Abstract
The estrogenic and antiestrogenic potential of perfluorooctanoic acid (PFOA) was assessed using an immature mouse uterotrophic assay and by histologic evaluation of the uterus, cervix and vagina following treatment. Female offspring of CD-1 dams were weaned at 18 days old and assigned to groups of equal weight, and received 0, 0.01, 0.1, or 1 mg PFOA/kg BW/d by gavage with or without 17-β estradiol (E2, 500 μg/kg/d) from PND18-20 (n=8/treatment/block). At 24 hr after the third dose (PND 21), uteri were removed and weighed. Absolute and relative uterine weights were significantly increased in the 0.01 mg/kg PFOA only group. Characteristic estrogenic changes were present in all E2-treated mice; however, they were minimally visible in the 0.01 PFOA only mice. These data suggest that at a low dose PFOA produces minimal histopathologic changes in the reproductive tract of immature female mice, and does not antagonize the cellular effects of E2.
Keywords: PFOA, immature mice, uterotrophic assay, uterus, cervix, vagina, pathology, estradiol
1. Introduction
Perfluorooctanoic acid (PFOA) is a synthetic, 8-carbon member of the perfluoroalkyl acid (PFAA) family of fluorinated organic compounds, and has become one of the most ubiquitous chemicals found in the environment [1]. PFOA is currently applied or is a final breakdown product of polymers used as surface protectors and coatings for textiles, and in numerous industrial products including lubricants, paints, polishes, surfactants, and flame-retardant foams [1–3]. It is ubiquitously detected in the environment and in the serum of wildlife and humans [4–6]. PFOA is also measurable in surface [7, 8] and drinking water [8], and is a global contamination problem of public health concern.
PFOA is found at very low levels in both the environment and the general U.S. population and individual exposure has been documented via inhalation, dermal absorption, and ingestion [1]. The compound transfers to the unborn offspring across the placenta and breast milk is thought to be a source of PFOA exposure for infants [9–11]. In lactating mice, milk has been shown to be a route of exposure for nursing pups [12, 13]. PFOA has a half-life of 2–4 years in humans and has been reported to cause developmental, toxic and long-term adverse outcomes in aquatic and laboratory animals [1, 4, 13–15].
The mechanism of PFOA-induced effects, primarily in the liver, has traditionally been thought to occur strictly through a peroxisome proliferator–activated receptor α (PPARα) mechanism [16]; however, recent animal and epidemiologic data suggest that PFOA may cause endocrine disruption [14, 15, 17–19]. PFOA has been reported to alter female pubertal timing in multiple strains of mice [12, 20], and has recently been reported to delay pubertal timing in girls, but not boys. In a report from the C8 Science Panel [21], data collected on the potential health effects in US regions once polluted with PFOA suggest that delayed puberty in girls (measured as either serum estradiol >20 pg/mL or self-reported menarche) was associated with the highest levels of serum PFOA. In a British cohort of girls, prenatal exposure to PFOA and a related compound was not associated with self-reported age at menarche [22]. Neither of these studies addressed pubertal breast timing, but disrupted mammary gland development of female mice following low-dose prenatal exposure to PFOA has been reported [23, 24]. PFOA treatment can up-regulate protein levels of estrogen receptor alpha (ERα) and proliferating cell nuclear antigen (PCNA) in the mammary glands of wild-type C57Bl/6 and PPARα knock-out mice [14]. Women with high serum concentrations of PFOA were reported to have an earlier onset of menopause compared to their counterparts who have lower serum PFOA levels [17]. PFOA can also increase the activity of estrogen-responsive genes in fish [25].
Because of these endocrine-related findings and others (reviewed in [26] ), we assessed the estrogenic or antiestrogenic potential of low doses of PFOA on female reproductive tissues using an immature mouse uterotrophic assay and by evaluating the histomorphology of the uterus, cervix and vagina following treatment.
2. Materials and Methods
2.1 Chemicals
PFOA, as its ammonium salt (>98% pure), was acquired from Fluka Chemical (Steinheim, Switzerland). PFOA was prepared fresh daily in deionized water. Mice received either distilled water as vehicle or 0.01, 0.1, 1 mg PFOA/kg body weight (BW)/d by oral gavage from PND 18–20.
17β-estradiol (E2) was acquired from Sigma-Aldrich (St. Louis, MO). E2 was prepared in corn oil and mice received either corn oil as vehicle or 500 µg/kg BW/d by subcutaneous injection from PND 18–20 [27].
2.2 Animals
Timed-pregnant CD-1 mice delivered their young on day 19 of gestation at Charles River Laboratories (Raleigh, NC). At birth (day 1), all pups were sorted by sex and randomly redistributed so that all litters were normalized to 12 female pups per dam (not necessarily their own). The dams and female pups were received at the U.S. EPA NHEERL Reproductive Toxicology Facility (RTP, NC) on postnatal day 12 (PND 12). Upon receipt, litters were standardized to 10 female pups per dam. Litters were housed in polypropylene cages lined with Alpha-dri bedding (Shepherd Specialty Papers, Kalamazoo, MI). All animals were housed under controlled lighting (12:12 hour light:dark), temperature (20–24°C), and relative humidity (40–60%) conditions. Fresh de-ionized water and NIH-31 rodent diet (an open formula, autoclavable, natural-ingredient rodent diet with estrogenic activity at ≤ 140 ppm, Zeigler Brothers, Inc., Gardners, PA) were provided ad libitum. All animal procedures complied with US EPA NHEERL Institutional Animal Care and Use Committee guidelines for care and euthanasia.
2.3 Uterotrophic Assay Study Design
Following the protocol of Padilla-Banks and co-workers [28], pups were weaned on the morning of PND 18 and randomly assigned to one of eight treatment groups (n=8 per treatment group per block). Replicate A (block1) included the treatment groups corn oil vehicle or E2 in corn oil (500 µg/kg/d, s.c.) alone, PFOA alone (0, 0.01, 0.1, 1 mg/kg BW/d, gavage), or PFOA (0, 0.01, 0.1, 1.0 mg/kg BW/d, gavage) + E2 (500 µg/kg/d, s.c.). Treatments were given for 3 consecutive days starting in the afternoon of PND 18 with uteri collected on PND 21. A second replicate of animals (block 2; B) was run in this identical design to confirm the effects in replicate A. A third replicate (block 3; C), in the identical dosing regimen of replicate A, was conducted to obtain the entire reproductive tracts from females in these treatment groups for further histological evaluation only (these uteri were not weighed).
