Abstract
Exposure to perfluorooctanoic acid (PFOA), a synthetic perfluorinated compound and an agonist of peroxisome proliferators-activated receptor α (PPARα), causes stunted mouse mammary gland development in various developmental stages. However, the underlying mechanisms remain poorly understood. We found that peripubertal PFOA exposure significantly inhibited mammary gland growth in both Balb/c and C57Bl/6 wild type mice, but not in C57Bl/6 PPARα knockout mice, and Balb/c mice were more sensitive to PFOA inhibition. PFOA caused 1) delayed or absence of vaginal opening and lack of estrous cycling during the experimental period; 2) decreases in ovarian steroid hormonal synthetic enzyme levels; and 3) reduced expression of estrogen- or progesterone-induced mammary growth factors. Supplementation with exogenous estrogen and/or progesterone reversed the PFOA inhibitory effect on mammary gland. These results indicate that PFOA effects on ovaries mediate its inhibition of mammary gland development in Balb/c and C57Bl/6 mice and that PPARα expression is a contributing factor.
Keywords: Perfluorooctanoic acid (PFOA), mammary gland development, puberty, steroid hormones, growth factors, peroxisome proliferator-activated receptor α (PPARα)
1. Introduction
Perfluorooctanoic acid (PFOA), a synthetic perfluorinated compound, has raised significant health concerns because of its widespread presence in the environment, in wildlife and humans, its persistence and accumulation properties, and its recently reported developmental toxicities in rodents [1–3]. Although blood concentrations of PFOA in the general population exposed to PFOA through air, food and drinking water seem to be low (<10 ng/ml), high blood concentrations of PFOA have been reported in humans exposed occupationally and through contaminated drinking water exposure have been reported, with a range of 0.25–22,412 ng/ml [4]. While previous classical toxicology studies using adult animals suggested that the acute toxicity of PFOA and other perfluorinated chemicals are low to moderate in mice, rats and rabbits [5,6], recent studies have revealed not only general development toxicity [1, 7–9] but also specific negative effects on mammary gland development in mice exposed to PFOA during various critical developmental stages [3, 10–14]. Only mice have been studied because the rapid excretion of PFOA in female rats (half life ~4 hours) precludes studies on mammary gland in that species [15].
Studies by White et al. [3,10] showed that either gestational or lactational PFOA exposure in CD-1 mice impairs the development of the mammary gland. However, the underlying mechanisms are not well understood. The peripubertal period is considered to be an important window of susceptibility of the developing mammary gland to environmental exposures [16,17]. Our recent studies showed that while Balb/c mice exposed to PFOA (5, 10 mg/kg) during the peripubertal period exhibited inhibition of mammary gland and uterine development, similarly exposed C57BL/6 mice exhibited stimulatory effects in both organs at the low dose (5 mg/kg) but inhibition at the higher dose (10 mg/kg) [12]. The mechanisms responsible for the strain-specific differences in effects of peripubertal exposure to PFOA on mammary gland development are not known.
Steroid hormones and growth factors play important roles in pubertal mammary gland development [18–21]. Estradiol (E) and progesterone (P), mainly produced by the ovaries, are the major steroid hormones that promote mammary gland development. In addition, growth factors produced in mammary glands are also involved in stimulating pubertal mammary gland development, including insulin-like growth factor I (IGF-I) [21], amphiregulin (Areg, a member of epidermal growth factor family and a ligand of epidermal growth factor receptor) [18–21] and hepatocyte growth factor (HGF) [22–24]. We have shown that 5 mg/kg of PFOA treatment that stimulated mammary gland growth significantly increased the ovarian protein levels of critical steroid hormone synthetic enzymes and also increased serum progesterone levels in C57Bl/6 mice [13]. Moreover, PFOA treatment also up-regulated the protein levels of epidermal growth factor receptor (EGFR), estrogen receptor α (ERα), Areg, HGF, cyclin D1 and proliferating cell nuclear antigen (PCNA) in C57Bl/6 mammary glands [13]. These results suggest that PFOA effects of increasing steroid hormone and growth factor levels mediate its stimulation of peripubertal mammary gland development in C57Bl/6 mice. Given the requirement of hormones and growth factors for pubertal mammary gland development, it was of interest to determine if PFOA exposure altered hormone and/or growth factor expression and whether they played a role in the observed inhibitory effects of PFOA on mammary gland development
The peroxisome proliferator-activated receptor α (PPARα) is a ligand-activated nuclear receptor critically involved in regulating inflammatory responses, cell proliferation, and differentiation [25–27]. PFOA is an agonist of PPARα and studies using PPARα knockout 129S1/SvlmJ mice have shown that the general developmental toxicity caused by PFOA depends on the expression of PPARα [1]. However, we found that 5 mg/kg of PFOA treatment promoted mammary gland development in both C57Bl/6 wild type mice and PPARα knockout mice [13], indicating that PFOA stimulation of mammary gland development in C57Bl/6 mice is independent of the expression of PPARα. Whether the inhibitory effect of PFOA on mouse mammary gland development depends on the expression of PPARα is not known.
This study was designed to investigate the potential mechanism(s) by which peripubertal PFOA exposure inhibits mouse mammary gland development. We found that PFOA treatment impaired ovary function and reduced protein levels of growth factors produced in mammary glands in Balb/c and C57BL/6 wild type mice. Supplementation with physiological levels of exogenous estradiol (E), progesterone (P) or E+P overcame the inhibition of PFOA on mammary gland growth. Balb/c and C57Bl/6 wild type mice showed different dose sensitivity to the PFOA inhibitory effect. However, C57Bl/6 PPARα knockout mice were resistant to the inhibitory effect of PFOA on peripubertal mammary gland development at all doses tested. This is likely due to the differences in PFOA plasma levels in wild type and knockout mice. These results suggest that PFOA effects on the ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice and that PPARα expression is a contributing factor.
2. Materials and Methods
2.1. Animals
Female 3-week-old Balb/c and C57Bl/6 wild type mice were obtained from Charles River Laboratories (Portage, MI). Animals were weighed upon arrival and randomly distributed into different treatment groups. The C57BL/6 PPARα knockout breeding mice were purchased from Taconic Farms, Inc., (Hudson, NY) to generate 3-week- old PPARα knockout female mice. All mice received food (8640 Harlan Teklad 22/5 Rodent Diet) and tap water ad libitum, and were housed in micro-isolator cages. Animal facilities were maintained on a 12:12 h light–dark cycle, at 20–24 °C and 40–50% relative humidity. All animal protocols were reviewed and approved by the Michigan State University Institutional Animal Care and Use Committee.
2.2. Study protocols
PFOA, as its ammonium salt (>98% pure), was obtained from Fluka Chemical (Steinheim, Switzerland). 17β-Estradiol (E) and progesterone (P) were purchased from Sigma Chemical Co. (St Louis, MO). The stock solutions of E (1 mg/ml) and P (5 mg/ml) were made in 100% ethanol or in 0.85% saline with gum Arabic, respectively. The stock solutions were diluted in 0.85% saline to the final concentrations for injection. PFOA dosing solution was prepared fresh daily in de-ionized water and given to animals as described previously [12,13]. Briefly, Balb/c, C57Bl/6 wild-type or PPARα knockout female mice received either vehicle control (de-ionized H2O) or PFOA at 2.5 mg/kg body weight (BW) (for Balb/c mice) and 7.5mg/kg BW (for C57Bl/6 wild type and PPARα knockout mice) by oral gavage, once daily, 5 days per week for 4 weeks starting at 21 days of age (5–10 mice per group). Because the half-life of PFOA is ~17 days in mice [8], the mice were not treated on the weekend. BW and appearance of vaginal opening were monitored daily. The animals were sacrificed after 4 weeks of treatment. The estrous cycle status was determined by vaginal smear at termination and additional histological examination of hematoxylin and eosin stained (H&E) sections from ovaries and uterus.
To study whether inhibition of PFOA treatment on mammary gland development could be rescued by exogenous hormones E, P, or E + P, mice were treated as follow: ovary-intact Balb/c or C57Bl/6 wild type mice were dosed with vehicle control or PFOA (2.5 mg/kg BW for Balb/c mice and 7.5 mg/kg BW for C57Bl/6 mice) for 2 weeks starting at 21 days of age. During the second week of dosing, the vehicle control- and PFOA-treated mice were also injected sc daily for 7 days with control (0.85% saline; 7dC), physiological levels of E (0.1 μg/0.2 ml per mouse; 7dE), P (0.1 mg/0.2 ml per mouse; 7dP), or E + P (0.1 μg + 0.1 mg/0.2 ml per mouse; 7dE+P) [20,28,29]. Thus, for each mouse strain there were 8 groups: 4 for control-treated mice (C, E, P, E+P) and 4 for PFOA-treated mice (C, E, P, E+P) with 5 mice in each group. The mice were terminated 24 h after the last PFOA and hormone dosing. BW and appearance of vaginal opening were monitored daily. The estrous cycle status was determined by vaginal smear at termination and additional histological examination of H&E sections from ovaries and uterus.
2.3. Necropsy and mammary gland wholemount analysis
Mammary glands, ovaries, uteri, livers, kidneys and blood were harvested at the time of termination. Tissues were fixed in 10% neutral formalin, paraffin embedded, sectioned (5μm) and stained with H&E. One abdominal and inguinal mammary gland from each animal was prepared as wholemount [12,13]. Whole-mount preparations of the inguinal glands from the same position for each mouse were scored for growth and developmental status as previously described [12,13].
