in sera by enzyme-linked immunosorbent assay (ELISA), as described previously [41]. Briefly, 96-well microtiter plates (Nunc-Immuno Plate MaxiSorp: Nunc Life Technologies, Basel, Switzerland) were coated with 100 µl of inactivated reassortant H5N1 virus (contained modified HA and NA from VN/04 virus and the remaining genes from A/PR/8/34 virus) [34] at a concentration of 4 µg/ml in coating buffer (0.1 M sodium carbonate, pH 9.5) at 4 °C overnight. The plates were then incubated with horseradish peroxidase-labeled goat anti-mouse IgG, IgG1, and IgG2a (Southern Biotechnology, Birmingham, AL) at 37 °C for 1.5 h and then the substrate O-phenylenediamine (Zymed, San Fransisco, CA) in citrate-phosphate buffer (pH 5.0) containing 0.03%(v/v) H2O2 (Sigma) was used to develop color. The optical density at 450 nm was read using an ELISA reader (model 680: Bio-Rad, Hercules, CA). Antibody levels were determined from a standard curve of purified mouse IgG antibody and presented in ng/ml concentrations.
2.5. Hemagglutination inhibition (HAI) titer
To determine hemagglutination-inhibition (HAI) titers, serum samples were first treated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) by incubation overnight at 37 °C, and then incubated 30 min at 56 °C. Sera were serially diluted, mixed with 4 HA units (HAU) of inactivated ΔH5N1 virus, and incubated for 30 min at room temperature prior to adding 0.5% horse red blood cells. The highest serum dilution preventing hemagglutination was scored as the HAI titer.
2.6. Challenge inoculation and virus loads in tissues
The highly pathogenic wild type avian influenza A/Viet Nam/1203/04 (VN/04) virus, subtype H5N1, was used for challenge studies. Virus was propagated in 10-days-old embryonated hen’s eggs and virus-containing allantoic fluid was used in the experiments.
For virus challenge studies, mice from each of the three study groups (n=9 per group) were transferred to the animal facilities at the CDC. Virus and infected animals were handled in biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and the Select Agents program (http://www.cdc.gov/od/ohs/biosfty/bmbl5/bmbl5toc.htm).
At 21 weeks post immunization, animals were anesthetized by isoflurane inhalation and inoculated intranasally with 50 µl of sterile PBS containing 200 plaque forming units (pfu) of wild-type VN/04 virus, which is approximately 20 times the 50% mouse lethal dose (20× LD50). Mice were observed and weighed daily for 14 days, starting immediately before challenge, to monitor health status (n=6 out of 9 challenged mice). Animals with more than 25% body weight loss were euthanized to minimize suffering. To determine virus titers in different organs, lung, brain and spleen were harvested at 4 days post challenge (n=3 out of 9 challenged mice) and homogenized in 1 ml of PBS for virus titration using a plaque assay in MDCK cells, as previously described [42].
2.7. Cell preparation and GFP DNA transfection for DNA stability test
To assess possible damage to DNA by microneedle coating, we determined DNA transfection efficiency in vitro after dissolving plasmid DNA from the coated stainless steel plate as the same material used for microneedle preparations. We measured the transfection of DNA encoded to express Green Fluorescent Protein (GFP-DNA) in DU145 cells. DNA plasmid coding for GFP (pEGFP-N1, 4.7 kb) was obtained from Clontech (Palo Alto, CA).
Transfection efficiency was measured using human prostate cancer cells (DU145, American Type Culture Collection, Manassas, VA) grown on T-150 flasks (BD Falcon, Franklin Lakes, NJ) as a monolayer in RPMI-1640 medium supplemented with 10% (v/v) penicillin-streptomycin (Cellgro, Mediatech, Herndon, VA) and 10% (v/v) heat inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, GA) in a humidified condition of 5% CO2 at 37°C. To maintain the culture, the cells were harvested, centrifuged, and resuspended in RPMI-1640 at a concentration of 2.5×106 cells/ml using the protocol described previously [43].