2.4 Tissue Collection
Mice were weighed daily and prior to tissue collection. Euthanasia was completed by decapitation on the morning of the fourth day (PND 21) and trunk blood was collected by funneling the blood into Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). For blocks A&B, an incision was made in the skin and abdominal muscle and cervix separated from the vaginal fornix. Because fluid imbibition is an estrogenic response, special care was taken to retain all uterine luminal fluid. The uterus was removed after cutting by gently lifting the tissue anteriorly and dissecting it from the mesometrium. An incision was made in the uterotubal junction to preserve the uterine horns and avoid loss of uterine fluid [27]. The uterus was immediately weighed, properly oriented in a histocassette, fixed in 10% buffered formalin (Fisher Scientific, Fair Lawn, NJ), and processed for sectioning and histopathologic examination. Replicate C tissue resections included the entire female reproductive tract (uterus, cervix, vagina) as a single unit. These tissues were fixed as stated above and trimmed so that longitudinal sections through the uterus, the utero-cervical junction, cervix and vagina could be prepared.
2.5 Histopathology
Fixed uterine samples were processed by routine methods and embedded in paraffin blocks. Five-micron tissue sections were cut, mounted on glass slides and stained with hematoxylin and eosin (H & E). Slides containing H & E stained tissue samples were evaluated for pathologic changes using conventional light microscopy.
Each H&E-stained tissue section was reviewed for histopathologic changes and semiquantitatively evaluated by assigning a severity score for changes observed in the uterus (endometrial edema; endometrial epithelial hyperplasia of the mucosa or glands; hypertrophy/hyperplasia/edema of the myometrium), and cervix and vagina (mucification, mucosa; squamous hyperplasia and cornificaton, mucosa; edema, submucosa/stroma) by a pathologist without knowledge of treatment groups. The cervix and vagina were scored together due to consistent and similar histological findings in the two regions. Severity scores ranged from 0–4. A score of 0 indicated an absence of demonstrable histopathologic changes and was considered to be within normal range. A score of 1 (minimal) denoted a subtle histopathologic change present that barely exceeded the normal range; 2 (mild) indicated the histopathologic change was present, however, it was not pronounced and of limited severity; 3 (moderate) denoted a histopathologic change was present and was pronounced; and 4 (severe) indicated a histopathologic change was present at its greatest extent and was very pronounced and significant.
2.6 Calculations