2.4. Measurement of mouse plasma PFOA levels
Samples from five mice of each stain treated with various doses of PFOA (Balb/c:1 and 5 mg/kg; C57Bl/6 wildtype: 1, 5, and 10 mg/kg; C57Bl/6 PPARα knockout, 5 mg/kg) collected from previous studies [12,13] along with samples collected in the current study (Balb/c: 2.5 mg/kg; C56Bl/6 wildtype and PPARα knockout: 7.5 mg/kg) were prepared according to the protocol described by Reiner et al. [30]. In brief, control animal plasma (25 μl) was denatured with 0.1 M formic acid (containing 13C2-PFOA) protein precipitated with cold acetonitrile and centrifuged to pelletize proteins. An aliquot of the acetonitrile extract was combined 50:50 with 2 mM ammonium acetate for UPLC/MS-MS analysis (Waters Acquity UPLC coupled with a Quatro Prmier XE MS/MS; Waters Corporation (Milford, MA). PFOA was monitored via the transition 413–369 and 413–169 and for the 13C2-PFOA 415–370. The standard curve and QA/QC samples were matrix matched by spiking PFOA in methanol from alternate stock solutions into control CD1 mouse serum (Pel-Freez) relating to 10 to 200,000 ng PFOA/ml serum and were likewise treated as all other samples. PFOA-dosed animal plasma (25 μl) was placed in a 15 ml polypropylene tube (BD Falcon) and diluted with 5 ml of 0.1 M formic acid. Diluted samples were shaken on a rotary shaker for 1 hour in the horizontal position, and then sonicated for 30 minutes. A 0.2 ml aliquot of the diluted plasma sample was placed in a new 15 ml PP tube and further diluted with 3 ml of methanol containing ~100 ng 13C2-PFOA. Samples were shaken (30 minutes) and vortexed before sampling. An aliquot of the methanol extract (200 μl) was combined with 200 μl of 2 mM ammonium acetate for LC/MS-MS analysis. As above all unknowns, replicates, method and matrix blanks, QA/QC and standard curve samples were prepared in this fashion. The standard curve was prepared by spiking in a corresponding mass of PFOA in methanol (25 – 5000 ng) relating to 10,000 to 200,000 ng PFOA/ml serum.
Appropriate QA/QC samples were selected for the standard curve range and analyzed in duplicate. Because the method was designed for serum sample analysis, 10% of the unknown plasma samples were randomly selected for standard addition analysis as a measure of method accuracy and 10% for replicate analysis as a measure of the methods precision performance. Samples were run in an analytical batch to include solvent blanks, a method blank, matrix blank (blank serum), standards, QA/QC samples, replicates, and unknowns in sequence. Standards were run at the beginning and end of the analytical batch, and QA/QC samples interspersed in the analytical batch. Samples were integrated using the equipment software and corrected if necessary by the operator.
2.5.Quantitative RT-PCR analysis (q-RT-PCR)
Total RNA was extracted from mouse kidney by TRIzol® reagent (Invitrogen, Carlsbad, CA), purified by RT2 qPCR-Grade RNA isolation kit (SAbiosciences, Frederick, MD) as described previously [13]. Three μg of total RNA was used for making the first strand complementary DNA (cDNA, in 20 μl volume) using RT2 First Strand kit (SAbiosciences) following the manufactures’ instructions. The generated first strand cDNAs (20 μl) was diluted to 150 μl with de-ionized H2O (ddH2O). Then 1 μl was used for q-PCR analysis (in a 96 well plate) for the selected genes for mouse kidney. The primers for q-PCR analysis of the following genes were purchased from SAbiosciences: organic anionic transporter 1 (OAT1) (Cat#: PPA27727E); OAT2 (Cat#: PPM30509A); OAT3 (Cat #: PPM37446A); organic anionic transporting polypeptide 1 (OATP1) (Cat#: PPM30654A); OATP2 (Cat #: PPM30541A); sodium-taurocholate cotransporting polypeptide (NTCP) (Cat #: PPM30690A); 18S ribosomal ribonucleic acid (rRNA) (Cat#: PPM57735E); Ribosomal protein L32 (RPL32) (Cat #: PPM03300B). Kidney specific organic anionic transporter (OAT-K) (Cat #: Rn00755673_m1) primers were obtained from Applied Biosystems Inc. (Forster city, CA). The q-PCR was performed with the ABI7500 Fast Real-time PCR system (Applied Biosystems Inc.). RNA samples from three mice (n=3) in control- or PFOA-treated group were analyzed.
2.6. Western blot analysis
Snap frozen mammary glands were homogenized in RIPA buffer containing the protease inhibitor cocktail, phenylmethylsulphonyl fluoride(PMSF) and sodium orthovanadate from Santa Cruz Biotechnology (Cat #: sc-24948). Ovarian proteins were isolated from the phenol solution after RNA extraction by TRIzol reagent following the manufacture’s instruction. Protein concentration was determined by Bio-Rad Dc protein assay kit. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. All following primary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA): rabbit polyclonal anti-steroidogenic acute regulatory protein (StAR) antibody (Ab) (Cat #: sc-25806); goat polyclonal anti-cytochrome P450 family11, subfamily A, polypeptide 1 (CYP11A1) Ab (Cat #: sc-18043); goat polyclonal anti-aromatase (CYP19A1) Ab (Cat #: sc-14245); rabbit polyclonal anti-HSD17β1 Ab (Cat #: sc-32872); goat polyclonal anti-HSD3β1 Ab (Cat #: sc-30820); mouse monoclonal anti-Areg Ab (Cat #: sc-74501); rabbit polyclonal anti-ERα Ab (Cat #: sc-542); rabbit polyclonal anti-EGFR Ab (Cat #: sc-03); goat polyclonal anti-IGF-1 Ab (Cat #: sc-1422); goat polyclonal anti-HGF Ab (Cat #: sc-1358); and mouse monoclonal anti-GAPDH Ab (Cat: sc-32233). Mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) Ab was purchased from Calbiochem® (Gibbstown, NJ; Cat #: NA-03). Rabbit polyclonal anti-PPARα Ab (Cat #: ab8934) was purchased from Abcam (Cambridge, MA). Thirty μg of total protein per sample was loaded to 12% SDS-PAGE gels for Western blot analysis as previously described [13]. The blots were incubated with primary Abs diluted with 1:1 of PBS:Odyssey block buffer (Cat #: 927-40000) (Abs final concentration 0.2–0.5 μg/ml) for 2 h at room temperature. After three washes with PBST, the blots were incubated with the Alexa Fluor–labeled secondary goat anti-mouse, goat anti-rabbit, or donkey anti-goat Ab for 1 h at room temperature. Then, the blots were scanned using the Odyssey Infrared Imaging System (Li-Cor, Inc., Lincoln, NE) after three washes. Three animals from control- or PFOA-treated group were analyzed. The resulting bands were quantified using Odyssey Infrared Imaging System software 3.0 following manufacturer’s instructions.
2.7. Double immunofluorescent staining of Areg and ERα in mouse mammary gland sections
Sections (5 μm) of paraffin-embedded mammary glands were subjected to antigen retrieval and immunofluorescent staining as previously described [13]. The stained sections were visualized with a Nikon Eclipse TE2000-U fluorescence microscope (Nikon Inc., Melville, NY), and the captured fluorescent images were analyzed using Metamorph software. A minimum of 1000 cells were counted for each section and a minimum of two to three tissue sections per animal were analyzed from three animals in control- or PFOA-treated groups. The number of Areg and/or ERα positive cells is expressed as the percentage of total luminal epithelial cells counted.
2.8. Statistical analysis
Values are presented as mean ± SD. Differences between control and treatment groups were determined using Student’s t-tests for comparison of two data sets or one-way analysis of variance (ANOVA) for multiple data sets and differences were considered significant at p < 0.05.
3. Results
3.1. Balb/c and C57Bl/6 wild type mice and C57Bl/6 PPARα knockout mice exhibit different dose sensitivity to the inhibitory effect of PFOA on peripubertal mammary gland development
We previously reported differential effects of peripubertal exposure to PFOA on mammary gland development in Balb/c and C57Bl/6 mouse strains: (i) exposure to 1 mg/kg of PFOA did not show a significant effect on mammary gland development in both Balb/c and C57Bl/6 wild type mice; (ii) exposure to 5 mg/kg of PFOA caused significant inhibition of mammary gland development in Balb/c mice. Thus we tested a dose between 1 & 5 on mammary gland inhibition and the lower dose of 2.5 mg/kg of PFOA caused inhibition. (ii) exposure to 5 mg/kg of PFOA caused stimulation and 10 mg/kg of PFOA resulted in severe inhibition of mammary gland development in C57Bl/6 wild type mice”. Thus we tested an intermediate dose of 7.5mg/kg and found this dose to be inhibitory [12,13]. The goals of this study were to investigate PFOA inhibition of mammary gland development in Balb/c and C57Bl/6 mice and investigate the mechanism of PFOA inhibitory effects. The lowest doses of PFOA 2.5 mg/kg for Balb/c mice and 7.5 mg/kg for C57Bl/6 mice that produced mammary gland inhibition were chosen.
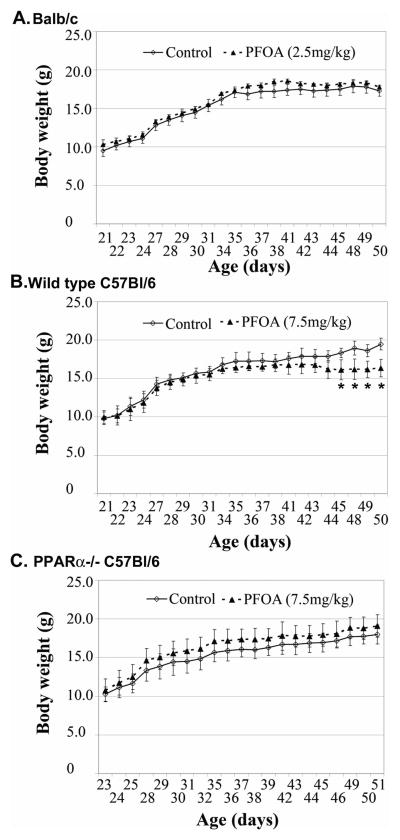
All mice displayed normal behavior and appeared healthy throughout the PFOA treatment period. The effects of peripubertal PFOA exposure on mouse body weight (BW) during the entire experiment treatment are shown in Fig. 1. No significant differences of BW were observed between Vehicle Control- and PFOA-treated Balb/c (2.5 mg/kg) or C57Bl/6 PPARα knockout (7.5 mg/kg) mice during the entire PFOA treatment period. In C57Bl/6 wild type mice treated with 7.5 mg/kg of PFOA, significant decreases of BW were observed only during the last week (the 4th week) of PFOA treatment. No obvious changes of food consumption behavior were observed between vehicle control- and PFOA-treated mice.
Fig. 1. Effect of peripubertal PFOA exposure on body weight in Balb/c, C57Bl/6 wild type and C57Bl/6 PPARα knockout mice.
