Abstract
Background
Obesity, which is described as excessive or abnormal body fat, increases the risk of diet-related diseases. In Korea and around the world, the prevalence of obesity has grown annually from 1998 to 2008. This growth has continued despite various therapeutic efforts. The discovery of new and alternative treatments for obesity should be considered an important priority. Taeumjowi-tang (TJ001), a traditional Korean medicinal extract consisting of eight herbs, is a widely used herbal remedy for obesity in Korea. However, the efficacy and safety of TJ001 have not been fully investigated in a clinical trial. The purpose of this pilot study is to estimate obesity-related parameters and to assess the efficacy and safety of TJ001.
Methods
Our study is a randomised, double-blind, placebo-controlled, multicentre clinical trial of Taeumjowi-tang (TJ001). For this study, we will recruit obese Korean patients of both sexes, ages 18 to 65 years, from four university hospitals. A total of 104 subjects will be recruited. The participants will receive either 7 g of TJ001 or a placebo three times daily for 12 weeks. The primary end point will be the rate of subjects who lose at least 5% of their baseline body weight. The secondary end points will be changes in body weight, body mass index, waist circumference, hip circumference, waist/hip circumference ratio, lipid profiles, body fat composition, blood pressure, fasting glucose concentration, C-reactive protein and questionnaires related to the quality of life. The outcomes will be measured every 4 weeks. The study period will be 12 weeks and will include a total of five visits with each subject (at screening and at 0, 4, 8 and 12 weeks).
Conclusions
The results of our study will inform various estimates of TJ001 and will serve as the basis for a larger-scale trial. This study will assess the efficacy and safety of TJ001 as an alternative herbal remedy for obesity.
Trial registration
Current Controlled Trials ISRCTN87153759
Keywords: Taeumjowi-tang, obesity, efficacy, safety, randomised controlled trial
Background
The World Health Organisation (WHO) defines "obesity" as abnormal or excessive fat accumulation that may impair health [1]. Treatment of obesity is important because this chronic, noncommunicable disease causes not only a range of health problems, such as cardiovascular disease, various metabolic syndromes and certain cancers [1-3], but also social problems. In addition to the global incidence of obesity, obesity has become one of the most life-threatening problems in Korea. The Korea National Health and Nutrition Examination Survey showed that the overall prevalence of Korean adult obesity in 2008 was 30.7%, compared with 21.8% in 1998 [4]. The survey defined adults as individuals who are at least 20 years old, and obesity was defined as a body mass index (BMI) 25 kg/m2 according to the definition of the International Association for the study of Obesity in the Western Pacific Region of the WHO [1,5].
Several nonpharmacological attempts to curb the increase in obesity, including dietary or exercise management, have been largely unsuccessful. These failures have encouraged specialists to develop treatments using pharmacotherapy [2]. Despite these therapeutic attempts, an efficacy and safety limit for conventional weight reduction therapies is apparent [6]. The demand for safe and effective anti-obesity agents is increasing, and herbal combinations are meeting those needs [7]. The relative importance and potential benefits of herbal preparations has extended their role as possible alternative methods for obesity therapies.
Taeumjowi-tang (TJ001; HANPOONG Pharm & Foods Co Ltd, Jeonju-si, South Korea) is a traditional Korean medicine preparation that originated from I Je-ma's Sasang constitutional medicine theory [8]. This theory is representative of the individualised medical approach, which is widely used to diagnose and treat disease in Korea. The Sasang typology explains specific disease susceptibility and drug response differences through distinctive pathology types. Taeumjowi-tang (TJ001) is a decoction consisting of eight herbal ingredients and is usually prescribed for Tae-Eum persons (Greater Yin person, or Tae-Eum-In) to regulate stomach-related symptoms such as jaundice, anhidrosis, stuffiness and the sensation of fullness [8-10]. Owing to the unique pathology of Tae-Eum, these individuals gain weight more easily than the other three constitutional types (Tae-Yang, So-Eum and So-Yang). The Sasang typological formulary for Tae-Eum persons contains many therapies related to obesity. Among the various preparations, TJ001 has become the treatment regimen for obesity most widely used by Korean medical professionals [11-15], and its use has been expanded to all types of obesity. Preclinical results have supported the antiobesity and hypolipidaemic effects of TJ001 [16-19]; however, the results of clinical studies have been insufficient [20,21]. Although many large, placebo-controlled trials of herbal combinations and dietary supplements showing antiobesity effects have been reported [22-27], large-scale trials of Taeumjowi-tang have not yet been conducted.
This study will be the first reported randomised, double-blind, placebo-controlled clinical trial of Taeumjowi-tang (TJ001) in obese adults in Korea. The main purpose of this study is to assess the efficacy and safety of medicinal herbal extract of Taeumjowi-tang through a 12-week randomised controlled trial (RCT) that produces obesity-related estimates.
