Abstract
Background
Cytosine methylation is involved in epigenetic control of gene expression in a wide range of organisms. An increasing number of examples indicate that changing the frequency of cytosine methylation in the genome is a feasible tool to engineer novel traits in plants. Although demethylating effects of compounds have been analyzed in human cultured cells in terms of suppressing cancer, their effect in plant cells has not been analyzed extensively. Here, we developed in planta assay systems to detect inhibition of cytosine methylation using plants that contain a transgene transcriptionally silenced by an epigenetic mechanism.
Results
Seeds of two transgenic plants were used: a petunia line that has been identified as a revertant of the co-suppression of the chalcone synthase-A (CHS-A) gene and contains CHS-A transgenes whose transcription is repressed; Nicotiana benthamiana plants that contain the green fluorescent protein (GFP) reporter gene whose transcription is repressed through virus-induced transcriptional gene silencing. Seeds of these plants were sown on a medium that contained a demethylating agent, either 5-azacytidine or trichostatin A, and the restoration of the transcriptionally active state of the transgene was detected in seedlings. Using these systems, we found that genistein, a major isoflavonoid compound, inhibits cytosine methylation, thus restoring transgene transcription. Genistein also restored the transcription of an epigenetically silenced endogenous gene in Arabidopsis plants.
Conclusions
Our assay systems allowed us to assess the inhibition of cytosine methylation, in particular of maintenance of methylation, by compounds in plant cells. These results suggest a novel role of flavonoids in plant cells and that genistein is useful for modifying the epigenetic state of plant genomes.
Keywords: Cytosine methylation, Demethylating agents, Genistein, RNA-directed DNA methylation, Transcriptional gene silencing
Background
Cytosine methylation is an epigenetic mark present in many eukaryotes, including plants, vertebrates and fungi [1], and plays an important role in various biological processes including regulation of gene expression, stability of the genome, cellular differentiation and development [2]. Transposons and repeats are frequently methylated in a wide range of species [3]. Loss of cytosine methylation induces reactivation and transposition of transposons [4-7], suggesting that cytosine methylation represents the primary mechanism of transposon suppression in host genomes [8]. Cytosine methylation also functions to maintain a repressed chromatin state and stably silence promoter activity [9]. A genome-wide analysis of Arabidopsis thaliana uncovered an interdependence between cytosine methylation and transcription [10].
Cytosine methylation in mammalian genomes occurs predominantly in the context of CG sequences. CG methylation is also the most common modification in plant genomes, but plant genomes also have cytosine methylation at CHG and CHH (where H is A, C or T) sequences. In plants, cytosine methylation can be established through RNA-directed DNA methylation (RdDM) [11], in which DOMAINS REARRANGED METHYLTRANSFERASE1 and 2 (DRM1 and DRM2), Arabidopsis orthologues of mammalian de novo methyltransferase DNMT3, are guided by small RNAs and targeted to DNA [12]. Whether all de novo cytosine methylation in Arabidopsis is guided by small RNAs is not known [12]. The maintenance of CG methylation requires METHYLTRANSFERASE1 (MET1), an orthologue of mammalian DNMT1, while that of non-CG methylation requires DRM1, DRM2 and the plant-specific methyltransferase CHROMOMETHYLASE3 (CMT3), which function redundantly [12]. On the other hand, a family of DNA glycosylases can remove cytosine methylation in plants [12].
Modification of the epigenetic state, in particular the frequency of cytosine methylation, has become a feasible tool to engineer novel traits in plants. Transcriptional gene silencing (TGS) has been induced by targeting small RNA to a transgene promoter via RdDM [11]. This approach has recently been applied to silence an endogenous gene using a viral vector, which led to the production of a plant that does not carry a transgene but has altered traits [13,14]. In addition to inducing changes via RdDM, changes in epigenetic state can also be induced randomly in the genome by inhibiting cytosine methylation. Although a method for targeted demethylation has not been developed for any organisms, transgenerational inheritance of a state of decreased methylation with an increased transcriptional activity has been observed for limited loci in plants [15]. These findings have prompted scientists to alter the cytosine methylation state of the genome that harbors novel epi-alleles. Examples of such attempts include a plant line that had a higher expression level of the Xa21G gene, which confers resistance against Xanthomonas oryzae, was produced in the progeny of rice plants treated with a demethylating agent [16]; various altered phenotypes were observed in a population of Arabidopsis recombinant inbred lines with epigenetically mosaic chromosomes consisting of wild-type and CG methylation-depleted segments that were derived from a cross between wild type and a met1 mutant [17]; exposure of Arabidopsis plants to various environmental stresses resulted in increased global genome methylation and a higher level of tolerance to stresses in the progeny [18]. Similarly, plants from an isogenic canola population, selected on the basis of respiration intensity, were used to generate sublines that have distinct characteristics and different epigenetic states [19].
One of the methods to modify the frequency of methylcytosine in the genome is to treat the organism with demethylating agents. 5-Azacytidine (5-azaC; trade name Vidaza) and its deoxy derivative 5-aza-2'-deoxycytidine (Decitabine; trade name Dacogen) were first synthesized over 40 years ago and are commonly applied inhibitors of cytosine methylation in plants and animals [20]. These reagents induce hypomethylation and reactivate transcriptionally repressed genes; hence, they have been used for cancer therapeutics to suppress hypermethylation of tumor suppressor genes in human. However, these toxic drugs are unstable in aqueous solution. More recently, 2-pyrimidine-1-β-D-riboside (Zebularine) induced the reactivation of hypermethylated genes in cultured human cells [21] and in plants [22]. Although this compound is stable in aqueous solutions, it raises considerable concerns about toxicity as a nucleoside analog. Besides nucleoside analogs, procainamide has been identified as an inhibitor of the human DNA methyltransferase DNMT1 [23]. Trichostatin A, known as a specific inhibitor of histone deacetylase, is also known to cause demethylation in Neurospora crassa [24] and plants [25,26] and downregulates DNA methyltransferase in human cells [27]. There is a growing list of cytosine methylation inhibitors in addition to these compounds (ref. [28]; and references therein).
