Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the most widely consumed pharmaceuticals, yet both the mechanisms involved in their therapeutic actions and side-effects, notably gastrointestinal (GI) ulceration/bleeding, have not been clearly defined. In this study, we have used a number of biochemical, structural, computational and biological systems including; Fourier Transform InfraRed (FTIR). Nuclear Magnetic Resonance (NMR) and Surface Plasmon Resonance (SPR) spectroscopy, and cell culture using a specific fluorescent membrane probe, to demonstrate that NSAIDs have a strong affinity to form ionic and hydrophobic associations with zwitterionic phospholipids, and specifically phosphatidylcholine (PC), that are reversible and non-covalent in nature. We propose that the pH-dependent partition of these potent anti-inflammatory drugs into the phospholipid bilayer, and possibly extracellular mono/multilayers present on the luminal interface of the mucus gel layer, may result in profound changes in the hydrophobicity, fluidity, permeability, biomechanical properties and stability of these membranes and barriers. These changes may not only provide an explanation of how NSAIDs induce surface injury to the GI mucosa as a component in the pathogenic mechanism leading to peptic ulceration and bleeding, but potentially an explanation for a number of (COX-independent) biological actions of this family of pharmaceuticals. This insight also has proven useful in the design and development of a novel class of PC-associated NSAIDs that have reduced GI toxicity while maintaining their essential therapeutic efficacy to inhibit pain and inflammation.
Keywords: NSAIDs, phospholipids, ulcer, membrane, anti-inflammatory, therapeutics
1. Introduction
NSAIDs represent a family of widely consumed pharmaceuticals which possess potent anti-inflammatory, analgesic and antipyretic actions [1]. In addition to these therapeutic actions, NSAIDs have been shown to prevent and/or treat a number of diseases such as heart disease, various cancers, and Alzheimer’s disease, in part due to the common inflammatory basis of these pathogenic conditions [2–12]. One of the major problems related to the widespread consumption of NSAIDs relates to their toxicity to a number of organ systems, notably the GI tract, where it has been estimated that chronic use of these drugs is associated with GI ulceration and bleeding that may result in life threatening hemorrhage in “at risk” subjects [13–15].
The molecular basis of both the therapeutic benefit and pathogenesis of NSAIDs has been attributed to the ability of these drugs to inhibit (either competitively or noncompetitively) cyclooxygenase (COX), the rate-limiting enzyme in the biosynthesis of a family of prostaglandins, which plays a central role in the mediation of inflammatory, nociceptive and alternative (cytoprotective) cellular pathways [16]. The discovery of a second COX isoform in the mid 1990’s led to the development and ultimate commercialization of COX-2 selective inhibitors (coxibs) [17, 18], whose GI benefits ultimately were overshadowed by their association with severe cardiovascular adverse events (e.g. thrombosis, myocardial infarction) leading to the recall of the more specific coxibs (Vioxx®, Bextra®) from the market [19–21].
One of the alternative mechanisms by which NSAIDs can affect both biological and cytotoxic responses is by interacting with cellular membranes and altering their biophysical properties. In support of this possibility, our and other laboratories have reported that NSAIDs can induce changes in the fluidity, permeability and biomechanical properties of cell membranes [22–29]. In this report we have extended these studies by investigating the interaction of NSAIDs (specifically aspirin, ibuprofen and naproxen) to chemically associate with the major organic component of membranes, phosphatidylcholine (PC), employing an array of biophysical and biochemical techniques including: Fourier Transform InfraRed (FTIR), Nuclear Magnetic Resonance (NMR) and Surface Plasmon Resonance (SPR) spectroscopy. These laboratory studies, which were first reported, in part as an abstract [30] provide compelling insight into the molecular/atomic association between PC and NSAIDs which were further confirmed by computational molecular dynamics (MD) simulation analysis. Lastly, we used confocal time-lapse image analysis on gastric cells in culture to demonstrate that NSAIDs interact with biological membranes and induce rapid alterations in their phospholipid distribution and patterning using a hydrophobic fluorescent probe.
2. Materials and methods
2.1 Test NSAIDs
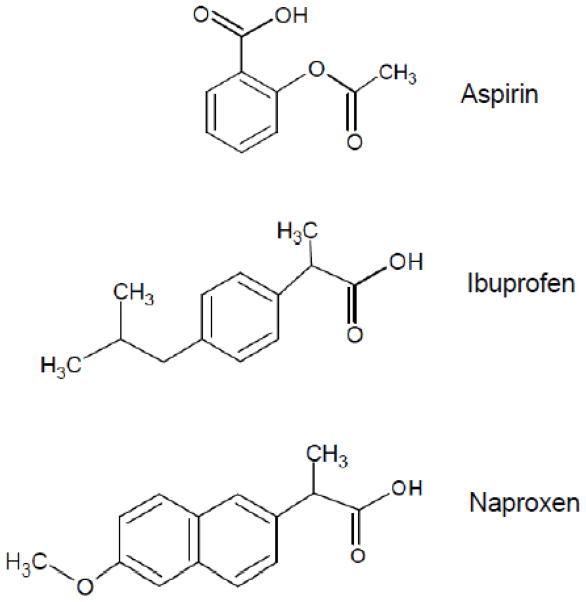
aspirin, ibuprofen and naproxen were purchased from Sigma-Aldrich (St. Luis, MO). Their chemical structures are depicted in Fig. 1.
Figure 1.
Chemical structure of test NSAIDs.
2.2 FTIR spectroscopic analysis
FTIR spectra were obtained using a Nicolet Nexus 870 spectrometer, using methods established in our laboratory [31]. For aqueous samples a 50-micron path length was used to obtain the spectra, and D2O was used instead of H2O. For the oil mixtures the FTIR spectra were collected in the Attenuated Total Refectance (ATR) mode. Both the aqueous and oil mixtures were collected at 4 cm−1 spectral resolution. The difference spectra were generated by subtracting the spectra of NSAID and PC from the spectrum of the NSAID-PC mixture.
2.3 Surface Plasmon Resonance (SPR)
SPR is a highly sensitive technique for characterizing interactions of two molecules, by measuring changes in the refractive index of a particular molecule or ligand that is adsorbed to a sensor chip [32]. Biacore has developed several chips that have the capacity to adsorb phospholipids as either a bilayer (L1 chip) or a monolayer (HPA chip) to which binding of NSAIDs can be monitored with SPR. All experiments were performed on a Biacore 2000 SPR sensor assayed at 37°C. For each run the chip was primed with running buffer (RB: 10 mM PIPES, 150 mM NaCl, pH 4.5), loaded with lipid (1 mM in RB) at a flow of 5 micro L per minute for 1 minute, then washed with RB at 100 micro L per minute. Analysis of binding of NSAIDs at defined concentrations (0 to 1 mM) was then performed in the normal manner (NSAID in pH adjusted RB) at a flow rate of 100 micro L per minute for 2 minutes or more. The chip was restored by first cleaning the chip with 2 M NaCl (flow rate: 30 micro L for 1 minute) and the procedure repeated for another binding experiment.
2.4 NMR spectroscopy and NMR-based r-MD calculations
NMR data were collected on Varian 750 or 800 MHz spectrometers with Direct Drive at 25 or 40 °C. 2D COSY spectra were collected at 800 MHz for both purified soy PC, Phospholipon 90G (90G, Lipoid Inc, Cologne, Germany) and for the PC(90G):ibuprofen oil, using 12.9 kHz sweep widths (SW), a 159 msec acquisition time and 2048 by 256 complex data points in f2 and f1, respectively. A 300 msec ROESY experiment [33] was acquired on PC(90G):ibuprofen oil using a 9.1 kHz SW. A 13C-attached proton test (APT) spectrum was acquired (800 MHz) to differentiate methyl, methylene and methine groups. 1D 13C and 2D 1H,13C-HMQC [34] spectra were acquired at 750 MHz. Restrained molecular dynamics calculations used SANDER within AMBER 6 [35]. To obtain MD parameters for ibuprofen and PC, the geometries were optimized using Gaussian98, Rev. A11 [36] at the restricted Hartree-Fock theory level using the 6–31g(d) basis set. Atomic charges were determined by RESP [37]. The ibuprofen:PC structure was determined using 5000 steps of r-MD followed by 5000 steps of energy minimization.
