Abstract
Aging is a physiological process characterized by progressive functional decline in various organs over time. To reveal possible molecular mechanisms of altered xenobiotic disposition and toxicity in elderly individuals, age-dependent mRNA profiles for 101 xenobiotic-processing genes (XPGs), including seven uptake transporters, 41 phase I enzymes, 36 phase II enzymes, 10 efflux transporters, and seven transcription factors, were characterized in livers of male and female mice from 3 to 27 months of age. Gender differences across the lifespan (significant at five ages or more) were observed for 52 XPGs, including 15 male-predominant genes (e.g., Oatp1a1, Cyp3a11, Ugt1a6a, Comt, and Bcrp) and 37 female-predominant genes (e.g., Oatp1a4, Cyp2b10, Sult1a1, Ugt1a1, and Mrp3). During aging, the mRNA levels for 44% of the 101 XPGs changed in male mice and 63% changed in female mice. In male mice, mRNA levels for 40 XPGs (e.g., Oatp1a1, Ces2c, Gstm4, Gstp1, and Ces1e) were lower in aged mice (more than 21 months of age), whereas mRNA levels for four XPGs (e.g., Oat2 and Gstm2) were higher in aged mice. In female mice, mRNA levels for 43 XPGs (e.g., Oatp1a1, Cyp1a2, Ces1f, Sult3a1, Gstt2, Comt, Ent1, Fmo3, and Mrp6) were lower in aged mice, whereas mRNA levels for 21 XPGs (e.g., Oatp1a4, Nqo1, Adh7, Sult2a1/2, Gsta1, and Mrp4) were higher in aged mice. In conclusion, 51% of the 101 XPGs exhibited gender differences in liver mRNA levels across the lifespan of mice; the mRNA levels for 40% of the XPGs were lower in aged male mice and 43% were lower in aged female mice.
Introduction
Aging is characterized by declining physiological functions of various organs and increased incidence of multiple concomitant diseases, such as diabetes mellitus, hypertension, and arthritis (Sandhiya and Adithan, 2008). The older population, described as people over the age of 65 years, made up 12.8% of the U.S. population in 2008. This population receives approximately 33% of all prescription drugs and 40% of all nonprescription drugs. Elderly individuals have been reported to experience changes in the absorption, distribution, metabolism, and excretion of many drugs, such as nonsteroidal anti-inflammatory, antihypertensive, anticonvulsant, and psychiatric drugs (Cusack, 2004). In rodent studies, increased susceptibility to environmental chemicals has been observed during aging (Birnbaum, 1991).
The liver is the major organ for the detoxification and elimination of xenobiotics, such as drugs and environmental chemicals. Diverse xenobiotic-processing genes (XPGs), including uptake transporters, phase I enzymes, phase II enzymes, and efflux transporters, are expressed at high levels in the liver. Uptake transporters remove xenobiotics from the portal blood and transfer them to the liver for metabolism. Organic anion-transporting polypeptides (Oatps) and organic anion transporter 2 (Oat2) (Slc22a7) are important transporters for organic anions, whereas organic cation transporter 1 (Oct1) (Slc22a1) is important for organic cations. Equilibrative nucleoside transporter 1 (Ent1) (Slc29a1) mediates the uptake of nucleosides. Xenobiotics can be oxidized, reduced, or hydrolyzed by various phase I enzymes, such as cytochromes P450 (P450s), P450 reductase (Por), NAD(P)H:quinone oxidoreductase 1 (Nqo1), flavin-containing monooxygenases (Fmos), alcohol dehydrogenases (Adhs), aldehyde dehydrogenases (Aldhs), carboxylesterases (Cess), and paraoxonases (Pons). The phase I metabolites usually are not hydrophilic enough to be excreted; therefore, they are conjugated to increase hydrophobicity. Phase II enzymes, namely, the sulfotransferases (Sults), UDP-glucuronosyltransferases (Ugts), glutathione transferases (Gsts), catechol-O-methyltransferase (Comt), and N-acetyltransferases (Nats), catalyze conjugating reactions with sulfate, glucuronic acid, glutathione, methyl, and acetyl groups, respectively. Xenobiotics and/or metabolites are excreted either into bile by efflux transporters, such as multidrug resistance-associated protein 2 (Mrp2) (Abcc2), breast cancer resistance protein (Bcrp) (Abcg2), multidrug and toxin extrusion 1 (Slc47a1), multidrug resistance protein 2 (Abcb4), and transporter heterodimer Abcg5/g8, or into blood by the multidrug resistance-associated proteins (Mrp3/Abcc3, Mrp4/Abcc4, and Mrp6/Abcc6) and transporter Abca1 (Klaassen and Lu, 2008). The changes in XPG expression during aging may help to explain differences in drug pharmacokinetics and toxicity for elderly individuals.
Many of the XPGs are regulated by transcription factors, such as the aryl hydrocarbon receptor (AhR), constitutive androstane receptor (Nr1l3), pregnane X receptor (Nr1l2), peroxisome proliferator-activated receptor α (Nr1c1), retinoid X receptor α (Nr2b1), and hepatocyte nuclear factors 1α and 4α (Klaassen and Aleksunes, 2010). Many of these transcription factors can be activated by xenobiotics to regulate the transcription of drug-metabolizing enzymes and transporters (Klaassen and Slitt, 2005).
Gender differences in the baseline expression of many XPGs are regulated by sex hormones or sex-dependent growth hormone patterns (Waxman and O'Connor, 2006). Gender-divergent expression regulated by growth hormone secretion was reported for P450s (Pampori and Shapiro, 1999), Sults (Liu and Klaassen, 1996; Alnouti and Klaassen, 2006), Ugts (Buckley and Klaassen, 2009), and Gsts (Srivastava and Waxman, 1993; Knight et al., 2007), as well as xenobiotic transporters (Tanaka et al., 2005; Cheng et al., 2006; Maher et al., 2006). Because of the altered sex hormone and growth hormone levels in old age (Rudman et al., 1990; Bjørnerem et al., 2004), it is important to investigate the gender differences in XPG expression during aging.
