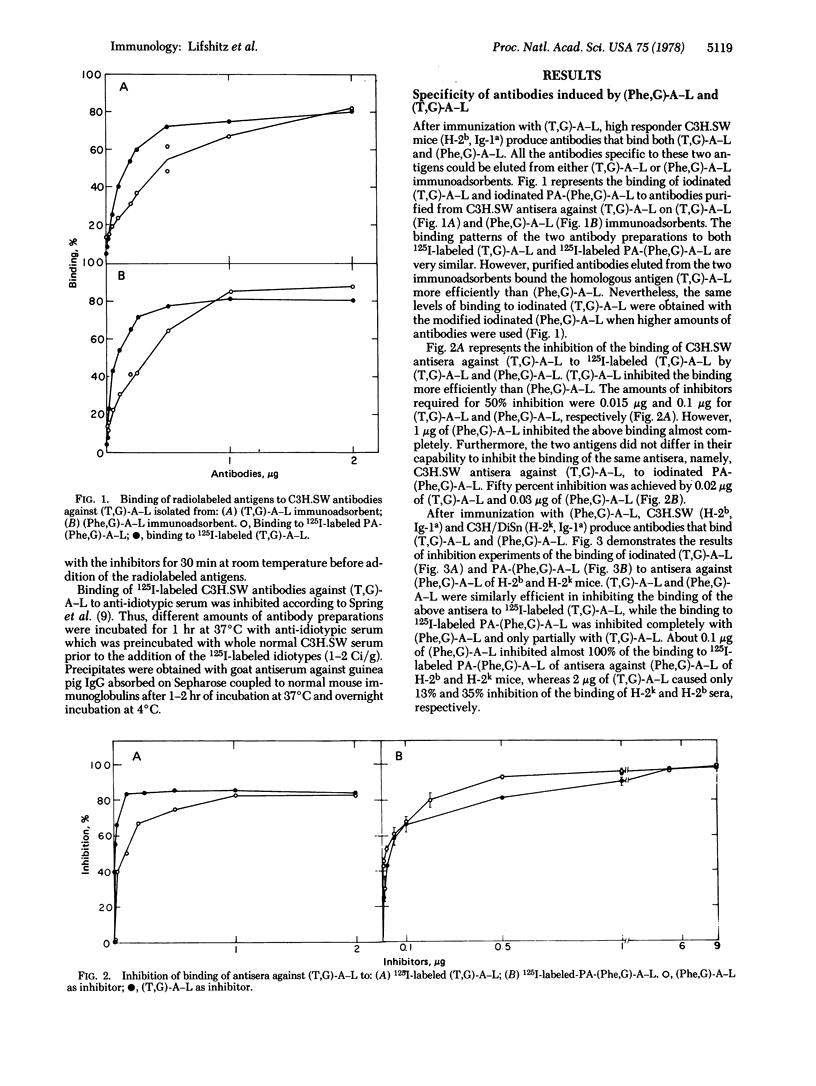
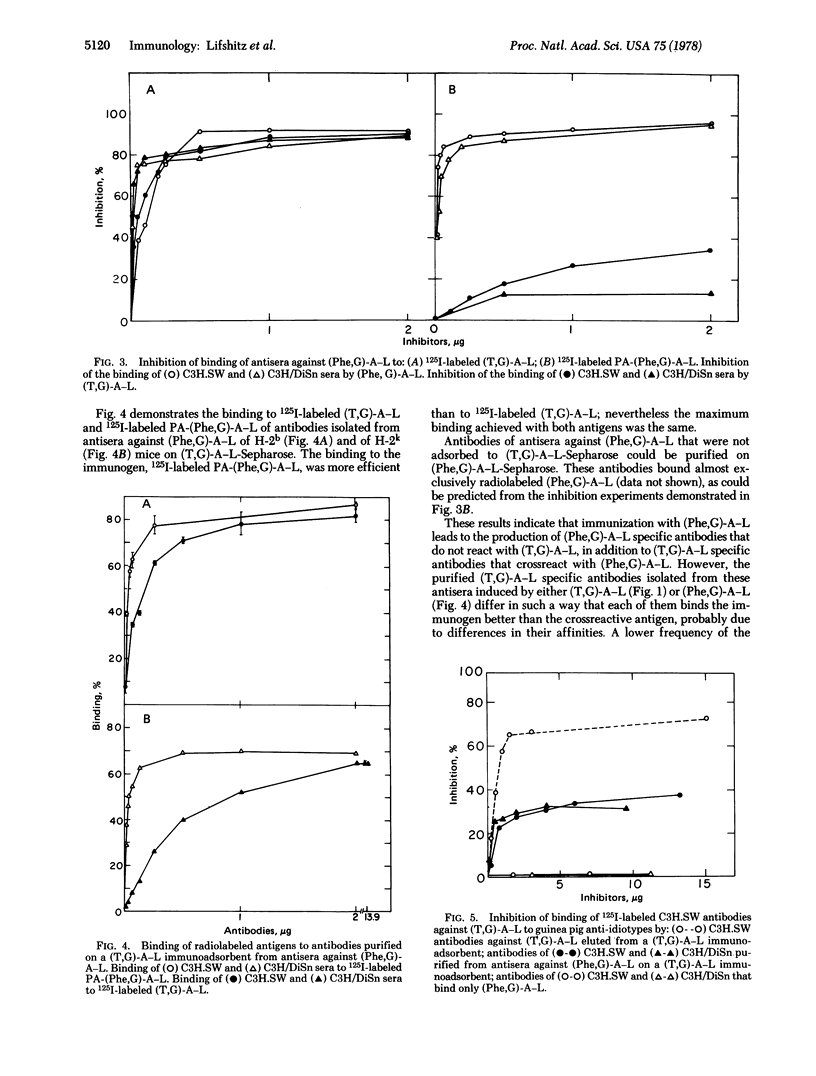
Abstract
Antibodies elicited against the two synthetic polypeptides, poly(Tyr,Glu)-poly(DLAla)-poly(Lys) [(T,G)-A-L] and poly(Phe,Glu)-poly(DLAla)-poly(Lys) [(Phe,G)-A-L], are crossreactive although the humoral responses to these immunogens are under different genetic controls. The fine specificity of the antibodies elicited by the two polypeptides was studied in the present work. Antisera against (Phe,G)-A-L bind both 125I-labeled (T,G)-A-L and iodinated modified (Phe,G)-A-L. However, while the binding to (T,G)-A-L could be inhibited completely with the two antigens, the binding to (Phe,G)-A-L was inhibited completely with (Phe,G)-A-L and only partially with (T,G)-A-L. The binding of 125I-labeled (T,G)-A-L to antisera against (T,G)-A-L was inhibted more efficiently by the homologous antigen than by (Phe,G)-A-L although both antigens completely inhibited the binding. (T,G)-A-L specific antibodies were purified on (T,G)-A-L immunoadsorbents from antisera of high and low responder mice to (T,G)-A-L immunized with (Phe,G)-A-L. (Phe,G)-A-L specific antibodies that did not bind (T,G)-A-L were isolated from the effluent of these columns. By use of anti-idiotypic antibodies of guinea pig against C3H.SW antibodies to (T,G)-A-L it was shown that (T,G)-A-L specific antibodies isolated from antisera against (Phe,G)-A-L of C3H.SW and C3H/DiSn mice possess part of the idiotypic determinants existing on antibodies of C3H.SW obtained by immunization with (T,G)-A-L. In contrast, antibodies to (Phe,G)-A-L that did not bind (T,G)-A-L did not share idiotypic determinants with C3H.SW antibody molecules against (T,G)-A-L. These results suggest that the B cell repertoire expressed by high and low responders to (T,G)-A-L after immunization with (Phe,G)-A-L is similar and represents only part of that of high responders immunized with (T,G)-A-L.