2.5 Molecular Dynamics Simulation
All simulations were performed using the GROMACS 3.3.3 package [38, 39]. For the initial structure of lipid bilayer, a well-equilibrated and widely used system containing 128 dipalmitoylphosphatidylcholine (DPPC) lipid molecules and 3655 water molecules was used [40, 41]. Periodic-boundary conditions were applied in all three directions. The lipid molecules were modeled based on Berger et al. [42] and the ffgmx as implemented in GROMACS, both based on GROMOS87 with improvements. The water molecules were modeled using the non-polarizable simple point charge (SPC) model in which the water molecule is considered to have point charges on oxygen and hydrogen atoms with interactive Lennard-Jones potential parameters applied only with respect to oxygen [43]. NSAID molecules were modeled (both the coordinates and partial charges) using freely available software PRODRG [44] which is based on the GROMOS87 force field and generates partial charges based on the concept of charge groups such that a single molecular group of bonded atoms (e.g., COO) is assigned an integer charge. United-atom model was used for both lipid and NSAIDs with explicit hydrogen atoms present only in the water model. The bonds between hydrogen and (non-water) heavy atoms were constrained using SHAKE [45] while using SETTLE for water [46]. A time step of 2 fs was used and the integration of Newton’s equations of motion was based on a leap-frog algorithm as implemented in GROMACS. The Lennard Jones (LJ) potential was switched at 1.0 nm to go smoothly to zero at 1.2 nm. Electrostatic interactions were computed using a Particle-Mesh Ewald (PME) sum [47] with a direct space cut-off at 1.4 nm and fast-Fourier grid space of 0.12 nm with fourth order interpolation and a tolerance of 1 × 10−5. In order to calculate the short range LJ and the electrostatic interactions, a neighbor list over 1.4 nm was maintained and updated every 50 fs. The trajectories were saved every 10 ps for further analyses. The pressure and temperature were maintained at 1 bar and 323K (well above the gel-liquid transition temperature of 314K for DPPC) using a weak coupling to an external barostat (time constant 1 ps) or thermostat (time constant 0.1 ps), respectively [48]. The simulation box was allowed to vary independently and isotropically in all directions to maintain the pressure, and the bilayer normal was fixed along the z-direction of the box. VMD software [49] was used for visualization and for drug insertion to create initial structures. A net charge of 0 and −1 for the neutral and charged NSAIDs were used to represent the NSAIDs below and above their pKa respectively. The NSAID molecules were placed within the lipid matrix approximately in a lattice and in about 8 layers along the bilayer normal. A lattice configuration for initial drug locations as opposed to randomly chosen locations was to ensure no clusters were present in the starting configuration. The total length of the run was 100 ns while the first 40 ns were ignored for equilibration. Equilibration was established by observing the system parameters such as potential energy, temperature, pressure, headgroup area and box volume. All the analyses were done on the last 60 ns of the data. The bilayer was assumed to be symmetric between two monolayers.
2.6 GI cell culture studies and confocal microscopy
AGS cells (purchased from ATCC (Manassas, VA) were cultured in appropriate growth media (HAM plus 10% FBS) in 24-well tissue culture plates to ~80–90% confluency. Media were replaced with fresh media (no FBS) prior to each of the following experiments and then were exposed to the NSAID-containing test compounds at the designated concentrations. For the confocal imaging experiments, gastric AGS cells were incubated in fresh HAM media (without serum)) containing 10 μg/ml fluorescent lipid analog di-8 ANEPPS (1 mg/ml stock in pure ethanol) at 37°C for 20 minutes. AGS cells were washed again using PBS and suspended in fresh HAM media for microscopy. A Nikon A1 confocal microscope (Nikon Instruments Inc., Melville, NY, USA) was utilized to study the fluorescently labeled AGS cells. The membrane probe di-8 ANEPPS was excited by an argon laser at 488 nm and images were obtained using a 40X Plan-Fluor/0.75 NA objective. Experiments started with imaging of control cells without tested agents and all the imaging parameters, such as laser power and gain, were kept constant throughout the entire experiment before and after addition of tested agents. This ensured proper indication of changes in fluorescent intensity and distribution of the probes within the cell plasma membrane.
3. RESULTS
3.1 FTIR Spectroscopic Analysis
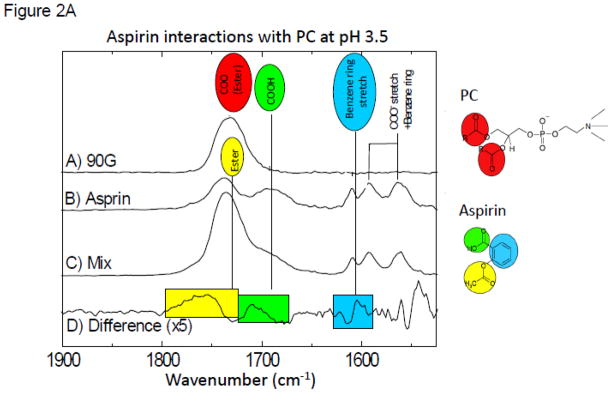
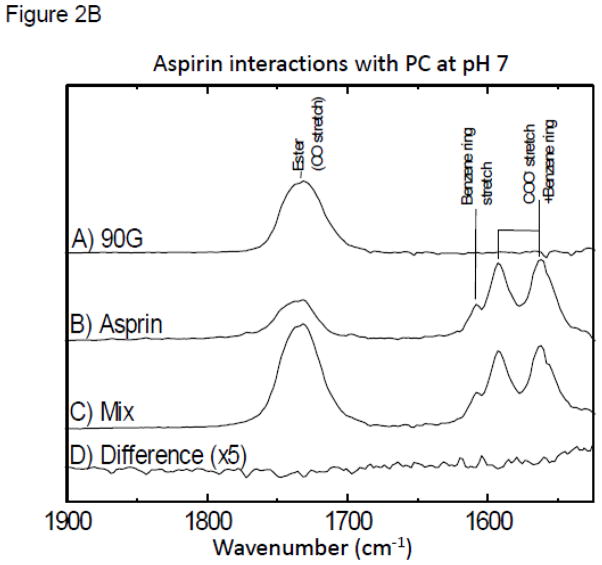
In these studies we investigated changes in the vibrational spectra of specific moieties within NSAIDs (aspirin, ibuprofen and naproxen) when combined with purified soy PC under aqueous conditions (where in the latter, the pH was systematically changed to values above and below the NSAIDs’ pKa) and in a PC-enriched soy lecithin oil using FTIR [31]. The FTIR spectra clearly indicated large shifts in the vibrations of aspirin and PC, when present in combination at or near the aspirin’s pKa of 3.5 (Fig. 2A), while no significant changes were observed at neutral pH (see Fig. 2B). These interactions are best observed by inspecting the Difference Spectra in which the contributions of the individual components to the mixture (Figure 2A-trace D) were subtracted out. These results thereby suggest that aspirin interacts stronger with the phospholipid under acidic conditions when the NSAID is in the neutral protonated form.
Figure 2.
Figure 2A. FTIR spectra at pH 3.5 for: (A) purified soy PC (90G); (B) aspirin; (C) mixture of aspirin and PC; (D) Difference FTIR spectra (which was calculated by subtracting out A&B from C) at pH 3.5. The color highlighted peaks represent the bonds affected by the intermolecular association.
Figure 2B. FTIR spectra at pH 7.0 for: (A) purified soy PC (90G); (B) aspirin; (C) mixture of aspirin and PC; (D) Difference FTIR spectra (which was calculated by subtracting out A&B from C) at pH 7.0.