Three-week old female Balb/c (A), C57Bl/6 wild type (B) or PPARα knockout (C) mice were treated with vehicle control (deionized H2O) or PFOA (2.5mg/kg for Balb/c mice, 7.5mg/kg for C57Bl/6 mice) for 4 weeks. Body weight was monitored daily during PFOA dosing period. Mouse body weight is presented as mean ± standard deviation (n=5). * p<0.05 compared to vehicle control-treated mice.
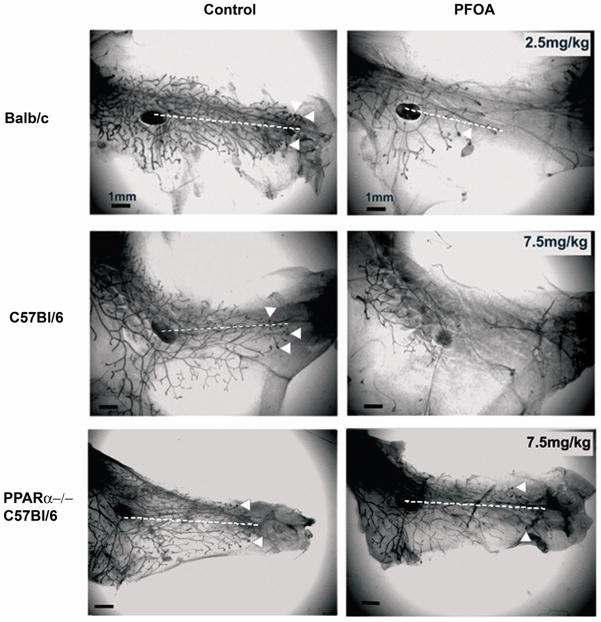
In striking contrast to a lack of effect of PFOA treatment on Balb/c BW, exposure to 2.5 mg/kg of PFOA significantly inhibited mammary gland growth as evidenced by reduced ductal length, decreased numbers of terminal end buds (TEBs) and stimulated ducts (STDs) (Fig. 2 and Table 1). Similarly, exposure to 7.5 mg/kg of PFOA caused significant inhibition of mammary gland growth in C57Bl/6 wild type mice (Fig. 2 and Table 1). However, no inhibition of mammary gland growth was observed in PFOA-treated (7.5 mg/kg) C57Bl/6 PPARα knockout mice (Fig. 2 and Table 1). Although PFOA treatment significantly inhibited mammary gland growth in Balb/c and C57Bl/6 wild type mice, PFOA had no significant effect on the weight of mammary glands/fat pads (Table 1).
Fig. 2. Peripubertal PFOA exposure inhibits mammary gland development in Balb/c and C57Bl/6 wild type mice but not in C57Bl/6 PPARα knockout mice.
Three-week old female Balb/c, C57Bl/6 wild type or PPARα knockout mice were treated with vehicle control (deionized H2O) or PFOA (2.5mg/kg for Balb/c mice, 7.5mg/kg for C57Bl/6 mice) for 4 weeks. Mice were sacrificed 24 h after the last treatment and mammary gland whole mounts were prepared as described in Materials and Methods. Representative photomicrographs of mammary gland whole mounts from vehicle control- and PFOA -treated mice. Note the reduced ductal length (dotted lines) and reduced number of TEB (arrow heads) in PFOA-treated wild type Balb/c and C57BL/6 mammary glands
Table 1.
Effects of PFOA treatment on mouse mammary gland ductal length, the number of terminal end buds (TEBs), the number of stimulated terminal ducts (TDs), absolute left inguinal mammary gland weight (AMGW) and relative left inguinal mammary gland weight (RMGW) (mean ± standard deviation, n=5)
| Treatment | Ductal length (relative units) | Number of TEBs | Stimulated TDs | AMGW (g) | RMGW (%) |
|---|---|---|---|---|---|
| Balb/c | |||||
| Vehicle Control | 8.17 ± 1.08 | 7.40 ± 1.14 | 6.11 ± 2.52 | 0.09±0.004 | 0.51±0.04 |
| PFOA (2.5mg/kg) | 4.99 ± 0.67* | 3.25 ± 0.95* | 2.63 ± 1.41* | 0.11±0.024 | 0.63±0.13 |
| C57Bl/6 Wild type | |||||
| Vehicle Control | 6.30 ± 0.96 | 6.25 ± 2.06 | 6.50 ± 2.89 | 0.15±0.035 | 0.77±0.18 |
| PFOA (7.5mg/kg) | 2.10 ± 1.98* | 1.00 ± 1.41* | 0.80 ± 1.31* | 0.11±0.026 | 0.66±0.13 |
| C57Bl/6 PPARα−/− | |||||
| Vehicle Control | 8.90 ± 1.04 | 10.00 ± 4.36 | 6.20 ± 1.64 | 0.17±0.035 | 0.88±0.21 |
| PFOA (7.5mg/kg) | 8.43 ± 1.08 | 9.43 ± 1.90 | 7.02 ± 2.16 | 0.14±0.026 | 0.78±0.11 |
Three-week old female Balb/c, C57Bl/6wild type and PPARα knockout mice were treated with vehicle control or PFOA for 4 weeks and sacrificed 24 h after the last treatment.
Relative mammary glands weight (RMGW) (%) = absolute mammary glands weight (AMGW)/Body weight
p< 0.05, compared with Vehicle Control-treated group in each mouse strain.
3.2. Balb/c mice have significantly higher plasma levels of PFOA than C57Bl/6 mice
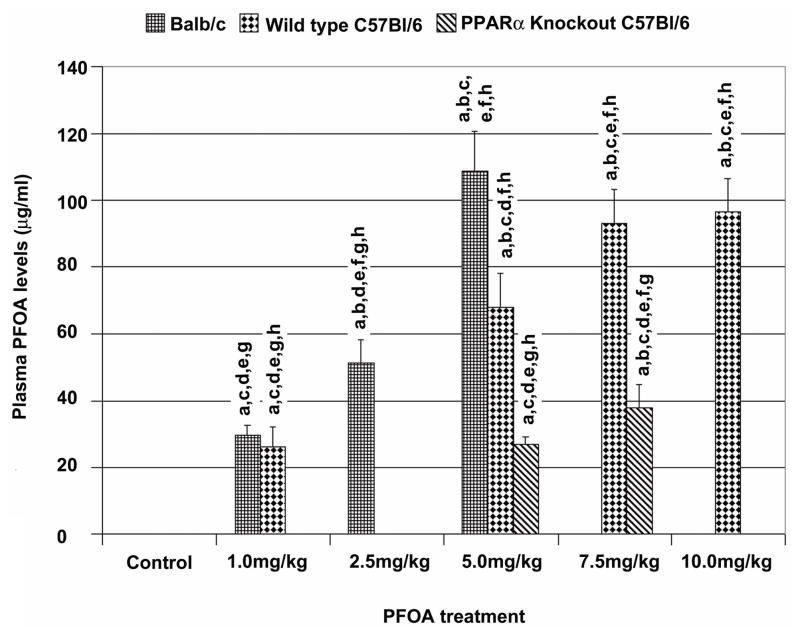
We considered the possibility that the strain-specific differences in sensitivity to the inhibitory effect of PFOA might be due to differences in PFOA body burden. For the purpose of comparing mouse plasma PFOA levels from different doses of PFOA treatment that caused different effects on mammary gland development, plasma PFOA levels from mice used in this and previous studies were measured using a UPLC-MS/MS method [30]. The mice in the previous PFOA studies were treated in the same way as those in the current study and PFOA level analysis from all plasma samples were carried out under the same conditions. As shown in Fig. 3, there was no significant difference of plasma PFOA levels between 1 mg/kg of PFOA-treated Balb/c and C57Bl/6 wild type mice. However, plasma PFOA levels of Balb/c mice were significantly higher than C57Bl/6 mice treated with 5 mg/kg PFOA dose for the same amount of time (4 weeks). Moreover, Balb/c mice treated with 2.5 mg/kg of PFOA had plasma PFOA levels similar to that of C57Bl/6 mice treated with 5 mg/kg of PFOA. Interestingly, C57Bl/6 PPARα knockout mice had the lowest plasma PFOA levels at the 5 mg/kg and 7.5 mg/kg doses. In addition, C57Bl/6 PPARα knockout mice were also given a higher dose (10 mg/kg) of PFOA, no significant differences of plasma PFOA levels between 7.5 mg/kg and 10 mg/kg of PFOA-treated mice were observed. Similarly, 10 mg/kg PFOA treatment showed no significant effect on mammary gland development in PPARα knockout mice (data not shown).
Fig. 3. Plasma PFOA levels in vehicle control- and PFOA-treated mice.
Three-week old female Balb/c, C57Bl/6 wild type and PPARα knockout mice were treated with vehicle control or indicated doses of PFOA for 4 weeks and sacrificed 24 h after the last treatment. Mouse plasma samples were collected for analyzing PFOA levels as described in Materials and Methods. Plasma samples of Balb/c mice treated with 1 or 5 mg/kg of PFOA; of C57Bl/6 wild type mice treated with 1, 5, or 10 mg/kg of PFOA; and of C57Bl/6 PPARα−/− mice treated with 5 mg/kg of PFOA were from previous studies (refs 12,13). All mice were treated the same way as those in current study and plasma PFOA levels were determined in the same laboratory under the same conditions. Mouse plasma PFOA levels are presented as mean ± standard deviation (n=5). PFOA levels in vehicle control-treated mice are lower than 0.01 μg/ml. a p<0.05, compared with vehicle control group; b p<0.05, compared with 1 mg/kg of PFOA-treated Balb/c or C57Bl/6 mice; c p<0.05, compared with 2.5 mg/kg of PFOA-treated Balb/c mice; d p<0.05, compared with 5 mg/kg of PFOA-treated Balb/c mice; e p<0.05, compared with 5 mg/kg of PFOA-treated C57Bl/6 wild type mice; f p<0.05, compared with 5 mg/kg of PFOA-treated C57Bl/6 PPARα knockout (PPARα−/−) mice; g p<0.05, compared with 7.5 or 10 mg/kg of PFOA-treated C57Bl/6 wild type mice; h p<0.05, compared with 7.5 mg/kg of PFOA-treated C57Bl/6 PPARα knockout (PPARα−/−) mice.