In terms of finding compounds effective in suppressing cancer, natural products have been screened for inhibitory activity of cytosine methylation using human cancer cells. Through such analysis, plant secondary metabolites, polyphenols, were shown to be antagonists of cytosine methylation in cultured human cells. These compounds include (-)-epigallocatechin-3-O-gallate (EGCG) from green tea [29,30], genistein from soybean [30-32], caffeic acid and chlorogenic acid from coffee [33], polyphenols from Annurca apples [34], and lycopene from tomato [32]. In vitro studies revealed that some of these compounds inhibit DNA methyltransferase activity of human DNMT1 [31,33,35]. However, whether these compounds inhibit methylation in plant cells has not been examined.
In the present study, we focused on genistein, a major pharmacologically active isoflavone abundant in soybean [36] and examined whether genistein can function as a methylation inhibitor in plant cells. For that purpose, we developed two different in planta assay systems, both involving an epigenetically silenced transgene. In these systems, an increase in the mRNA level of the transgene, which is otherwise transcriptionally repressed, indicates inhibition of cytosine methylation. Through these assays, we demonstrated that genistein functions as a methylation inhibitor, thus identifying the first methylation inhibitor of plant origin that acts in plant cells.
Results
Detection of inhibition of cytosine methylation by genistein in transgenic petunia plants that have a transcriptionally silenced transgene
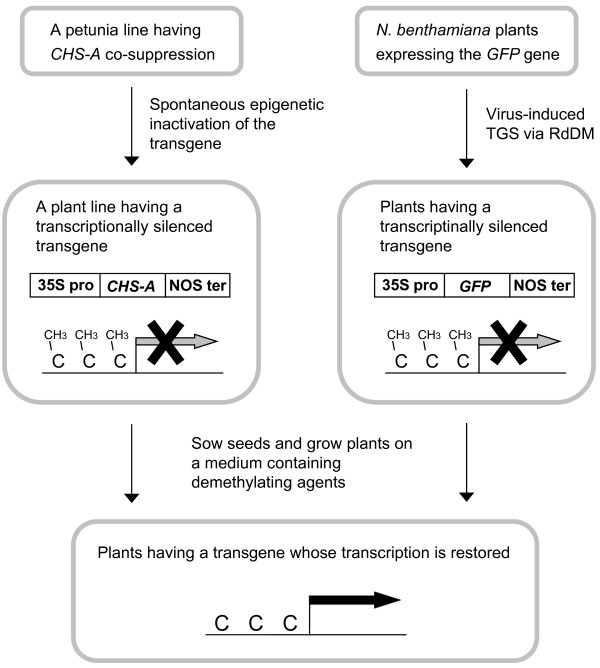
We have been maintaining plants of petunia C002 line that carry the chalcone synthase-A (CHS-A) transgene whose transcription is controlled by the Cauliflower mosaic virus (CaMV) 35S promoter. In this plant line, transcription of the CHS-A transgene is stably repressed through a spontaneous epigenetic change involving cytosine methylation of the CaMV 35S promoter [26]. We have shown previously that treatment of C002 plants with 5-azaC or TSA reduces cytosine methylation and restores transcription of the transgene. We came up with the idea of growing C002 plants on a medium that contained a potential demethylating agent and analyzed its effect based on restoration of transcriptional activity and reduction in the frequency of cytosine methylation in the transgene promoter. The scheme of this analytical system is shown in Figure 1 with the system using the green fluorescent protein (GFP) gene, which is described later.
Figure 1.
Scheme of the system to detect inhibition of cytosine methylation. Petunia plant line C002, which has transcriptionally silenced CHS-A transgenes, has been generated through spontaneous epigenetic changes involving cytosine methylation on CaMV 35S promoter (left; ref. [26]). N. benthamiana plants that have a transcriptionally silenced GFP gene were produced through virus-induced RNA-directed DNA methylation of the CaMV 35S promoter (right; ref. [37]). Seeds of these plants were sown on a medium that contained a potential demethylating agent. If the agent is inhibitory of cytosine methylation, the frequency of cytosine methylation in the promoters is reduced, resulting in the restoration of transcription from the promoters.
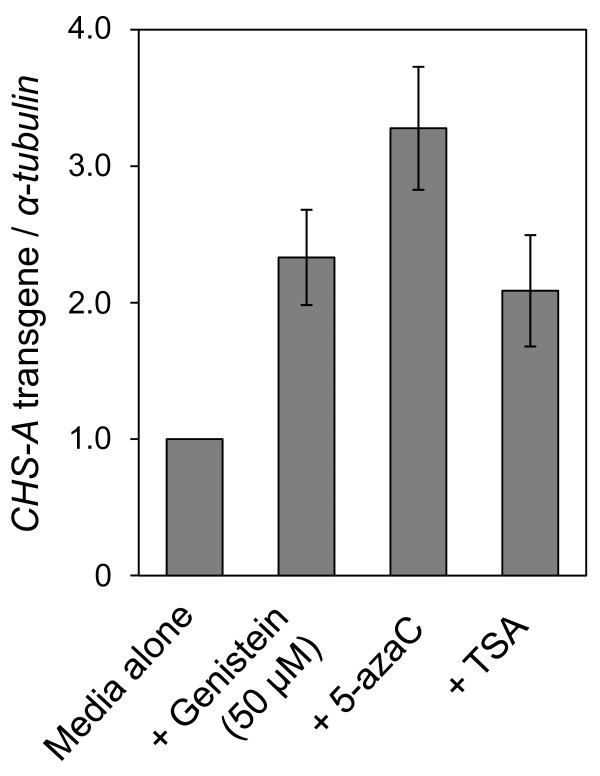
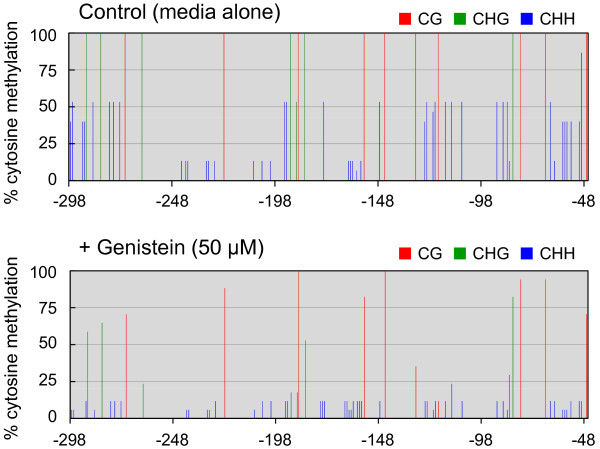
Seeds of the C002 line were sown on a medium that contained genistein. For a positive control, plants were also grown in a medium supplemented with 5-azaC or TSA. RNA was isolated from bulked plants after growing for 1 month on the same plate, to analyze the level of mRNA transcribed from the CHS-A transgene using quantitative RT-PCR. The mRNA levels of the CHS-A transgene increased as a consequence of growing plants in the presence of genistein, indicating that the TGS is suppressed (Figure 2). In bisulfite sequencing analysis of cytosine methylation of the CaMV 35S promoter in plants treated with genistein, the methylation level was actually reduced (Figure 3). The frequency of methylcytosine was reduced in all sequence contexts, namely, CG, CHG and CHH (Additional file 1: Figure S1).