Methods
Objectives and hypothesis
Objectives
The main objectives of this trial are to (1) evaluate the efficacy and safety of TJ001 in obese Korean adults, (2) explore estimates of obesity-related variables, including the amount of weight reduction, (3) discover the precise target population, (4) establish an appropriate primary end point and (5) estimate the proper treatment period.
Hypothesis
We hypothesise that the TJ001 responder rates with a 5% or greater weight reduction will be higher than those in the placebo group. We also expect that use of TJ001 will be relatively safe.
Study design and period
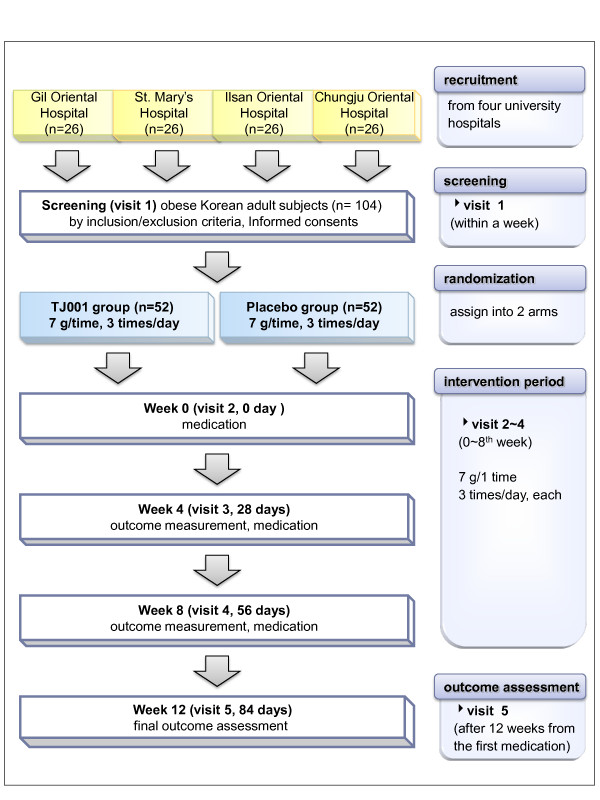
This study is a 12-week randomised, double-blind, placebo-controlled, multicentre trial at four tertiary university hospitals. Figure 1 shows the schematic flow of the study.
Figure 1.
Flowchart of the Taeumjowi-tang (TJ001) clinical trial.
Study groups
The study will include the following two arms: the TJ001 group (treatment arm) and the placebo group (control arm).
Population
The subjects will be obese Koreans of both sexes and ages 18 to 65 years.
Eligibility criteria
Inclusion and exclusion criteria
The inclusion and exclusion criteria are shown in Table 1.
Table 1.
Inclusion and exclusion criteriaa
| Criteria |
|---|
| Inclusion criteria |
| 1. Men and women ages 18 to 65 years |
| 2. Individuals who meet one of the following criteria: |
| 2.1. BMI ≥ 30 kg/m2 |
| 2.2. BMI 27 to 30 kg/m2 with hypertension at a proper treatment- and blood pressure-controlled 95 to 145 mmHg |
| 2.3. BMI 27 to 30 kg/m2 with non-insulin-dependent diabetes mellitus and fasting blood glucose < 7.8 mmol/L (140 mg/dl) |
| 2.4. BMI 27 to 30 kg/m2 with hyperlipidaemia in a proper treatment regimen |
| 2.5. BMI 27 to 30 kg/m2 and ≥ 236 mg/dl total cholesterol or ≥ 150 mg/dl triglycerides at screening |
| 3. Agreed to low-calorie diet during the trial |
| 4. Written informed consent for participation in the trial |
| 5. Written informed consent for the genetic test |
| Exclusion criteria |
| 1. Endocrine disease such as hypothyroidism or Cushing syndrome |
| 2. Heart disease (heart failure, angina pectoris and/or myocardial infarction) |
| 3. Uncontrolled hypertension (SBP > 145 mmHg or DBP > 95 mmHg) |
| 4. Malignant tumour or lung disease |
| 5. Cholelithiasis |
| 6. Severe renal disability (sCr > 2.0 mg/dl) |
| 7. Severe liver disability (2.5-fold the normal high range value for ALT, AST and ALP) |
| 8. Non-insulin-dependent diabetes mellitus and fasting blood sugar 7.8 mmol/L (140 mg/d or over |
| 9. Narrow-angle glaucoma |
| 10. History or existence of neurological or psychological disease (schizophrenia, epilepsy, alcoholism, drug addiction, anorexia, bulimia and so on) |
| 11. History of stroke or temporary ischaemic cardioplegia |
| 12. History or existence of eating disorder such as anorexia nervosa or bulimia nervosa |
| 13. Use of medication within the past 3 months that could have an effect on weight (appetite suppressant, laxative, oral steroid, thyroid hormone, amphetamine, cyproheptadine, phenothiazine or medication having an effect on absorption, metabolism and excretion) |
| 14. Use of β-blocker or diuretic as hypertension medication within the past 3 months |
| 15. Use of central nervous system medications or central nervous system stimulating weight reduction medications |
| 16. Forbidden treatments (insulin, hypoglycaemic agent, antidepressant, antiserotonin agent, barbiturate, antipsychotic and concerns related to medication abuse) |
| 17. Difficult-to-measure anthropometric dimensions due to anatomical changes such as resection surgery |
| 18. History of weight reduction surgery, bariatric surgery and so on |
| 19. Unable to follow instructions during the trial as judged by the investigator |
| 20. Women who are pregnant, lactating, planning a pregnancy or women of child-bearing age who do not agree to proper contraception (birth control pill, hormone implant, IUD, spermicide, condom, abstinence and so on) (women of child-bearing age indicated to be within 2 years of menopause who did not receive hysterectomy, bilateral tubal ligation, bilateral oophorectomy, and so on) |
| 21. Use of other investigational products within the past month |
| 22. Weight reduction > 10% within the past 6 months |
| 23. Cessation of smoking within past 3 months or an irregular smoking habit |
aALP alkaline phosphatase, ALT alanine aminotransferase, ASTaspartate aminotransferase, BMI body mass index, DBP diastolic blood pressure, IUD intrauterine device; SBP systolic blood pressure, sCr, serum creatinine
Subject withdrawal criteria
The subjects who meet the criteria listed in Table 2 will be discontinued from treatment. The participants who will be withdrawn after randomisation will be followed for outcomes.