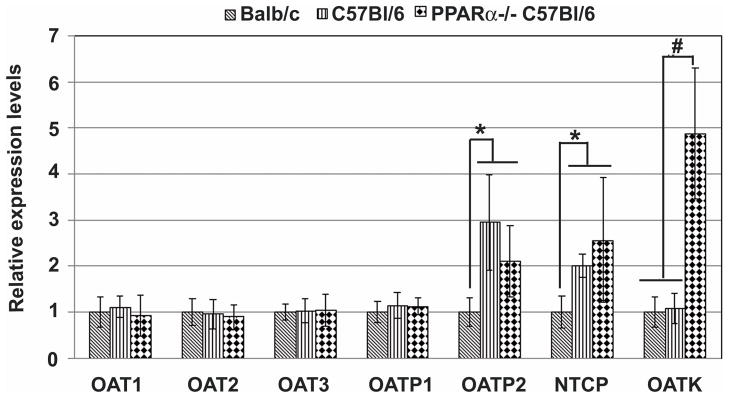
It has been reported that PFOA is excreted mainly through the kidney by organic anionic transporters (OATs) in rats [31]. The expression levels of 7 OATs in Balb/c and C57Bl/6 kidneys were analyzed using Q-PCR. As shown in Fig. 4, there were no significant differences in expression levels of 4 OATs [OAT1, OAT2, OAT3 and OATP1 (organic anionic transporting polypeptide 1)] between the two mouse strains. However, Balb/c mice had significantly lower expression levels than C57Bl/6 mice of OATP2 and NTCP (sodium-taurocholate cotransporting polypeptide). C57Bl/6 PPARα knockout mice had significantly higher expression levels of OAT-K (kidney specific organic anionic transporter) than Balb/c and C57Bl/6 wild type mice. PFOA treatment did not change the expression levels of OATs in Balb/c and C57Bl/6 PPARα knockout mice (data not shown). In C57Bl/6 wild type mice, 7.5 mg/kg of PFOA treatment significantly reduced the expression levels of OATP2 (−3.20±1.43), but had no effect on the levels of other OATs (data not shown).
Fig. 4. Relative expression levels of kidney organic anionic transporters (OATs) in wild type Balb/c, C57Bl/6 and C57BL/6 PPARα knockout mice.
Balb/c and C57Bl/6 mice were sacrificed 24 h after the last vehicle control or PFOA treatment. RNA samples were prepared from frozen kidneys and used for quantitative RT-PCR analysis of OAT expression levels as described in Materials and Methods. The OAT levels were expressed relative to Balb/c mice (mean ± standard deviation, n=3). * p<0.05 compared to Balb/c mice; # p<0.05 compared to Balb/c and C57Bl/6 wild type mice.
3.3. PFOA treatment alters ovary functions
The ovaries are the major source of estrogen and progesterone required for normal pubertal mammary gland development. Thus, we next examined the effect of inhibitory doses of PFOA on ovarian function. Vaginal opening is dependent upon estrogen and is an indicator of initiation of ovarian function and estrous cycling [32]. As shown in Table 2, the mean age at vaginal opening was significantly later in PFOA-treated Balb/c mice and vaginal opening was absent in PFOA-treated C57Bl/6 wild type mice at the time of termination. These results indicate delayed and absence of ovarian function in Balb/c and C57BL/6 wild type PFOA-treated mice. In contrast and consistent with a lack of inhibition of mammary gland development, PFOA treatment had no significant effect on the mean age at vaginal opening in C57BL/6 PPARα knockout mice. Histological analysis of ovaries revealed absence of estrous cycling as determined by the absence of corpora lutea (CL) from both ovaries along with the uterine alterations (thinning of the endometrium and myometrium, similar findings as reported in ref. 12) in PFOA-treated Balb/c and C57Bl/6 wild type mice (Table 2). However, both control- and PFOA-treated C57BL/6 PPARα knockout mice exhibited uterine histology indicative of normal estrous cycling with all stages of the cycle seen. Together, these results suggest that PFOA treatment significantly altered ovary function in Balb/c and C57Bl/6 wild type mice.
Table 2.
Effect of PFOA treatment on mouse age at vaginal opening (AVO) (mean ± standard deviation, n=5), and ovarian corpora lutea (CL) status.
| Treatment | AVO (Day) | Presence of CL |
|---|---|---|
| Balb/c | ||
| Vehicle Control | 40.5±2.12 | Yes |
| PFOA (2.5mg/kg) | 46.4±4.04 * | No |
| C57Bl/6 Wild type | ||
| Vehicle Control | 40.2±2.25 | Yes |
| PFOA (7.5mg/kg) | No opening | No |
| C57Bl/6 PPARα−/− | ||
| Vehicle Control | 38.98±2.56 | Yes |
| PFOA (7.5mg/kg) | 39.21±2.97 | Yes |
Three-week old female Balb/c, C57Bl/6wild type and PPARα knockout mice were treated with vehicle control or PFOA for 4 weeks and sacrificed 24 h after the last treatment.
p< 0.05, compared with Vehicle Control-treated group in each mouse strain.
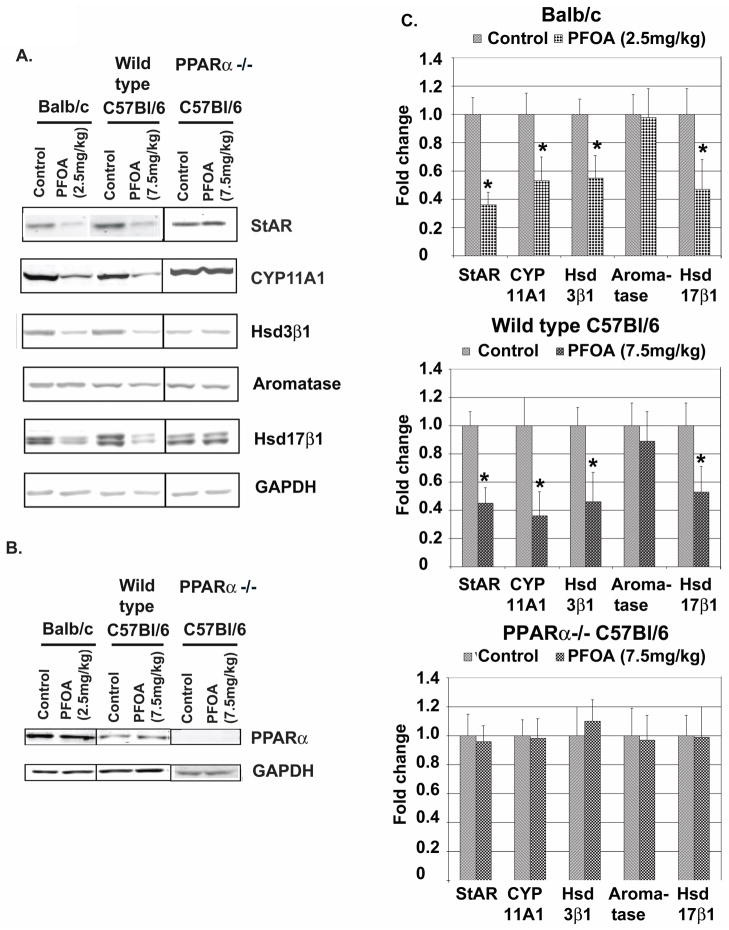
The steroid hormones estradiol (E) and progesterone (P) are mainly produced by the ovaries. E plays dominant role during pubertal development responsible for the production of two important paracrine acting growth factors Areg and HGF. P acting through progesterone receptors can also up-regulate Areg expression [33,34]. E is also required for the induction of progesterone receptors [35,36]. Thus, we next analyzed mouse serum E and P levels. However, we did not have a sufficient number of control-treated mice at the various estrous cycle stages to determine significant differences in estrogen and progesterone levels for each cycle stage. Thus, to further investigate the potential effect of PFOA treatment on ovarian function, the expression levels of five ovarian proteins/enzymes (StAR, CYP11A1, HSD3β1, aromatase, HSD17β1) were analyzed based on their important roles in producing estradiol and progesterone. StAR is an essential protein in transferring cholesterol into mitochondria, providing the raw material for steroid hormone biosynthesis [37]. CY11A1 initiates steroid hormone biosynthesis by converting cholesterol to pregnenolone [37,38]. HSD3β1 is a critical enzyme for conversion pregnenolone to progesterone and androstenediol to testosterone, the precursor for the production of estradiol [39]. Aromatase is a key enzyme for the production of estrogens, converting testosterone to estradiol and androstenedione to estrone [39]. HSD17β1 is an important enzyme in the production of estradiol, mainly converting estrone to estradiol [39]. Western blot analysis showed that PFOA treatment significantly reduced ovarian protein levels of StAR, CYP11A1, HSD3β1 and HSD17β1 in Balb/c and C57Bl/6 wild-type mice; but there was no effect of PFOA treatment on aromatase level (Fig. 5A and quantifications of Western blots in Fig. 5C). In contrast, no changes in these protein levels were detected in PFOA-treated C57Bl/6 PPARα knockout mice (Fig. 5A and C).
Fig. 5. PFOA treatment decreases ovarian protein levels of steroid hormone synthetic enzymes in Balb/c and C57Bl/6 wild type mice but not in C57Bl/6 PPARα knockout mice.
A. Effect of PFOA treatment on ovarian protein levels of steroid hormone synthetic enzymes. Balb/c, C57Bl/6 wild type and PPARα knockout mice were sacrificed 24 h after the last vehicle control or PFOA treatment. Protein samples prepared from frozen ovaries were used for Western blot analysis as described in Materials and Methods. Representative Western blots of protein levels of StAR, CYP11A1, HSD3β1, HSD17β1, aromatase and PPARα in vehicle control- or PFOA-treated ovaries. GAPDH served as a protein loading control. B. Western blot analysis of PPARα protein levels in mouse ovaries. Representative Western blots of protein levels of PPARα in vehicle control- or PFOA-treated ovaries. GAPDH served as a protein loading control. C. Quantification of Western blot analysis for the effect of PFOA treatment on ovarian protein levels of steroid hormone synthetic enzymes in Balb/c and C57Bl/6 wild type and PPARα knockout mice (mean ± standard deviation, n=3). * p< 0.05, compared with Vehicle Control-treated group in each mouse strain.