Previous studies concerning age-dependent expression of xenobiotic metabolism genes were restricted to only a few age groups (Handler and Brian, 1997; Wauthier et al., 2004; Mori et al., 2007; Lee et al., 2008); therefore, possible changes in XPG expression might have been missed. Previous studies were primarily with rats, with limited data available for mice. Mice are a common laboratory model because of the availability of the well characterized mouse genome and genetically engineered mice. Previous studies seldom reported gender differences in XPG mRNA levels during aging. The present study was designed to investigate the comprehensive, age-dependent, mRNA profiles for XPGs in the livers of both male and female C57BL/6 mice with aging.
Materials and Methods
Animals.
Male (M) and female (F) C57BL/6 mice of various ages were purchased from the National Institutes of Health National Institute of Aging (Bethesda, MD) and were acclimated for at least 1 month before tissue collections. Mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility, with a 14-h light/10-h dark cycle and a temperature- and humidity-controlled environment, and were given ad libitum access to water and standard rodent chow (Harlan Teklad 8604; Harlan Teklad, Madison, WI). At 3, 6, 9, 12, 15, 18, 21, 24, and 27 months of age, mice (n = 5–7) were anesthetized with pentobarbital (50 mg/kg i.p.). Approximately 10 min later, when each mouse was well anesthetized, the liver was removed, snap-frozen in liquid nitrogen, and stored at −80°C. To decrease the mRNA variations in drug-processing genes attributable to circadian rhythm (Zhang et al., 2009), livers were collected between 9:00 AM and 12:00 noon. These studies were approved by the institutional animal care and use committee at the University of Kansas Medical Center.
Total RNA Isolation.
Total RNA was isolated from liver tissue by using RNA Bee reagent (Tel-Test Inc., Friendswood, TX), following the manufacturer's protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm.
Multiplex Suspension Assay.
The mRNA expression of genes of interest in the liver was determined with Panomics 2.0 QuantiGene Plex technology (Panomics/Affymetrix, Fremont, CA), following the manufacturer's protocol. Individual gene information can be found at the Panomics Web site (http://www.panomics.com) for panels 21095, 21152, 21153, 21174, and 21175. The mRNA levels for target genes were normalized to levels of the housekeeping gene Gapdh.
Branched DNA Assay.
The branched DNA assay (QuantiGene high-volume branched DNA signal amplification kit; Panomics/Affymetrix) was used to quantify mRNA levels for Comt (because this gene was not included in any predesigned panels for the multiplex suspension assay). The assay was performed as described previously (Cheng et al., 2005). Probe sets (containing capture extenders, label extenders, and blockers) specific to Comt were designed by using ProbeDesigner software (Bayer Corp., Emeryville, CA), as shown in Supplemental Table 1.
Real-Time, Quantitative, Reverse Transcription-PCR Analysis.
Total RNA was transcribed to single-stranded cDNA by using high-capacity cDNA reverse transcription kits (1001073; Applied Biosystems, Foster City, CA). Reverse transcription products were then amplified with PCR, by using Power SYBR Green PCR Master Mix (Applied Biosystems) and PCR primers (Integrated DNA Technologies, Coralville, IA). The levels of Gapdh mRNA in livers of male and female mice at 3 and 27 months of age were quantified. The primer sequences for Gapdh (National Center for Biotechnology Information reference sequence, NM_008084.2) were 5′-aactttggcattgtggaagg-3′ (forward) and 5′-ggatgcagggatgatgttct-3′ (reverse).
Statistical Analysis and Hierarchical Clustering.
Data are presented as mean ± S.E.M. Gender differences between male and female mice were determined with Student's t test (p < 0.05). Age differences, compared with 3 months of age, for male and female mice were determined with one-way ANOVA followed by Duncan's post hoc test (p < 0.05). Hierarchical clustering of XPGs that exhibited mRNA changes with aging (p < 0.05, ANOVA) was performed by using JMP 8.0 software (SAS Institute, Cary, NC). High mRNA abundance is represented in red, whereas low mRNA abundance is in blue. The mRNAs were normalized for each gene; therefore, relative color intensities are not comparable between genes.
Results
Gapdh Normalization.
In the present study, data at 3 and 27 months of age were compared most often, because they represent young adult and senescent ages, respectively. The mRNA levels for XPGs were normalized to levels of Gapdh, which is the most commonly used housekeeping gene. The levels of mRNA for Gapdh were quantified with real-time, quantitative, reverse transcription-PCR analyses. As shown in Supplemental Fig. 1, Gapdh mRNA levels remained relatively constant during aging and did not exhibit gender differences.
Uptake Transporters during Aging.
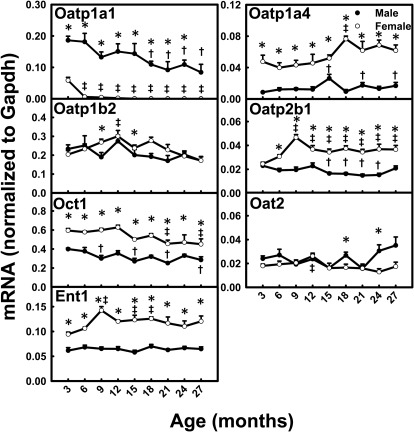
Among the uptake transporters that exhibited decreased mRNA levels with aging, Oatp1a1 mRNA levels decreased most markedly between 3 and 27 months of age (M, 55%; F, 100%) and Oct1 mRNA levels decreased slightly between 3 and 27 months of age (M, 28%; F, 24%) (Fig. 1). In contrast, Oatp1a4 mRNA levels increased 96% between 3 and 27 months in male mice and 30% between 3 and 18 months in female mice. Oatp2b1 mRNA levels at 9 to 27 months were higher than those at 3 months for female mice, whereas changes were not obvious for male mice. Ent1 mRNA levels increased from 3 to 9 months and tended to decrease thereafter in female mice, whereas levels remained constant in male mice. The mRNA levels for Oatp1b2 and Oat2 remained relatively constant with aging. The mRNA levels for most uptake transporters exhibited significant gender differences across the lifespan, including male-predominant Oatp1a1 and female-predominant Oatp1a4, Oatp2b1, Oct1, and Ent1. Oatp1a1 mRNA levels were more than 300% higher in male than female mice. In contrast, Oatp1a4 mRNA levels were more than 300% higher in female than male mice. The mRNA levels for Oatp2b1, Oct1, and Ent1 were all twice as high in female mice as in male mice.