Table 2.
Subject withdrawal criteria
| Subject withdrawal criteria |
|---|
| 1. Protocol violation: detection of eligibility violations, poor compliance (mean compliance < 70% at the last estimation) or noncompliance, use of any forbidden medication or treatment during the trial that could affect the study results, occurrence of other significant protocol violation during the trial |
| 2. Occurrence of a serious adverse event |
| 3. Subject has an acute reaction (allergy, shock and so on) to the investigational product |
| 4. Detection of a systemic disease that was not discovered at the screening stage |
| 5. Unable to progress because of worsening of preexisting disease |
| 6. Subject's withdrawal of consent |
| 7. Subject is uncooperative |
| 8. Investigator's decision to terminate the process for the sake of the subject's health |
Interventions
Participating subjects assigned to either the treatment group or the placebo group will be instructed to take 7 g of TJ001 or placebo three times daily (a total of 21 g) for 12 weeks (84 days). TJ001 (Taeumjowi-tang granule extract, HANPOONG Pharm & Foods Co Ltd) is a compound of the powdered extract of several medicinal herbs, and the placebo will be similar to TJ001 in form, colour and odour. The components of TJ001 and the placebo are listed in Table 3.
Table 3.
Constituents of interventions (TJ001 and placebo)a
| TJ001 granule extractb | |||
|---|---|---|---|
| Name of herb | Raw material code | Dose (g) | Content (%) |
| Semen Coicis | M040487 | 3.75 | NA |
| Semen Castaneae | M089646 | 3.75 | NA |
| Semen Raphani | M210181 | 2.5 | NA |
| Schisandrae Fructus | M040445 | 1.25 | NA |
| Liriopis tuber | M040139 | 1.25 | NA |
| Herba Ephedrae | M040135 | 1.25 | NA |
| Radix platycodi | M040048 | 1.25 | NA |
| Acori Tatarinowii Rhizoma | M051377 | 1.25 | NA |
| Placebo | |||
| Composition | Raw material code | Dose | Content (%) |
| Lactose | NA | NA | 87.99% |
| Starch | NA | NA | 11.73% |
| Food colouring | NA | NA | 0.28% |
aNA not applicable. bKorea Ministry for Health and Welfare classification 233 (ATC code A16AX).
Lifestyle management
Dietary intake and the type and intensity of exercise will be recorded by the research coordinator at every visit but will not be managed strictly.
Dietary instructions
For dietary counselling, the subjects will be counselled to consume 20 to 25 kcal/kg. In other words, the participants will be asked to keep their dietary intake approximately at 1,500 kcal/day for men and 1,200 kcal/day for women. A dietary intake diary will be distributed to the subjects, and they will use a diary to calculate the number of calories consumed.
Exercise
As the trial will be conducted without lifestyle management, the participants will be allowed to maintain their usual exercise levels. However, intense, unusual workouts will not be permitted.
Concomitant treatments and forbidden drugs
The subjects will be permitted to use medications only with the investigator's permission. The product name, use, dosage and duration of these medications will be recorded. The following concomitant treatments will be prohibited: central nervous system medications; central nervous system stimulating weight reduction medications; medications that could have an effect on weight (insulin, hypoglycaemic agents, oral steroids, thyroid hormones, cyproheptadine and phenothiazine); medications that affect absorption, metabolism or excretion; β-blockers and diuretics for hypertension treatment; amphetamines; antidepressants; antiserotonin agents; barbiturates; antipsychotics; and medicines that increase blood pressure or pulse, including decongestants that contain phenylpropanolamine, ephedrine or pseudoephedrine as well as medicines for coughs, common colds and allergies.