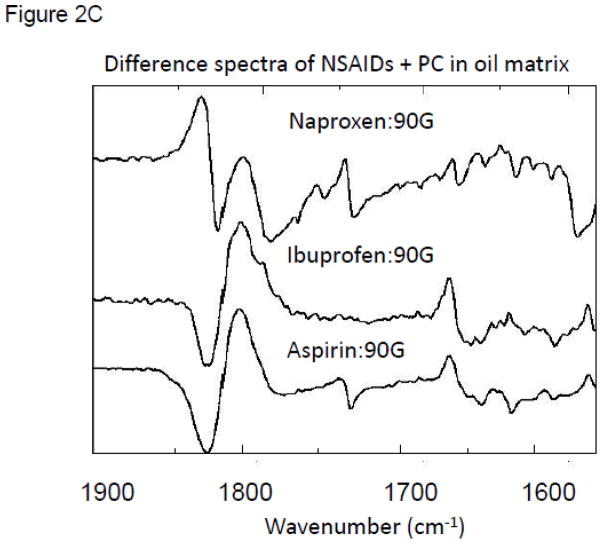
Figure 2C. Comparison of the difference spectra using ATR-FTIR analyses of naproxen, ibuprofen, and aspirin and purified soy PC (Phospholipon 90G) when the NSAID and PC are combined at equimolar concentration in an oil matrix indicating PC has high affinity to associate with the test NSAIDs in a non-aqueous solvent system.
In subsequent studies we also compared the changes in FTIR spectra when aspirin was combined with different analogues of PC, and demonstrated that little or no interaction occurred when we used just the phosphorylcholine head-group of the molecule (Figure SA of the Supplemental data section). Furthermore, changing the alkyl group linker to the glycerol backbone of PC from an ester to an ether bond made little difference in the molecular interaction between the NSAID and the phospholipid. To dissect the individual changes and assign the specific interactions that give rise to the difference features, we performed studies, described in the Supplemental Data section, employing aspirin labeled with a stable 13C isotope at the ester group (Figure SB). which allows for the selective investigation of the environment of this moiety. The results of these experiments can be best accounted for placing the aspirin near the ester group of the phospholipid.
A parallel series of aqueous FTIR analyses were performed to investigate interactions between ibuprofen and soy PC, and in confirmation with the above observations, the strongest associations between the NSAID and phospholipid were seen as the ibuprofen was being protonated (pH~5.2), as depicted in Fig. SC.
We also used Attenuated Total Reflectance (ATR) FTIR analysis to investigate the molecular association between the protonated NSAIDs (aspirin, ibuprofen and naproxen) and PC when the latter was present in a soy lecithin oil. As can be appreciated in the FTIR difference spectra shown in Figure 2C, ATR demonstrated strong non-covalent interactions between the test NSAIDs and PC. It appears likely that this association occurs between the polar carboxyl group of the NSAID and the polar head group of PC. Further, multiple interactions were detected between the constituent atoms present in the aromatic rings of the NSAID and PC, most likely representing the acyl side-chains of the phospholipid. A comparison of the difference spectra of aspirin, naproxen and ibuprofen employing ATR-FTIR confirms that PC associates with the NSAIDs in an oil matrix to simulate a membrane microenvironment. It should be noted, that it is likely that the molecular interactions observed between the NSAIDs and PC in this and subsequent sections may well be due to the chemical attraction of the two classes amphipathic molecules due their respective charge state and hydrophobic characteristics which would be promoted by their proximity, as opposed to being driven by a specific chemical association.
3.2 Surface Plasmon Resonance
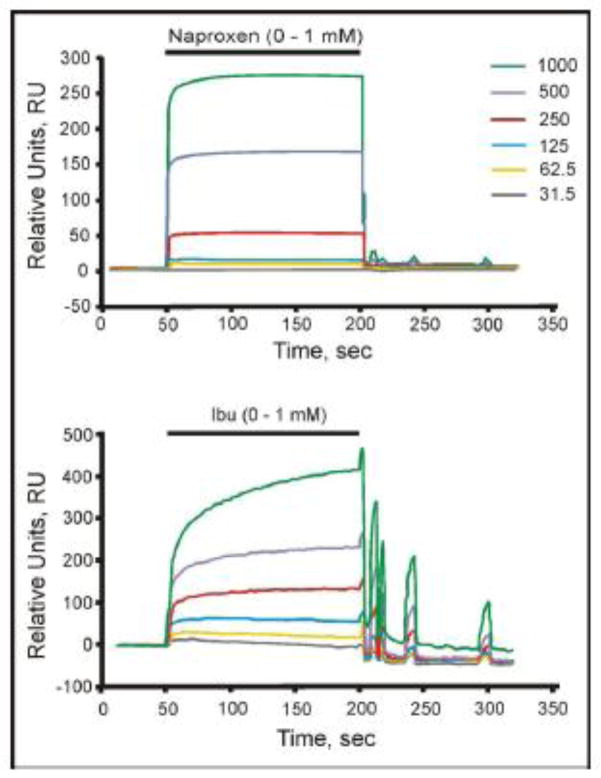
SPR is a highly sensitive technique for characterizing surface interactions of two molecules by measuring changes in refractive index of a particular molecule or ligand that is adsorbed to a sensor chip [32]. In our studies, we investigated the interaction of both naproxen and ibuprofen to purified (soy) PC that had previously been adsorbed to an L1 chip. This study indicated that both NSAIDs chemically associate with adsorbed PC, in a pH- and concentration-dependent manner with optimal binding occurring at a pH near the pKa of the molecules with little or no association being detected at neutrality. Furthermore, our sensorgrams demonstrated that the NSAIDs interact with the adsorbed phospholipid with variable KD values, with the 1st KD being in the mid μM range and the 2nd in the low mM range [50]. This technique also indicated that the association was reversible as the refractive index changes can be returned to baseline after the NSAID-containing solution is replaced with buffer in the microfluidic solution perfusing the chips. Typical SPR tracings, demonstrating a dose-dependent increase in signal from the PC-associated L1 Biacore chip by stepwise increases in NSAID concentration in the perfusion buffer is demonstrated in Figure 3. The off rates upon washout of the NSAIDs typically displayed a rapid decrease in signal as the NSAID quickly dissociated from the lipid layer. However, in some cases, such as shown for ibuprofen (Fig. 3), wash out of the NSAID displayed a cyclical signal that dissipated over time. The reason for this cyclical behavior is not known, but likely reflects some rebinding of the NSAID, or mass transport into the lipid layer, followed by cycles of release and and mass transport (rebinding) as the NSAID is flushed from the chip [51]. It should be noted that we did not include aspirin in the depicted SPR spectra due to the high non-specific binding of this NSAID to the L1 chip, even in the absence of a PC coating, making the results uninterpretable.
Figure 3.
SPR analysis of interaction of naproxen (top)and ibuprofen (bottom)with PC bound to a L1 Biacore chip. Sensorgram (Biacore) showing the Naproxen and Ibuprofen (Ibu) response (Response Units, RU) on a sensorchip coated with 90G lecithin liposomes. Liposomes (100 nm diameter) were applied to the surface of one channel (Ch2) of the L1 sensorchip and the effect of addition of various doses of the two test NSAIDs (31.2, 62.5, 125, 250, 500 and 1000 μM; shown in ascending order on the sensorgram) tested as indicated (pH 4.5, PIPES buffer, 37 °C). These results demonstrate that the interaction of the NSAIDs with PC bilayer was both dose-dependent and reversible.