To coat DNA vaccine on the stainless steel surface, 1 µL of GFP-DNA (5 mg/ml) was mixed with 1 µL of standard coating solution or deionized water in situ on a piece of stainless steel (3 mm × 3 mm), and air dried at room temperature overnight. Then, the DNA was dissolved off the metal piece in 50 µL of Opti-MEMI Reduced Serum Medium (Invitrogen, Carlsbad, CA) without serum for 12 h at 4 °C. To assess the effect of coating solution on DNA, 1 µL of standard coating solution was mixed with 1 µL of GFP DNA (5 mg/ml) in a safe-lock micro test tube (Eppendorf AG, Hamburg, Germany) and incubated for 12 h at 4 °C, after which is was added to 100 µl Opti-MEM® I Reduced Serum Medium. 4 µL of Lipofectamine 2000 (Invitrogen) was diluted in 100 µl Opti-MEM® I Reduced Serum Medium and incubated for 5 min at room temperature. The resulting 100 µl GFP DNA was combined with 100 µl of diluted Lipofectamine 2000 and incubated for 20 min at room temperature. This was repeated, such that each 200 µl mixture was added to a well in a 12-well plate containing 1 ml of DU140 cells in RPMI medium and mixed well. Finally, cells were incubated at 37°C in a CO2 incubator for 24 h before subsequent analysis of DNA transfection efficiency (see below).
2.8. Dynamic light scattering
DNA particle size was determined by dissolving DNA coatings from metal chips or using aqueous mixtures of CMC and DNA, and analyzed by dynamic light scattering (DynaPro, Wyatt, Santa Barbara, CA).
2.9. Imaging for microneedle coating and cell transfection
Fluorescence micrographs of coated microneedles were collected using an Olympus IX70 fluorescent microscope (Olympus, Tokyo, Japan) with a CCD camera (RT Slider, Diagnostic Instruments, Sterling Heights, MI). Bright-field micrographs were collected using an Olympus SZX12 stereo microscope with a CCD camera (Leica DC 300, Leica Microsystems, Bannockburn, IL).
To visualize GFP DNA transfection in cells, imaging was carried out at room temperature using a Zeiss LSM 510 multiphoton microscope (Zeiss, Thornwood, NY) with an oil-immersion lens of 40× magnification. A cell sample of 5 µl was placed on a 25 mm glass microscope cover slip (Fisher Scientific, Waltham, MA).
2.10. Statistical Analysis
Every assay was measured using at least three samples, from which the arithmetic mean and standard error of the mean were calculated. A two-tailed Student’s t-test (α=0.05) was performed when comparing two different conditions. When comparing three or more conditions, a one-way analysis of variance (ANOVA; α=0.05) was performed. In all cases, a value p<0.05 was considered statistically significant.
3. Results
3.1. Coating and delivery of fluorescently stained influenza H5 DNA vaccine
A coating solution formulation containing CMC and surfactant was previously developed to coat metal microneedles [44] and used to coat inactivated viral influenza vaccines onto microneedles [31]. We used the same coating approach to coat fluorescently labeled H5 HA DNA onto microneedles and imaged the coated microneedles by bright-field and fluorescence microscopy, which showed a uniform distribution of DNA localized onto the microneedles (Fig. 2A). To assess delivery of coated DNA from microneedles into skin, microneedles were imaged by fluorescence microscopy after different times of insertion into mouse skin in vitro up to 3 minutes. As shown in Fig. 2B, coated DNA vaccine was efficiently released from the microneedles after insertion into mouse skin within minutes.
Figure 2.
Microneedles coated with Cy3-stained HA DNA vaccine. (A) Representative bright-field (i) and fluorescence (ii) micrographs of microneedles coated with HA DNA vaccine (scale bar= 1 mm). (B) Representative bright-field (i) and fluorescence (ii–vi) micrographs of a microneedle coated with HA DNA vaccine (i, ii) before insertion and (iii) 30 s (iv) 60 s (v) 120 s and (vi)180 s after insertion into mouse skin in vitro (scale bar = 200 µm).
To control the dose of DNA vaccine coated on microneedles, we determined the effects of the number of microneedle dip-coating cycles and the DNA concentration in the coating solution on the amount of H5 DNA vaccine coated onto microneedles. As shown in Fig. 3A, when the dip-coating cycles were increased from 3 to 9 dips using 5 mg/ml DNA solution, the amount of coated DNA linearly increased from 0.9 to 3.6 µg per array of 5 microneedles (ANOVA, p<0.05). In addition, increasing DNA concentration in the coating solution from 1 to 5 mg/ml with 10 dips increased the amount of coated DNA from 0.6 to 3.9 µg (Fig. 3B, ANOVA, p<0.05). Coated DNA increased linearly from 1 to 4 mg/ml, but there was a disproportionally large increase from 4 to 5 mg/ml. When the DNA concentration was close to 5 mg/ml, the coating solution viscosity was significantly increased (data not shown), which may explain the disproportionate increase in coated dose at 5 mg/ml. In this way, the coated dose can be controlled by adjusting the number of dip-coating cycles and the DNA concentration in the coating solution.
Figure 3.