Sample size calculation
A sample size of 104 subjects was estimated for 80% power at a significance level of 0.05 and an attrition rate of 20%. Participants will be selected according to the eligibility criteria.
No preliminary studies of TJ001 were available to aid in estimating the percentage of subjects with 5% or greater weight loss [21]. Therefore, we set the rate at 40% for the TJ001 group and 13% for the placebo group on the basis of a reference paper [28]. The formula for calculating the sample size when allocating subjects at a ratio of 1:1 (TJ001:placebo) is as follows:
pt
The 5% or greater responder rate of the initial weight in TJ001 group = 40%.
pc
The 5% or greater responder rate of the initial weight in the placebo group = 13%.
Allowing for an attrition rate of 20%, the number of subjects in each group is 52.
Therefore, a total of 104 subjects are needed for this trial.
Randomisation method
The study subjects who satisfy the eligibility criteria will be randomised using a web-based randomisation program at an independent centre (Medical Research Collaborating Center of Seoul National University Hospital http://mrcc.snuh.org/). This program will be set to allocate participants equally to each site at a ratio of 1:1. Each of the four sites will be allocated 26 participants. The TJ001 and placebo groups will each be allocated 52 participants. The randomisation program will be designed with a four-patient block randomisation.
Blinding
Both the investigator and the subject will be blinded regarding the assignment of the study drugs. The contract research organisation (CRO; Kyung Hee University, Center for Clinical Research and Genomics) of the sponsor will label the investigational drugs by the randomisation code number. The labelled experimental products will be provided to the trial sites by the CRO.
Recruitment
Participants will be recruited through posted notes on the bulletin boards at four hospitals: Catholic University of Korea Seoul St Mary's Hospital (located in Seoul, South Korea), Dongguk University Ilsan Oriental Hospital, Kyungwon Gil Oriental Medical Hospital (Ilsan and Incheon, South Korea, both satellite cities near Seoul) and Semyung University Oriental Medicine Hospital (Chungju, a midsized city located in central South Korea).
Study schedule
The measurements that need to be carried out at each visit are listed in Table 4.
Table 4.
Study schedule of TJ001 clinical trial (12 weeks)a
| Initial screening b | Treatment period | ||||
|---|---|---|---|---|---|
| Measurement items | Visit 1 (-7 days) | Visit 2, week 0 (0 days) | Visit 3, 4 weeks (28 days) | Visit 4, 8 weeks (56 days) | Visit 5, 12 weeks (84 days) |
| Informed consent | Yes | ||||
| Demographic characteristicsc | Yes | ||||
| Vital signsd | Yes | Yes | Yes | Yes | Yes |
| Medical/drug use history | Yes | ||||
| Smoking/drinking status | Yes | Yes | Yes | Yes | |
| Physical examinatione | Yes | Yes | Yes | Yes | Yes |
| Laboratory testsf | Yes | Yes | |||
| Thyroid hormonesg | Yes | ||||
| Lipid test, CRP levelh | Yes | Yes | |||
| Abdominal computed tomography for body fat | Yes | Yes | |||
| Blood sample for genetic test | Yes | ||||
| Electrocardiography | Yes | ||||
| Pregnancy test | Yes | Yes | Yes | ||
| Inclusion/exclusion criteria check | Yes | ||||
| Dietary intake measurementi | Yes | Yes | Yes | Yes | |
| Concomitant medication | Yes | Yes | Yes | Yes | |
| Adverse event | Yes | Yes | Yes | ||
| Questionnairesj | Yes | Yes | |||
| Questionnaire for Sasang | |||||
| Constitution Classification | Yes | ||||
| II | |||||
| Exterior Cold | |||||
| Disease induced from the | Yes | Yes | Yes | Yes | |
| Esophagus affected by | |||||
| Cold' questionnaire in Tae-Eum-In persons Compliance calculation | Yes | Yes | Yes | ||
aCRP C-reactive protein. bThe initial screening is performed within 1 week of the start of the study period. The visit windows for each participant are ± 3 days. cSex, date of birth, age, contact address and telephone number. dBlood pressure (mmHg), pulse (beats/minute) and body temperature (°C). eBody weight (kg), height (cm), waist circumference (cm) and hip circumference (cm). fBlood tests are performed for haemoglobin, hematocrit, red blood cell count, white blood cell count, platelet count, total protein, albumin, total bilirubin, alanine transaminase, aspartate transaminase, alkaline γ-glutamyltransferase, uric acid, blood urea nitrogen/creatinine ratio, fasting plasma glucose, creatinine kinase and urine test for colour, specific gravity, pH, protein, glucose, ketone, urobilinogen, bilirubin, nitrite, red blood cells and white blood cells. gThyroid-stimulating hormone and free thyroxine are measured at the time of screening. hTotal cholesterol (mg/dl), high-density lipoprotein cholesterol (mg/dl), triglyceride (mg/dl) and CRP. iRecorded in kilocalories using 24-hour dietary recall methods by a dietician or clinical research coordinator. jKorean obesity-related quality of life (KOQOL) scale and Korean version of Eating Attitudes Test-26 (KEAT-26).