3.3 NMR Spectroscopy and r-MD Structure Calculations
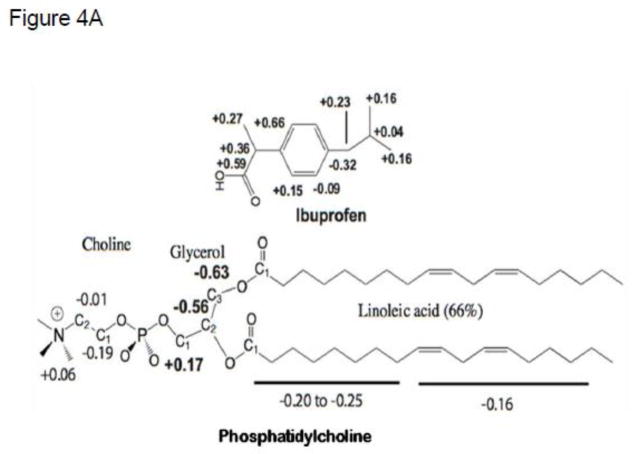
The interactions of NSAIDs with PC were probed with 1D 1H and 13C NMR spectroscopy, and chemical shift changes were identified. Data were collected for the NSAIDs and PC, separately, in acetone at 40 °C. Resonance assignments were determined by 2D COSY [52], 2D ROESY [33, 53], and 2D 1H-13C-HMQC correlations, and by 1H-1H coupling. Significant changes for many resonances were observed. Changes observed with ibuprofen-PC conjugate (Figure 4A and Table S1) demonstrate the greatest upfield changes for PC occurred near the headgroup in the presence of ibuprofen, indicating increased electron density.
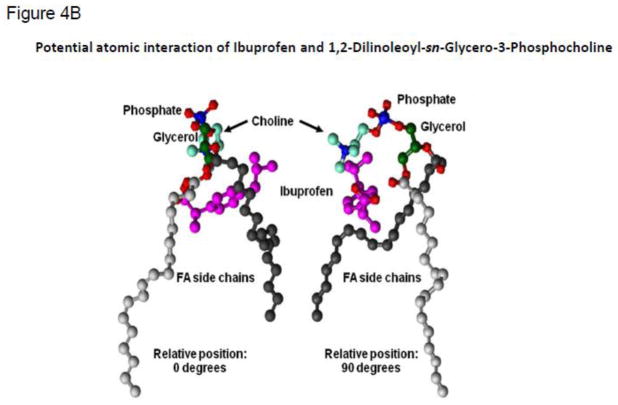
Figure 4.
(A) Changes in 13C chemical shifts of ibuprofen:PC in acetone at 40 °C relative to the individual spectra as determined by NMR. (B) Structure of the ibuprofen:PC complex determined by r-MD calculations based on direct 1H-1H interactions observed in a 300 ms ROESY NMR experiment. Two views, rotated by 180°.
Chemical shift changes for PC mixed with ibuprofen or naproxen suggests similar interactions. As depicted in Table S2, the direction of chemical shift change is the same when ibuprofen or naproxen is added. The magnitudes of changes are mostly smaller when naproxen is added, except in the headgroup of PC, where larger changes occur for carbons Cho1 and Cho2, suggesting naproxen interacts more tightly (vs. ibuprofen) with the phospholipid headgroup. Focusing on the NSAIDs (ibuprofen and naproxen), the carboxyl groups underwent the greatest downfield chemical shift changes indicating loss of electron density, likely due to strong interactions with the headgroup of PC. The aromatic nucleus of ibuprofen was shifted upfield, indicative of hydrophobic interaction with the PC acyl chains. The chemical shift changes of aspirin, when mixed with PC, suggest a similar interaction. While most of the aromatic carbon atoms are more shielded, the carbon atoms on the polar side are deshielded, indicating electron loss (Table S3). Interestingly, although the acid protons of NSAIDs are readily observable by NMR, in the presence of PC they disappear completely or are greatly diminished, suggesting that PC may alter the pKa of NSAIDs. This shift in the apparent pKa of NSAIDs when intercalated in a lipidic microenvironment agrees with the findings of Chakraborty and associated who compared the physico-chemical properties of NSAIDs of the oxicam class in free solution and when incorporated into synthetic micellar and liposomal systems, as will be discussed in more detail [54, 55].
A 300 ms 2D ROESY experiment of ibuprofen:PC in acetone provided 43 total proton-proton correlations and 23 intermolecular correlations (Table S4). These included ibuprofen interactions with all choline and glycerol protons, as well as with linoleic acid protons H2 through H5 [56]. The interaction between choline H1 and ibuprofen H10 was critical. The ROESY correlations were used in restrained MD calculations. The NMR data could not distinguish if ibuprofen interacts with a single PC molecule (or one acid group) or multiple PC molecules. [56]. For the r-MD calculation, a 1:1 interaction was assumed. The NMR structure derived from the MD calculations indicates that ibuprofen is cradled by the carboxyl-terminal half of the lipids on the bottom and by the glycerol and choline functional groups at the top (Fig. 4B).
3.4 Molecular Dynamics Simulations of NSAIDs in a PC Bilayer
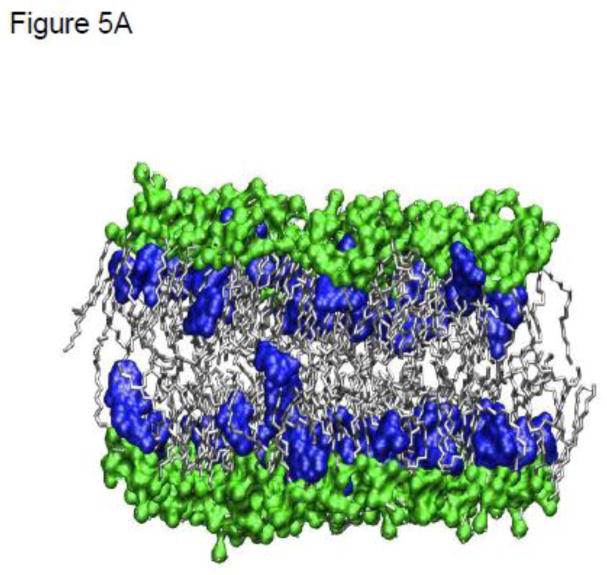
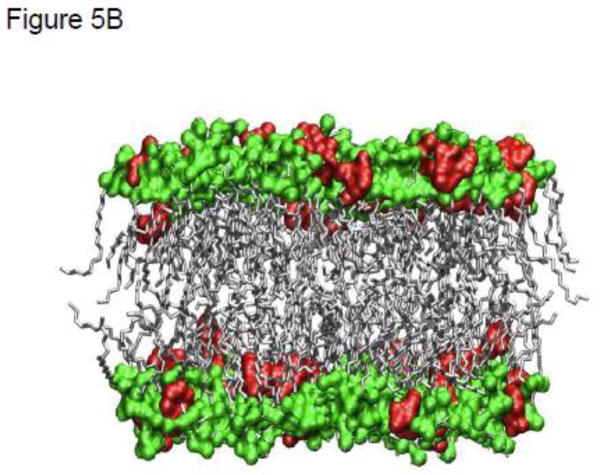
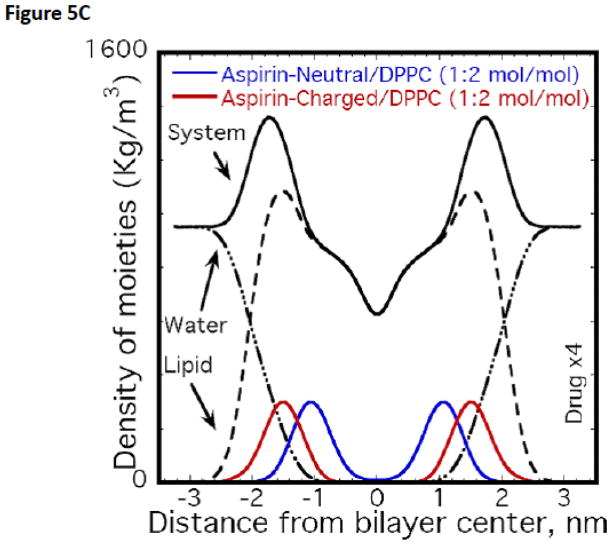
Atomistic Molecular Dynamics (MD) simulations were used to probe the interaction of various NSAIDs, including aspirin, naproxen and ibuprofen, and PC based lipid molecules. The effect of pH on the charge state of the drug was qualitatively addressed by simulating systems with two distinct charge states for the drug molecules i.e., neutral and anionic. The lipid itself was modeled as a zwitterionic moiety and therefore representative of the dominant state observed over pH values of 2 and 10 for PC based lipids. These well equilibrated MD simulations indicated that the NSAIDs, irrespective of their charge state, partition strongly into the PC membrane [57, 58]. However, the molecular details vary depending on the drug charge state. The partitioning of aspirin in DPPC bilayers from these well equilibrated MD simulations is shown in Figs. 5A–C. From Fig. 5A we observe that aspirin in an uncharged protonated state (depicted as blue spheres), as would occur when the pH<pKa, partitions to the high-density alkyl tail region, as a result of hydrophobic/enthalpic interactions between the aspirin and the lipid molecule. In contrast, the anionic form of aspirin (depicted as red spheres in Fig. 5B), as would occur when the pH>pKa, prefers to partition predominantly to the high-density headgroup region of the phospholipid, possibly due to electrostatic interactions between the aspirin and the PC headgroup (Fig. 5B). The quantitative distribution of aspirin molecules in both their charged and neutral state (and contrasted to the density distribution of lipid and water molecules), as determined by MD simulations of bilayers, is shown in Fig. 5C. Similar results have been observed for the other two NSAIDs, and on the basis of these simulations it was determined that the affinity of an NSAID to chemically associate with PC under both conditions (pH above or below pKa) followed the rank order of: naproxen > ibuprofen > aspirin.