Keywords: synthetic polypeptide antigens, antibody specificity, idiotypic determinants, genetic control of immune responses
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuchs S., Sela M. Antigenicity of some new synthetic polypeptides and polypeptidyl gelatins. Biochem J. 1964 Dec;93(3):566–572. doi: 10.1042/bj0930566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Chinitz A. Genetic control of the antibody response: relationship between immune response and histocompatibility (H-2) type. Science. 1969 Mar 14;163(3872):1207–1208. doi: 10.1126/science.163.3872.1207. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Sela M. Genetic control of the antibody response. II. Further analysis of the specificity of determinant-specific control, and genetic analysis of the response to (H,G)-A--L in CBA and C57 mice. J Exp Med. 1967 Nov 1;126(5):969–978. doi: 10.1084/jem.126.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Tyan M. L. Genetic control of the antibody response in inbred mice. Transfer of response by spleen cells and linkage to the major histocompatibility (H-2) locus. J Exp Med. 1968 Jul 1;128(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H., Horton C. L., Paul W. E. T-lymphocyte-enriched murine peritoneal exudate cells. IV. Genetic control of cross-stimulation at the T-cell level. J Exp Med. 1977 Feb 1;145(2):327–343. doi: 10.1084/jem.145.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver S. S., Brown A., Hämmerling G., McDevitt H. O. Genetic control of the immune response: ability of antigens of defined amino acid sequence to be recognized by the Ir-1 gene system. Eur J Immunol. 1976 Jul;6(7):502–507. doi: 10.1002/eji.1830060711. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Schroeder K. W., Nisonoff A. Quantitative investigations of idiotypic antibodies. V. Factors affecting the persistence and replacement of clones of antibody-producing cells. J Exp Med. 1971 Sep 1;134(3 Pt 1):765–785. doi: 10.1084/jem.134.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]