Measurement tools
Anthropometric measuring for height, weight, waist circumference and hip circumference
All measurements will be taken by well-trained examiners using standard procedures. Measurements will be made while participants are dressed in light garments and with bare feet. Height and weight will be measured in the standing position to the nearest 0.1 cm and 0.1 kg, respectively. A measuring rod with a movable headpiece will be mounted on a balance beam scale. The waist circumference (WC), recorded to the nearest 0.1 cm, will be measured midway between the lower margin of the last rib and the top of the pelvic bone in a horizontal plane using plastic tape. The hip circumference (HC), recorded to the nearest 0.1 cm, will be measured at the horizontal level of the widest part of the hip. BMI is calculated by dividing weight in kilograms by height in square meters [29]. We will record the subjects' weight loss in kilograms at every visit and calculate BMI and the rate of monthly weight loss in both groups [30].
Questionnaires
Questionnaire for the Sasang Constitution Classification II
The Questionnaire for the Sasang Constitution Classification II Sasang Constitution Classification II classifies a person into one of four types of constitution according to personal traits based on I Je-ma's Sasang constitutional medicine theory [31]. We will use this questionnaire to classify obese Korean adults. Additionally, we will use this questionnaire to evaluate whether there is an association between each constitutional type and the effect of TJ001.
Exterior Cold Disease induced from the Oesophagus affected by Cold questionnaire in Tae-Eum persons
Exterior Cold Disease induced from the Esophagus affected by Cold questionnaire is used to evaluate symptoms that can occur in people of the Tae-Eum constitutional type according to the Sasang constitutional medicine theory [8]. We will apply this questionnaire to examine whether there is a relationship between the tendency toward obesity in Tae-Eum persons and their response to TJ001.
Compliance
All subjects will be asked to return the remaining investigational products at their next visit, and the rate of compliance (percentage) will be calculated on the basis of the returned products.
Investigational drugs will be distributed in packs of 100 at each visit.
Outcomes
Both primary and secondary end points will be measured at each visit according to the study schedule (Table 4).
Primary outcome
The primary outcome is the rate of subjects who have lost 5% or more of their baseline body weight [28,30,32].
Secondary outcomes
Both within-group and between-group analyses will be performed for each outcome. The differences in the following variables between the baseline (visit 1) and the last visit (visit 5) will be calculated.
• Body weight (kg)
• BMI (kg/m2)
• WC (cm)
• HC (cm)
• Waist/hip circumference ratio (WHR) change
Lipid profile: total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol levels (mg/dl) (LDL cholesterol will be estimated using the Friedewald equation [33].)
Body fat composition: visceral fat area (cm2) and subcutaneous fat area (cm2) (Body composition will be evaluated by abdominal computed tomography.)
• Blood pressure (mmHg)
• Fasting plasma glucose concentration (mg/dl)
• C-reactive protein (mg/L) Questionnaires scores: Korean Obesity-related Quality of Life (KOQOL) and Korean version of Eating Attitudes Test-26 (KEAT-26)
Safety outcomes
All variables related to the safety assessment such as vital signs, general physical examinations, various test results (hematologic tests, biochemical tests, and urine tests) and adverse events (AEs) will be documented on the case report form (CRF) at every visit.
Statistical analysis
Efficacy assessment
The primary outcome will be analysed using the intention-to-treat (ITT) method. The secondary outcomes will be assessed by both the ITT and the per-protocol (PP) methods. The full analysis set for ITT method will include all randomised subjects, regardless of their subsequent withdrawal after enrolment. The PP analysis will include patients who have completed the 12-week study term without any major protocol violations and have a compliance rate > 70%. Missing values will be replaced by using the last observation carried forward method. Continuous variables will be reported as means ± SD, and categorical variables will be reported as percentages. The baseline characteristics will be compared by either Student's t-test for continuous variables or the χ2 test (Fisher's exact test when the expected value is < 5) for categorical data. Alternatively, McNemar's test will be used if the normality assumption is not satisfied for the continuous variables.
For the within-group analyses, primary and secondary outcome variables will be evaluated by using a paired t-test. Alternatively, for nonnormal distribution data, the Wilcoxon test will be performed. Analysis of covariance will be applied to analyse differences in each group at every visit, adjusting for age, baseline weight and BMI as covariates. Between-group comparisons for each variable will be performed using Student's t-test. Statistical significance will be defined as P < 0.05. PASW for Windows version 18.0 software (SPSS, Inc, Chicago, IL, USA) will be used for the analyses.
Safety assessment
Safety analyses will be performed for all subjects, who will be randomised and visited more than once after the initial screening. Safety-related variables will be analysed using the ITT method. Safety data will be stratified according to symptoms.