Figure 5.
MD simulations of partitioning of aspirin into PC bilayer when the NSAID is: (A) uncharged (pH<pKa, blue) or (B) charged (pH>pKa, red). Only lipid bilayer and drug are shown with water above and below the bilayer, not shown for clarity. Heavy atoms (P,N,O) in the headgroup are shown in green color while lipid tails are shown in gray. (C) Favorable location of aspirin in the bilayer along with density of moieties (total system, lipid and water). Drug distribution is scaled by a factor of 4 for clarity.
3.5 Effect of NSAIDs on Biological Membranes
3.5.1 Imaging Studies on Gastric (AGS) cells in culture
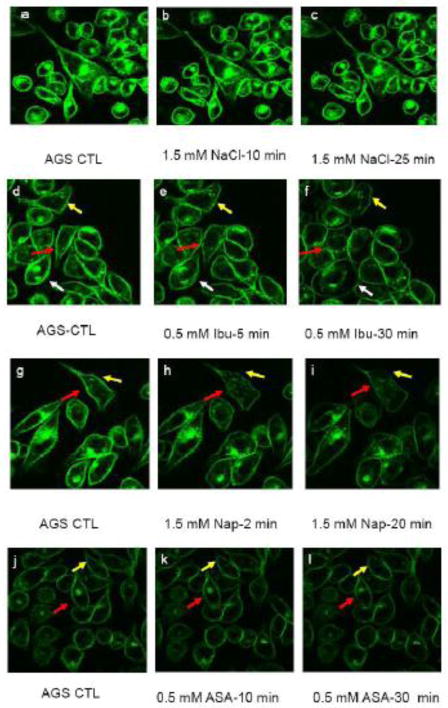
We then examined the ability of NSAIDs to alter cell membrane properties using confocal imaging of gastric AGS cells in culture (Figs. 6a–l). We found that untreated AGS cells had smooth labeling of the lipid probe, di-8 ANEPPS, within the plasma membrane. Further, the dye’s fluorescence showed no indication of bleaching throughout the 30 minute experimental time of imaging. The probe di-8 ANEPPS partitions deep inside of the phospholipid bilayer. The intercalation depth and the fluorescence intensity di-8 ANEPPS depend on the lateral packing density and the overall stability of the membrane [59–63]. Thus, the probe nicely indicates the overall integrity of the cell plasma membrane. Additionally, changes in local fluorescence intensity in certain areas of the plasma membrane demonstrate change in fluidity and packing density in such areas. Addition of 1.5 mM NaCl to the suspending media (to represent the highest NSAID concentration tested) had little effect on the intensity and lateral distribution of the probe (Fig. 6a–c). Depending on the NSAID studied, all cultured cells demonstrated a rapid dose-dependent change in the intensity and lateral distribution of di-8 ANEPPS within the AGS cell plasma membrane with aspirin and ibuprofen having an effect at a concentration of 0.5 mM and naproxen inducing changes at 1.5 mM (Fig. 6d-l). Within 5 minutes, the fluorescent intensity of di-8 ANEPPS in some areas of the plasma membrane significantly decreased while the probe in other areas maintained high intensity. Because the intensity of di-8 ANEPPS depends on the hydrophobicity and fluidity of the membrane environment, the observed heterogeneous labeling suggests marked changes in plasma membrane fluidity and also points to the possibility of increased heterogeneity within the cell plasma membrane, i.e. portions of the membrane were still hydrophobic while other parts became more hydrophilic. Further, in the presence of the test NSAIDs the morphology of some cells began to change and cells became round. However, no cell detachment was found. While the changes in cell morphology could potentially be an effect of COX-dependent event, we think this is unlikely. This is because the phenomenon observed in our study occurs within minutes of being exposed to NSAIDs, the COX-dependent events usually occur on a time scale of hours [64–66].
Figure 6.
Confocal Images of AGS cells labeled with lipid analogue, di-8 ANEPPS, acutely exposed to NSAIDs. The plasma membrane of AGS cells in culture were pre-labeled with the hydrophobic dye and then the cells were exposed to: 1.5 mM NaCl (control, a–c); 0.5 mM ibuprofen, (Ibu, d–f); 1.5 mM naproxen (Nap, g–i); or 0.5 mM aspirin (ASA, j–l) for the indicated lengths of time. The different colored arrows trace the changes in fluorescent patterning of a cell over time after NSAID exposure.
4. Discussion
In this study, we systematically examined the potential association between NSAIDs and zwitterionic phospholipids, specifically PC molecules using SPR, FTIR and NMR, which demonstrate the interaction at a molecular and atomistic resolution. These techniques clearly illustrated experimentally that the physical-chemical properties of both the NSAID and PC molecules undergo profound changes when mixed, indicating that the NSAID biochemically associates with the PC and alters the phospholipid micro-environment, either extracellularly or within the bilayer membrane. Detailed MD simulations provided strong confirmatory evidence to support the distribution of NSAIDs in PC bilayers. Interestingly, using FTIR spectroscopy, we found that the chemical association between NSAIDs and PC was both electrostatic and hydrophobic and the localization of the NSAID and the nature of the interaction has a clear pH-dependence, with the uncharged lipidic species which predominates at acidic pH values < pKa, inserting deeper into the bilayer and undergoing hydrophobic interactions with the aliphatic fatty acid side-chains of the phospholipid. This pH-dependence is completely consistent with previous studies in animal models as well as clinical studies [22, 29, 67–69]. Similar experimental (NMR and IR) associations between NSAIDs and DPPC have recently been reported by Husch et al [70], although this group questioned the physiological significance of these “so-called complexes in physiologically relevant aqueous media.” In the present study, detailed MD simulations demonstrated the same pattern on the theoretical basis. In the MD simulations, anionic NSAIDs (pH > pKa) partition most favorably into the headgroup region of the phospholipid bilayer, due to electrostatic interactions with the positively-charged quarternary ammonium component of choline. In these computer simulations, we chose not to incorporate additional electrolytes into the system in order to investigate the native interactions between NSAIDs (in their charged and neutral state) and the membrane phospholipids, with the intention of performing more complex ionic strength–dependent simulations in the future. Surface Plasmon Resonance and FTIR studies also demonstrate that the association is reversible providing additional evidence that it is non-covalent.
We then extended our study by examining the effect of NSAIDs on the hydrophobic characteristics of biological membranes. Confocal imaging demonstrated that NSAID molecules significantly altered the hydrophobicity and induced heterogeneity within the cell plasma membrane. This is completely consistent with our data in model systems and simulations in this study.