Figure 2.
Effects of genistein on reactivation of the transcriptionally silenced CHS-A transgene in petunia plants. Real-time RT-PCR was conducted to analyze the mRNA levels of the CHS-A transgene in plants treated with genistein (50 μM), 5-azaC (20 μM) and TSA (6 μM). The mRNA levels were quantified relative to the mRNA level of α-tubulin. The value for control plants grown in a medium with no supplement ('Media alone') was set at 1. Data represent means and standard errors obtained from three replicates. Statistic analysis using Student's t-test indicated that the mRNA levels of CHS-A transgene in plants treated with these compounds were significantly higher than those of non-treated plants (P < 0.05).
Figure 3.
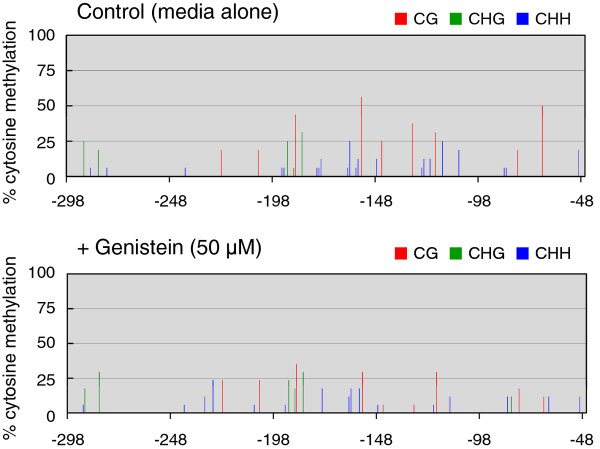
Changes in frequency of methylcytosine in the CaMV 35S promoter as a consequence of treatment of C002 petunia plants with genistein. Sequencing data for PCR products amplified from bisulfite-treated DNA have been compiled. The height of the vertical lines shows the frequency of methylcytosine at respective positions per total PCR clones sequenced. Red, green, and blue lines indicate frequencies of methylcytosine at CpG, CpHpG, and CpHpH sites, respectively. For control and genistein-treated plants, 15 and 16 clones were sequenced, respectively. Numbers below the line indicate nucleotide positions relative to transcription start site of the CaMV 35S promoter. The analyzed sequences cover the -298 to -47 region of the promoter.
Detecting inhibition of cytosine methylation by genistein in transgenic Nicotiana benthamiana plants that have a transcriptionally silenced transgene
We also analyzed the inhibitory effect of genistein on cytosine methylation using a different assay system (Figure 1, right). We previously developed a system that induces RNA-mediated heritable TGS using a virus vector [13,14,37]. In this system, a virus vector carrying a portion of a gene promoter efficiently induces cytosine methylation and TGS by targeting double-stranded RNA to the promoter, and the epigenetically silenced state of the gene is transmitted to subsequent generations. On the other hand, the virus that induced TGS is not transmitted to the next generation [13,37]. We used seeds produced on transgenic N. benthamiana plants, in which TGS of the GFP gene was systemically induced by infecting the plants with a recombinant virus that contained a portion of the CaMV 35S promoter, which controlled transcription of the GFP gene (ref. [37]; for details, see also Methods). Seeds produced on such plants were sown on a medium for germination, and the resultant plants were grown in the medium for 1 month.
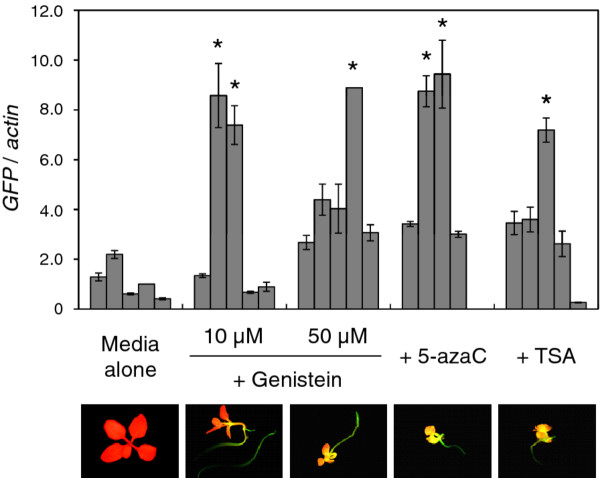
Both GFP fluorescence and the mRNA level of the GFP gene indicated that the GFP gene expression was restored in plants grown in a medium that contained genistein as well as that contained 5-azaC or TSA (Figure 4). The advantage of this system is that GFP fluorescence allowed us to visually detect changes in the level of transgene expression in an individual plant. The extent of both growth inhibition and restoration of GFP fluorescence induced by each of these compounds differed between individual plants: the treated plant population comprised individuals that were highly affected and those that were not (for GFP mRNA level, see Figure 4; for GFP fluorescence, see Additional file 1: Figure S2). Such a difference among individual plants could be ascribed to the short half-life of these compounds in aqueous solution: e.g., the half-life of 5-azaC in mice is less than 6 h, and its residual effect lasts 1-2 days after administration [38]. If these compounds are not absorbed by proliferating cells of germinating plants while these compounds are active, then their full effect would not be realized. In addition, the low level of solubility of the compounds, especially genistein and TSA, in aqueous solution may also limit their uptake by plants. On the other hand, restoration of GFP fluorescence was not detected in any control plants grown in a medium with no supplement, which indicated that the effect was due to these compounds.