HA DNA vaccine coated on microneedles as a function of coating parameters. Amount of DNA vaccine coated on an array of five stainless-steel microneedles according to (A) the number of dip-coating cycles (5 mg/ml DNA solution) and (B) the initial concentration of DNA in the coating solution (with 10 dipings). Data represent the average of n = 9 replicates. Error bars represent the standard error.
3.2. IgG antibody responses and HAI titers
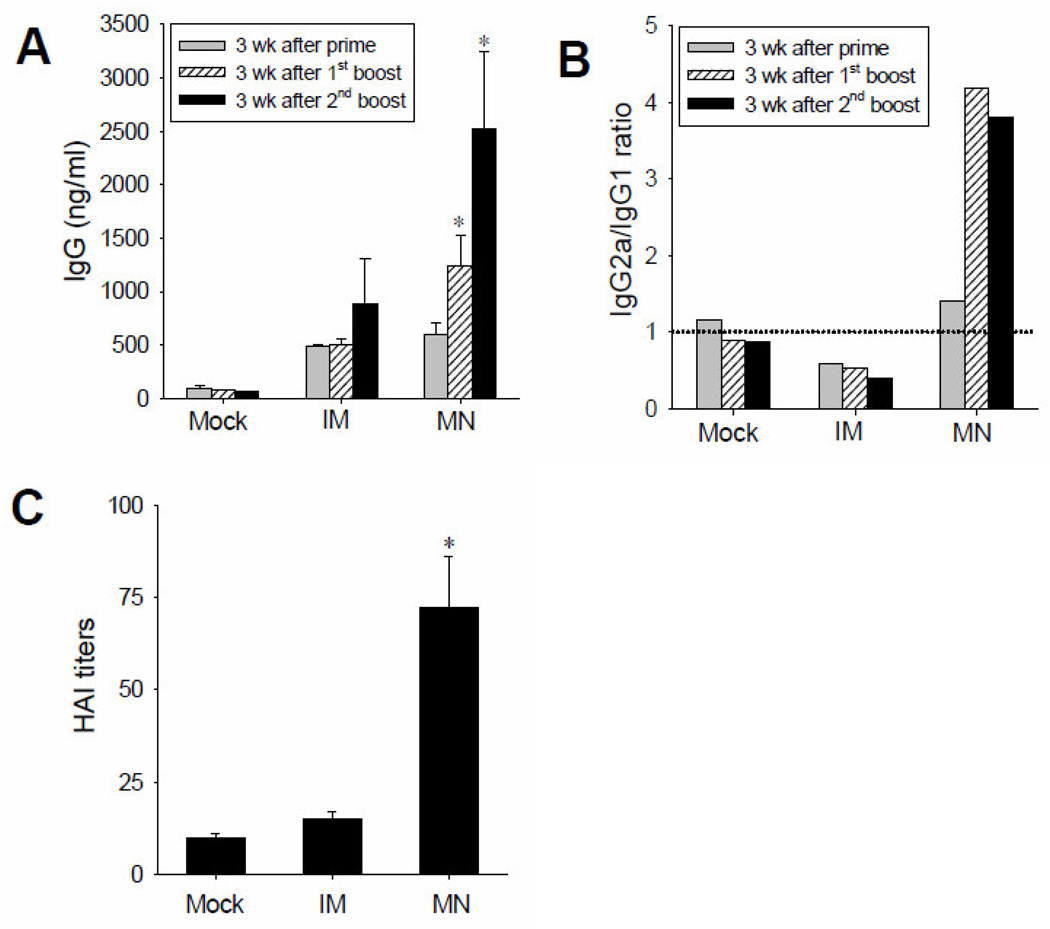
BALB/c mice (n=9) were vaccinated with H5 DNA vaccine at a dose of 3 µg per animal using a microneedle array or by intramuscular injection (IM). A “mock” group of animals (n=9) was also included, in which microneedles coated only with coating solution (i.e., no vaccine) were used. Three vaccination doses were given to each mouse with an interval of 5 weeks. Serum samples were collected 3 weeks after each dose. As shown in Fig 4A, levels of IgG antibody responses specific to H5N1 virus hemagglutinin after prime immunization using microneedles and IM injection were similar to each other, and higher than those in the mock group. After 1st and 2nd boosting, levels of IgG in the group immunized using microneedles were significantly higher than those in the IM immunization and mock-immunization groups. Specifically, IgG levels in the microneedle group were 2.5 and 2.8 times higher than the IM group after 1st and 2nd boosting, respectively.
Figure 4.