Since PFOA treatment did not reduce the levels of critical ovarian proteins/enzymes involved in the production of two major steroid hormones in C57Bl/6 PPARα knockout mice, we examined ovarian PPARα protein levels in Balb/c and C57Bl/6 mice. As shown in Fig. 5B, significantly higher ovarian protein levels of PPARα were detected in Balb/c mice compared to C57Bl/6 mice (1.00±0.20 vs 0.43±0.19, p<0.05). However, PFOA treatment did not change PPARα protein levels in either mouse strain (Fig. 5B). These results suggest that in addition to higher PFOA plasma levels, higher ovarian PPARα protein levels may also contribute to the higher sensitivity of Balb/c mice to the inhibitory effect of PFOA on mammary grand development.
3.4. PFOA treatment decreases the protein levels of Areg, HGF, ERα, EGFR and PCNA in Balb/c and C57Bl/6 wild type mammary glands
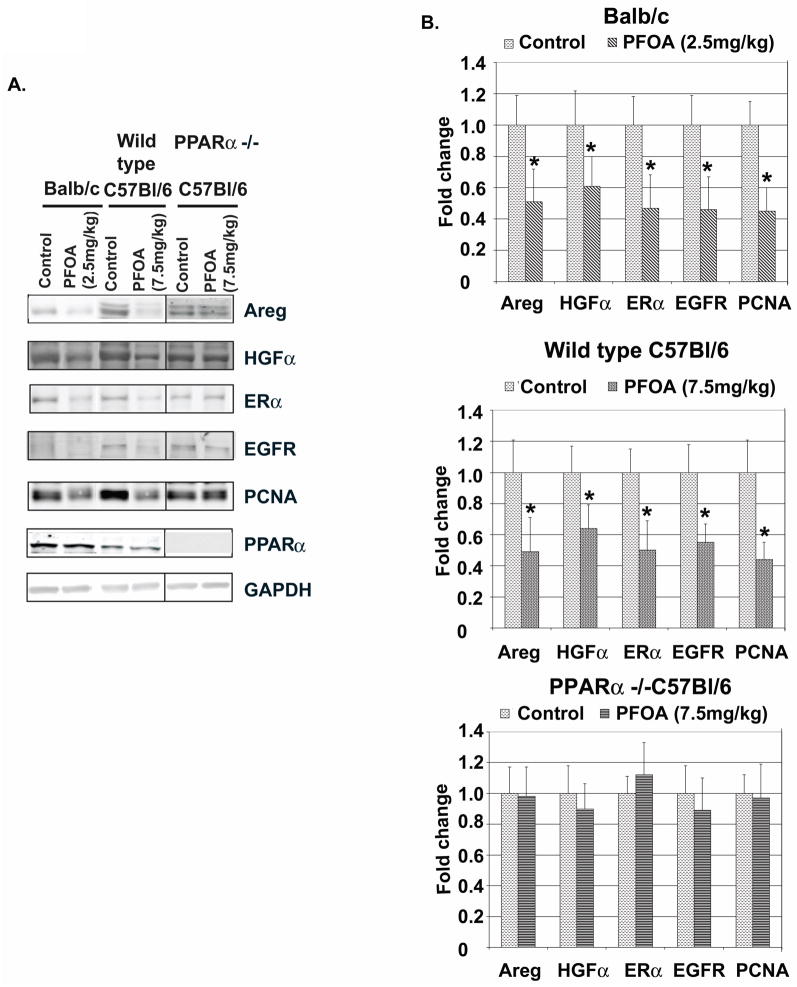
In addition to steroid hormones, local growth factors also play important roles in mammary gland development [18]. Thus, we examined the effect of PFOA treatment on the expression levels of Areg, IGF-I and HGF, growth factors critically involved in mammary gland development. Western blot analysis showed that PFOA treatment significantly decreased the protein levels of Areg and HGF in Balb/c and C57Bl/6 wild type mammary glands (Fig. 6A and quantifications of Western blots in Fig. 6B). PFOA treatment did not change the protein levels of IGF-I (data not shown). Because the expression of Areg can be induced by E through ERα, and Areg is an essential mediator of ERα function in mammary gland development [20], and an EGFR ligand, we also examined the effect of PFOA on the expression levels of ERα and EGFR in mammary glands. PFOA treatment caused significant decreases of ERα and EGFR protein levels in both Balb/c and C57Bl/6 wild type mammary glands (Fig. 6A and B). We also analyzed mammary gland protein levels of proliferating cell nuclear antigen (PCNA), one of the most commonly used markers reflecting active cell proliferation. PFOA treatment greatly decreased PCNA protein levels in Balb/c and C57Bl/6 wild type mammary glands (Fig. 6A and B). In contrast, PFOA treatment had no effect on the protein levels of Areg, HGF, ERα, EGFR or PCNA in C57Bl/6 PPARα knockout mammary glands (Fig. 6A and B). Similar to the observation that Balb/c mice had higher PPARα protein levels in their ovaries than C57Bl/6 mice, significantly higher PPARα protein levels were also detected in their mammary glands (1.00±0.17 vs 0.56±0.21, p<0.05) (Fig. 6A).
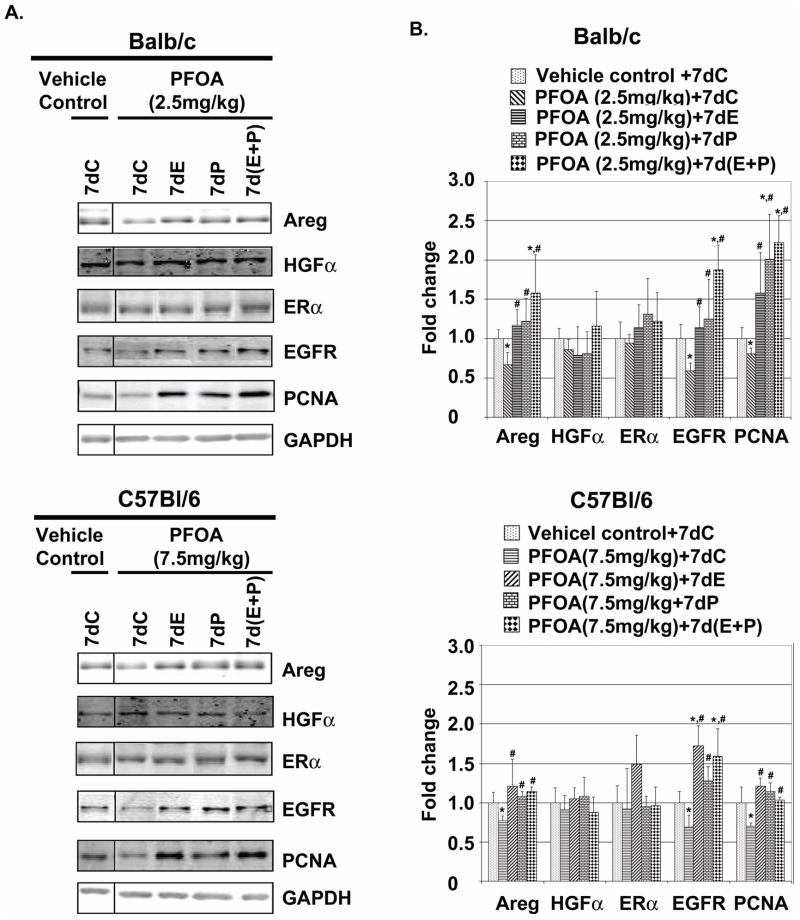
Fig. 6. PFOA treatment reduces mammary gland protein levels of Areg, HGF, ERα, EGFR and PCNA in Balb/c and C57Bl/6 wild type mice but not in C57Bl/6 PPARα knockout mice.
A. Effect of PFOA treatment on mammary gland protein levels of Areg, HGF, ERα, EGFR and PCNA. Balb/c, C57Bl/6 wild type and PPARα knockout mice were sacrificed 24 h after the last vehicle control or PFOA treatment. Protein samples were prepared from frozen mammary glands and used for Western blot analysis as described in Materials and Methods. Representative Western blots of protein levels of Areg, HGF, ERα, EGFR, PCNA and PPARα in vehicle control- or PFOA-treated mammary glands. GAPDH served as a protein loading control. B. Quantification of Western blot analysis for the effect of PFOA treatment on mammary gland protein levels of Areg, HGF, ERα, EGFR and PCNA in Balb/c and C57Bl/6 wild type and PPARα knockout mice (mean ± standard deviation, n=3). * p< 0.05, compared with Vehicle Control-treated group in each mouse strain.
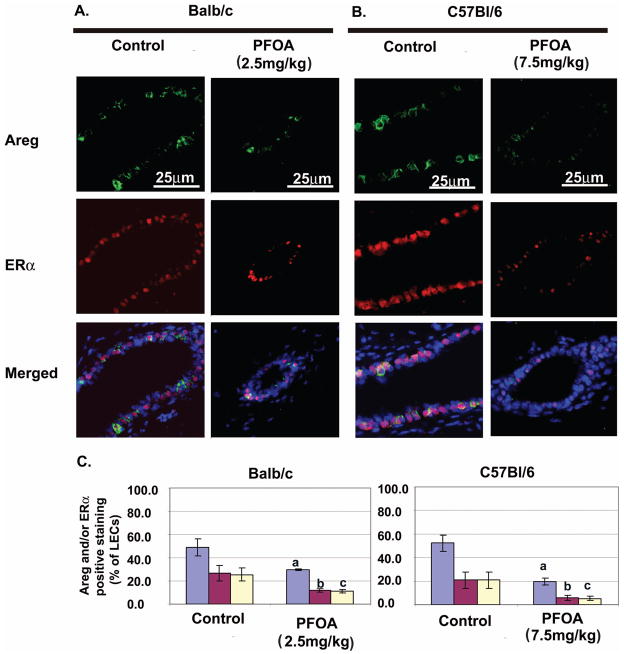
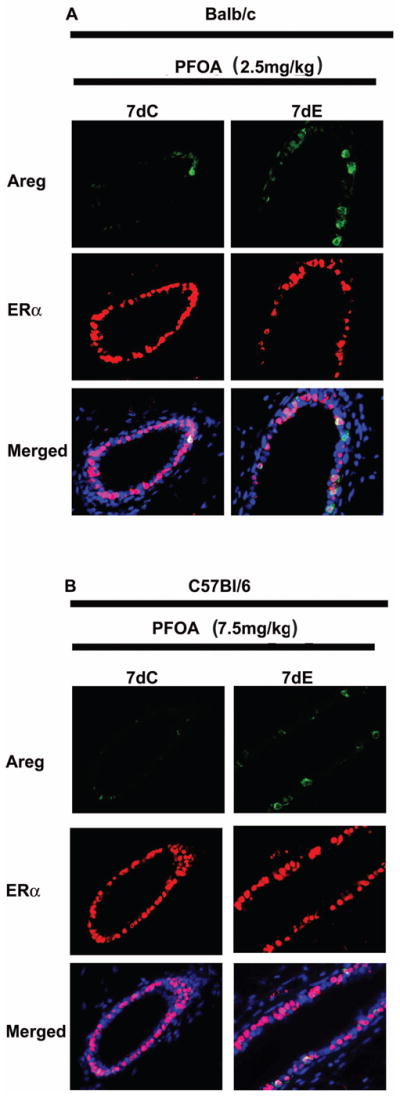
The decreases in Areg and ERα protein levels in PFOA-treated Balb/c and C57Bl/6 wild type mammary glands were further confirmed by double immunofluorescent staining of ERα and Areg (Fig. 7A and B). There were significant decreases in the numbers of Areg positive-, ERα positive-, and Areg plus ERα double positive luminal epithelial cells in PFOA-treated mammary glands compared to those in vehicle control-treated mice (Fig. 7C). Consistent with a recent report showing that the expression of Areg can be induced by E through ERα [20], co-localization of Areg and ERα indicated that Areg was produced by ERα positive cells.