Figure 4.
Effects of genistein on reactivation of the transcriptionally silenced GFP gene in N. benthamiana plants. Real-time RT-PCR was conducted to analyze the mRNA levels of the GFP gene in plants treated with genistein (10 μM or 50 μM), 5-azaC (20 μM) and TSA (2 μM). The mRNA levels were quantified relative to the mRNA level of the actin gene. Data for four or five individuals for each treatment are shown. The value for one of the control plants grown in a medium with no supplement ('Media alone') was set at 1. The data represent the means and standard errors obtained from three replicates. Statistic analysis was done using Bonferroni/Dunn test. Means that are indicated by asterisk are significantly different from those of all the five plants grown in a medium with no supplement (P < 0.05). A representative image of plants for each treatment is shown below the chart. Fluorescence was analyzed using a long-pass filter that allows detection of autofluorescence of chloroplasts colored in red.
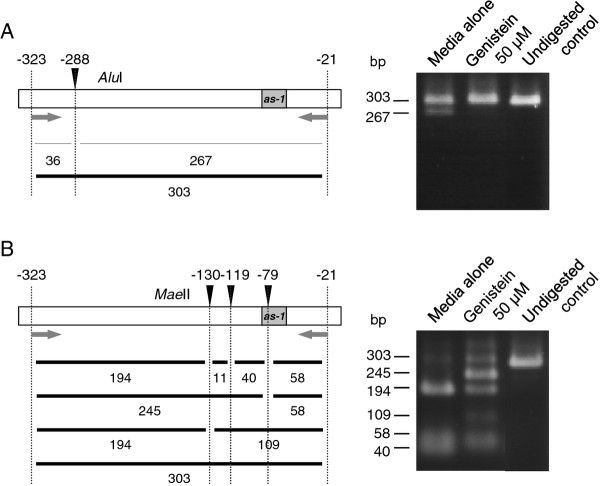
Bisulfite sequencing analysis showed that the frequency of cytosine methylation was reduced in the GFP-restored plants grown in a medium supplemented with genistein compared with plants grown in a medium with no supplement (Figure 5). Changes in the frequency of methylcytosine were also analyzed by digesting the DNA fragments amplified by PCR from bisulfite-treated DNA (Figure 6). In this experiment, tolerance of PCR products to digestion indicates lack of methylation of the cytosine at the restriction sites because of the conversion of cytosine by the bisulfite treatment. The results clearly indicated that genistein-treated plants had a lower frequency of methylation at the AluI and MaeII sites in the CaMV 35S promoter than the control plants.
Figure 5.
Changes in frequency of methylcytosine in CaMV 35S promoter as a consequence of treatment of N. benthamiana plants with genistein. For both control and genistein-treated plants, 17 clones were sequenced. Representative data of the analysis of DNAs isolated from a single control plant and three genistein-treated plants are shown. For other information, see the legend to Figure 3.
Figure 6.
Analysis of methylation status of CaMV 35S promoter in genistein-treated N. benthamiana plants by restriction digestion of DNA fragments amplified with PCR from bisulfite-treated DNA. (A) Analysis of cytosine at position -288 (relative to the transcription initiation site) of the promoter using AluI. (B) Analysis of cytosines at positions -130, -119, and -79 of the promoter using MaeII. Note that treatments of PCR-amplified fragments with AluI and MaeII both resulted in lower levels of digestion when DNA isolated from genistein-treated plants was used for the analysis, indicating that genistein-treated plants have a lower frequency of cytosine methylation in the promoter. Sizes of DNA fragments (in bp) predicted by complete or partial digestions are indicated below the maps of the promoter. Arrows indicate primers for PCR. The position of the cis-acting as-1 element, to which binding of protein factor(s) is inhibited by cytosine methylation [60], is shown.
Genistein reactivates a transcriptionally silenced endogenous gene in Arabidopsis
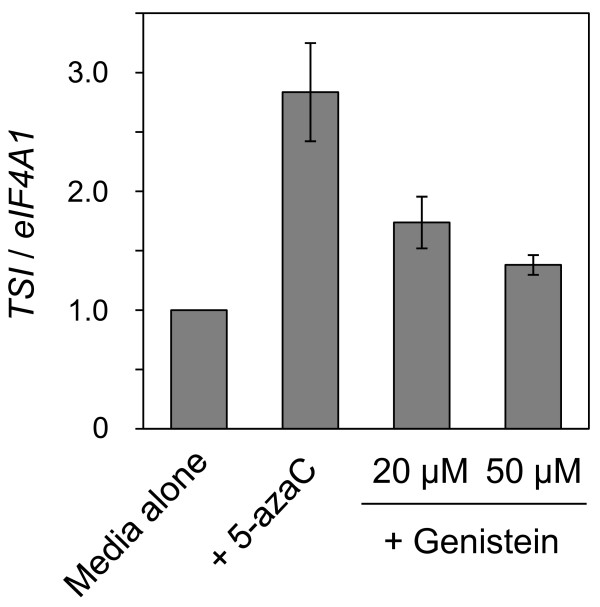
To determine whether genistein affects silenced state of an endogenous gene that is transcriptionally silenced, we analyzed Arabidopsis plants grown on medium supplemented with genistein. We analyzed changes in the mRNA level of TRANSCRIPTIONALLY SILENT INFORMATION (TSI), which is known to be silenced transcriptionally by an epigenetic mechanism involving cytosine methylation in vegetative tissues but reactivated by treatment with demethylating agents or in hypomethylation mutants [22,39]. In plants grown in medium that contained genistein, the mRNA level of TSI increased, although not as much as in 5-azaC-treated plants (Figure 7).