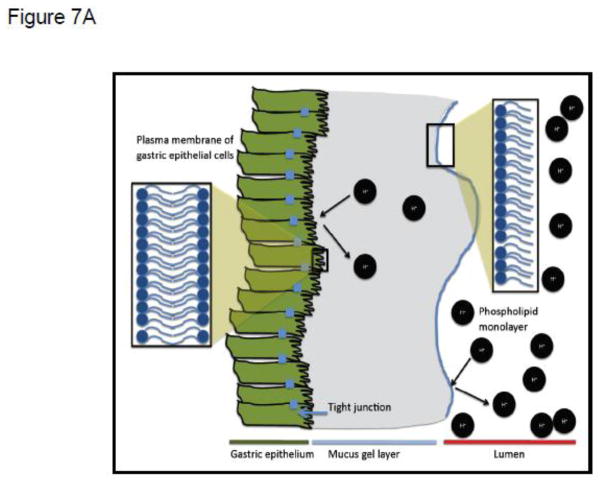
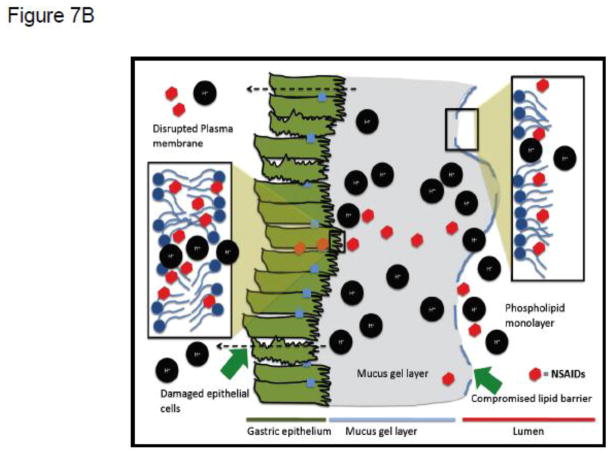
The observations described above are also consistent with our previous studies, which demonstrated that, in the gastrointestinal tract, NSAIDs interacted with a putative extracellular lining of PC and related zwitterionic phospholipids, possibly a monolayer or multilayer, localized on the surface of the mucus gel layer. We have previously found that NSAIDs decreased the hydrophobicity of the luminal interface of the mucus gel layer [22, 29, 67–69] that provides the upper GI tract with hydrophobic, non-wettable barrier properties to luminal acid [69, 71, 72]. This hydrophobic biophysical characteristic, depicted in Figure 7A as an adsorbed phospholipid monolayer, can be measured by contact angle analysis, and is rapidly attenuated in a pH-dependent manner by aspirin and other NSAIDs, as shown schematically in Figure 7B. [22, 29, 67–69].
Figure 7.
(A) Schematic depiction of extracellular (putative phospholipid monolayer at interface of mucus gel layer and gastric luminal fluid) and membrane barriers of the gastric mucosa to attack by luminal acid, indicated by H+. (B) Depiction of the disruptive effect of NSAIDs on both extracellular and membrane barriers to luminal acid, due to the drugs’ association with phospholipid constituents of the monolayer and membrane bilayer respectively.
Evidence from our and other laboratories have also supported the concept that the interaction of NSAIDs with PC lipids and the ability of the drugs to partition into the membranes can induce marked changes in the permeability, fluidity and biomechanical properties of these membranes [22, 24–29, 73, 74]. This effect of NSAIDs on the membrane bilayer is also depicted in schematic form in Figure 7A & B. These NSAID-phospholipid changes will not only increase the permeability of biological membranes to potentially damaging ions (H+) and macromolecules (e.g. enterotoxins) but also induce an instability of the membranes due to the formation of unstable pores as indicated by a decrease in lysis tension [75]. Thus, this chemical association between NSAIDs and zwitterionic phospholipids provide us with a molecular understanding of the pH-dependent effects of this potent class of anti-inflammatory drugs to induce injury to the GI mucosa, resulting in ulcer formation and bleeding, which may lead to an increased risk of developing potentially life-threatening hemorrhage. As described below this molecular understanding may also provide us with a new avenue to limit the GI toxicity of NSAIDs.
Our study demonstrates that the interaction of NSAIDs with membranes could be a potential mechanism for NSAID-induced GI toxicity irrespective of the NSAID’s inhibitory effect on COX-1 and COX-2, which would be expected to have a longer time course. Significant amount of work has been done by Chakraborty et al [54, 55] and Nunes et al [76, 77] on probing NSAID-membrane interactions under conditions such as different pH and membrane phase. NSAIDs have complex structures as well as charge-state behavior. Work by Chakraborty et al [54, 55] clearly demonstrated that NSAIDs could have complex charge-state behavior not just dictated by pH but the microenvironment of the membranes, promoting membrane fusion and that the drugs’ effect on membranes appears to be concentration-dependent. Nunes et al [76, 77] used several biophysical techniques to probe NSAID-membrane interactions at the molecular level. Their efforts demonstrated that in general NSAIDs interact with polar headgroups of the membrane phosphospholipids when the drugs are charged and penetrate deeper into the membrane when the drugs are neutral and may result in the development of whole of pores in the bilayer, confirming our earlier observations and theoretical predictions [75, 78, 79]. Further, they demonstrated that these interactions appear to be dependent on lipid phase.
One approach of attenuating the damaging action of NSAIDs on the surface of the GI mucosa, that is caused by the drugs interacting with phospholipids present either in the bilayer or the presumptive monolayer coating the mucus gel lining, is to pre-associate the NSAID with either synthetic or natural PC. This approach of pre-associating an NSAID with PC, thereby reduces the affinity of a luminal NSAID to interact with intrinsic phospholipids of the GI mucosa, fortifying the tissue’s barrier properties, while promoting the transit of the drug across the mucosa into the blood due the increased lipophilicity of the conjugate. To date, this appears to be an effective strategy to reduce the GI toxicity of a number of NSAIDs (notably aspirin, ibuprofen, naproxen and indomethacin), while maintaining their bioavailability and therapeutic efficacy in both rodent models and pilot clinical endoscopic trials [22, 72, 80–82].
The NSAID-phospholipid interactions demonstrated here may thus provide a novel perspective to explain the well-documented surface damaging actions of NSAIDs that have been dissociated from COX inhibition. It is also conceivable that the direct effect of NSAIDs on membrane fluidity, biomechanics and the distribution of lipidic micro-domains within the bilayer as demonstrated recently in both synthetic and biological membranes, may affect in a noncompetitive manner, the activity of critical enzymes and the signaling pathways that they regulate [83, 84]. If so, this membrane action may underlie a number of the diverse biological activities of this class of molecules which are not tightly linked to the competitive inhibition of COX.
In summary, we have used a number of biochemical, structural, computational and biological systems to demonstrate that NSAIDs have a strong affinity to form ionic and hydrophobic associations with zwitterionic phospholipids (specifically PC) that are reversible and non-covalent in nature. We propose that the pH-dependent partitioning of these potent anti-inflammatory drugs into the phospholipid bilayer; and possibly extracellular mono/multilayers present on the luminal interface of the mucus gel layer; may result in profound changes in the hydrophobicity, fluidity, permeability, biomechanical properties and stability of these membranes and barriers. Although the biophysical significance of these findings is compelling, the true pathophysiological importance of the proposed mechanism must await accurate measurements of the local concentration of a given NSAID within the microenvironment of the mucosal membrane. The NSAID-phospholipid interactions demonstrated here may thus provide a novel perspective to explain the well documented surface damaging actions of NSAIDs, which have been dissociated from COX inhibition. These changes may also potentially provide an explanation for a number of (COX-independent) biological actions of this family of pharmaceuticals. This insight also has proven useful in drug design and the development of a novel class of PC-associated NSAIDs that have reduced GI toxicity while maintaining the drugs’ essential therapeutic efficacy to inhibit pain and inflammation.
Supplementary Material
Highlights.
NSAIDs attenuate GI barrier properties by associating with phospholipids.
NSAID interaction with membrane phospholipids alters cell function.
NSAIDs affect cell signaling by changing the membrane micro-environment.