Fig. 1.
mRNA profiles for uptake transporters with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
Phase I Xenobiotic-Metabolizing Enzymes during Aging.
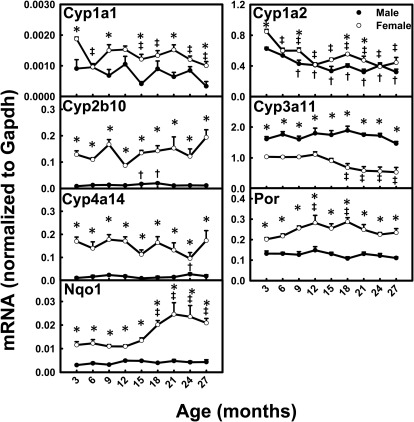
With aging, the mRNA levels for Cyp1a1 (M and F) and Cyp1a2 (M and F) decreased ∼50% between 3 and 27 months of age (Fig. 2). Cyp3a11 mRNA levels decreased ∼50% between 3 and 27 months in female mice, whereas they remained relatively constant with aging in male mice. Interestingly, Nqo1 mRNA levels remained relatively constant with aging in male mice but doubled between 3 and 27 months in female mice. The mRNA levels for Cyp2b10, Cyp4a14, and Por remained relatively constant with aging in both genders. Most P450s had female-predominant mRNA patterns across the lifespan; the mRNA levels for Cyp2b10 (10-fold) and Cyp4a14 (8-fold) were higher in female than male mice. The mRNA levels for Cyp1a1, Por, and Nqo1 were also higher in female mice. Cyp3a11 was the only P450 gene with a male-predominant mRNA pattern.
Fig. 2.
mRNA profiles for major P450s, Por, and Nqo1 with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
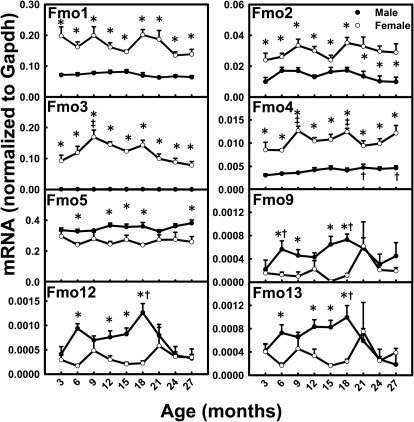
Fmo mRNA levels did not change much with aging (Fig. 3). The mRNA levels for Fmo2, Fmo3, and Fmo4 exhibited moderate increases between 3 and 27 months in female mice. Fmo4 mRNA levels in male mice also increased moderately between 3 and 27 months of age. The mRNAs of a number of Fmos had female-predominant patterns across the lifespan. Remarkably, Fmo3 mRNA levels were ∼200-fold higher in female than male mice. Higher mRNA levels in female mice were also observed for Fmo1, Fmo2, and Fmo4. In contrast, Fmo5 mRNA was male-predominant across the lifespan.
Fig. 3.
mRNA profiles for Fmos with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
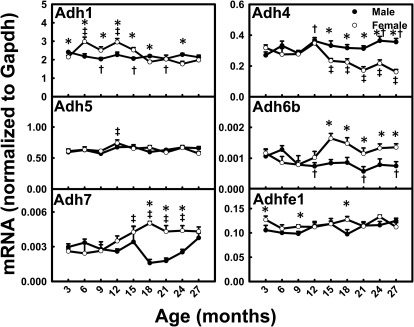
Adh4 mRNA levels decreased ∼50% between 3 and 27 months of age in female mice (Fig. 4). Interestingly, Adh6b levels decreased 29% in male mice and increased 16% in female mice between 3 and 27 months. Adh7 mRNA levels increased 93% in female mice between 3 and 18 months. The mRNA levels for Adh1, Adh5, and Adhfe1 did not change much with aging. Most Adh mRNA levels did not show a consistent gender-divergent pattern across the lifespan. Adh4 mRNA was male-predominant, whereas Adh6b was female-predominant after 1 year of age.
Fig. 4.
mRNA profiles for Adhs with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
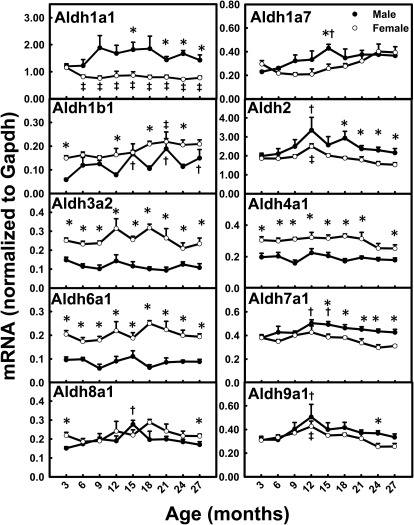
Aldh1a1 mRNA levels were 33% lower at 6 to 27 months than at 3 months in female mice (Fig. 5). Aldh1a7 mRNA levels increased between 3 and 15 months of age in male mice. The mRNA levels for Aldh2 and Aldh9a1 increased between 3 and 12 months and decreased thereafter in both genders. The mRNA levels for the remaining Aldhs remained relatively constant with aging. A number of the Aldh mRNAs had a female-predominant pattern across the lifespan, including Aldh3a2, Aldh4a1, and Aldh6a1, which had levels 100% higher in female than male mice. Interestingly, Aldh7a1 mRNA levels were higher in male than female mice only between 15 and 27 months.
Fig. 5.
mRNA profiles for Aldhs with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
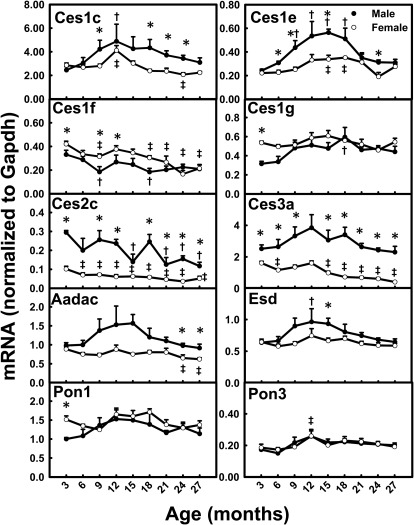
For many Cess, such as Ces1c, Ces1e, and esterase D, the highest mRNA levels were observed when the mice were ∼12 months of age, and levels decreased thereafter (Fig. 6). Decreased mRNAs were observed for Ces1f (F, 49%), Ces2c (M, 60%; F, 49%), and Ces3a (F, 75%) between 3 and 27 months of age. The mRNA levels of Pon1 and Pon3 remained relatively constant with aging. Most of the Ces and Pon mRNAs did not show gender-divergent patterns except for Ces2c and Ces3a, which had male-predominant mRNA patterns, with levels 200% higher in male than female mice.