The uterine wet weight (UWW):body weight (BW) ratios were calculated for each animal by dividing the uterine wet weight by the body weight. Data were evaluated for exposure-related effects using general linear model analysis of variance (ANOVA) in SAS 9.1 (SAS Institute, Inc. Cary, NC), with statistical significance at p<0.05. All data were assessed via one- or two-way analysis of variance with Dunnett’s posthoc test. Block effect was evaluated. The average body weight and the average uterine wet weight for each treatment group were also calculated and compared.
For histopathology severity score comparisons in the uterus, cervix and vagina, the Mann-Whitney test with a one-sided p-value was used to determine statistical significance (p<0.01 or p<0.05) between the mean severity scores of individual and overall histopathological changes for all treatment groups and to compare each treatment group with respective controls.
3. Results
3.1 Absolute and Relative Uterine Wet Weight (UWW)
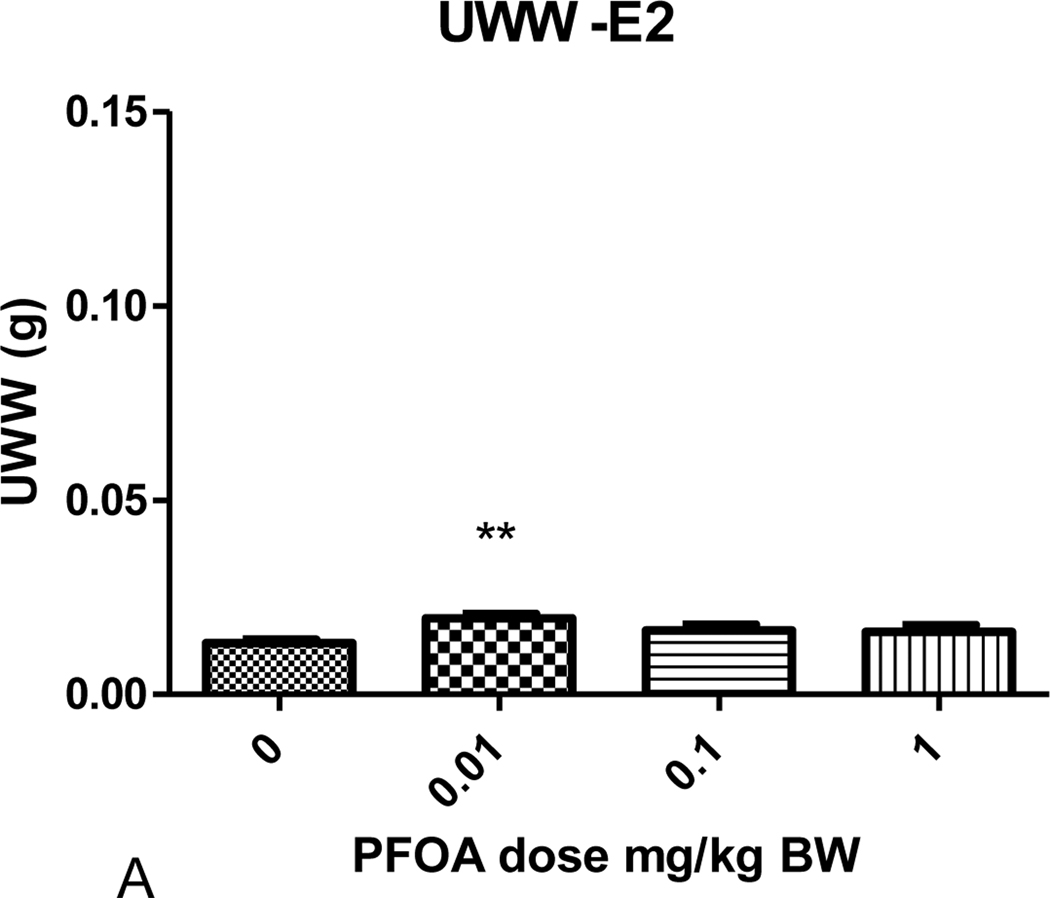
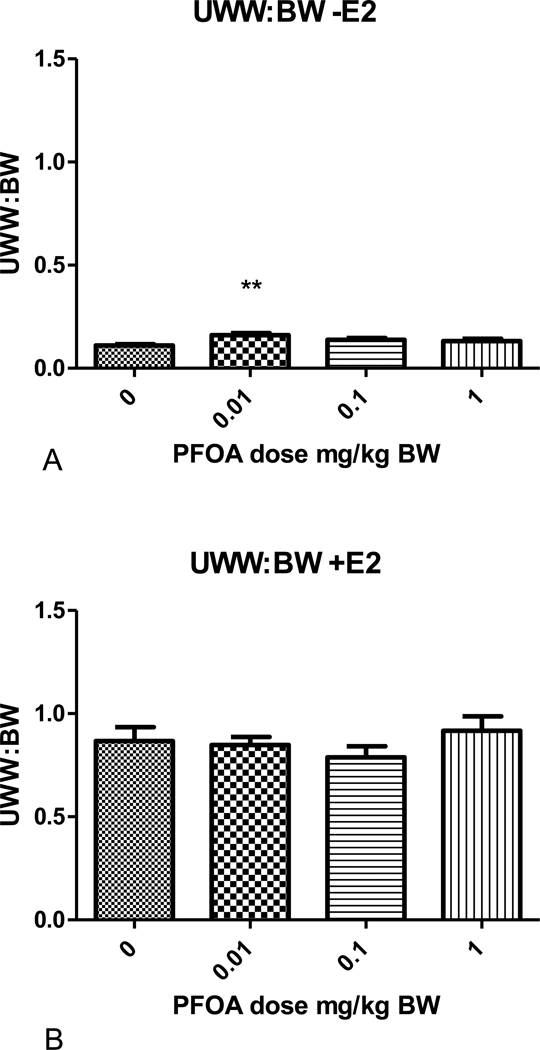
There was no effect of PFOA or estradiol on body weight in these studies (Table 1). With the exception of mice administered 0.01 mg/kg PFOA, the UWW (absolute) and UWW:BW ratios (relative uterine weight) for mice administered varying doses of PFOA alone, once daily for 3 days from PND 18–20, were not significantly different from those of the control group. The absolute and relative UWWs of mice administered 0.01 mg/kg PFOA were significantly greater than those of the PFOA control group (p<0.05; Figs. 1 and 2; Table 1). Similar effects were evident in each block (A and B; no block effect). The 0.01 mg/kg PFOA exposure produced a nearly 50% increase over control in UWW and UWW:BW ratio. The absolute and relative UWW of groups administered PFOA + E2 were not significantly different from the respective controls. The increase in weight due to estradiol administration is shown for UWW in Table 1, and the fold over control varied from 7.14 to 8.30 for the UWW:BW ratios, as expected.
Table 1.
Effects of PFOA and 17β-estradiol in the uterotrophic bioassay.
| No 17β-estradiol | 500 µg/kg/d 17β-estradiol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFOA (mg/kg/d) |
Body weight (g) |
UWW(g) | Fold over Control |
UWW:BW | Fold over Control |
Body weight (g) |
UWW(g) | Fold over Control |
UWW:BW | Fold over Control |
| 0 | 11.8±0.3 | 0.013+0.001 | 1.0 | 0.111+0.006 | 1.0 | 13.4±0.6 | 0.117+0.013 | 8.85 | 0.867+0.067 | 7.80 |
| 0.01 | 12.1±0.3 | 0.020+0.001 | 1.48* | 0.161+0.009 | 1.46* | 12.8±0.6 | 0.109+0.008 | 8.22 | 0.848+0.039 | 7.67 |
| 0.1 | 11.7±0.4 | 0.016+0.002 | 1.24 | 0.138+0.009 | 1.25 | 13.3±0.7 | 0.105+0.010 | 7.97 | 0.786+0.052 | 7.14 |
| 1.0 | 11.9±0.4 | 0.016+0.002 | 1.21 | 0.132+0.012 | 1.19 | 12.9±0.6 | 0.117+0.008 | 8.89 | 0.917+0.069 | 8.30 |
Weights are shown as mean+SEM. UWW=uterine wet weight, PFOA=perfluorooctanoic acid.
denotes statistically different from control (p<0.05)
Figure 1.