Figure 7.
Effects of genistein on mRNA level of a transcriptionally silenced endogenous gene in Arabidopsis plants. Real-time RT-PCR was conducted to analyze mRNA level of TSI gene in Arabidopsis plants treated with genistein (20 μM or 50 μM) and 5-azaC (20 μM). The mRNA levels were quantified relative to the mRNA level of eIF4A1. The value of control plants grown in a medium with no supplement ('Media alone') was set at 1. Data represent the means and standard errors obtained from three replicates. Statistic analysis using Student's t-test indicated that the mRNA levels of TSI gene in plants treated with these compounds were significantly higher than those of non-treated plants (P < 0.05).
Discussion
In the in planta assay systems that we have established, we can detect the restoration of the transcriptionally active state of a gene whose transcription is epigenetically repressed. Petunia C002 line arose as a spontaneous revertant plant from a CHS-A co-suppressed plant line in which transcripts of both the CHS-A transgene and endogenous CHS-A gene are degraded. Because transcription of the CHS-A transgene is repressed in C002 plants and small RNAs corresponding to both the CHS-A coding region and CaMV 35S promoter are not detected in this plant line [26], it is not likely that cytosine methylation of the CaMV 35S promoter is established in each generation of this plant line through de novo methylation. Such a lack of de novo methylation is unequivocally true of the promoter of the silenced GFP transgene in N. benthamiana plants. In this plant, the virus that induced cytosine methylation of the CaMV 35S promoter is eliminated during the meiosis and is not transmitted to the subsequent generation. As a consequence, the progeny plants that were used for the assay in the present study do not have viral RNAs that trigger de novo methylation on the CaMV 35S promoter [37]. Accordingly, reduction in the frequency of methylcytosine detected in the genistein-treated plants indicates inhibition of the process to maintain cytosine methylation rather than inhibition of de novo methylation. This notion is consistent with the fact that genistein inhibits the in vitro activity of human DNA methyltransferase DNMT1, an orthologue of Arabidopsis MET1 [31,32]. Thus, this assay system allows the detection of a compound's inhibitory activity that is specific to the maintenance process of the cytosine methylation. In this regard, our in planta assay systems can be used to dissect the functions of demethylating compounds. In addition, our assay systems may be useful for screening natural or artificial compounds that can inhibit cytosine methylation.
Using these assay systems, we found that genistein induced the reduction of the methylation of cytosines in all sequence contexts, namely, CG, CHG and CHH. Genistein may affect the catalytic function of more than one DNA methyltransferase that controls the maintenance of cytosine methylation. In Arabidopsis, in addition to controlling CG methylation, MET1 is required to maintain non-CG methylation: pre-existing CG methylation, or a chromatin mark associated with it, might be able to attract non-CG methylation [12]. Therefore, it is also possible that non-CG methylation is reduced as an indirect effect of inhibition of MET1 orthologue(s) by genistein. Alternatively, histone modification may be a target of genistein. Genetic studies have identified a link between histone and cytosine methylation: mutations in CMT3 and histone H3 lysine 9 (H3K9) methyltransferase gene KYP cause a reduction in CHG methylation (ref. [40]; and references therein). The CMT3 protein can interact with histone H3 when it is methylated at lysines 9 and 27, suggesting that these modifications recruit the CMT3 protein to target loci [40]. In human cancer cells, genistein can induce demethylation and acetylation of H3K9 [41]. Taking into account these observations, it is also conceivable that the observed reduction in cytosine methylation in plants treated with genistein is mediated by changes in histone modification, which result in failure to recruit DNA methyltransferase(s) to the targets. Such a link between cytosine methylation and histone modification is consistent with the restoration of transgene transcription in plants treated with TSA, an inhibitor of histone deacetylase. In fact, this compound can induce demethylation of cytosine in organisms including plants [24-26].
Genistein may serve as an agent to modify the epigenetic state of a plant genome
In terms of producing plants with novel traits, changes in methylation levels can be induced by crossing plants with methyltransferase mutants. However, cytosine methylation affected by a mutation in a single methyltransferase gene is confined to a particular sequence context because a particular methyltransferase is responsible for methylation of cytosine in a specific sequence context. Moreover, mutants in cytosine methylation are available only in limited plant species. Therefore, genistein, like cytidine analogs such as 5-azaC or zebularine, has the potential advantage over the use of mutants in efficiently producing a novel epi-allele in plants. In addition, polyphenols such as genistein have a potential advantage over cytidine analogs. The mechanism of inhibition of methyltransferase by cytidine analogs involves incorporation of these compounds into DNA [42,43]. Incorporation of cytidine analogs results in a permanent alteration of the genome, often leading to mutation, whose process is also mediated by a DNA methyltransferase [44]. On the other hand, polyphenols are not incorporated into DNA and, hence, likely inhibit cytosine methylation without inducing mutation. This notion is consistent with the result of computational modeling, which suggests that EGCG directly interacts with the catalytic site of human DNMT1 to inhibit its activity [35]. Likewise, genistein might directly interact with MET1 orthologues and/or other methyltransferases without being incorporated into DNA in plants. Accordingly, in terms of inducing an epigenetic change rather than a mutation, the use of genistein may be preferred over nucleoside analogs, although the efficiency of the demethylation between genistein and nucleoside analogs may differ.
Genistein inhibition of cytosine methylation suggests a novel role for flavonoids in plant cells
The reduction in the frequency of methylation suggests that exogenously applied genistein was active in the nucleus of the plant cell. Isoflavonoids are mostly present as glycosides or malonyl glycosides in plant tissues such as soybean seeds [45]. Whether these isoflavone glycoconjugates (e.g., genistin) or isoflavone malonyl glycoconjugates (e.g., malonylgenistin) affect cytosine demethylation the same as isoflavone aglycone (e.g., genistein) remains to be examined. Flavonoids, a major class of polyphenols, include flavonols, anthocyanins, proanthocyanidins (condensed tannins), and isoflavonoids. These compounds have diverse functions in plants, e.g., in flower pigmentation, UV protection, signaling, male fertility, and defense against pathogens [46-48].