Fig. 6.
mRNA profiles for Cess and Pons with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
Phase II Xenobiotic-Metabolizing Enzymes during Aging.
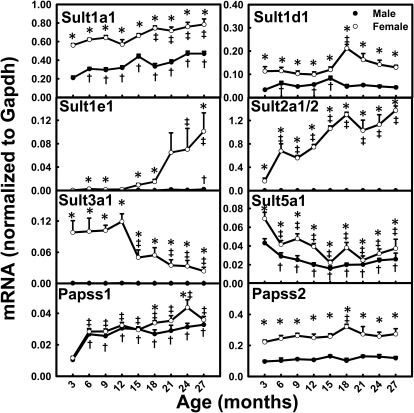
Sults catalyze the conjugation of xenobiotics with sulfate groups, and 3′-phosphoadenosine 5′-phosphosulfate synthase (Papss) catalyzes the synthesis of the sulfate donor for all Sults. In contrast to the mRNA level changes for most XPGs during aging, the mRNA levels for a number of Sults increased with aging in mice (Fig. 7). Increased mRNA levels with aging were observed for Sult1a1 (M, 123%; F, 40%), Sult1e1 (M, 9.8-fold; F, 315-fold), Sult2a1/2 (F, 732%), and Papss1 (M, 211%; F, 209%). In contrast, Sult3a1 mRNA levels decreased 76% in aged female mice, and Sult5a1 levels decreased ∼50% between 3 and 27 months of age in both genders. The mRNA levels for Sult1d1 and Papss2 peaked at 18 months of age in female mice. The mRNA levels for the majority of Sults, namely, Sult1a1, Sult1d1, Sult1e1, Sult2a1/2, Sult3a1, and Sult5a1, exhibited marked female-predominant patterns across the lifespan. In addition, Papss2 mRNA exhibited a female-predominant pattern.
Fig. 7.
mRNA profiles for Sults and Papss with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
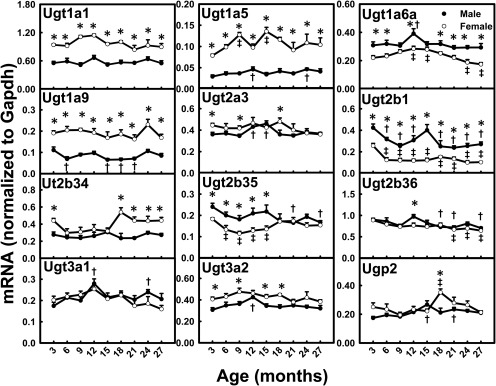
Ugts catalyze the conjugation reaction with glucuronic acids, and UDP-glucose pyrophosphorylase 2 (Ugp2) catalyzes the synthesis from glucose-1-phosphate to UDP-glucose, which is an important precursor for the glucuronidation cosubstrate UDP-glucuronic acid. The mRNA levels for Ugt2b1 (M, 36%; F, 60%) and Ugt2b35 (M, 30%) decreased markedly between 3 and 27 months of age (Fig. 8). Ugt2b36 mRNA levels also decreased in aging male and female mice. Ugt1a6a mRNA levels reached peaks at 12 months of age in both genders. The mRNA levels for Ugt3a1 and Ugt3a2 reached peaks at 12 months in male mice. Ugp2 mRNA levels decreased between middle age and old age in both genders. The mRNA levels for Ugt1a1, Ugt1a5, Ugt1a9, Ugt2a3, and Ugt2b34 did not change much with aging. The mRNAs of some Ugts, namely, Ugt1a1, Ugt1a5, Ugt1a9, and Ugt2b34, exhibited female-predominant patterns across the lifespan. In contrast, Ugt1a6a, Ugt2b1, and Ugt2b35 exhibited male-predominant patterns.
Fig. 8.
mRNA profiles for Ugts and Ugp2 with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
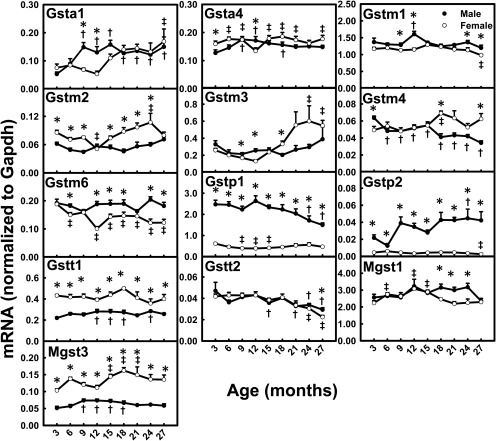
Gsta1 mRNA levels increased markedly (M, 183%; F, 124%) between 3 and 27 months of age in both genders (Fig. 9). Gstm3 mRNA levels increased 112% between 3 and 27 months in female mice. Decreases of 35 to 50% in mRNA levels were observed between 3 and 27 months for Gstm4 (M), Gstm6 (F), Gstp1 (M), and Gstt2 (M and F). The mRNA levels for Gstp2 increased 95% between 3 and 27 months in male mice, whereas they decreased 43% in female mice. Mgst3 mRNA levels increased slightly between 6 and 18 months in both genders. Some of the Gst mRNAs (namely, Gstm2, Gstt1, and Mgst3) had female-predominant patterns across the lifespan, whereas some (namely, Gstm6, Gstp1, and Gstp2) had male-predominant patterns. Gstp1 and Gstp2 mRNA levels were ∼300% higher in male than female mice, whereas Gstt1 and Mgst3 mRNA levels were 200% higher in female than male mice.