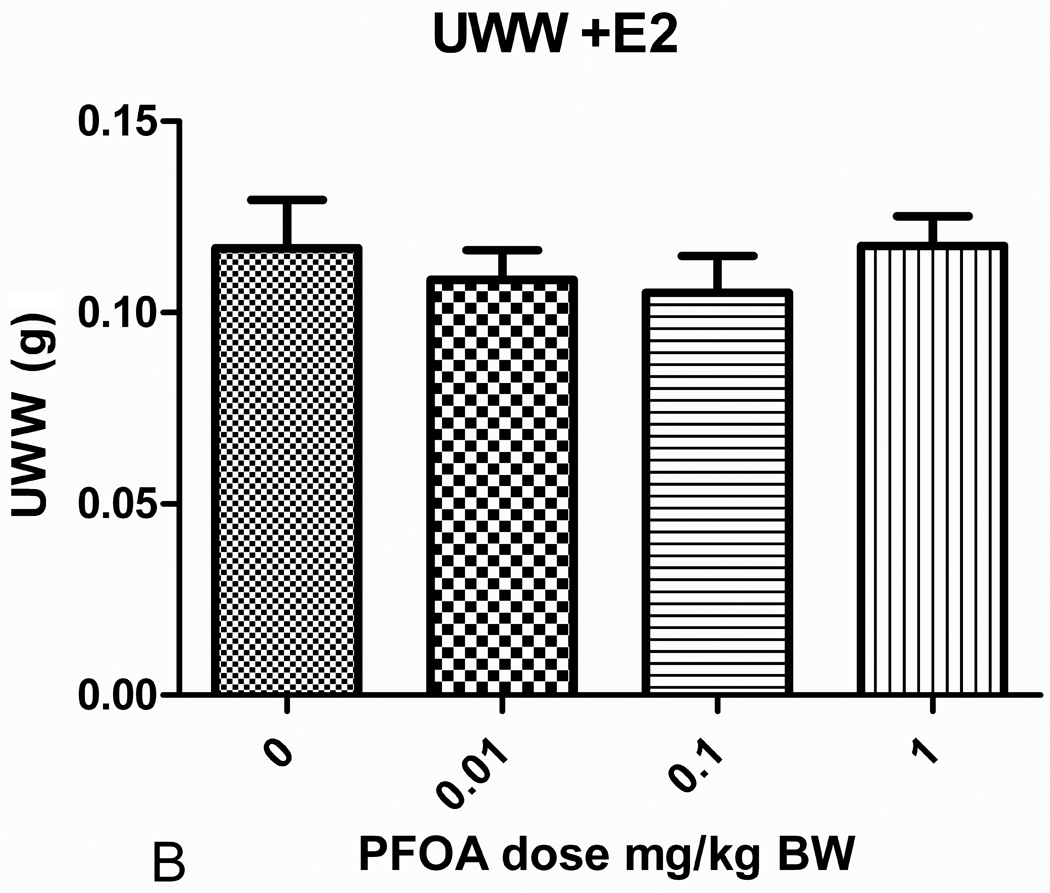
Uterine wet weight (UWW) for mice receiving varying doses of PFOA treatments once daily for 3 days from PND18-20. (A) The PFOA dose response on uterine wet weight for mice not treated with 17 β-estradiol. (B) The PFOA dose response on uterine wet weight for mice treated with 17 β-estradiol (500 μg/kg/day) for 3 days from PND18-20. **Statistically significant different from control p<0.05.
Figure 2.
Uterine wet weight to body weight (UWW:BW) ratio of mice that received varying doses of PFOA once daily for 3 days from PND18–20. (A) The PFOA response on the ratio of mice not treated with 17 β-estradiol. (B) The PFOA response on the ratio of mice treated with 17 β-estradiol (500 μg/kg/day) for 3 days. **Statistically significant different from control p<0.05.
3.2 Histopathology
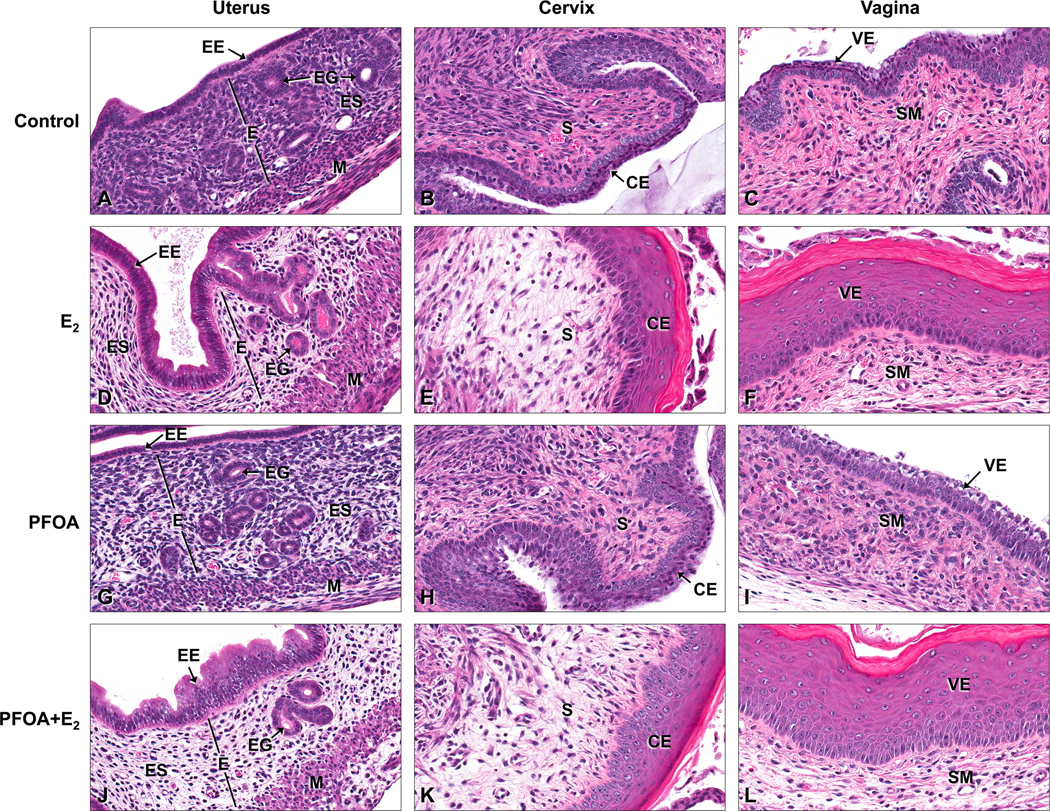
The uterus, cervix and vagina of E2-treated mice showed several characteristic, estrogenic changes. In the uterus, there was moderate edema of the endometrium characterized by separation of the endometrial stromal cells, moderate thickening (hyperplasia) of the uterine mucosal and endometrial glandular epithelia, and hypertrophy of the smooth muscle cell layers (Fig. 3) Squamous hyperplasia and cornification of the vaginal and cervical epithelium in response to E2 was also observed. There were no significant differences in the mean severity scores for individual or overall histopathologic changes in the uterus, cervix and vagina between the groups of E2-treated mice (Figs. 4 and 5).
Figure 3.