Antibody responses after vaccination with HA DNA. (A) Total virus-specific antibody responses and (B) antibody isotype ratio (IgG2a/IgG1) and (C) hemagglutination inhibition (HAI) titers in mouse sera after vaccination with microneedles or IM injection at 3 weeks after a three-dose vaccination regimen. Mock = negative control immunization using microneedles coated only with coating solution only; IM = intramuscular immunization with HA DNA coated on microneedles, dissolved off and injected; MN = microneedle immunization. *p<0.05 compared to Mock and IM.
In subtype IgG assay, there was a clear difference in isotype profiles between microneedle and IM immunization (Fig 4B, Supplementary Figure). Microneedle delivery showed IgG2a dominant (IgG2a/IgG1 ratio is almost 4 after 1st and 2nd boosting immunization). In contrast, IgG1 was the dominant antibody isotype after IM immunization (IgG2a/IgG1 ratio is ≤0.5).
Sera were also tested for functional antibodies by measuring hemagglutination inhibition (HAI) activity, which provides a better serological correlate for protection and is typically accepted by regulatory agencies to establish vaccine efficacy; an HAI titer greater than 40 is generally associated with protection in humans [45]. HAI titers in the microneedle group after third immunization (we could not observe significant HAI value after first and second immunization) were significantly increased to over 70 and significantly higher than the mock and IM groups by 7.2 and 4.8 times, respectively (Student’s t-test, p<0.05) (Fig. 4C). Therefore, these data show that immunization with H5 DNA vaccine using microneedles produced significantly stronger HA-specific antibody responses and HAI activities compared to those by IM immunization of the same vaccine.
3.3. Protection against lethal challenge and tissue virus titers
To determine protective efficacy of microneedle DNA vaccination, immunized and mock-immunized mice (no vaccine) were challenged by intranasal inoculation with 20× LD50 (200 pfu) of VN/04 (H5N1) virus at 21 weeks after the 2nd boost. The challenged mice were monitored daily to record changes in body weight and survival for 14 days (Fig. 5A, B). Mice that were intramuscularly immunized or mock immunized showed continued body weight loss to over 25% and died or were euthanized by days 7 to 8 post challenge (Fig. 5B). In contrast, mice immunized in the skin using microneedles had a 60% survival rate after lethal challenge infection and animals that did die lived longer than in the IM and mock groups (Fig. 5A). The microneedle group exhibited less body weight loss compared to IM and mock control groups, although these mice were sick as shown by a significant loss up to 18% in body weight. Overall, microneedle DNA immunization induced improved protection compared to IM injection.
Figure 5.
Protection of mice after immunization with HA DNA vaccine. (A) Survival rate, (B) body weight change in infected mice. Mice were challenged by intranasal inoculation with H5N1 influenza virus (20 LD50) 21 days after a three-dose vaccination regimen. Survival rate and body weight change were monitored in n=6 mice.
Highly pathogenic avian influenza viruses are known to spread systemically in infected hosts [32, 46]. To determine the efficacy of controlling virus replication, viral titers were determined in the lung, spleen, and brain, which were collected at day 4 post challenge (3 out of 9 challenged mice). In the mock group, high viral titers were detected in all organs tested, including lung (over 107.5 pfu per ml), brain (104 pfu per ml), and spleen (105 pfu per ml) after infection with 200 PFU of VN/04 virus. In contrast, mice that received microneedle DNA vaccination showed about 300-fold lower lung viral titers compared to the mock control group. Also, virus was not detected in the brain tissue and very low levels of virus were found in the spleen from the microneedle vaccination group (data not shown). These results indicate that microneedle vaccination decrease viral replication in lower respiratory tract and spleen of infected mice and prevent virus spread to central neurons system.
3.4. DNA stability assay
Although microneedle vaccination exhibited improved immune responses, there was still incomplete protection even after three vaccine doses. We therefore wanted to determine if the DNA vaccine may have been damaged during the process of microneedle coating. To facilitate straightforward assay, we prepared coatings of GFP-encoding DNA as a model DNA molecule and then assayed transfection efficiency of the DNA after coating, reconstitution in media and incubation with DU145 cells and Lipofectamine 2000 (Fig. 6A and 6B).
Figure 6.
Effect of coating solution formulation on DNA stability. Lipofectamine-mediated transfection of DU-145 cells was determined using GFP-encoding DNA after formulation and coating under different conditions. (A) Representative multiphoton microscope images of cell transfection (green cells have been transfected). (i) no DNA added (negative control). (ii) DNA in DI water added (positive control), (iii) DNA dried in coating solution (CS), (iv) DNA in coating solution, (v) DNA dried in DI water. Scale bar = 100 µm. (B) Quantitative cell transfection rates determined by flow cytometry. Data represent the average of n=6 replicates. Error bars represent the standard error. (C) DNA molecule size determined by dynamic light scattering. Data represent the average of n=4 replicates. Error bars represent the standard error. DIW : deionized water, CS : coating solution.