Adverse event reporting
AEs will be recorded in medical diagnostic terminology. Detailed symptoms, duration, severity, causal relationships, actions taken, results and other information will be recorded for each AE. All AEs must be observed and recorded in the CRF in the AE report section. When an AE occurs, investigators should notify both the IRB and the regulatory authorities within 24 hours.
Data quality control, data collection and data management
Data quality control will be achieved through monitoring during the trial. After checking the written CRF, well-trained clinical research associates of the CRO will collect the data. Data management will be performed by the CRO, Center for Clinical Research and Genomics, Seoul, Republic of Korea. All processes will be conducted using standard operating procedures.
Ethical issues
This study has been approved by the institutional review boards (IRBs) at each of the four institutions: the IRB of the Catholic University of Korea Seoul St Mary's Hospital (reference KC09MNME0032), the IRB of the Dongguk University Ilsan Oriental Hospital (ref: SR-09), the IRB of the Semyung University Oriental Medicine Hospital (reference 2008-03) and the IRB of the Kyungwon Gil Oriental Medical Hospital (reference 08-101)). Written informed consent will be obtained from each individual prior to enrolment. Research will be performed in compliance with the Helsinki Declaration and with the Good Clinical Practice Guidelines.
Discussion
By exploring estimated obesity-related variables, our aim in this pilot study is to determine the precise target population, the primary end point and the intervention period. Throughout the trial we will evaluate the efficacy and safety of Taeumjowi-tang. Korea has a dual medical treatment system, and herbal medicine assessments are under special regulations as described in the WHO legal status review of traditional medicine. For traditional Korean medicinal herbs, the Korea Food and Drug Administration rules specify that they are permitted to be produced [34,35] if herbal preparations are listed in the 11 classic traditional Korean and Chinese medicine books [36]. As these preparations are considered to have historical evidence of efficacy and safety, clinical or toxicological results can be exempted. Our intervention, Taeumjowi-tang, is described in one of these classic medical books, the Donguisusebowon [8]. Despite its long history and widespread usage, few Taeumjowi-tang trials have been published [21,27,37]. Therefore, our trial could be considered a pilot study, and the efficacy and safety of Taeumjowi-tang should be assessed in a RCT.
We encountered difficulties in deciding several issues. First, we had difficulty in selecting the target population. We considered using stratified randomisation, because weight reduction is affected by sex, age and physical baseline characteristics such as initial weight and BMI [3,38,39]. However, these various strata would decrease the statistical power. We first needed to determine the general patterns of Korean obesity. To maximise both the power and the results within given research budgets, we will include adults over age 18 years and both sexes. We will carry out stratified analyses with sex as a stratum. Other covariates, such as age, weight and BMI at the initial screening visit, will be adjusted.
The second question was what the primary end point would be. Among various outcomes [40,41], we set a 5% or greater weight loss responder rate as the primary outcome because 5% or greater weight loss is considered to be clinically significant [28,30,32]. As the amount of weight loss can vary due to initial weight, our study team concluded that the rate of the subjects who achieved a weight loss of 5% or more from their baseline weight would be reasonable compared to the absolute weight change. Other parameters, such as BMI, weight, body fat composition and WHR, were set as secondary outcomes.
Third, we had difficulty in determining the treatment duration. Though a short-term medication period for chronic treatment may not be sufficient to observe accurate long-term effects [22,42], we prioritised feasibility. We decided on a 12-week treatment for the trial after reflecting on the opinions of the clinicians' general experiences and considering both the attrition rate and the effectiveness of the preparation. Attrition rate will increase if the treatment duration is prolonged for more than 3 months. Additionally, according to data reported in the literature [21,27,32,43,44], the intervention period of weight reduction ranges from 1 day to 18 months for complementary therapy RCTs. Among complementary therapy RCTs, 8-week and 12-week trials are the most common [23,26,27,44].
Conclusions
We will assess the efficacy and safety of TJ001 in this trial. On the basis of our research results, we will be able to determine an adequate target population, a valid primary end point and an adequate treatment duration for TJ001. These results will be used to guide a future large-scale study. In addition, we expect that the results regarding the relationship between Sasang typology and obesity will lead us closer to personalised medicine.
Trial status
The trial was first designed in 2009, and the study began the same year. The study is ongoing, and subject recruitment has not been completed.
Abbreviations
AE: Adverse event; ATC: Anatomical therapeutic chemical classification; BMI: Body mass index; CRF: Case report form; CRO: Contract research organisation; CRP: C-reactive protein; ECDEC: Exterior cold disease induced from the oesophagus affected by cold; HC: Hip circumference; HDL: High-density lipoprotein; IRB: Institutional Review Board; ITT: Intention to treat; KEAT-26: Korean version of Eating Attitudes Test-26; KOQOL: Korean Obesity-related Quality of Life; LDL: Low-density lipoprotein; PP: Per protocol; RCT: Randomised controlled trial; TJ001: Taeumjowi-tang; WC: waist circumference; WHO: World Health Organization; WHR: Waist/hip circumference ratio.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SGK substantially contributed to the general idea and design of the study. SGK and YKS directed the overall project and take responsibility for the project. SJP, HYG, BHJ, YCS, CHC, JSP, YJY, CSA, JAL and YKS took part in designing the protocol. SJP, BHJ and HYG planned the data analysis. SJP drafted the manuscript. All authors read and consented to the publication of the manuscript.