Fig. 7. Effect of PFOA treatment on the expression of Areg and ERα in Balb/c and C57Bl/6 wild type mammary gland luminal epithelial cells (LECs).
Mice were sacrificed 24 h after the last vehicle control or PFOA treatment and mammary glands were processed for dual immunofluorescent staining of Areg and ERα as described in Materials and Methods. A. and B. Representative images of immunoflourescence staining of Areg (green) and ERα (red) in mammary gland sections from vehicle control- or PFOA-treated Balb/c (A) or C57Bl/6 wild type (B) mice. Nuclei were counterstained with DAPI (blue). C. Quantitation of Areg and/or ERα positive mammary LECs in vehicle control- or PFOA-treated mice. Data (mean ± standard deviation, n=3) are presented as percent of total LECs. Blue bar = ERα positive cells; red bar = Areg positive cells; and yellow bar = Areg and ERα double positive cells. a, b. c p<0.05, compared with vehicle control-treated mice.
3.5. Supplementing E, P or E+P overcomes the inhibitory effect of PFOA treatment on mammary gland development
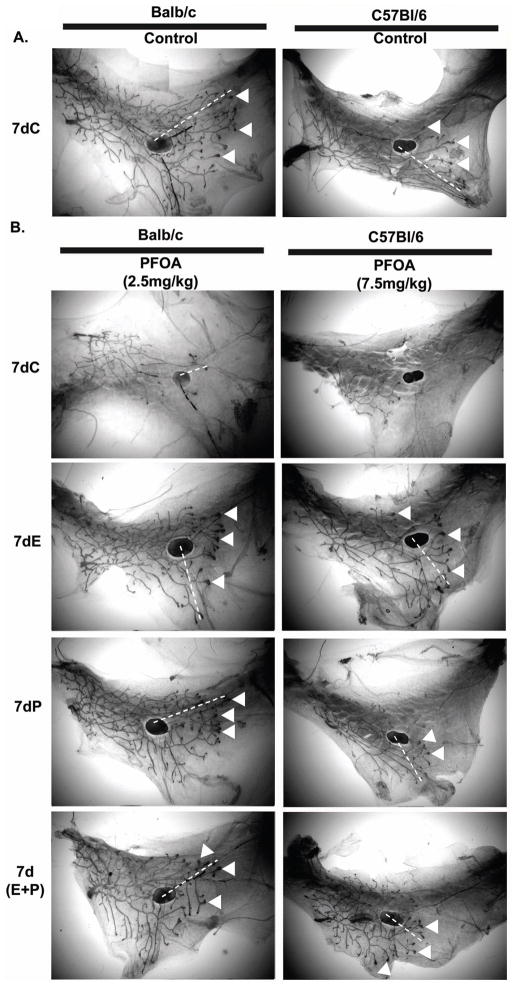
To further determine the mechanism by which PFOA treatment inhibits mouse mammary gland development, we investigated whether steroid hormone supplementation could overcome the inhibitory effect of PFOA treatment on mouse mammary gland development. Three-week old of Balb/c and C57Bl/6 wild type mice were first treated with vehicle control or PFOA for one week, followed by treatment with vehicle control, PFOA, or control or PFOA plus physiological doses of E, P or E+P for another week as described in Materials and Methods. Two weeks of PFOA treatment significantly inhibited mammary gland development in both Balb/c and C57Bl/6 wild type mice evidenced by decreases in the ductal length, the number of TEBs, although PFOA had no significant effect on the weight of mammary glands/fat pads (Fig. 8 and Table 3). In striking contrast, Balb/c and C57Bl/6 wild type mice treated with PFOA plus E, P or E+P all showed mammary gland development comparable to control-treated mammary glands (Fig. 8 and Table 3). These results demonstrated that supplementing E, P, or E+P overcame the inhibitory effect of PFOA treatment in both Balb/c and C57Bl/6 wild type mice. No significant synergistic effect in E+P treatment was observed. This was not surprising since the effect of E+P to synergistically induce side branching and alveologenesis is restricted to the adult gland and not observed in pubertal mice (unpublished observations, Aupperlee & Haslam). Supplementing E, P, or E+P had no significant effect on control-treated mammary gland morphology (data not shown). This may be because control mice have normal functioning ovaries and that the levels of exogenous supplemented hormones did not have an additional stimulatory effect.
Fig. 8. Supplement of exogenous estradiol (E), progesterone (P) or E+P overcomes PFOA mammary gland inhibition in Balb/c and C57Bl/6 mice.
Three-week old female Balb/c or C57Bl/6 wild type mice were treated with vehicle control or PFOA for 1 week, then the mice were treated with vehicle control plus hormone C (vehicle) (A) or PFOA plus C (vehicle), E, P or E+P (B) for another week as described in Materials and Methods. Mice were sacrificed 24 h after the last PFOA/hormones treatment and mammary gland whole mounts were prepared as described in Materials and Methods. Representative photomicrographs of mammary gland whole mounts from vehicle control-, PFOA-, or PFOA plus hormone-treated mice. Note the reduced ductal length (dotted lines) and reduced number of TEB (arrow heads) in PFOA-treated wild type Balb/c and C57BL/6 mammary glands (B 7dC) and the increased ductal length and numbers of TEBs in 7dE, 7dP and 7dE+P supplemented PFOA-treated glands.
Table 3.
Supplement of exogenous estradiol (E), progesterone (P) or E+P overcomes PFOA mammary gland inhibition in Balb/c and C57Bl/6 wild type mice (mean ± standard deviation, n=5)
| Treatment | Ductal length (relative units) | Number of TEBs | AMGW (g) | RMGW (%) |
|---|---|---|---|---|
| Balb/c | ||||
| Vehicle Control | 3.24 ± 1.32 | 5.40 ± 2.61 | 0.096±0.018 | 0.55±0.09 |
| PFOA (2.5mg/kg)+7dC | 1.28 ± 1.33a | 1.67 ± 1.03a | 0.110±0.019 | 0.64±0.08 |
| PFOA (2.5mg/kg)+7dE | 2.76 ± 0.57b | 9.40 ± 1.95a,b | 0.098±0.019 | 0.55±0.06 |
| PFOA (2.5mg/kg)+7dP | 3.13 ± 0.94b | 5.20 ± 1.79b | 0.094±0.024 | 0.56±0.14 |
| PFOA (2.5mg/kg)+7d(E+P) | 2.90 ± 0.53b | 7.40 ± 2.07b | 0.094±0.013 | 0.56±0.09 |
| C57Bl/6 Wild type | ||||
| Vehicle Control | 2.60 ± 0.53 | 4.60 ± 2.51 | 0.106±0.011 | 0.66±0.07 |
| PFOA (7.5mg/kg)+7dC | 0.72 ± 0.80a | 0.67 ± 0.82a | 0.110±0.020 | 0.77±0.14 |
| PFOA (7.5mg/kg)+7dE | 2.53 ± 0.45b | 7.00 ± 1.41b | 0.116±0.026 | 0.72±0.17 |
| PFOA (7.5mg/kg)+7dP | 1.60 ± 0.46 | 2.67 ± 0.58b | 0.116±0.015 | 0.75±0.12 |
| PFOA (7.5mg/kg)+7d(E+P) | 2.13 ± 023b | 7.20 ± 1.10b | 0.118±0.011 | 0.73±0.06 |
Three-week old female Balb/c or C57Bl/6 wild type mice were treated with vehicle control or PFOA for 1 week, then the mice were treated with vehicle control or PFOA plus C (vehicle), E, P or E+P for another week as described in Materials and Methods. Mice were sacrificed 24 h after the last PFOA/hormone treatment and mammary gland whole mounts were prepared as described in Materials and Methods.
Relative mammary glands weight (RMGW) (%) = absolute mammary glands weight (AMGW)/BW
p< 0.05, compared with Vehicle Control-treated group in each mouse strain.
p< 0.05, compared with PFOA-treated group + 7dC in each mouse strain.
3.6. Effect of supplemented E, P or E+P on decreases of growth factor levels in mammary gland by PFOA
Since supplemented E, P or E+P overcame the inhibitory effect of PFOA treatment on mammary gland development, we examined the effect of the hormone supplements on mammary gland protein levels of growth factors and receptors in PFOA-treated mice. The 2-week PFOA treatment decreased mammary gland protein levels of Areg, EGFR and PCNA, but did not change HGF and ERα protein levels (Fig. 9A and quantifications of Western blots in Fig. 9B). The supplement of E, P or E+P increased protein levels of Areg, EGFR and PCNA in PFOA-treated mammary glands (Fig. 9A and B). The effect of E supplement on PFOA-treated mammary gland protein levels of Areg and ERα was further examined by immunofluorescent staining of ERα and Areg. As shown in Fig. 10, and consistent with Western blot analysis (Fig. 9A and B) the E supplement significantly increased the number of Areg positive staining luminal epithelial cells but did not change the number of ERα positive staining luminal epithelial cells in PFOA-treated mammary glands. These results suggest that decreased Areg protein levels play an important role in PFOA inhibition of mammary gland development.