In the negative control cells incubated without DNA, there was essentially no transfection (Fig. 6A-i) and in the positive control cells incubated with uncoated DNA in Opti-MEM media, there were bright green fluorescent spots, indicating GFP expression due to DNA transfection (Fig 6A-ii). However, transfection efficiency was weak among cells incubated with DNA that was either dried as a coating in a CMC solution or simply dissolved in coating solution including CMC (Fig 6A-iii, iv). As a final control, DNA was dried as a coating in de-ionized water, which yielded a DNA expression efficiency almost as high as the positive control (Fig. 6A-v) and significantly higher than CMC-containing solution. These results demonstrate that CMC, in the dried or solution state, damaged the DNA in a way that reduced gene expression. We hypothesize that the H5 DNA used in the vaccination study may have been similarly damaged and thereby reduced its immunogenicity.
We further hypothesized that this loss in DNA transfection caused by CMC could be partially due to aggregation of DNA molecules. In Fig. 6C, dynamic light scattering showed that drying or mixing in coating solution significantly increased the size of DNA vaccine particles (Student’s t-test, p<0.05), while drying in DI water also increased the size of DNA particles (Student’s t-test, p<0.05) relative to the negative control, but less so that the CMC-containing samples (Student’s t-test, p<0.05). These results indicate that the presence of CMC in the coating formulation inhibited the expression of DNA upon transfection.
4. Discussion
The spread of highly pathogenic H5N1 avian influenza viruses and sporadic cases of human infection has raised significant global health concern and warnings of a future pandemic [47]. The effectiveness of H5N1 viral vaccines to induce protective antibody titers is relatively low [48]. Therefore, many studies have been carried out to improve vaccine immunogenicity. DNA vaccines have been considered a next-generation vaccine suitable for highly pathogenic H5N1 avian influenza viruses, because they have several advantages, including the ability to express diverse antigens, rapid production, inability to revert into virulent forms, easy storage, long shelf life, and possible avoidance of the cold chain [5]. In spite of these advantages, DNA vaccines have not shown sufficient levels of immunity in non-human primate models due to low DNA transfection efficiency [49]. In order to overcome this problem, various approaches have been applied to improve DNA vaccination efficacy.
Intradermal (ID) immunization has been proposed as a solution, because epidermis and dermis, the main targeting site for antigen delivery, is replete with antigen-presenting cells such as Langerhans and dermal dendritic cells. Direct delivery of plasmid antigen into these cells has been shown to induce strong immune responses [50]. For efficient vaccine delivery to the skin, microneedle-mediated immunization in the skin has been studied previously by coating viral influenza antigens, such as inactivated whole viral vaccines and VLPs, on the shaft of microneedles [31, 33–38, 51].
To obtain effective coating, coating formulations have been evaluated previously in detail for coating influenza virus and VLP vaccines on metal microneedles [27, 28]. In the current study, influenza H5 DNA vaccine was coated onto microneedles using a similar approach. However, in contrast to previous coating formulations, the stabilizer trehalose was not used because DNA vaccine is expected to be stable during drying, unlike virus particles. The DNA vaccine was successfully coated onto microneedles and, upon insertion into mouse skin, the coated DNA was released into the skin within 3 min.
In previous studies with inactivated virus and VLP vaccines, skin vaccination with microneedles showed similar or even better immune responses compared to IM injection. The findings reported here demonstrate that DNA vaccination in the skin using microneedles similarly induced higher IgG levels as well as stronger HAI titers than those by IM immunization.
In case of IgG subtypes, DNA vaccines delivered by microneedles induced IgG2a dominant antibody responses, and conferred enhanced protection as compared to conventional IM delivery of the same dose of the DNA vaccine. In microneedle vaccination study using inactivated virus of virus-like particle, IgG2a antibody responses derived more protective immunity than IgG1 dominant antibody responses [33, 38].
Protection, as measured by mouse survival and viral titers in mouse organs, was also better using microneedles compared to IM immunization. This improvement may be attributed to a possibility that DNA vaccine administered to the skin can be taken up by antigen-presenting cells residing in epidermis and dermis due to direct delivery of DNA into the skin. In contrast, IM immunization delivers DNA vaccine to the muscle where muscle cells reside. Therefore, only transfected protein or cells may be able to cross-transfer DNA vaccine-expressed protein antigen to the nearby antigen presenting cells. While microneedles were used in this study as a means to target vaccination to the skin, there may also be differences between skin vaccinations using a microneedle patch as opposed to intradermal injection. Future studies that analyze the method of vaccination, as opposed to this study that was focused on the route of administration, are needed to determine possible immunologic differences between vaccinations in the skin using microneedles compared to intradermal injection.