Flavonoids are synthesized in the cytoplasm and are then deposited in a variety of cellular sites including the vacuole and cell wall or secreted to outside [47]. In addition to these locations, flavonoids can also be present in plant cell nuclei [49-52]. However, the significance of the accumulation of flavonoids in nuclei has not been clear, although anthocyanin and DNA have been presumed to form a protective complex against oxidative damage [53]. Here we found that genistein inhibited transcriptional repression not only of the transgene but also of an endogenous plant gene. The present results thus indicate the involvement of a flavonoid in the epigenetic control of transcription, a novel role for flavonoids in plant cells.
Conclusions
We developed an in planta assay systems using plants of transgenic petunia and N. benthamiana that contain epigenetically silenced transgenes, which allow the detection of inhibition of cytosine methylation by a particular compound. Transcriptional repression of the GFP gene in N. benthamiana plants was induced by a recombinant virus, which is not transmitted to the subsequent generation. Therefore, the assay for restoration of GFP expression in the progeny plants in our system is particularly useful for detecting the inhibition of the maintenance process of cytosine methylation. Using these assay systems, we found that genistein has such activity. Genistein also had restoring effects on an epigenetically silenced endogenous gene in Arabidopsis, suggesting that the compound is useful for modifying epigenetic state of plant genomes.
Methods
Plant materials
Seeds of a transgenic petunia (Petunia hybrida) line C002 [26,54-56], designated CHS38P, were used. C002 is a line derived from a spontaneous revertant plant from the C001 line (designated as CHS38W), which was obtained by the transformation of wild-type plant V26 with the CHS-A transgene controlled by the CaMV 35S promoter and the NOS terminator [57]. The C001 line produces white flowers as a consequence of stable co-suppression of the CHS-A genes [56], while revertant line C002 produces pigmented wild-type flowers as a consequence of transcriptional repression of the CHS-A transgene, which occurred via an epigenetic mechanism involving cytosine methylation [26].
Seeds of transgenic N. benthamiana line 16c plants infected with a recombinant Cucumber mosaic virus containing a segment of the transgene promoter [37] were also used. The N. benthamiana 16c line contains a single copy of the GFP transgene whose transcription is controlled by the CaMV 35S promoter [58]. Plants of this line were infected with the recombinant virus containing the -208 to -89 region (positions are relative to the transcriptional initiation site) of the promoter. We have found that the resultant plants systemically lose GFP fluorescence through the induction of TGS mediated by RdDM of the promoter, and the silenced state is stably transmitted to the subsequent generations [37]. In addition, seeds of A. thaliana ecotype Columbia (Col-0) were used for analyzing changes in the expression of endogenous genes.
Treatment of plants with demethylating agents
Surface-sterilized seeds of P. hybrida, N. bentahamiana or A. thaliana were sown on plates of medium containing 1/2 concentration of MS salts [59] and 10 g/l sucrose. The pH of the medium was adjusted to 5.7 before autoclaving. Media were solidified with 0.8% (w/v) agar, amended with final concentrations of 20 μM aqueous 5-azaC, 2-6 μM TSA in methanol or 10-50 μM genistein in 80% methanol just before plates were poured. After 10 or 20 seeds were sown per plate, plates were incubated at a photoperiod of 16-h light/8-h dark, 26°C. Development of plants treated with these compounds was slightly affected (e.g., delay in germination). Each treatment was done in triplicate.
Gene expression analysis
Isolation of total RNA and quantitative RT-PCR were done essentially as described previously [26]. The following primer pairs were used for the PCR: for the CHS-A transgene, CHS-trans + 12 F (5'-CTCATTTCTCTATTACTTCAGCC-3') and 2350 (5'-GTGCTTTGATCAACACAGTTTG-3'); for the petunia α-tubulin gene, tub1110F (5'-GCCACCATCAAGACCAAGC-3') and tub201R (5'-ACCTCAGCAACACTGGTTGA-3'); for the GFP gene, mGFP + 148 F (5'-ACTGGAAAACTACCTGTTCC-3') and mGFP + 344R (5'-TCAAACTTGACTTCAGCACG-3'); for the N. benthamiana actin gene, Nb-actF (5'-GAAGATACTCACAGAAAGAGG-3'); and Nb-actR2 (5'-GGAGCTAATGCAGTAATTTGG-3'); for the Arabidopsis TSI gene, TSI-F1 5'-TAGAGCAGTTAACCCGAACC-3') and TSI-R1 (5'-TAGCTTACTTCACCTAGAGTC-3'); for the Arabidopsis eukaryotic initiation factor 4A-1 (eIF4A1) gene (At3g13920), eIF4A-F1 (5'-CATGTTGAAGAGGCAGTCTC-3') and eIF4A-R1 (5'-GAAGAACACACCAACTTGGA-3'). Differences in mRNA level between plants were statistically analyzed using Student's t-test or Bonferroni/Dunn test. GFP fluorescence was examined with an epifluorescence microscope (MVX10; Olympus, Tokyo, Japan) equipped with a GFP mirror unit (U-MGFP HQ/XL and U-MGFP/XL; Olympus).
Analysis of cytosine methylation
Cytosine methylation of the CaMV 35S promoter was analyzed by bisulfite sequencing. DNA was isolated from young seedlings grown on plates using Nucleon PhytoPure DNA extraction kit (Amersham Biosciences, Piscataway, NJ, USA). Bisulfite treatment of DNA, subsequent PCR amplification, and a control experiment to ensure the completion of the treatment were done as described previously [26].
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AK conceived and planned the study. SA, MK and AK carried out the experiments and drafted the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Figure S1. Summary of bisulfite sequencing analysis of CaMV 35S promoter in control and genistein-treated petunia C002 plants. Red, green, and blue bars indicate frequencies of methylcytosine at CpG, CpHpG, and CpHpH sites, respectively. Figure S2 GFP fluorescence of plants grown in a medium with no supplement and plants treated with genistein (10 μM or 50 μM), 5-azaC (20 μM), and TSA (2 μM). Images of five N. benthamiana plants are shown for each treatment. Fluorescence was analyzed using a long-pass filter that allows detection of the red autofluorescence of chloroplasts.