Histopathologic changes in the uterus, cervix and vagina of mice following 0 PFOA (control), PFOA alone, 17 β-estradiol (E2) alone or PFOA+E2 treatment for 3 days from PND18–20. (A–C) Control Tissues: Uterus (A); Cervix (B); and Vagina (C). Note no estrogenic changes in the uterus, cervix and vagina of a control mouse. (D–F) E2 (500 μg/kg/day) treatment: Uterus (D), note moderate edema of the endometrium (E), endometrial epithelial (EE) and glandular (EG) hyperplasia, and myometrial (M) hypertrophy; Cervix (E), note moderate squamous hyperplasia and cornification of the cervical epithelium (CE) and edema of the stroma (S); and Vagina (F), note moderate squamous hyperplasia and cornification of the vaginal epithelium (VE) and edema of the submucosa (SM). (G–I) PFOA treatment (0.01 mg/kg/day): Uterus (G), note minimal edema of the endometrium (E) with minimal endometrial epithelial (EE) and glandular (EG) hyperplasia and myometrial (M) hypertrophy and edema; Cervix (H), note absence of squamous hyperplasia and cornification of the CE and minimal stromal (S) edema; and Vagina (I), note absence of squamous hyperplasia and cornification of the vaginal epithelium (VE), but presence of minimal mucification and minimal submucosal (SM) edema.
(J–L) PFOA (0.01 mg/kg/day) + E2: Uterus (J), note moderate edema of the endometrium (E), endometrial epithelial (EE) and glandular (EG) hyperplasia and myometrial (M) hypertrophy and edema; Cervix (K) note moderate squamous hyperplasia and cornification of the cervical epithelium (CE) and edema of the stroma (S); and Vagina (L), note moderate squamous hyperplasia and cornification of the vaginal epithelium (VE) and edema of the submucosa (SM). H & E. 40x, original magnification.
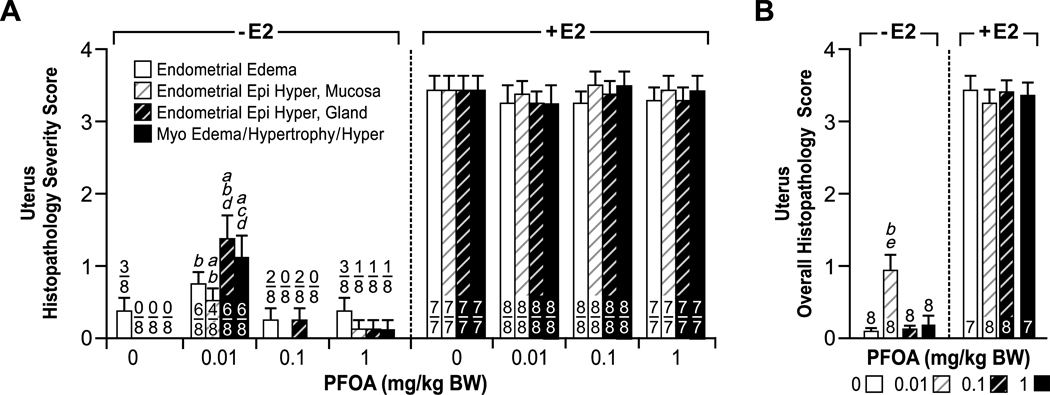
Figure 4.
Comparison of histopathology severity scores for estrogenic changes in the uterus of mice following 0 (control) to 1.0 PFOA with or without 17 β-estradiol (E2) treatment for 3 days from PND18–20. H & E stained sections of uterus from immature mice were scored in a blinded and unbiased manner from 0 to 4 for the extent of estrogenic changes, as described in the Materials and Methods section. 0–1, indicates no or minimal estrogenic changes, and 4 represents severe estrogenic changes. (A) Endometrial edema, hyperplasia of the endometrial mucosa (Endometrial Epi Hyper, Mucosa), and glands (Endometrial Epi Hyper, gland) and edema, hypertrophy and hyperplasia of the myometrium (Myo Edema/Hypertrophy/Hyper) were scored separately and individually compared between groups. The numbers indicate mice with lesion over total evaluated. Each category of histopathologic changes indicated above was compared independently between groups and significant differences are as indicated: ap<0.01 vs. control; bp< 0.05 vs. 0.1 PFOA alone; cp<0.01 vs. 0.1 PFOA alone; dp<0.05 vs. 1.0 PFOA alone. (B) A mean overall score for estrogenic changes in the uterus was generated for each group. The numbers indicate total number of mice evaluated. Significant differences in overall histopathologic changes between groups are as indicated: bp< 0.05 vs. 0.1 PFOA alone; ep<0.05 vs. control. There were no statistically significant differences between the E2 treated groups.
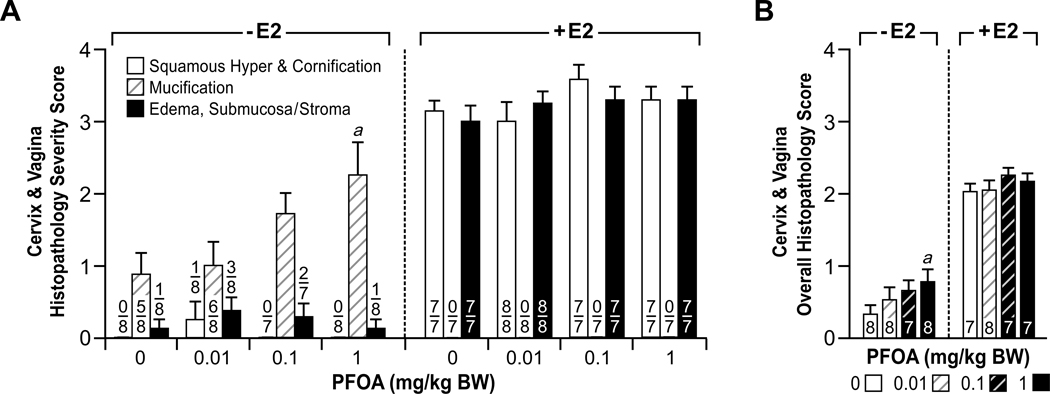
Figure 5.