Highly pathogenic avian influenza viruses have been reported to spread systemically in the mouse model, beyond the respiratory tract, in contrast to seasonal human influenza viruses, which mainly infect the respiratory organs [32]. In this study, high virus titers were measured in the lung, brain and spleen of negative-control mice. Microneedle vaccination significantly suppressed virus infection in the lungs and spleen and prevented spreading of virus to the brain. The high levels of HAI activity induced by microneedle vaccination may have contributed to this improved control of viral replication. As a result, we conclude that microneedle vaccination with H5 DNA vaccines provides improved protection compared to IM vaccination route.
Despite these improvements, DNA immunization with microneedles showed high IgG responses only after three immunizations, which differs from previous viral vaccine immunizations against seasonal H1N1 influenza by microneedles [31, 33]. It was previously reported that vaccination using viral antigens, such as inactivated virus and VLP vaccines, induced strong IgG responses after a single immunization. Additionally, DNA immunizations by microneedles did not fully protect the mice from lethal viral challenge. It protected mice partially with a 60% survival rate. In contrast, previous single microneedle immunizations with viral antigens induced 100% protection against lethal challenge infection with seasonal H1N1 virus [52] or H5N1 pandemic potential virus [34]. In order to improve DNA vaccination, heterologous prime/boost strategies would be an important alternative approach [53–55].
There may be several reasons for inferior immunogenicity by microneedle DNA vaccination. First, for vaccination with inactivated viral vaccines, the vaccine antigens are recognized by immune cells on cell surfaces. Internalization of protein vaccine antigens such as whole virus or VLP vaccines and their presentation on cell surfaces may not be a required process for inducing immune responses [56]. In contrast, for DNA vaccination, the DNA plasmid vaccine must be internalized by cells and protein antigens would be expressed by transfected cells. Second, except for gene gun delivery of DNA, typical doses in previous studies of influenza DNA vaccination ranged from 20 to 100 µg DNA [57–59]. In this study, only 3 µg of DNA was coated onto microneedles and less than 3 µg of DNA vaccine might have actually been delivered into skin. Third, our data suggest that CMC may inhibit DNA transfection efficiency. As shown in Fig 6, the coating process used here changed DNA integrity due to inclusion of CMC in the coating formulation, suggesting that DNA aggregation by CMC might play a role in lowering transfection efficacy. We hypothesize that an improved coating process without CMC that retains DNA integrity will enable a better immune response by DNA vaccination. On-going studies are assessing that hypothesis.
In conclusion, vaccination of mice with H5 influenza HA-encoding DNA vaccine using coated microneedles elicited significantly higher levels of virus-specific antibody responses as well as superior protection compared to IM immunization, as shown by higher survival rate and less viral replication in the internal organs after lethal viral challenge. These findings demonstrate that microneedle vaccination in the skin can be a promising approach for H5 DNA vaccine delivery compared to IM immunization. Future research should focus on improving DNA vaccine coating methods for microneedles that retain high transfection efficacy of DNA vaccines delivered to the skin.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants EB006369 (M.R.P.), AI0680003 (R.W.C.), AI081385 (S.M.K.) and AI093772 (S.M.K.), and Korea Research Foundation Grant KRF-2007-357-C00088 (J.M.S). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry. We thank Dr. Vladimir Zarnitsyn for microneedle fabrication, Dr. Fu-shi Quan for discussion of immunization studies, Mr. Dae-goon Yoo for technical help, Dr. Mark Allen for use of his laser microfabrication facilities and Ms. Donna Bondy for administrative support. M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
References
- 1.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monto AS. The threat of an avian influenza pandemic. N.Engl.J.Med. 2005;352:323–325. doi: 10.1056/NEJMp048343. [DOI] [PubMed] [Google Scholar]