Fig. 9. Effect of supplement of exogenous hormones on mammary gland protein levels of Areg, HGF, ERα, EGFR and PCNA in vehicle control- and PFOA-treated mice.
A. Effect of supplement of exogenous hormones on mammary gland protein levels of Areg, HGF, ERα, EGFR and PCNA. Balb/c and C57Bl/6 Wild type mice were sacrificed 24 h after the last PFOA+hormone treatments. Representative Western Blots from protein samples prepared from frozen mammary glands as described in Materials and Methods. GAPDH served as a protein loading control. B. Quantification of Western blot analysis for the effect of supplement of exogenous hormones on mammary gland protein levels of Areg, HGF, ERα, EGFR and PCNA in vehicle control- and PFOA-treated mice (mean ± standard deviation, n=3). * p<0.05 compared with vehicle control + 7dC in each mouse strain; # p<0.05 compared with PFOA + 7dC in each mouse strain.
Fig. 10. Effect of supplement of exogenous estradiol (E) on the expression of Areg and ERα in PFOA-treated Balb/c and C57Bl/6 wild type LECs.
Mice were sacrificed 24 h after the last PFOA/hormones treatment and mammary glands were processed for dual immunofluorescent staining of Areg and ERα as described in Materials and Methods. Representative images of immunoflourescence staining of Areg (green) and ERα (red) in mammary gland sections from Balb/c (A) or C57Bl/6 wild type (B) mice. Nuclei were counterstained with DAPI (blue).
4. Discussion
Inhibitory effects on mammary gland development have been reported in animals exposed to PFOA during various critical developmental stages including gestational, lactational and peripubertal periods [3, 10–12]. However, the mechanisms by which PFOA exposure inhibits mammary gland development remain poorly understood. PFOA is used extensively in various industries and PFOA is widely present in the bodies of both the general and occupationally-exposed human population. Most studies of PFOA effects in humans have focused on adult exposures. However, less is known about potential effects of exposure at other ages. The pubertal mammary gland in humans and mice is thought to be particularly sensitive to changes caused by environmental exposures that can produce long-term effects. For example, irradiation exposure in young Japanese girls but not older women has resulted in increased breast cancer incidence [40]; exposure to DDT early in life may increase breast cancer risk [41]. Thus, we have investigated the underlying mechanisms of the PFOA inhibitory effects on the murine mammary gland at the sensitive developmental stage of pubertal development. In this study, we found that PFOA effects on the ovaries may mediate its inhibition on peripubertal mammary gland development in Balb/c and C57Bl/6 mice.
Differential effects of peripubertal exposure to PFOA on mammary gland development in Balb/c and C57Bl/6 mouse strains were observed in our recent studies [12,13]. Combining the findings from this and our previous studies [12,13], it is concluded that while 2.5 mg/kg or higher doses of PFOA treatment caused significant inhibition of mammary gland growth in Balb/c mice, mammary gland inhibition was observed only at 7.5 mg/kg or higher dose of PFOA treatment in C57Bl/6 wild type mice. However, 7.5 mg/kg of PFOA treatment had no inhibitory effect on mammary gland in C57Bl/6 PPARα knockout mice, suggesting that PPARα is critically involved in PFOA inhibitory effect on the mammary gland. The greater sensitivity to the inhibitory effects of PFOA in Balb/c mice is likely due to the observations that Balb/c mice had significantly higher protein levels of PPARα (~2-fold greater than C57Bl/6 mouse PPARα levels in ovaries and mammary glands) and higher plasma PFOA levels. It is interesting to note that while plasma PFOA levels of Balb/c mice treated with 2.5 mg/kg of PFOA were comparable to that of C57Bl/6 mice treated with 5 mg/kg of PFOA, the opposite effects on mammary glands were observed. Significantly higher PPARα protein levels in Balb/c mice may be one contributing factor to the observed opposite effects in two mouse strains.
Studies have shown that PFOA is excreted mainly through the kidney and the higher retention of PFOA in some species or sexes within species may be partially due to the differential expression of organic anionic transporters (OATs) [31,42]. It was suggested that differential expression levels of renal OAT2 and OAT3 may contribute to different biological half-life of PFOA in male and female rats [31]. However, no significant differences of OAT2 and OAT3 expression levels were detected in Balb/c and C57Bl/6 mice, suggesting that OAT2 and OAT3 may function differently in different species. Instead, significantly higher levels of OATP2 and NTCP were detected in C57Bl/6 mice, and significantly higher levels of OAT-K were found in C57Bl/6 PPARα knockout mice. It has been reported that renal OATs are involved in both secretion (OAT1, OAT2 and OAT3) and reabsorption (OATP1a1) of PFOA in rats [43]. It is currently not clear whether differential expression levels of renal OATs may contribute to the differential plasma PFOA levels in Balb/c and C57Bl/6 mice.
PFOA is an agonist of PPARα, a nuclear receptor being capable of regulating inflammatory responses, cell proliferation, and differentiation [27]. It was reported that the general development toxicity caused by PFOA depends on the expression of PPARα in 129S1/SvlmJ mice [1]. The findings from this study indicate that PPARα plays a role in mediating PFOA mammary gland inhibition. Compared with C57Bl/6 wild type mice, significantly lower plasma PFOA levels were detected in C57Bl/6 PPARα knockout mice when similarly treated with 5 mg/kg or 7.5 mg/kg of PFOA. Thus, either decreased PFOA blood levels and/or deletion of the PPARα gene may explain the lack of inhibitory effect on mammary gland in PPARα knockout mice. It remains to be determined whether PPARα directly mediates the inhibitory effects of PFOA on the mammary gland or if those effects are related to the differences in PFOA serum levels achieved in the wild type and knockout mice. It is interesting to note that no significant differences of serum PFOA levels were found between wild type and PPARα knockout 129S1/SvlmJ mice treated with 0.1 to 20 mg/kg of PFOA [1]. The reasons for the differential effects of PPARα knockout on PFOA blood levels in C57Bl/6 and 129S1/SvlmJ mice are not known. Given the fact that Balb/c and C57Bl/6 mice showed differential expression pattern of renal OATs, it is possible that C57Bl/6 and 129S1/SvlmJ mice may also have differences in OATs expression, and that PPARα may have different effects on OAT expression in these two mouse strains. These differences may in part contribute to the observed different effects of PPARα knockout on PFOA blood levels in C57Bl/6 and 129S1/SvlmJ mice.
Pubertal mammary gland development is largely controlled by E and P produced by the ovary. Both E and P can induce Areg which is an important paracrine factor for pubertal development [20,33,34]. Thus normal ovarian function is critical for pubertal mammary gland growth. We recently reported that peripubertal PFOA exposure (5 mg/kg) stimulates C57Bl/6 mouse mammary gland development probably by enhancing ovary function as evidenced by elevated protein levels in the ovaries of two important steroid hormone synthetic enzymes (HSD3β1 and HSD17β1) and increased serum levels of P [13]. In this study we found that PFOA treatment at inhibitory doses altered ovary function in Balb/c and C57Bl/6 wild type mice evidenced by delayed or absent vaginal opening and lack of corpora lutea (CL), an indication of lack of estrous cycling. We also found significant decreases in ovarian protein levels of StAR, CYP11A1, HSD3β1 and HSD17β1 that are critically involved in E and P synthesis. The importance of altered ovary function in the mammary gland inhibitory effect of PFOA was further demonstrated by the “rescue” experiment through supplementation with exogenous E and P. Supplementing with physiological levels of E or P reversed stunted mammary gland development in PFOA-exposed Balb/c and C57Bl/6 wild type mice. While we observed effects of PFOA indicative of altered ovarian function, we can not conclude if PFOA acts directly on the ovary or may interfere with the hypothalamic/pituitary/ovarian axis resulting in altered leutinizing hormone and follicle stimulating hormone signaling.
In addition to steroid hormones, growth factors produced in mammary glands also play important roles for mammary gland growth [18–21]. The expression of Areg, a ligand of EGFR, can be induced by E. Areg is an essential mediator of ERα function in pubertal mammary gland development acting in a paracrine manner by binding to the EGFR in stromal cells to produce growth factors that increase epithelial proliferation, terminal end bud formation, and ductal elongation [20]. HGF, synthesized in the stroma, is important for normal mammary ductal development by stimulating the proliferation, motility, and morphogenesis of nearby epithelium [22–24]. We recently found significantly increased protein levels of Areg, HGF, EGFR, and ERα in PFOA-stimulated C57Bl/6 mammary glands [13]. In contrast, in this study we found that the protein levels of Areg, HGF, EGFR, and ERα were significantly decreased in PFOA-inhibited Balb/c and C57Bl/6 mammary glands. Supplementation with E or P that overcome the PFOA inhibition of mammary gland development also restored the protein levels of Areg and EGFR in PFOA-treated mammary glands to the levels of control-treated mammary glands. Together, these results along with our previous findings suggest that decreased levels of these growth factors also play an important role in PFOA inhibition of mammary gland growth.
It is well-known that PFOA is capable of increasing the expression of PPARα responsive genes, especially those involved in lipid metabolism [44,45]. Bjork et al. [45] found that exposure to PFOA stimulated the expression of genes involved in fatty acid uptake and oxidation in rat heptocytes, suggesting that an early metabolic response to PFOA exposure in rats is an increase in lipid catabolism. Increased oxidation of fat may be responsible for lower serum cholesterol and triacylglycerols levels as well as weight loss in PFOA-treated rats [46]. Lack of fat and weight loss may also contribute to impaired mammary gland development. Although we did not analyze mouse blood cholesterol and triacylglycerols levels in this study, we do not think that fat availability was one of the major factors that caused stunned mammary gland development in PFOA-treated mice as no significant differences of mammary gland fat pad weights were observed. In addition, body weight loss may not contribute significantly to the inhibitory effect of PFOA on mammary gland because (i) PFOA treatment that inhibited mammary gland growth had no significant effect on Balb/c mouse body weight during the entire treatment period; (ii) significant body weight decreases were observed only during the 4th week of PFOA treatment whereas a two-week PFOA treatment caused significant mammary gland growth inhibition in C57Bl/6 wild type mice without affecting body weight.