Fig. 9.
mRNA profiles for Gsts with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
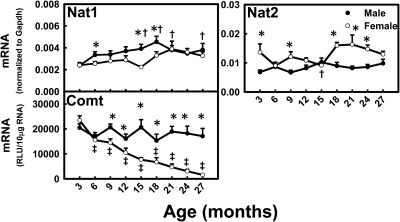
Nat1 mRNA levels increased (M, 60%; F, 35%) between 3 and 27 months of age, whereas Nat2 mRNA levels did not change much with aging (Fig. 10). Comt mRNA levels decreased markedly (93%) between 3 and 27 months of age in female mice, whereas they remained relatively constant in male mice. The mRNA levels for Nats did not exhibit marked gender-divergent patterns across the lifespan. Comt mRNA had a male-predominant pattern in aging mice.
Fig. 10.
mRNA profiles for Nats and Comt with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
Efflux Transporters during Aging.
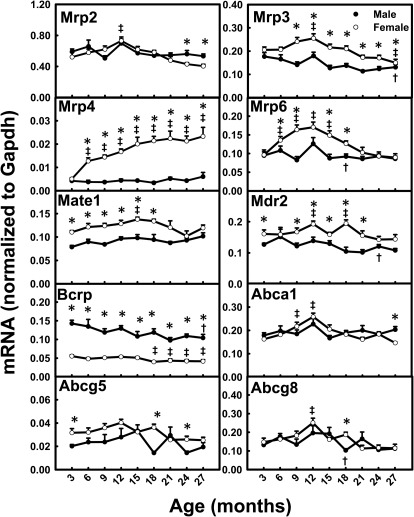
Mrp4 mRNA levels increased 363% between 3 and 27 months of age in female mice, whereas they remained relatively constant in male mice (Fig. 11). Significant decreases in mRNA levels between 3 and 27 months were observed for Mrp3 (26%) and Bcrp (27%) in male mice. Mrp2, Mrp3, Mrp6, Abca1, and Abcg8 mRNA levels peaked at 12 months of age in female mice. The mRNAs for some efflux transporters, such as Mrp3, Mrp4, multidrug and toxin extrusion 1 (Mate1), and multidrug resistance protein 2 (Mdr2), exhibited female-predominant mRNA patterns. In contrast, Bcrp had a male-predominant mRNA pattern.
Fig. 11.
mRNA profiles for efflux transporters with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test.
Transcription Factors during Aging.
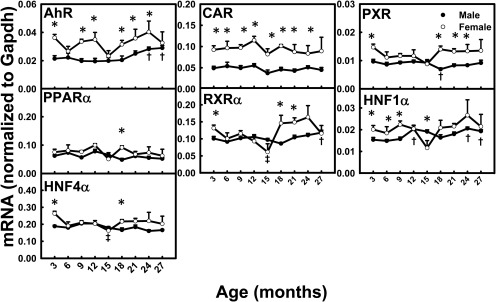
With aging, the mRNA levels for all of the transcription factors remained relatively constant (Fig. 12). The mRNA levels for AhR and hepatocyte nuclear factor 1α were slightly higher at 24 and 27 months of age than at 3 months in male mice. The mRNA levels for AhR and constitutive androstane receptor (CAR) were female-predominant across the lifespan. The mRNAs for the remaining transcription factors did not exhibit gender differences.
Fig. 12.
mRNA profiles for major transcription factors with aging in livers of male and female mice. *, gender differences, determined with Student's t test (p < 0.05). †, male; ‡, female age differences (p < 0.05), compared with 3 months of age, determined with one-way ANOVA, followed by Duncan's post hoc test. CAR, constitutive androstane receptor; PXR, pregnane X receptor; PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor; HNF, hepatocyte nuclear factor.
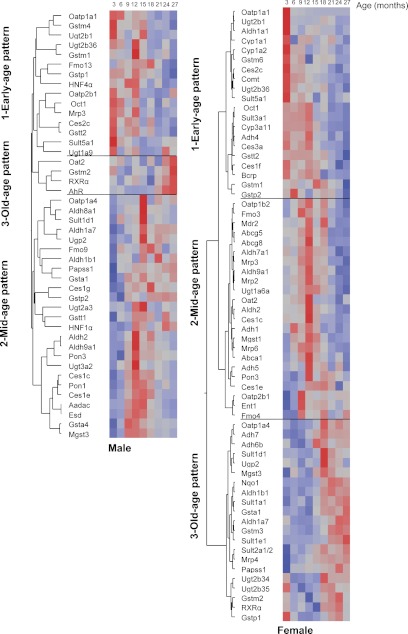
Hierarchical Cluster Analysis of Age-Dependent mRNA Profiles for XPGs.
The mRNA levels for 44% of the 101 XPGs changed with aging in male mice and 63% changed in female mice. The mRNA profiles for these XPGs with aging were analyzed through hierarchical cluster analysis, with red representing higher and blue lower mRNA abundance (Fig. 13). The age-dependent mRNA changes in these XPGs could be classified into three clusters for both genders (Table 1), namely, young-age pattern (highest levels observed at 3–9 months of age), middle-age pattern (highest levels observed at 12–18 months of age), and old-age pattern (highest levels observed at 21–27 months of age). In male mice, mRNA levels for 40 XPGs (e.g., Oatp1a1, Ces2c, Gstm4, Gstp1, and Ces1e) were lower in aged mice (more than 21 months of age), whereas mRNA levels for four XPGs (e.g., Oat2 and Gstm2) were higher in aged mice. In female mice, mRNA levels for 43 XPGs (e.g., Oatp1a1, Cyp1a2, Ces1f, Sult3a1, Gstt2, Comt, Ent1, Fmo3, and Mrp6) were lower in aged mice, whereas mRNA levels for 21 XPGs (e.g., Oatp1a4, Nqo1, Adh7, Sult2a1/2, Gsta1, and Mrp4) were higher in aged mice. In conclusion, the mRNA levels for 40% of the XPGs were lower in aged male mice and 43% were lower in aged female mice.
Fig. 13.