- 3.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. Delivery systems for intradermal vaccination. Curr. Top. Microbiol. Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson JS, Nicolson C, Harvey R, Johnson R, Major D, Guilfoyle K, Roseby S, Newman R, Collin R, Wallis C, Engelhardt OG, Wood JM, Le JH, Manojkumar R, Pokorny BA, Silverman J, Devis R, Bucher D, Verity E, Agius C, Camuglia S, Ong C, Rockman S, Curtis A, Schoofs P, Zoueva O, Xie H, Li X, Lin ZS, Ye ZP, Chen LM, O'Neill E, Balishe A, Lipatov AS, Guo Z, Isakova I, Davis CT, Rivailler P, Gustin KM, Belser JA, Maines TR, Tumpey TM, Xu XY, Katz JM, Klimov A, Cox NJ, Donis RO. The development of vaccine viruses against pandemic A(H1N1) influenza. Vaccine. 2011;29:1836–1843. doi: 10.1016/j.vaccine.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat.Rev.Genetics. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perdue ML, Arnold F, Li S, Donabedian A, Cioce V, Warf T, Huebner R. The future of cell culture-based influenza vaccine production. Exp. Rev. Vaccines. 2011;10:1183–1194. doi: 10.1586/erv.11.82. [DOI] [PubMed] [Google Scholar]
- 7.Davis HL, McCluskie MJ. DNA vaccines for viral diseases. Microbes.Infect. 1999;1:7–21. doi: 10.1016/s1286-4579(99)80009-4. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly JJ, Friedman A, Martinez D, Montgomery DL, Shiver JW, Motzel SL, Ulmer JB, Liu MA. Preclinical efficacy of a prototype DNA vaccine - Enhanced protection against antigenic drift in influenza-virus. Nat.Med. 1995;1:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 9.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, Dewitt CM, Friedman A, Hawe LA, Leander KR, Martinez D, Perry HC, Shiver JW, Montgomery DL, Liu MA. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 10.Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006;24:4475–4481. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Kodihalli S, Haynes JR, Robinson HL, Webster RG. Cross-protection among lethal H5N2 Influenza viruses induced by DNA vaccine to the hemagglutinin. J. Virol. 1997;71:3391–3396. doi: 10.1128/jvi.71.5.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodihalli S, Kobasa DL, Webster RG. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine. 2000;18:2592–2599. doi: 10.1016/s0264-410x(99)00485-5. [DOI] [PubMed] [Google Scholar]
- 13.Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–2989. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalor PA, Webby RJ, Morrow J, Rusalov D, Kaslow DC, Rolland A, Smith LR. Plasmid DNA-based vaccines protect mice and ferrets against lethal challenge with A/Vietnam/1203/04 (H5N1) influenza virus. J. Infect. Dis. 2008;197:1643–1652. doi: 10.1086/588431. [DOI] [PubMed] [Google Scholar]
- 15.Littel-van den Hurk SV, Babiuk SL, Babiuk LA. Strategies for improved formulation and delivery of DNA vaccines to veterinary target species. Immunol. Rev. 2004;199:113–125. doi: 10.1111/j.0105-2896.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 16.Babiuk LA, Pontarollo R, Babiuk S, Loehr B, Little-van den Hurk SV. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21:649–658. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- 17.MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, Chattergoon MA, Baine Y, Higgins TJ, Ciccarelli RB, Coney LR, Ginsberg RS, Weiner DB. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 18.Haensler J, Verdelet C, Sanchez V, Girerd-Chambaz Y, Bonnin A, Trannoy E, Krishnan S, Meulien P. Intradermal DNA immunization by using jet-injectors in mice and monkeys. Vaccine. 1999;17:628–638. doi: 10.1016/s0264-410x(98)00242-4. [DOI] [PubMed] [Google Scholar]
- 19.Laddy DJ, Yan J, Khan AS, Andersen H, Cohn A, Greenhouse J, Lewis M, Manischewitz J, King LR, Golding H, Draghia-Akli R, Weiner DB. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J. Virol. 2009;83:4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yager EJ, Dean HJ, Fuller DH. Prospects for developing an effective particle-mediated DNA vaccine against influenza. Exp.Rev.Vaccines. 2009;8:1205–1220. doi: 10.1586/erv.09.82. [DOI] [PubMed] [Google Scholar]
- 22.Falo LD. Targeting the skin for genetic immunization. Proc. Assoc. Am. Physicians. 1999;111:211–219. doi: 10.1046/j.1525-1381.1999.99227.x. [DOI] [PubMed] [Google Scholar]
- 23.Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiraishi Y, Nandakumar S, Choi SO, Lee JW, Kim YC, Posey JE, Sable SB, Prausnitz MR. Bacillus Calmette-Guerin vaccination using a microneedle patch. Vaccine. 2011;29:2626–2636. doi: 10.1016/j.vaccine.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 26.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 27.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11:1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hehme N, Colegate T, Palache B, Hessel L. Influenza vaccine supply: Building long-term sustainability. Vaccine. 2008;26:D23–D26. doi: 10.1016/j.vaccine.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 30.Huang HN, Li TL, Chan YL, Chen CL, Wu CJ. Transdermal immunization with low-pressure-gene-gun mediated chitosan-based DNA vaccines against Japanese encephalitis virus. Biomaterials. 2009;30:6017–6025. doi: 10.1016/j.biomaterials.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu XH, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J. Infect. Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JM, Kim YC, Barlow PG, Hossain MJ, Park KM, Donis RO, Prausnitz MR, Compans RW, Kang SM. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010;88:244–247. doi: 10.1016/j.antiviral.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan SP, Koutsonanos DG, Martin MD, Lee JW, Zarnitsyn V, Choi SO, Murthy N, Compans RW, Skountzou I, Prausnitz MR. Dissolving polymer microneedle patches for influenza vaccination. Nat.Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weldon WC, Martin MP, Zarnitsyn V, Wang B, Koutsonanos D, Skountzou I, Prausnitz MR, Compans RW. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin. Vaccine Immunol. 2011;18:647–654. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu QY, Zarnitsyn VG, Ye L, Wen ZY, Gao YL, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang CL, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. USA. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 2010;84:7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen XF, Kask AS, Crichton ML, McNeilly C, Yukiko S, Dong LC, Marshak JO, Jarrahian C, Fernando GJP, Chen DX, Koelle DM, Kendall MAF. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J. Control. Release. 2010;148:327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J. Control. Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quan FS, Huang CZ, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J.Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang SM, Yoo DG, Lipatov AS, Song JM, Davis CT, Quan FS, Chen LM, Donis RO, Compans RW. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS ONE. 2009;4:e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canatella PJ, Karr JF, Petros JA, Prausnitz MR. Quantitative study of electroporation-mediated molecular uptake and cell viability. Biophys.J. 2001;80:755–764. doi: 10.1016/S0006-3495(01)76055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, Frey SE. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–6763. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hobson D, Curry RL, Beare AS, Wardgard A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hygiene. 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HHT, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subbarao K, Luke C. H5N1 viruses and vaccines. Plos Pathogens. 2007;3:e40. doi: 10.1371/journal.ppat.0030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Sahly HM, Keitel WA. Pandemic influenza vaccine development: an update. Exp. Rev. Vaccines. 2008;7:241–247. doi: 10.1586/14760584.7.2.241. [DOI] [PubMed] [Google Scholar]
- 49.Donnelly JJ, Wahren B, Liu MA. DNA vaccines: Progress and challenges. J.Immunol. 2005;175:633–639. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 50.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: Transfection and activation of dendritic cells as key events for immunity. J.Exp.Med. 1999;189:169–177. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J. Control. Release. 2010;147:326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm. Res. 2011;28:135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ledgerwood JE, Wei C-J, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, Bailer R, Tumpey TM, Koup RA, Mascola JR, Nabel GJ, Graham BS. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect. Dis. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu S. Heterologous prime–boost vaccination. Curr. Opin. Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Parker C, Taaffe J, Solorzano A, Garcia-Sastre A, Lu S. Heterologous HA DNA vaccine prime-inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. 2008;26:3626–3633. doi: 10.1016/j.vaccine.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bridges CB, Katz JM, Levandowski RA, Cox NJ. Inactivated influenza vaccines. In: Plotkin SA, Orenstein WA, Offit P, editors. Vaccines. Philadelphia: Saunders Elsevier; 2008. pp. 259–290. [Google Scholar]
- 57.Garg S, Oran AE, Hon H, Jacob J. The Hybrid Cytomegalovirus Enhancer/Chicken β-Actin promoter along with woodchuck hepatitis virus posttranscriptional regulatory element enhances the protective efficacy of DNA vaccines. J. Immunol. 2004;173:550–558. doi: 10.4049/jimmunol.173.1.550. [DOI] [PubMed] [Google Scholar]
- 58.Johnson PA, Conway MA, Daly J, Nicolson C, Robertson J, Mills KHG. Plasmid DNA encoding influenza virus haemagglutinin induces Th1 cells and protection against respiratory infection despite its limited ability to generate antibody responses. J. Gen. Virol. 2000;81:1737–1745. doi: 10.1099/0022-1317-81-7-1737. [DOI] [PubMed] [Google Scholar]
- 59.Patel A, Tran K, Gray M, Li Y, Ao Z, Yao X, Kobasa D, Kobinger GP. Evaluation of conserved and variable influenza antigens for immunization against different isolates of H5N1 viruses. Vaccine. 2009;27:3083–3089. doi: 10.1016/j.vaccine.2009.03.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.