Reduction of GI injury by association of NSAIDs with phosphatidylcholine
Acknowledgments
This work was supported in part by NIH grants 1RC1 DK086304 and P30 DK56338.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furst DE, Paulus HE. Aspirin and Other Nonsteroidal Anti-inflammatory Drugs. In: McCarty DJ, Koopman WJ, editors. Arthritis and Allied Conditions. Lea & Febiger; Philadelphia: 1993. pp. 567–602. [Google Scholar]
- 2.Chan AR, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin Dose and Duration of Use and Risk of Colorectal Cancer in Men. Gastroenterology. 2008;134:21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole BR, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA. Aspirin for the Chemoprevention of Colorectal Adenomas: Meta-analysis of the Randomized Trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 8.Hebert PR, Hennekens CH. An overview of the 4 randomized trials of aspirin therapy in the primary prevention of vascular disease. Arch Intern Med. 2000;160:3123–3127. doi: 10.1001/archinte.160.20.3123. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier J-P. Pathological Pathways of Osteoarthritis, Non-steroidal Anti-imflammatory Drugs: A Research and Clinical Perspective. Royal Society of Medicine Press; London: 1994. pp. 1–14. [Google Scholar]
- 10.Samil A, Etiminan M, Wiens MO, Jafari S. NSAID Use and the Risk of Parkinson’s Disease: Systematic Review and Meta-analysis of Observational Studies. Drugs Aging. 2009;26:769–779. doi: 10.2165/11316780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Veld BA, Ruitenberg A, Hofman A. Nonsteroidal Anti-inflammatory Drugs and the Risk of Alzheimer’s Disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 12.Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- 13.Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992;327:749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787–796. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 15.Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000;119:521–535. doi: 10.1053/gast.2000.9561. [DOI] [PubMed] [Google Scholar]
- 16.Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol) Nature. 1972;240:410–411. doi: 10.1038/240410a0. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–1528. 1522. doi: 10.1056/NEJM200011233432103. p following 1528. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 21.Garcia Rodriguez LA, Gonzalez-Perez A, Bueno H, Hwa J. NSAID use selectively increases the risk of non-fatal myocardial infarction: a systematic review of randomised trials and observational studies. PLoS One. 6:e16780. doi: 10.1371/journal.pone.0016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenberger LM, Wang ZM, Romero JJ, Ulloa C, Perez JC, Giraud MN, Barreto JC. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- 23.Lichtenberger LM, Wang ZM, Giraud MN, Romero JJ, Barreto JC. Effect of naproxen (NAP) on gastric mucosal hydrophobicity: possible interaction with surface phospholipids. Gastroenterology. 1995;10:A149. [Google Scholar]
- 24.Ferreira H, Lucio M, DeCastro B, Gameriro P, Lima JLFC, Reis S. Partition and localization of nimesulide in EPC liposomes: a spectrophotometric and fluorescence study. Anal Bioanal Chem. 2003;377:293–298. doi: 10.1007/s00216-003-2089-5. [DOI] [PubMed] [Google Scholar]
- 25.Kyrikou I, Hadjikakou SK, Kovala-Demertzi D, Viras K, Mavromoustakos T. Effects of non-steroid anti-inflammatory drugs in membrane bilayers. Chem Phys Lipids. 2004;132:157–169. doi: 10.1016/j.chemphyslip.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenberger LM, Dial EJ, Jayaraman V, Zhou ZH, Krishnamorti, Volk R, Gorenstein DE, Marathi DGU. Evaluation of the structural properties and activities of a purified PC-NSAID preparation. Gastroenterology. 2006;130:A270. [Google Scholar]
- 27.Lucio M, Bringezu, Reis S, Lima JLFC, Brezinsinski G. Binding of nonsteroidal anti-inflammatory drugs to DPPC: structure and thermodynamic aspects. Langmuir. 2008;24:4132–4139. doi: 10.1021/la703584s. [DOI] [PubMed] [Google Scholar]
- 28.Moreno MM, Garidel P, Suwalsky M, Howe J, Brandenburg K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: a physico-chemical study with lecithin phospholipids. Biochim Biophys Acta. 2009;1788:1296–1303. doi: 10.1016/j.bbamem.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Giraud MN, Motta C, Romero JJ, Bommelaer G, Lichtenberger LM. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: a possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs) Biochem Pharmacol. 1999;57:247–254. doi: 10.1016/s0006-2952(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenberger LM, Dial EJ, Jayaraman V, Zhou Y, Krishnamorti R, Volk DE, Gorenstein DG, Marathi U. Evaluation of the structural properties and activities of a purified PC-NSAID preparation. Gastroenterology. 2006;130:A-270. [Google Scholar]
- 31.Jayaraman V, Thiran S, Madden DR. Fourier transform infrared spectroscopic characterization of a photolabile precursor of glutamate. FEBS Lett. 2000;475:278–282. doi: 10.1016/s0014-5793(00)01690-2. [DOI] [PubMed] [Google Scholar]
- 32.Abdiche YN, Myszka DG. Probing the mechanism of drug/lipid membrane interactions using Biacore. Anal Biochem. 2004;328:233–243. doi: 10.1016/j.ab.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Bothner-By AA, Stephens RL, Lee J, Warren CD, Jeanloz RW. Structure determination of a tetrasaccharide: transient nuclear Overhauser effects in the rotating frame. J Am Chem Soc. 1984;106:811–813. [Google Scholar]
- 34.Mueller L. Sensitivity enhanced detection of weak nuclei using heteronuclear multiple quantum coherence. J Am Chem Soc. 1979;101:4481–4484. [Google Scholar]
- 35.Case DA, Pearlman DA, Caldwell JW, Cheatham TE, III, Rose WS, Simmerling CL, Darden KM, Merz RV, Stanton AL, Cheng JJ, Vincent M, Crowley DM, Ferguson RJ, Radmer GL, Seibel UC, Singh PK, Weiner SJ, Kollman PA. AMBER6. University of California; San Francisco, CA: 1999. [Google Scholar]
- 36.Frisch MJ, Trucks GW, Schegel HB, Scuseria GE, Robb JRMA, Cheeseman VG, Zakrzewski JA, Montgomery J, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Salvador P, Da(30)nnenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Lui G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Gomalez C, Head-Gordon M, Replogle ES, Pople JA. GAUSSIAN 98 (Revision A.11) Gaussian, Inc; Pittsburgh, PA: 2001. [Google Scholar]
- 37.Bayly CI, Cieplak P, Cornell W, Kollman PA. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem. 1993;97:10269–10280. [Google Scholar]
- 38.Berendsen HJC, van der Spoel D, van Drunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Computer Physics Communications. 1995;91:43. [Google Scholar]
- 39.Lindahl E, Hess B, van der Spoel D. GROMACS 3.0: a package for molecular simulation and trajectory analysis. Journal of Molecular Modeling. 2001;7:306–317. [Google Scholar]
- 40.Tieleman DP, Berendsen HJC. Molecular dynamics simulations of a fully hydrated dipalmitoylphosphatidylcholine bilayer with different macroscopic boundary conditions and parameters. J Chem Phys. 1996;105:4871–4880. [Google Scholar]
- 41.Tieleman DP. 2002 http://moose.bio.ucalgary.ca/index.php?page=Structures_and_Topologies.