Contributor Information
Sunju Park, Email: sjpark422@khu.ac.kr.
Jeong-Su Park, Email: suyahpark@gmail.com.
ChunHoo Cheon, Email: pm.thehoo@gmail.com.
Yong Joon Yang, Email: imagineer@nate.com.
Changsuk An, Email: sunnydoctor@naver.com.
Bo-Hyoung Jang, Email: jbh8284@gmail.com.
Yun-Kyung Song, Email: lyricsong@kyungwon.ac.kr.
Hoyeon Go, Email: kohoyeon@semyung.ac.kr.
Ju Ah Lee, Email: motoong@kiom.re.kr.
Yongcheol Shin, Email: syc99@khu.ac.kr.
Seong-Gyu Ko, Email: epiko@khu.ac.kr.
Acknowledgements
This study will be supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health (B080037). Additionally, the study will receive partial support from a Korea Science and Engineering Foundation (KOSEF) grant that is funded by the Korean government (Ministry of Education, Science and Technology grant 2011-0063436).
References
- World Health Organisation. International Association for the Study of Obesity, International Obesity Task Force: The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications; 2000. [Google Scholar]
- Hofbauer KG, Nicholson JR, Boss O. The obesity epidemic: current and future pharmacological treatments. Annu Rev Pharmacol Toxicol. 2007;47:565–592. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- Jee S, Sull J, Park J, Lee S, Ohrr H, Guallar E, Samet J. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- Korea Centers for Disease Control and Prevention (KCDC) National Health and Nutrition Survey Microdata 2008: Prevalence of Obesity. Chungcheongbuk-do: Korea Centers for Disease Control and Prevention; 2009. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation (WHO Technical Report Series 894) Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA. 1998;280:1596–1600. doi: 10.1001/jama.280.18.1596. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Traditional Medicine: Growing Needs and Potential. Geneva: World Health Organization; 2002. http://apps.who.int/medicinedocs/pdf/s2293e/s2293e.pdf (WHO Policy Perspectives on Medicines No. 002) [Google Scholar]
- Lee JM. Donguisusebowon [Longevity and Life Preservation in Oriental Medicine]. Seoul. 1894.
- World Health Organization. Regional Office for the Western Pacific: WHO International Standard Terminologies on Traditional Medicine in the Western Pacific Region. Manila: WHO Regional Office for the Western Pacific; 2007. [Google Scholar]
- Song I, Koh B, Lee E, Kim K, Kim D, Park S. Sasang Constitutional Medicine. Seoul: Jipmoondang; 2004. [Google Scholar]
- Korean Index of Medical Specialties (KIMS) Drug Information. Seoul: UBM Medica; 2011. [Google Scholar]
- Lee JI, Park YK, Kim YJ, Kim KS, Kim KS. Effects of Taeyeumjowi-tang water extract on the mouse fed high-fat diet. Korean J Orient Int Med. 2003;24:497–507. [Google Scholar]
- Gurley BJ, Wang P, Gardner SF. Ephedrine-type alkaloid content of nutritional supplements containing Ephedra sinica (ma-huang) as determined by high performance liquid chromatography. J Pharm Sci. 1998;87:1547–1553. doi: 10.1021/js9801844. [DOI] [PubMed] [Google Scholar]
- Hwang MJ, Shin HD, Song MY. Review of literature on herbal medicines for the treatment of obesity in Korea: mainly papers since 2000. J Oriental Rehab Med. 2006;16:65–81. [Google Scholar]
- Kim SH, Kim Y, Kim H, Jeong K, Lee S. Research on the usage frequency of Sasang constitutional herbal formula with case record form. J Korea Inst Orient Med. 2011;17:101–106. [Google Scholar]
- Han JS, Shin YO, Oh JK, Keum DH. Anorexigenic effect of Taeyeumjowui-tang (Taiyintiaowei-tang) in obese Zucker rat. J Orient Rehab Med. 2005;15:131–145. [Google Scholar]
- Cho SW, Park SS. Effects of Taeyeumjowee-tang on loss in body weight, plasma lipids and UCP I Revelation of fated white rats. J Korean Orient Med. 2004;25:87–97. [Google Scholar]
- Park SM, Ahn IS, Kim DS, Kang SA, Kwon DY, Yang HJ. Anti-obesity effects of Tae-Um-Jo-Wee-Tang and Do-Dam-Tang in female rats with diet-induced obesity. J Appl Biol Chem. 2010;53:44–50. doi: 10.3839/jabc.2010.008. [DOI] [Google Scholar]
- Choi CH, Yang DY, Kim CH, Jung JG, Jung HW. The effects of Taeyeumjowee-tang and Taeyeumjoweebaemahwang-tang on obese rats. Korean J Herbol. 2010;25:103–109. [Google Scholar]
- Lee JA, Kong KH, Ko HY, Bae KH, Park SY, Park KM, Song YK, Park JH, Kim HJ, Park SJ, Park JS, Ko SG. Recent topics of clinical trials in obesity and metabolic study. J Soc Korean Med Obes Res. 2009;9:15–22. [Google Scholar]