Contributor Information
Sachiko Arase, Email: arase3sachiko@yahoo.co.jp.
Megumi Kasai, Email: m.kasai1027@gmail.com.
Akira Kanazawa, Email: kanazawa@res.agr.hokudai.ac.jp.
Acknowledgements
We thank Shungo Otagaki for technical advice and seeds produced on virus-infected N. benthamiana plants. We also thank David Baulcombe for the original 16c N. benthamiana plants, and Neal Gutterson and Richard Jorgensen for transgenic petunia plants. We are grateful to Keisuke Kitamura and Chikara Masuta for helpful discussion on the roles of flanovoids in soybean and virus-induced gene silencing, respectively. Our work is supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Zemach A, McDaniel IE, Silva P, Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science. 2010;328:916–919. doi: 10.1126/science.1186366. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Ecker JR. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 2006;126:1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kato M, Miura A, Bender J, Jacobsen SE, Kakutani T. Role of CG and non-CG methylation in immobilization of transposons in Arabidopsis. Curr Biol. 2003;13:421–426. doi: 10.1016/S0960-9822(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Miura A, Yonebayashi S, Watanabe K, Toyama T, Shimada H, Kakutani T. Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature. 2001;411:212–214. doi: 10.1038/35075612. [DOI] [PubMed] [Google Scholar]
- Singer T, Yordan C, Martienssen RA. Robertson's Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1) Genes Dev. 2001;15:591–602. doi: 10.1101/gad.193701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Kobayashi A, Kawabe A, Mathieu O, Miura A, Kakutani T. Bursts of retrotransposition reproduced in Arabidopsis. Nature. 2009;461:423–426. doi: 10.1038/nature08351. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet. 2007;39:61–69. doi: 10.1038/ng1929. [DOI] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ. RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol. 2009;21:367–376. doi: 10.1016/j.ceb.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Chan SW, Henderson IR, Jacobsen SE. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet. 2005;6:351–360. doi: 10.1038/nrg1601. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Inaba J, Shimura H, Otagaki S, Tsukahara S, Matsuzawa A, Kim BM, Goto K, Masuta C. Virus-mediated efficient induction of epigenetic modifications of endogenous genes with phenotypic changes in plants. Plant J. 2011;65:156–168. doi: 10.1111/j.1365-313X.2010.04401.x. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Inaba J, Kasai M, Shimura H, Masuta C. RNA-mediated epigenetic modifications of an endogenous gene targeted by a viral vector: a potent gene silencing system to produce a plant that does not carry a transgene but has altered traits. Plant Signal Behav. 2011;6:1090–1093. doi: 10.4161/psb.6.8.16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski J, Grossniklaus U. Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Curr Opin Plant Biol. 2011;14:195–203. doi: 10.1016/j.pbi.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Akimoto K, Katakami H, Kim HJ, Ogawa E, Sano CM, Wada Y, Sano H. Epigenetic inheritance in rice plants. Ann Bot. 2007;100:205–217. doi: 10.1093/aob/mcm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders J, Wulff BB, Mirouze M, Marí-Ordóñez A, Dapp M, Rozhon W, Bucher E, Theiler G, Paszkowski J. Compromised stability of DNA methylation and transposon immobilization in mosaic Arabidopsis epigenomes. Genes Dev. 2009;23:939–950. doi: 10.1101/gad.524609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, Hollunder J, Hollander J, Meins F, Kovalchuk I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben M, Haesendonckx B, Standaert E, Van Der Kelen K, Azmi A, Akpo H, Van Breusegem F, Guisez Y, Bots M, Lambert B. et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc Natl Acad Sci USA. 2009;106:20109–20114. doi: 10.1073/pnas.0908755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Cheng JC, Matsen CB, Gonzales FA, Ye W, Greer S, Marquez VE, Jones PA, Selker EU. Inhibition of DNA methylation and reactivation of silenced genes by zebularine. J Natl Cancer Inst. 2003;95:399–409. doi: 10.1093/jnci/95.5.399. [DOI] [PubMed] [Google Scholar]
- Baubec T, Pecinka A, Rozhon W, Mittelsten Scheid O. Effective, homogeneous and transient interference with cytosine methylation in plant genomic DNA by zebularine. Plant J. 2009;57:542–554. doi: 10.1111/j.1365-313X.2008.03699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Yegnasubramanian S, Lin X, Nelson WG. Procainamide is a specific inhibitor of DNA methyltransferase 1. J Biol Chem. 2005;280:40749–40756. doi: 10.1074/jbc.M505593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker EU. Trichostatin A causes selective loss of DNA methylation in Neurospora. Proc Natl Acad Sci USA. 1998;95:9430–9435. doi: 10.1073/pnas.95.16.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RJ, Earley K, Pontes O, Silva M, Chen ZJ, Neves N, Viegas W, Pikaard CS. A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell. 2004;13:599–609. doi: 10.1016/S1097-2765(04)00064-4. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, O'Dell M, Hellens RP. Epigenetic inactivation of chalcone synthase-A transgene transcription in petunia leads to a reversion of the post-transcriptional gene silencing phenotype. Plant Cell Physiol. 2007;48:638–647. doi: 10.1093/pcp/pcm028. [DOI] [PubMed] [Google Scholar]
- Januchowski R, Dabrowski M, Ofori H, Jagodzinski PP. Trichostatin A down-regulates DNA methyltransferase 1 in Jurkat T cells. Cancer Lett. 2007;246:313–317. doi: 10.1016/j.canlet.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, Jones PA. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2'-deoxycytidine. Mol Cancer Ther. 2005;4:1515–1520. doi: 10.1158/1535-7163.MCT-05-0172. [DOI] [PubMed] [Google Scholar]
- Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–7041. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- King-Batoon A, Leszczynska JM, Klein CB. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ Mol Mutagen. 2008;49:36–45. doi: 10.1002/em.20363. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Zhu BT. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis. 2006;27:269–277. doi: 10.1093/carcin/bgi206. [DOI] [PubMed] [Google Scholar]
- Fini L, Selgrad M, Fogliano V, Graziani G, Romano M, Hotchkiss E, Daoud YA, De Vol EB, Boland CR, Ricciardiello L. Annurca apple polyphenols have potent demethylating activity and can reactivate silenced tumor suppressor genes in colorectal cancer cells. J Nutr. 2007;137:2622–2628. doi: 10.1093/jn/137.12.2622. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Shim JY, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005;68:1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004;150:43–56. doi: 10.1016/j.toxlet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Otagaki S, Kawai M, Masuta C, Kanazawa A. Size and positional effects of promoter RNA segments on virus-induced RNA-directed DNA methylation and transcriptional gene silencing. Epigenetics. 2011;6:681–691. doi: 10.4161/epi.6.6.16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisler JA. Isolation, characterization, and properties of a labile hydrolysis product of the antitumor nucleoside, 5-azacytidine. J Med Chem. 1978;21:204–208. doi: 10.1021/jm00200a012. [DOI] [PubMed] [Google Scholar]
- Mathieu O, Probst AV, Paszkowski J. Distinct regulation of histone H3 methylation at lysines 27 and 9 by CpG methylation in Arabidopsis. EMBO J. 2005;24:2783–2791. doi: 10.1038/sj.emboj.7600743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroth AM, Shultis D, Jasencakova Z, Fuchs J, Johnson L, Schubert D, Patnaik D, Pradhan S, Goodrich J, Schubert I, Jenuwein T, Khorasanizadeh S, Jacobsen SE. Dual histone H3 methylation marks at lysines 9 and 27 required for interaction with CHROMOMETHYLASE3. EMBO J. 2004;23:4286–4296. doi: 10.1038/sj.emboj.7600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Majid S, Igawa M, Dahiya R. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123:552–560. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- Christman JK. 5-Azacytidine and 5-aza-2'-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- Gowher H, Jeltsch A. Mechanism of inhibition of DNA methyltransferases by cytidine analogs in cancer therapy. Cancer Biol Ther. 2004;3:1062–1068. doi: 10.4161/cbt.3.11.1308. [DOI] [PubMed] [Google Scholar]
- Jackson-Grusby L, Laird PW, Magge SN, Moeller BJ, Jaenisch R. Mutagenicity of 5-aza-2'-deoxycytidine is mediated by the mammalian DNA methyltransferase. Proc Natl Acad Sci USA. 1997;94:4681–4685. doi: 10.1073/pnas.94.9.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaubhadel S, Farhangkhoee M, Chapman R. Identification and characterization of isoflavonoid specific glycosyltransferase and malonyltransferase from soybean seeds. J Exp Bot. 2008;59:981–994. doi: 10.1093/jxb/ern046. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Steele CL. Flavonoids and isoflavonoids - a gold mine for metabolic engineering. Trends Plant Sci. 1999;4:394–400. doi: 10.1016/S1360-1385(99)01471-5. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. J Integr Plant Biol. 2010;52:98–111. doi: 10.1111/j.1744-7909.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- Hutzler P, Fischbach R, Heller W, Jungblut T, Reuber S, Schmitz R, Veit M, Weissenbock G, Schnitzler J. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J Exp Bot. 1998;49:953–965. doi: 10.1093/jexbot/49.323.953. [DOI] [Google Scholar]
- Feucht W, Treutter D, Polster J. Flavanol binding of nuclei from tree species. Plant Cell Rep. 2004;22:430–436. doi: 10.1007/s00299-003-0705-7. [DOI] [PubMed] [Google Scholar]
- Buer CS, Muday GK. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell. 2004;16:1191–1205. doi: 10.1105/tpc.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslowsky DE, Warek U, Winkel BS. Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem. 2005;280:23735–23740. doi: 10.1074/jbc.M413506200. [DOI] [PubMed] [Google Scholar]
- Sarma A, Sharma R. Anthocyanin-DNA copigmentation complex: mutual protection against oxidative damage. Phytochemistry. 1999;52:1313–1318. doi: 10.1016/S0031-9422(99)00427-6. [DOI] [Google Scholar]
- Metzlaff M, O'Dell M, Cluster PD, Flavell RB. RNA-mediated RNA degradation and chalcone synthase A silencing in petunia. Cell. 1997;88:845–854. doi: 10.1016/S0092-8674(00)81930-3. [DOI] [PubMed] [Google Scholar]
- O'Dell M, Metzlaff M, Flavell R. Post-transcriptional gene silencing of chalcone synthase in transgenic petunias, cytosine methylation and epigenetic variation. Plant J. 1999;18:33–42. doi: 10.1046/j.1365-313X.1999.00425.x. [DOI] [Google Scholar]
- Kasai M, Koseki M, Goto K, Masuta C, Ishii S, Hellens RP, Taneda A, Kanazawa A. Coincident sequence-specific RNA degradation of linked transgenes in the plant genome. Plant Mol Biol. 2012;78:259–273. doi: 10.1007/s11103-011-9863-0. [DOI] [PubMed] [Google Scholar]
- Napoli C, Lemieux C, Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MT, Voinnet O, Baulcombe DC. Initiation and maintenance of virus-induced gene silencing. Plant Cell. 1998;10:937–946. doi: 10.1105/tpc.10.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Kanazawa A, O'Dell M, Hellens RP. The binding of nuclear factors to the as-1 element in the CaMV 35S promoter is affected by cytosine methylation in vitro. Plant Biol. 2007;9:435–441. doi: 10.1055/s-2006-924633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary of bisulfite sequencing analysis of CaMV 35S promoter in control and genistein-treated petunia C002 plants. Red, green, and blue bars indicate frequencies of methylcytosine at CpG, CpHpG, and CpHpH sites, respectively. Figure S2 GFP fluorescence of plants grown in a medium with no supplement and plants treated with genistein (10 μM or 50 μM), 5-azaC (20 μM), and TSA (2 μM). Images of five N. benthamiana plants are shown for each treatment. Fluorescence was analyzed using a long-pass filter that allows detection of the red autofluorescence of chloroplasts.