Hierarchical cluster analysis of the mRNA profiles for XPGs during aging in livers of male and female mice. Clustering analysis results are shown in the dendrogram, with the y-axis representing the XPGs that change with aging and the x-axis representing the nine age groups of mice (from 3 to 27 months of age). Red, higher mRNA abundance; blue, lower mRNA abundance. The spectrum of each gene is standardized and specific to the scale for its own mRNA. Therefore, it is not valid to compare mRNA levels among different genes according to color.
TABLE 1.
Summary of three patterns of age-dependent mRNA changes for XPGs
The numbers of XPGs that did not change with aging were as follows: male, 57 of 101; female, 37 of 101.
| Pattern | Male | Female |
|---|---|---|
| no./total | ||
| Early age | 15 of 101 | 20 of 101 |
| Middle age | 25 of 101 | 23 of 101 |
| Old age | 4 of 101 | 21 of 101 |
Discussion
The present study characterizes comprehensive mRNA profiles for XPGs with aging in the livers of mice. Nine groups of mice from 3 to 27 months of age were examined, which yielded many more details of age-dependent changes than in previous reports (Peng et al., 2005; Mori et al., 2007; Lee et al., 2008). By using both male and female mice, the present study uniquely reveals gender-divergent mRNA profiles for XPGs across the lifespan.
Levels of many uptake transporters are altered during aging. Oatps are sodium-independent uptake transporters whose substrates are diverse, being mainly amphipathic organic compounds, including bile acids, hormones and their conjugates, toxins, and various drugs (Hagenbuch and Gui, 2008). The mRNA levels for liver-enriched Oatp1a1 decreased more than 50% in aged male mice and surprisingly decreased almost 100% in aged female mice (Fig. 1). The mRNA levels for liver-specific Oatp1b2 decreased from 12 to 27 months of age in female mice. The mRNA levels for Oct1 decreased during aging in both genders. Taken together, these findings suggest that the liver removes xenobiotics from the blood more slowly in elderly individuals.
The phase I enzymes whose levels are most altered during aging are the P450 and Ces families. P450s are heme-containing enzymes that catalyze monooxygenase reactions. The Cyp1, Cyp2, and Cyp3 families are the P450s primarily involved in the metabolism of drugs, xenobiotics, and steroids (Monostory and Dvorak, 2011). Most of the P450s quantified in the present study exhibited age-dependent decreases in mRNA levels, markedly for Cyp1a1 (F) and Cyp1a2 (M and F) and to a lesser degree for Cyp3a11 (F) (Fig. 2). This suggests a possible mechanism for decreased metabolism of some xenobiotics in elderly individuals. Cess catalyze the hydrolysis of many clinically useful drugs with ester moieties, which results mainly in the inactivation of drugs (such as heroin, cocaine, and flumazenil), as well as the activation of prodrugs (such as the anticancer drugs irinotecan and capecitabine) (Redinbo and Potter, 2005). The nomenclature used for mouse Cess in the present study was reported previously (Holmes et al., 2010). The decreased mRNA levels for Cess1f, Cess2c, and Cess3a during aging in female mice (Fig. 6) indicate that it may take longer for the anticancer prodrugs to take effect and ester drugs may have longer half-lives in elderly individuals. Aging had little effect on the mRNAs for other phase I enzymes, such as Fmos, Adhs, Aldhs, and Pons (Figs. 3–6).
Aging has profound effects in altering the mRNA levels for phase II enzymes. All of the Sults quantified in the present study remained female-predominant during aging. The mRNA levels for Sult1a1 and Papss1 increased during aging in both genders. Sult1e1 mRNA levels increased 9.8-fold in male mice and 315-fold in female mice between 3 and 27 months of age (Fig. 7). Sult1e1 sulfonates a variety of estrogens (Falany et al., 1995) and is the predominant determinant of the active unconjugated estrogen/inactive estrogen sulfate ratio. In addition to the aging-induced decreases in ovarian function and female sex hormone secretion, the increased Sult1e1 levels and possibly increased inactivation of estrogens might contribute to decreased levels of biologically active estrogens in aged female individuals. Sult2a1/2 mRNA was abundant in female mice but levels were extremely low in male mice in young adulthood (3 month of age), which is consistent with our previous publication (Alnouti and Klaassen, 2006). The 5-dehydroepiandrosterone sulfotransferase Sult2A is known as a rat senescence marker protein, the expression of which is increased in aged male rats (Echchgadda et al., 2004). The present study showed that Sult2a1/2 mRNA levels increased 732% in aged female mice but remained at extremely low levels in male mice during aging (Fig. 7). This discrepancy may result from species differences. Additional work is necessary to determine definitely the mechanisms underlying the age- and sex-dependent regulation of Sult2a1/2 in mice. In contrast to the markedly elevated mRNA levels for Sult1a1, Sult1e1, and Sult2a1/2, Sult3a1 mRNA levels decreased 76% in aged female mice. Sult3a1 is the only Sult isozyme that catalyzes N-sulfonation, rather than O-sulfonation, of amines such as phenyltetrahydropyridine, aniline, 4-chloroaniline, 2-naphthylamine, and desipramine (Yoshinari et al., 1998). 4-Chloroaniline is carcinogenic in male rats and mice (Chhabra et al., 1991), mainly because of the toxic intermediate N-phenylhydroxylamine. The C-hydroxylated product of 4-chloroaniline is sulfated and ready for excretion. The decreased mRNA level for Sult3a1 after 12 months of age in male mice (Fig. 7) suggests that the detoxification of 4-chloroaniline might decrease with aging, which might favor cancer development with 4-chloroaniline.
The mRNA levels for most Ugts remained relatively constant with aging (Fig. 8). However, Ugt2b1 mRNA levels decreased between 3 and 27 months of age in both genders. A previous study (Buckley and Klaassen, 2009) reported that the regulation of Ugt2b1 expression in mouse liver was attributable to male-pattern growth hormone secretion. This may explain the dramatic decreases in Ugt2b1 levels for male mice with aging and less dramatic decreases for female mice.
Gsts catalyze the biotransformation and disposition of a wide range of chemical carcinogens, therapeutic drugs, products of oxidative stress, and steroid hormones such as Δ5-androstenedione (Johansson and Mannervik, 2001). Gstp1 mRNA levels decreased markedly with aging (Fig. 9). Gstp2 mRNA levels increased with aging, but Gstp2 was expressed at a much lower level than Gstp1. The glutathione conjugation by Gstp is a pathway for cisplatin detoxification (Townsend et al., 2009). The decrease in Gstp1 mRNA levels with aging in the present study provides a possible mechanism for changes in cisplatin toxicity during aging.