Comparison of histopathology severity scores for estrogenic changes in the cervix and vagina of mice following 0 (control) to 1.0 PFOA with or without 17 β-estradiol (E2) treatment for 3 days from PND18–20. H & E stained sections of cervix and vagina from immature mice were scored in a blinded and unbiased manner from 0 to 4 for the extent of estrogenic changes, as described in the Materials and Methods section. 0–1, indicates no or minimal estrogenic changes, and 4 represents severe changes. (A) Squamous hyperplasia and cornification of the mucosa (Squamous Hyperplasia & Cornification), mucification of the mucosa (Mucification), and edema of the submucosa or stroma (Edema, Submucosa/Stroma) were scored separately. The numbers indicate mice with lesion over total number evaluated. Each category of histopathologic changes indicated above was compared independently between groups and significant differences are as indicated: ap<0.05 vs. control. (B) A mean Page overall score for changes in the vagina and cervix was generated for each group. The numbers indicate total number of mice evaluated. Significant differences in overall histopathologic changes between groups are as indicated: ap<0.05 vs. control. There were no statistically significant differences between the E2 treated groups.
Mice treated with PFOA alone had minimal histopathologic changes in the uterus, cervix and vagina that were similar to but far less severe than those observed in E2-treated mice (Fig. 3). In the uterus, there was minimal to mild endometrial and myometrial edema in addition to minimal thickening (hyperplasia) of the uterine mucosal and endometrial glandular epithelia, and smooth muscle layers. These uterine changes were present in some, but not all sections from the PFOA treatment groups. There was also focal minimal stromal edema of the cervix, and the vagina had focal areas of mucification, but cornification and squamous hyperplasia of the cervical and vaginal mucosae were not present to any significant degree. All vaginal and cervical changes noted were present in some, but not all sections from the PFOA treatment groups. The mean severity scores for the uterus showed the 0.01 PFOA alone group had significantly increased histopathologic changes in the endometrium and myometrium compared to the controls and the 0.1 and 1.0 PFOA alone groups (Fig. 4). The mean severity score for overall estrogenic changes in the uterus was significant in the 0.01 PFOA group compared to controls and the 0.1 PFOA group. These hsitopathologic alterations may have contributed to the increased uterine weight detected in the 0.01 PFOA group. In the cervix and vagina, the mean severity scores for mucification and overall changes were significantly increased in the 1.0 PFOA only group compared to the controls (Fig. 5). PFOA induced varied dose effects in the uterus versus the vagina and cervix; whereby there was a nonmonotonic (inverted-U) response for overall histopathologic changes in the uterus, and a linear response was observed in the vaginal and cervical tissues (Figs. 4B and 5B).
PFOA had no antiestrogenic effects when co-administered with E2 and characteristic estrogenic changes were observed in the uterus, cervix and vagina as described above (Figs. 3, 4 and 5).
4. Discussion
This study, to our knowledge, is the first to describe the histopathologic changes in the immature mouse uterus, cervix and vagina following low doses of PFOA administration with and without E2. Earlier carcinogenicity studies in adult rats, in which there is descriptive histopathology, have shown that PFOA induces tumors of the liver and pituitary gland in males and females. In the same study, increased incidences of mammary gland tumors in females, and testicular Leydig cell tumors in males were also noted [29]. However, a reexamination of mammary tissues from that study showed that the incidence of mammary gland neoplasms was similar to that of historical controls for female SD rats [30]. Nevertheless, these earlier findings suggest that hormone responsive tissues in both male and female adult rats may be the targets for the effects of PFOA following long-term adult exposures.
PFOA is a synthetic perfluorinated compound that does not occur naturally in the environment, yet it is a global contamination problem and a public health concern [1–3]. Recent studies suggest that PFOA may pose a threat as a weak environmental xenoestrogen following prenatal exposures [25, 31, 32]. Using an immature mouse uterotrophic study that has been directly compared to a similar assay in Sprague-Dawley rats [28], we found a slight albeit significant increase in uterine weight in the lowest exposure group (0.01 mg PFOA/kg) that coincided with gross enlargement at the utero-cervical junction in most 0.01 and a smaller fraction of 0.1 and 1.0 PFOA-exposed animals in blocks A and B and minimal, but significant histopathologic changes compared to respective controls in (the histopathology only) block C. The effects of stress on the young mice used in this study could not be ruled out; although, we did not see much variation in uterine weight data between blocks A and B, and the histopathology findings (block C) in the uterus of the 0.01 PFOA-exposed only group correlated well with the increased uterine weight data observed in mice in blocks A and B.
The E2-induced uterine weight change reported in previous studies [28] using the immature CD-1 mouse was 6.5-fold between control and 500 µg/kg E2, whereas that same dose in the current study induced nearly 9-fold stimulation of uterine weight gain. The mean UWW for 20 day old control mice in the previous study [28] was 0.011±0.001 g and in our study on PND 21, it was a similar, 0.013±0.001 g (mean±SEM). These studies reported low variability in uterine wet weight. Thus, in the present study, a nearly 7 mg increase in uterine wet weight between the 0 and 0.01 mg PFOA/kg exposure groups represents a significant 50% increase, whereas 20–25% change from control were evident in the 0.1 and 1.0 mg PFOA/kg only groups. The nonmonotonic or inverted-U dose response observed in the uterus of the PFOA/kg only groups, may possibly be attributed to the ability of some environmental agents at low doses to mimic the normal mechanisms controlling a response. Also, when a low dose of an agent rises into a higher range it may function to activate different pathways and create a negative feedback loop which then may in turn shut down the original response, or if at high enough level result in overt toxicity and/or cell death. Further molecular and mechanistic studies, beyond the scope of this paper, are needed to delineate the mode of action of PFOA in the uterus.
It has been shown that PFOA is a potent inducer of vitellogenin in vivo, weakly binds the ER similar to other environmental estrogens, and can enhance human ERα-dependent transcriptional activity in rainbow trout [31]. Previously, we and other investigators have reported that PFOA treatment could disrupt development or alter gene expression in mammary gland tissue, another estrogen responsive organ [14, 15]. Additionally, male and female freshwater minnows exposed to PFOA developed testicular oocytes and ovarian degeneration, respectively, both of which are indicators of estrogenic activity [33].