- Park KM, Song YK, Lim HH, Lee JA, Ko HY, Park JH, Kim HJ, Park SJ, Park JS, Ko SG. Review on the research relative to Taeeumjowui-Tang (Taiyintiaowei-tang) J Soc Korean Med Obes Res. 2009;9:23–36. [Google Scholar]
- Hackman RM, Havel PJ, Schwartz HJ, Rutledge JC, Watnik MR, Noceti EM, Stohs SJ, Stern JS, Keen CL. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: a randomized controlled trial. Int J Obes. 2006;30:1545–1556. doi: 10.1038/sj.ijo.0803283. [DOI] [PubMed] [Google Scholar]
- Boozer CN, Nasser JA, Heymsfield SB, Wang V, Chen G, Solomon JL. An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial. Int J Obes Relat Metab Disord. 2001;25:316–324. doi: 10.1038/sj.ijo.0801539. [DOI] [PubMed] [Google Scholar]
- Boozer CN, Daly PA, Homel P, Solomon JL, Blanchard D, Nasser JA, Strauss R, Meredith T. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int J Obes Relat Metab Disord. 2002;26:593–604. doi: 10.1038/sj.ijo.0802023. [DOI] [PubMed] [Google Scholar]
- Greenway FL, De Jonge L, Blanchard D, Frisard M, Smith SR. Effect of a dietary herbal supplement containing caffeine and ephedra on weight, metabolic rate, and body composition. Obes Res. 2004;12:1152–1157. doi: 10.1038/oby.2004.144. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Liu Z, Martin CK, Kai-yuan W, Nofziger J, Rood JC, Yu Y, Amen RJ. Safety and efficacy of NT, an herbal supplement, in treating human obesity. Int J Obes (London) 2006;30:1737–1741. doi: 10.1038/sj.ijo.0803343. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Ernst E. Complementary therapies for reducing body weight: a systematic review. Int J Obes (London) 2005;29:1030–1038. doi: 10.1038/sj.ijo.0803008. [DOI] [PubMed] [Google Scholar]
- Park CY, Kim YS, Ryu MS, Nam SY, Park HS, Kim SM. A phase 3 double-blind, parallel-group, placebo-controlled trial of the efficacy and safety of sibutramine (Reductil) in the treatment of obese patients. Korean J Obes. 2001;10:336–347. [Google Scholar]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee (WHO Technical Report Series 854) Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagné J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003;289:1537–1545. doi: 10.1001/jama.289.12.1537. [DOI] [PubMed] [Google Scholar]
- Chae H, Lyoo IK, Lee SJ, Cho S, Bae H, Hong M, Shin M. An alternative way to individualized medicine: psychological and physical traits of Sasang typology. J Alternative Compl Med. 2003;9:519–528. doi: 10.1089/107555303322284811. [DOI] [PubMed] [Google Scholar]
- Coffey CS, Steiner D, Baker BA, Allison DB. A randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. Int J Obes Relat Metab Disord. 2004;28:1411–1419. doi: 10.1038/sj.ijo.0802784. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- World Health Organization. Legal Status of Traditional Medicine and Complementary/Alternative Medicine: A Worldwide Review. Geneva: World Health Organization; 2001. [Google Scholar]
- Korea Food and Drug Administration (KFDA); Herbal Medicine Research Division. General Considerations of Clinical Trials for Herbal Medicine. Osong-eup, Chungcheongbuk-do, Korea: KFDA; 2007. [Google Scholar]
- Korea Ministry of Health & Welfare. Notification 1995-15. Seoul: Korea Ministry of Health & Welfare; 1995. [Google Scholar]
- Yoo JH, Lee EJ, Kwak CK, Sohn EH, Koh BH, Song IB, Lee KS. Clinical trial of herbal formula on weight loss in obese Korean children. Am J Chin Med. 2005;33:713–722. doi: 10.1142/S0192415X0500334X. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl 7):S120–S126. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes Relat Metab Disord. 2003;27:1437–1446. doi: 10.1038/sj.ijo.0802475. [DOI] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Donahue M, Wilson PW, Califf RM. Clinical trial issues in weight-loss therapy. Am Heart J. 2006;151:633–642. doi: 10.1016/j.ahj.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Opala T, Rzymski P, Pischel I, Wilczak M, Wozniak J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects-a randomised double-blind placebo-controlled clinical trial. Eur J Med Res. 2006;11:343–350. [PubMed] [Google Scholar]
- Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009;15:3073–3085. doi: 10.3748/wjg.15.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]