Comt catalyzes the methylation of catecholamine neurotransmitters, l- DOPA, catecholestrogens (2- and 4-hydroxylated estrogen), and drugs such as carbidopa and dobutamine. The highest Comt activity has been found in liver and kidney. Comt activity is also detected in several glands, muscle tissue, adipose tissue, blood cells, and other tissues. One important function of Comt is to detoxify catecholestrogens, which appears to be important in initiating some estrogen-dependent cancers, possibly through generation of reactive oxygen species and subsequent DNA damage (Weisz et al., 1998). The decreased Comt levels with aging in female mice (Fig. 10) suggest that detoxification of catecholestrogens in livers might decrease in aged female individuals.
Levels of most efflux transporters are altered during aging. The mRNA levels for Mrp3 and Bcrp decreased during aging in both genders (Fig. 10). Mrp3 is important in transporting bilirubin glucuronides and bile acids from the liver into blood, whereas Bcrp is important in the liver for transporting sulfate and glucuronide conjugates of xenobiotics into bile. The decreased expression of Mrp3 and Bcrp with aging suggests that glucuronide conjugates of xenobiotics and bilirubin might accumulate in hepatocytes with aging. Interestingly, Mrp4 mRNA levels increased between 3 and 27 months of age in both genders but more markedly in female mice (Fig. 10). A previous study showed that higher mRNA levels for Mrp4 in female kidneys were attributable to repression by both 5α-dihydroxytesteosterone and male-pattern growth hormone secretion in male mice (Maher et al., 2006). With the hypothesis that similar mechanisms of hormonal regulation of female-predominant expression of Mrp4 exist in liver, the markedly elevated Mrp4 mRNA levels during aging possibly result from decreases in sex hormone and growth hormone levels with aging.
All of the data collected in the present study on the expression of XPGs were at the mRNA level. Caution is needed in interpretation of the data, because mRNA levels do not always correlate with protein levels and protein activities. On the basis of previous reports that decreased protein contents (Peng et al., 2005; Mori et al., 2007) and enzyme activities (Warrington et al., 2004) of many phase I and phase II enzymes are regulated at the transcription level, the mRNA results from this study should provide a good indication for most enzymes and transporters. It is especially difficult to quantify protein and activity levels for transporters, because of the lack of specific antibodies and specific substrates for activity assays.
In summary, the current study investigated the effects of aging on mRNA changes for 101 XPGs in livers of male and female mice. Gender differences across the lifespan (significant at five ages or more) were observed for 52 XPGs, including 15 male-predominant genes (e.g., Oatp1a1, Cyp3a11, Ugt1a6a, Comt, and Bcrp) and 37 female-predominant genes (e.g., Oatp1a4, Cyp2b10, Sult1a1, Ugt1a1, and Mrp3). The mRNA levels for 44% of the XPGs changed with aging in male mice and 63% changed in female mice. In male mice, mRNA levels for 40 XPGs (e.g., Oatp1a1, Ces2c, Gstm4, Gstp1, and Ces1e) were lower in aged mice (more than 21 months of age), whereas mRNA levels for four XPGs (e.g., Oat2 and Gstm2) were higher in aged mice. In female mice, mRNA levels for 43 XPGs (e.g., Oatp1a1, Cyp1a2, Ces1f, Sult3a1, Gstt2, Comt, Ent1, Fmo3, and Mrp6) were lower in aged mice, whereas mRNA levels for 21 XPGs (e.g., Oatp1a4, Nqo1, Adh7, Sult2a1/2, Gsta1, and Mrp4) were higher in aged mice. In conclusion, 51% of the 101 XPGs exhibited gender differences in liver mRNA levels across the lifespan of mice; the mRNA levels for 40% of the XPGs were lower in aged male mice and 43% are lower in aged female mice. With the roles of XPGs in the detoxification and elimination of drugs and environmental chemicals, the present results may improve the interpretation of long-term toxicity studies with old animals and aid in determining effective and safe doses for elderly individuals.
Supplementary Material
Acknowledgments
We acknowledge Dr. Nancy Berman for help in obtaining mice for the study, Dr. Rachel Chennault for technical assistance with the multiplex suspension assay, Dr. Hong Lu for designing branched DNA probes for Comt, and all of the graduate students and fellows in Dr. Klaassen's laboratory group for help in tissue collection and critical review of the manuscript.
This work was supported by the National Institutes of Health National Institute of Environmental Sciences [Grant ES-009649]; and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK-081461].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- XPG
- xenobiotic-processing gene
- Abc
- ATP-binding cassette
- Adh
- alcohol dehydrogenase
- AhR
- aryl hydrocarbon receptor
- Aldh
- aldehyde dehydrogenase
- ANOVA
- analysis of variance
- Bcrp
- breast cancer resistance protein
- Ces
- carboxylesterase
- Comt
- catechol-O-methyltransferase
- P450
- cytochrome P450
- Ent
- equilibrative nucleoside transporter
- Fmo
- flavin-containing monooxygenase
- Gapdh
- glyceraldehyde-3-phophate dehydrogenase
- Gst
- glutathione transferase
- Mgst
- microsomal glutathione transferase
- Mrp
- multidrug resistance-associated protein
- Nat
- N-acetyltransferase
- Nqo1
- NAD(P)H:quinone oxidoreductase
- Oat
- organic anion transporter
- Oatp
- organic anion-transporting polypeptide
- Oct
- organic cation transporter
- Papss
- 3′-phosphoadenosine 5′-phosphosulfate synthase
- PCR
- polymerase chain reaction
- Pon
- paraoxonase
- Por
- cytochrome P450 reductase
- Slc
- solute carrier
- Sult
- sulfotransferase
- Ugt
- UDP-glucuronosyltransferase
- M
- male
- F
- female
- Nr
- nuclear receptor
- Ugp2
- UDP-glucose pyrophosphorylase 2.
Authorship Contributions
Participated in research design: Fu, Csanaky, and Klaassen.
Conducted experiments: Fu and Csanaky.