PFOA upregulates protein levels of ERα and PCNA in mammary glands of wild-type C57Bl/6 and PPARα knock-out mice, which supports a possible non-peroxisome proliferators mechanism of action in reproductive organs [14]. This concept of PFOA-induced non-PPARα mediated toxicity has been demonstrated in an in vivo rainbow trout model in which the liver tumor promoting activity was related to an estrogenic signaling mechanism rather than to the ability of PFOA to function as a peroxisome proliferator [32]. Following PFOA administration, hepatic microarray profiles in rare minnows showed significant induction of estrogen-responsive genes [25] and confirmed earlier findings of increased expression of vitellogenin and ERβ, and induction of histopathologic changes in the testes and ovary suggestive of endocrine disruption [33].
The above studies all support the concept that the effects of PFOA may be endocrine-mediated, possibly through an estrogen signaling mechanism. In the present study, we found that exposure of immature mice to low doses of PFOA had minimal but visible estrogenic effects which occurred primarily in the uterus in the 0.01 PFOA group with fewer effects observed in the cervix and vagina. Vaginal mucification was noted; however, squamous hyperplasia and keratinization of the epithelial layers of the cervix and vagina, lesions that typically occur following exposure to estrogens, such as estradiol and DES [34, 35] were not observed to any significant extent in the PFOA alone groups. Vaginal mucification has been reported in neonatal suckling mice exposed to estradiol through the milk [36], in the offspring of mice transplacentally exposed to estrogens [37], and in rats exposed to the phytoestrogen, genistein, which is weakly estrogenic [38]. Also, vaginal mucification occurs in response to exposure to potent androgens [39] or progesterone receptor ligands, such as the selective progesterone receptor modulator, asoprisnil [40], or following administration of a combination of E2 and progesterone [41]. PFOA is reported to increase progesterone levels in mice, and enhance mammary gland receptivity to the effects of exogenous E2 with upregulation of growth factor expression in the mammary gland [14]. Additional mechanistic studies are underway to determine the mode of action of PFOA in relation to the effects observed in this study and to determine if the histopathologic changes observed are indeed attributable to an estrogen- or progesterone- mediated mechanism, or through indirect hormonal regulation of other signaling pathways, and/or a hormone independent pathway.
In conclusion, we have shown that PFOA produces minimal, but significant changes in the reproductive organs of immature mice. These data suggest that the immature reproductive tract may be a target for endocrine disrupting compounds that could result in perturbations in development or may manifest as an adverse outcome later in life. PFOA, when co-administered with E2, does not appear to interfere with E2-induced effects in the immature reproductive tract of female CD-1 mice. However, it has been reported that a combination of PFOA and E2 produced anti-estrogenic effects in cultured tilapia hepatocytes [42]. Future studies are needed to define the mechanism(s) by which PFOA induces histopathologic changes in the reproductive organs of immature mice. In addition, because of several animal studies and the advent of human exposure data that indicate that PFOA can cause endocrine disruption, more research is needed to determine the importance of time of exposure, low dose exposures, and the molecular mechanisms by which the endocrine disrupting effects may occur.
Highlights.
We assess whether perfluorooctanoic acid (PFOA) is estrogenic or antiestrogenic.
We use an immature mouse uterotrophic assay and histopathology.
Uterine weights were significantly increased in the 0.01 mg/kg PFOA only group.
Characteristic estrogenic changes were minimally visible in the 0.01 PFOA only mice.
A low dose PFOA does not antagonize the histopathologic effects of E2.
Acknowledgements
This research was supported by the Division of the National Toxicology Program of the NIH, National Institute of Environmental Health Sciences and the U.S. EPA’s National Health and Environmental Effects Laboratory. The authors would like to thank Drs. Ronald Herbert and Mark Cesta for their critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare there are no financial conflict of interest issues.
Disclaimer
The information in this document has been subjected to review by the National Institute of Environmental Health Sciences, NIH and the U.S. EPA’s National Health and Environmental Effects Laboratory and approved for publication. This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
References
- 1.EPA US. Perfluoroctanoic Acid (PFOA) and Fluorinated Telomers. http://epagov/oppt/pfoa/
- 2.Hekster FM, Laane RW, de Voogt P. Environmental and toxicity effects of perfluoroalkylated substances. Rev Environ Contam Toxicol. 2003;179:99–121. doi: 10.1007/0-387-21731-2_4. [DOI] [PubMed] [Google Scholar]
- 3.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol. 2006;40:3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- 4.Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jorgensen EH, Sonne C, et al. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci Total Environ. 2010;408:2995–3043. doi: 10.1016/j.scitotenv.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Keller JM, Calafat AM, Kato K, Ellefson ME, Reagen WK, Strynar M, et al. Determination of perfluorinated alkyl acid concentrations in human serum and milk standard reference materials. Anal Bioanal Chem. 2010;397:439–451. doi: 10.1007/s00216-009-3222-x. [DOI] [PubMed] [Google Scholar]
- 6.Vestergren R, Cousins IT. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol. 2009;43:5565–5575. doi: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- 7.Betts KS. Perfluoroalkyl acids: what is the evidence telling us? Environ Health Perspect. 2007;115:A250–A256. doi: 10.1289/ehp.115-a250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skutlarek D, Exner M, Farber H. Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res Int. 2006;13:299–307. doi: 10.1065/espr2006.07.326. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Li J, Zhao Y, Wang Y, Zhang L, Wu Y. The occurrence of perfluorinated alkyl compounds in human milk from different regions of China. Environ Int. 2010;36:433–438. doi: 10.1016/j.envint.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds - Comparing exposure estimates with biomarkers of exposure. Environ Int. 2011 doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Lee KT, Kang CS, Tao L, Kannan K, Kim KR, et al. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ Pollut. 2011;159:169–174. doi: 10.1016/j.envpol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90:510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 13.Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, et al. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol. 2009;27:365–372. doi: 10.1016/j.reprotox.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Tan YS, Haslam SZ, Yang C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol Sci. 2010;115:214–224. doi: 10.1093/toxsci/kfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96:133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 16.Elcombe CR, Elcombe BM, Foster JR, Farrar DG, Jung R, Chang SC, et al. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats following dietary exposure to ammonium perfluorooctanoate occurs through increased activation of the xenosensor nuclear receptors PPARalpha and CAR/PXR. Arch Toxicol. 2010;84:787–798. doi: 10.1007/s00204-010-0572-2. [DOI] [PubMed] [Google Scholar]