- 42.Berger O, Edholm O, Jahnig F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophysical Journal. 1997;72:2002–2013. doi: 10.1016/S0006-3495(97)78845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J. Interaction models for water in relation to protein hydration. Intermolecular Forces. 1981;331 [Google Scholar]
- 44.Schuettelkopf AW, Aalten DMFv. PRODRG - a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallographica D. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. ( http://davapc1.bioch.dundee.ac.uk/) [DOI] [PubMed] [Google Scholar]
- 45.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]
- 46.Miyamoto S, Kollman PA. SETTLE: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. Journal of Computational Chemistry. 1992;13 [Google Scholar]
- 47.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A Smooth Particle Mesh Ewald Method. Journal of Chemical Physics. 1995;103:8577–8593. [Google Scholar]
- 48.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. The Journal of Chemical Physics. 1984;81:3684. [Google Scholar]
- 49.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 50.Lichtenberger LM, Barron M, Marathi U. Association of phosphatidylcholine and NSAIDs as a novel strategy to reduce gastrointestinal toxicity. Drugs Today (Barc) 2009;45:877–890. doi: 10.1358/dot.2009.45.12.1441075. [DOI] [PubMed] [Google Scholar]
- 51.Ober RJ, Ward ES. The choice of reference cell in the analysis of kinetic data using BIAcore. Anal Biochem. 1999;271:70–80. doi: 10.1006/abio.1999.4129. [DOI] [PubMed] [Google Scholar]
- 52.Bax A, Freeman R. Investigation of complex networks of spin-spin coupling by two-dimensional NMR. Journal of Magnetic Resonance. 1981;44:542–561. [Google Scholar]
- 53.Bax AD, Davis DG. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J magn Reson. 1985;63:207–213. [Google Scholar]
- 54.Chakraborty H, Banerjee R, Sarkar M. Incorporation of NSAIDs in micelles: implication of structural switchover in drug-membrane interaction. Biophys Chem. 2003;104:315–325. doi: 10.1016/s0301-4622(02)00389-7. [DOI] [PubMed] [Google Scholar]
- 55.Chakraborty H, Roy S, Sarkar M. Interaction of oxicam NSAIDs with DMPC vesicles: differential partitioning of drugs. Chem Phys Lipids. 2005;138:20–28. doi: 10.1016/j.chemphyslip.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, Mackerell AD., Jr CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem. 2009 doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boggara MB. PhD. University of Houston; Houston, USA: 2009. Lipid-based drug delivery system – molecular dynamics simulations and neutron scattering studies, Chemical and Biomolecular Engineering; p. 238. [Google Scholar]
- 58.Boggara MB, Krishnamoorti R. Partitioning of nonsteroidal antiinflammatory drugs in lipid membranes: a molecular dynamics simulation study. Biophys J. 2010;98:586–595. doi: 10.1016/j.bpj.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clarke RJ. Effect of lipid structure on the dipole potential of phosphatidylcholine bilayers. Biochim Biophys Acta. 1997;1327:269–278. doi: 10.1016/s0005-2736(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 60.Clarke RJ. The dipole potential of phospholipid membranes and methods for its detection. Adv Colloid Interface Sci. 2001;89–90:263–281. doi: 10.1016/s0001-8686(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 61.Starke-Peterkovic T, Turner N, Vitha MF, Waller MP, Hibbs DE, Clarke RJ. Cholesterol effect on the dipole potential of lipid membranes. Biophys J. 2006;90:4060–4070. doi: 10.1529/biophysj.105.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Starke-Peterkovic T, Clarke RJ. Effect of headgroup on the dipole potential of phospholipid vesicles. Eur Biophys J. 2009;39:103–110. doi: 10.1007/s00249-008-0392-y. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Y, Doyen R, Lichtenberger LM. The role of membrane cholesterol in determining bile acid cytotoxicity and cytoprotection of ursodeoxycholic acid. Biochim Biophys Acta. 2009;1788:507–513. doi: 10.1016/j.bbamem.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams JL, Nath N, Chen J, Hundley TR, Gao J, Kopelovich L, Kashfi K, Rigas B. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction, beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res. 2003;63:7613–7618. [PubMed] [Google Scholar]
- 65.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 66.Vane JR, Botting RM. Control of the circulation by vasoactive mediators from endothelial cells. J Lipid Mediat Cell Signal. 1994;9:1–8. [PubMed] [Google Scholar]
- 67.Goddard PJ, Hills BA, Lichtenberger LM. Does aspirin damage canine gastric mucosa by reducing its surface hydrophobicity? Am J Physiol. 1987;252:G421–430. doi: 10.1152/ajpgi.1987.252.3.G421. [DOI] [PubMed] [Google Scholar]
- 68.Goddard PJ, Kao YC, Lichtenberger LM. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990;98:361–370. doi: 10.1016/0016-5085(90)90826-m. [DOI] [PubMed] [Google Scholar]
- 69.Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 70.Husch J, Dutagaci B, Glaubitz C, Geppert T, Schneider G, Harms M, Muller-Goymann CC, Fink L, Schmidt MU, Setzer C, Zirkel J, Rebmann H, Schubert-Zsilavecz M, Abdel-Tawab M. Structural properties of so-called NSAID-phospholipid-complexes. Eur J Pharm Sci. 44:103–116. doi: 10.1016/j.ejps.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244:G561–568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- 72.Lanza FL, Marathi UK, Anand BS, Lichtenberger LM. Clinical trial: comparison of ibuprofen-phosphatidylcholine and ibuprofen on the gastrointestinal safety and analgesic efficacy in osteoarthritic patients. Aliment Pharmacol Ther. 2008;28:431–442. doi: 10.1111/j.1365-2036.2008.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y, Raphael RM. Effect of salicylate on the elasticity, bending stiffness, and strength of SOPC membranes. Biophys J. 2005;89:1789–1801. doi: 10.1529/biophysj.104.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boggara MB, Faraone A, Krishnamoorti R. Effect of pH and ibuprofen on the phospholipid bilayer bending modulus. J Phys Chem B. 114:8061–8066. doi: 10.1021/jp100494n. [DOI] [PubMed] [Google Scholar]
- 75.Lichtenberger LM, Zhou Y, Dial EJ, Raphael RM. NSAID injury to the gastrointestinal tract: evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J Pharm Pharmacol. 2006;58:1421–1428. doi: 10.1211/jpp.58.10.0001. [DOI] [PubMed] [Google Scholar]
- 76.Nunes C, Brezesinski G, Lima JL, Reis S, Lucio M. Synchrotron SAXS and WAXS study of the interactions of NSAIDs with lipid membranes. J Phys Chem B. 115:8024–8032. doi: 10.1021/jp2025158. [DOI] [PubMed] [Google Scholar]
- 77.Nunes C, Brezesinski G, Pereira-Leite C, Lima JL, Reis S, Lucio M. NSAIDs interactions with membranes: a biophysical approach. Langmuir. 27:10847–10858. doi: 10.1021/la201600y. [DOI] [PubMed] [Google Scholar]
- 78.Boggara MB, Krishnamoorti R. Small-angle neutron scattering studies of phospholipid-NSAID adducts. Langmuir. 26:5734–5745. doi: 10.1021/la903854s. [DOI] [PubMed] [Google Scholar]
- 79.Boggara MB, Krishnamoorti R. Partitioning of nonsteroidal antiinflammatory drugs in lipid membranes: a molecular dynamics simulation study. Biophys J. 98:586–595. doi: 10.1016/j.bpj.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lichtenberger LM, Romero JJ, Dial EJ, Moore JE. Naproxen-PC: A GI Safe and Highly Effective Anti-inflammatory. Inflammopharmacology. 2008;16:1–5. doi: 10.1007/s10787-008-8047-2. [DOI] [PubMed] [Google Scholar]
- 81.Anand BS, Romero JJ, Sanduja SK, Lichtenberger LM. Phospholipid association reduces the gastric mucosal toxicity of aspirin in human subjects. Am J Gastroenterol. 1999;94:1818–1822. doi: 10.1111/j.1572-0241.1999.01211.x. [DOI] [PubMed] [Google Scholar]
- 82.Cryer B, Bhatt DL, Lanza FL, Dong JF, Lichtenberger LM, Marathi UK. Low-Dose Aspirin-Induced Ulceration Is Attenuated by Aspirin-Phosphatidylcholine: A Randomized Clinical Trial. Am J Gastroenterol. 2011;106:272–277. doi: 10.1038/ajg.2010.436. [DOI] [PubMed] [Google Scholar]
- 83.Zhou Y, Hancock JF, Lichtenberger LM. The nonsteroidal anti-inflammatory drug indomethacin induces heterogeneity in lipid membranes: potential implication for its diverse biological action. PLoS One. 5:e8811. doi: 10.1371/journal.pone.0008811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Y, Plowman SJ, Lichtenberger LM, Hancock JF. The anti-inflammatory drug indomethacin alters nanoclustering in synthetic and cell plasma membranes. J Biol Chem. 285:35188–35195. doi: 10.1074/jbc.M110.141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.