One interesting finding from the present and our previous study is the PFOA dose-response on mammary gland in C57Bl/6 wild type mice. There was a change from a stimulatory effect at 5 mg/kg PFOA dose to an inhibitory effect on mammary gland at 7.5 mg/kg PFOA dose. Plasma PFOA levels were increased by ~ 37% (68.1±9.8 vs 93.1±10.1 μg/ml) between the two doses. This does not conform to a typical linear dose-response but rather is indicative of a threshold dose-response. Furthermore, this response may be complicated by the status of PPARα expression, since PPARα knockout mice exhibited a different dose-response.
There are clear limitations in extrapolating the findings of animal studies to the potential effects of PFOA on human mammary gland health. One major concern is that mouse plasma PFOA levels detected in the present and other animal studies are ~ 10 to 100 times higher than the typical serum PFOA levels in humans through occupational or contaminated drinking water exposure. However, it should be recognized that although humans have a much lower blood PFOA levels than mice, the biological half-life of PFOA in humans (3.0–4.1 years) is much longer than in mice (~ 17 days) [8,47]. Thus, it remains to be determined whether the effects of short term higher levels of PFOA in mice may be relevant to effects of persistent lower levels of PFOA in humans. Moreover, although no reports have so far shown the adverse effects of adult occupational PFOA exposure on human mammary gland health, this does not negate the potential importance of the findings from this and previous animal studies that examine different stages of mammary gland development that may be more sensitive to adverse effects. In this regard, inhibition of mammary gland development in mice has previously been observed at various critical developmental stages including gestational, lactational and peripubertal periods [3, 10–12]. Future studies that focus on populations exposed to PFOA during these critical developmental stages are warranted.
5. Conclusion
Peripubertal PFOA exposure significantly inhibited mammary gland growth in both Balb/c and C57Bl/6 wild type mice, but not in C57Bl/6 PPARα knockout mice, and Balb/c mice were more sensitive to PFOA inhibition. This may be due in part to the fact that Balb/c mice had the highest plasma PFOA levels whereas C57Bl/6 PPARα knockout mice had the lowest plasma PFOA levels. PFOA at the inhibitory doses in both Balb/c and C57BL/6 wild type mice altered ovary functions as evidenced by delayed or absence of vaginal opening and lack of estrus cycling during the experimental period and decreases in ovarian steroid hormonal synthetic enzyme levels. In addition, PFOA also reduced the expression of estrogen- or progesterone-induced mammary growth factors (amphiregulin, hepatocyte growth factor). Supplementation with exogenous estrogen and/or progesterone reversed the PFOA inhibitory effect on mammary gland. Based on these findings, it is concluded that PFOA effects on the ovaries, likely through reduction of estrogen and progesterone production, mediate its inhibition of mammary gland development in Balb/c and C57Bl/6 mice and that PPARα expression is a contributing factor.
Research Highlight.
Peripubertal PFOA exposure inhibited mammary gland growth in Balb/c and C57Bl/6 wild type mice
Balb/c mice were more sensitive to PFOA inhibition
C57Bl/6 PPARα knockout mice were resistant to PFOA inhibition
PFOA at the inhibitory doses impaired Balb/c and C57BL/6 wild type mouse ovary functions
Supplementation with exogenous estrogen and/or progesterone reversed the PFOA inhibitory effect
Acknowledgments
This work was supported by the Breast Cancer and the Environment Research Centers Grant U01 ES/CA 012800 from the National Institute of Environment Health Science (NIEHS) and the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, NIH. The authors would like to thank Jeffery Leipprandt, Jessica Bennett, Lisa Ann Zustiak and Dr. Jianwei Xie for their excellent technical assistance in animal model studies.
Abbreviations
- Areg
amphiregulin
- CYP11A1
cytochrome P450 family 11, subfamily A, polypeptide1
- E
estradiol
- EGFR
epidermal growth factor receptor
- ERα
estrogen receptor alpha
- HGF
hepatocyte growth factor
- HSD3β1
hydroxysteroid (3-β) dehydrogenase 1
- HSD17β1
hydroxysteroid (17-β) dehydrogenase 1
- P
progesterone
- PCNA
proliferating cell nuclear antigen
- PFOA
perfluorooctanoic acid
- PPARα
peroxisome proliferator-activated receptor alpha
- StAR
Steroidogenic acute regulated protein
- STDs
stimulated terminal ducts
- TEBs
terminal end buds
Footnotes
Conflict of interest: the authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yong Zhao, Email: yzhao@msu.edu.
Ying S. Tan, Email: tanying@msu.edu.
Mark J. Strynar, Email: strynar.mark@epa.gov.
Gloria Perez, Email: perezg@msu.edu.
References
- 1.Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, et al. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol Sci. 2007;98(2):571–581. doi: 10.1093/toxsci/kfm110. [DOI] [PubMed] [Google Scholar]
- 2.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 3.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96(1):33–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 4.Innes KE, Ducatman AM, Luster MI, Shankar A. Association of osteoarthritis with serum levels of the environmental contaminants perfluorooctanoate and perfluorooctane sulfonate in a large appalachian population. Am J Epidemiol. 2011;174(4):440–450. doi: 10.1093/aje/kwr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;28 (2):49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy GL, Jr, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34(4):351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 7.Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198(2):231–41. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90(2):510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 9.Wolf CJ, Fenton SE, Schmid JE, Calafat AM, Kuklenyik Z, Bryant XA, et al. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci. 2007;95(2):462–473. doi: 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- 10.White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, et al. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod Toxicol. 2009;27(3–4):289–298. doi: 10.1016/j.reprotox.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127(1–2):16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C, Tan YS, Harkema JR, Haslam SZ. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol. 2009;27(3–4):299–306. doi: 10.1016/j.reprotox.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Tan YS, Haslam SZ, Yang C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol Sci. 2010;115(1):214–224. doi: 10.1093/toxsci/kfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, et al. Prenatal perfluorooctanoic acid exposure in CD-1 mice: low dose developmental effects and internal dosimetry. Toxicol Sci. 2011;122(1):134–45. doi: 10.1093/toxsci/kfr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol. 1991;6(2):83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- 16.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(Suppl 6):S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 17.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imagawa W, Pedchenko VK, Helber J, Zhang H. Hormone/growth factor interactions mediating epithelial/stromal communication in mammary gland development and carcinogenesis. J Steroid Biochem Mol Biol. 2002;80(2):213–230. doi: 10.1016/s0960-0760(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 19.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8(1):201–211. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104(13):5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinberg DL, Ruan W. IGF-I, GH, and sex steroid effects in normal mammary gland development. J Mammary Gland Biol Neoplasia. 2008;13(4):353–360. doi: 10.1007/s10911-008-9103-7. [DOI] [PubMed] [Google Scholar]
- 22.Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, et al. HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development. 1995;121(9):2897–2908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- 23.Soriano JV, Pepper MS, Orci L, Montesano R. Roles of hepatocyte growth factor/scatter factor and transforming growth factor-beta1 in mammary gland ductal morphogenesis. J Mammary Gland Biol Neoplasia. 1998;3(2):133–150. doi: 10.1023/a:1018790705727. [DOI] [PubMed] [Google Scholar]
- 24.Accornero P, Miretti S, Starvaggi Cucuzza L, Martignani E, Baratta M. Epidermal growth factor and hepatocyte growth factor cooperate to enhance cell proliferation, scatter and invasion in murine mammary epithelial cells. J Mol Endocrinol. 2010;44(2):115–125. doi: 10.1677/JME-09-0035. [DOI] [PubMed] [Google Scholar]
- 25.Mandard S, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61(4):393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Curr Opin Pharmacol. 2006;6(4):421–427. doi: 10.1016/j.coph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod Toxicol. 2009;27(3–4):246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214(2):456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 29.Gibson CL, Coomber B, Murphy SP. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain. 2011;134(Pt 7):2125–2133. doi: 10.1093/brain/awr132. [DOI] [PubMed] [Google Scholar]
- 30.Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Fenton SE, Lindstrom AB, et al. Analysis of PFOA in dosed CD1 mice. Part 1. Methods development for the analysis of tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol. 2009;27(3–4):360–364. doi: 10.1016/j.reprotox.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact. 2002;139(3):301–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 32.Bronson FH, Dagg PCP, Snell GD. Green EL, editor. Reproduction. Biology of the Laboratory Mouse. Online: http://www.informatics.jax.org/greenbook/
- 33.Curtis SW, Clark J, Myers P, Korach KS. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor alpha knockout mouse uterus. Proc Natl Acad Sci USA. 1999;96(7):3646–3651. doi: 10.1073/pnas.96.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9(6):691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 35.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 36.Hewitt SC, Korach KS. Progesterone action and responses in the alphaERKO mouse. Steroids. 2000;65(10–11):551–557. doi: 10.1016/s0039-128x(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 37.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007;1771(6):663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Seto-Young D, Avtanski D, Strizhevsky M, Parikh G, Patel P, Kaplun J, et al. Interactions among peroxisome proliferator activated receptor-gamma, insulin signaling pathways, and steroidogenic acute regulatory protein in human ovarian cells. J Clin Endocrinol Metab. 2007;92(6):2232–2239. doi: 10.1210/jc.2006-1935. [DOI] [PubMed] [Google Scholar]
- 39.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 40.Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat Res. 2003;160(6):707–717. doi: 10.1667/rr3082. [DOI] [PubMed] [Google Scholar]
- 41.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersen ME, Clewell HJ, 3rd, Tan YM, Butenhoff JL, Olsen GW. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys--probing the determinants of long plasma half-lives. Toxicology. 2006;227(1–2):156–164. doi: 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol Sci. 2010;113(2):305–314. doi: 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen ME, Butenhoff JL, Chang SC, Farrar DG, Kennedy GL, Jr, Lau C, et al. Perfluoroalkyl acids and related chemistries--toxicokinetics and modes of action. Toxicol Sci. 2008;102(1):3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- 45.Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology. 2011;288(1–3):8–17. doi: 10.1016/j.tox.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Haughom B, Spydevold O. The mechanism underlying the hypolipemic effect of perfluorooctanoic acid (PFOA), perfluorooctane sulphonic acid (PFOSA) and clofibric acid. Biochim Biophys Acta. 1992;1128(1):65–72. doi: 10.1016/0005-2760(92)90258-w. [DOI] [PubMed] [Google Scholar]
- 47.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]