- 17.Knox SS, Jackson T, Javins B, Frisbee SJ, Shankar A, Ducatman AM. Implications of Early Menopause in Women Exposed to Perfluorocarbons. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2010-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Z, Zhang H, Liu Y, Xu M, Dai J. Alterations in gene expression and testosterone synthesis in the testes of male rats exposed to perfluorododecanoic acid. Toxicol Sci. 2007;98:206–215. doi: 10.1093/toxsci/kfm070. [DOI] [PubMed] [Google Scholar]
- 19.Shi Z, Zhang H, Ding L, Feng Y, Xu M, Dai J. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod Toxicol. 2009;27:352–359. doi: 10.1016/j.reprotox.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Tan YS, Harkema JR, Haslam SZ. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol. 2009;27:299–306. doi: 10.1016/j.reprotox.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, et al. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with Age of Puberty among Children Living near a Chemical Plant. Environ Sci Technol. 2011 doi: 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
- 22.Christensen KY, Maisonet M, Rubin C, Holmes A, Flanders WD, Heron J, et al. Progression through puberty in girls enrolled in a contemporary British cohort. J Adolesc Health. 2010;47:282–289. doi: 10.1016/j.jadohealth.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. Gestational and Chronic Low-Dose PFOA Exposures and Mammary Gland Growth and Differentiation in Three Generations of CD-1 Mice. Environ Health Perspect. 2011;119:1070–1076. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, et al. Prenatal Perfluorooctanoic Acid Exposure in CD-1 Mice: Low-Dose Developmental Effects and Internal Dosimetry. Toxicol Sci. 2011;122:134–145. doi: 10.1093/toxsci/kfr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Liu Y, Wang J, Tao Y, Dai J. Toxicogenomic analysis of the hepatic effects of perfluorooctanoic acid on rare minnows (Gobiocypris rarus) Toxicol Appl Pharmacol. 2008;226:285–297. doi: 10.1016/j.taap.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 26.White SS, Fenton SE, Hines EP. Endocrine Disrupting Properties of Perfluorooctanoic Acid. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newbold RR, Jefferson WN, Padilla-Banks E. The mouse uterotrophic assay: other end points. Environ Health Perspect. 2001;109:A569–A570. doi: 10.1289/ehp.109-a569a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padilla-Banks E, Jefferson WN, Newbold RR. The immature mouse is a suitable model for detection of estrogenicity in the uterotropic bioassay. Environ Health Perspect. 2001;109:821–826. doi: 10.1289/ehp.01109821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sibinski L. Two-Year oral (diet) toxicity/carcinogenicity study of fluorochemical FC-143 (perfluorooctane ammonium carboxylate) in rats. 1987:226–1137. [Google Scholar]
- 30.Hardisty JF, Willson GA, Brown WR, McConnell EE, Frame SR, Gaylor DW, et al. Pathology Working Group review and evaluation of proliferative lesions of mammary gland tissues in female rats fed ammonium perfluorooctanoate (APFO) in the diet for 2 years. Drug Chem Toxicol. 2010;33:131–137. doi: 10.3109/01480541003667610. [DOI] [PubMed] [Google Scholar]
- 31.Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci. 2011;120:42–58. doi: 10.1093/toxsci/kfq379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilton SC, Orner GA, Benninghoff AD, Carpenter HM, Hendricks JD, Pereira CB, et al. Genomic profiling reveals an alternate mechanism for hepatic tumor promotion by perfluorooctanoic acid in rainbow trout. Environ Health Perspect. 2008;116:1047–1055. doi: 10.1289/ehp.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Y, Dai J, Liu M, Wang J, Xu M, Zha J, et al. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus) Environ Toxicol Chem. 2007;26:2440–2447. doi: 10.1897/07-008R1.1. [DOI] [PubMed] [Google Scholar]
- 34.Aoyama H, Couse JF, Hewitt SC, Haseman JK, He H, Zheng X, et al. Upregulation of estrogen receptor expression in the uterus of ovariectomized B6C3F1 mice and Ishikawa cells treated with bromoethane. Toxicol Appl Pharmacol. 2005;209:226–235. doi: 10.1016/j.taap.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Newbold RR, McLachlan JA. Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 1982;42:2003–2011. [PubMed] [Google Scholar]
- 36.Eroschenko VP, Osman F. Scanning electron microscopic changes in vaginal epithelium of suckling neonatal mice in response to estradiol or insecticide chlordecone (Kepone) passage in milk. Toxicology. 1986;38:175–185. doi: 10.1016/0300-483x(86)90118-6. [DOI] [PubMed] [Google Scholar]
- 37.Holderegger C. Ultrastructural study of the mucification of the stratified epithelium of the mouse vagina. Cell Tissue Res. 1980;213:475–482. doi: 10.1007/BF00237892. [DOI] [PubMed] [Google Scholar]
- 38.McClain R, Wolz E, Davidovich A, Pfannkuch F, Edwards J, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44:56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Mori T, Mills KT, Bern HA. Sensitivity of the vagina and uterus of mice neonatally exposed to estrogen or androgen to postnatal treatment with estrogen or androgen. Proc Soc Exp Biol Med. 1992;199:466–469. doi: 10.3181/00379727-199-43382. [DOI] [PubMed] [Google Scholar]
- 40.DeManno D, Elger W, Garg R, Lee R, Schneider B, Hess-Stumpp H, et al. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 2003;68:1019–1032. doi: 10.1016/j.steroids.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi K, Hayashi M, Boutin E, Cunha GR, Bernfield M, Trelstad RL. Hormonal modification of epithelial differentiation and expression of cell surface heparan sulfate proteoglycan in the mouse vaginal epithelium. An immunohistochemical and electron microscopic study. Lab Invest. 1988;58:68–76. [PubMed] [Google Scholar]
- 42.Liu C, Du Y, Zhou B. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquat Toxicol. 2007;85:267–277. doi: 10.1016/j.aquatox.2007.09.009. [DOI] [PubMed] [Google Scholar]