Contributed new reagents or analytic tools: Fu and Klaassen.
Performed data analysis: Fu and Klaassen.
Wrote or contributed to the writing of the manuscript: Fu, Csanaky, and Klaassen.
References
- Alnouti Y, Klaassen CD. (2006) Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci 93:242–255 [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. (1991) Pharmacokinetic basis of age-related changes in sensitivity to toxicants. Annu Rev Pharmacol Toxicol 31:101–128 [DOI] [PubMed] [Google Scholar]
- Bjørnerem A, Straume B, Midtby M, Fønnebø V, Sundsfjord J, Svartberg J, Acharya G, Oian P, Berntsen GK. (2004) Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromsø Study. J Clin Endocrinol Metab 89:6039–6047 [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. (2009) Mechanism of gender-divergent UDP-glucuronosyltransferase mRNA expression in mouse liver and kidney. Drug Metab Dispos 37:834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. (2005) Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab Dispos 33:1062–1073 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Lu H, Klaassen CD. (2006) Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol 70:1291–1297 [DOI] [PubMed] [Google Scholar]
- Chhabra RS, Huff JE, Haseman JK, Elwell MR, Peters AC. (1991) Carcinogenicity of p-chloroaniline in rats and mice. Food Chem Toxicol 29:119–124 [DOI] [PubMed] [Google Scholar]
- Cusack BJ. (2004) Pharmacokinetics in older persons. Am J Geriatr Pharmacother 2:274–302 [DOI] [PubMed] [Google Scholar]
- Echchgadda I, Song CS, Oh TS, Cho SH, Rivera OJ, Chatterjee B. (2004) Gene regulation for the senescence marker protein DHEA-sulfotransferase by the xenobiotic-activated nuclear pregnane X receptor (PXR). Mech Ageing Dev 125:733–745 [DOI] [PubMed] [Google Scholar]
- Falany CN, Krasnykh V, Falany JL. (1995) Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol 52:529–539 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38:778–801 [DOI] [PubMed] [Google Scholar]
- Handler JA, Brian WR. (1997) Effect of aging on mixed-function oxidation and conjugation by isolated perfused rat livers. Biochem Pharmacol 54:159–164 [DOI] [PubMed] [Google Scholar]
- Holmes RS, Wright MW, Laulederkind SJ, Cox LA, Hosokawa M, Imai T, Ishibashi S, Lehner R, Miyazaki M, Perkins EJ, et al. (2010) Recommended nomenclature for five mammalian carboxylesterase gene families: human, mouse, and rat genes and proteins. Mamm Genome 21:427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AS, Mannervik B. (2001) Human glutathione transferase A3-3, a highly efficient catalyst of double-bond isomerization in the biosynthetic pathway of steroid hormones. J Biol Chem 276:33061–33065 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Lu H. (2008) Xenobiotic transporters: ascribing function from gene knockout and mutation studies. Toxicol Sci 101:186–196 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. (2005) Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab 6:309–328 [DOI] [PubMed] [Google Scholar]
- Knight TR, Choudhuri S, Klaassen CD. (2007) Constitutive mRNA expression of various glutathione S-transferase isoforms in different tissues of mice. Toxicol Sci 100:513–524 [DOI] [PubMed] [Google Scholar]
- Lee JS, Ward WO, Wolf DC, Allen JW, Mills C, DeVito MJ, Corton JC. (2008) Coordinated changes in xenobiotic metabolizing enzyme gene expression in aging male rats. Toxicol Sci 106:263–283 [DOI] [PubMed] [Google Scholar]
- Liu L, Klaassen CD. (1996) Ontogeny and hormonal basis of female-dominant rat hepatic sulfotransferases. J Pharmacol Exp Ther 279:386–391 [PubMed] [Google Scholar]
- Maher JM, Cheng X, Tanaka Y, Scheffer GL, Klaassen CD. (2006) Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (Mrp3 and Mrp4) in mice. Biochem Pharmacol 71:1470–1478 [DOI] [PubMed] [Google Scholar]
- Monostory K, Dvorak Z. (2011) Steroid regulation of drug-metabolizing cytochromes P450. Curr Drug Metab 12:154–172 [DOI] [PubMed] [Google Scholar]
- Mori K, Blackshear PE, Lobenhofer EK, Parker JS, Orzech DP, Roycroft JH, Walker KL, Johnson KA, Marsh TA, Irwin RD, et al. (2007) Hepatic transcript levels for genes coding for enzymes associated with xenobiotic metabolism are altered with age. Toxicol Pathol 35:242–251 [DOI] [PubMed] [Google Scholar]
- Pampori NA, Shapiro BH. (1999) Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology 140:1245–1254 [DOI] [PubMed] [Google Scholar]
- Peng FC, Jian WC, Edwards RJ. (2005) Profile of territrem metabolism and cytochrome P-450 3A expression in liver microsomes from Wistar rats of both genders as a function of age. J Toxicol Environ Health A 68:1871–1888 [DOI] [PubMed] [Google Scholar]
- Redinbo MR, Potter PM. (2005) Mammalian carboxylesterases: from drug targets to protein therapeutics. Drug Discov Today 10:313–325 [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. (1990) Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- Sandhiya S, Adithan C. (2008) Drug therapy in elderly. J Assoc Physicians India 56:525–531 [PubMed] [Google Scholar]
- Srivastava PK, Waxman DJ. (1993) Sex-dependent expression and growth hormone regulation of class alpha and class mu glutathione S-transferase mRNAs in adult rat liver. Biochem J 294:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. (2005) Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun 326:181–187 [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, He L, King JB, Hanigan MH. (2009) Role of glutathione S-transferase pi in cisplatin-induced nephrotoxicity. Biomed Pharmacother 63:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JS, Greenblatt DJ, von Moltke LL. (2004) Age-related differences in CYP3A expression and activity in the rat liver, intestine, and kidney. J Pharmacol Exp Ther 309:720–729 [DOI] [PubMed] [Google Scholar]
- Wauthier V, Verbeeck RK, Buc Calderon P. (2004) Age-related changes in the protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as in their hepatic activities in Wistar rats. What role for oxidative stress? Arch Toxicol 78:131–138 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C. (2006) Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Weisz J, Fritz-Wolz G, Clawson GA, Benedict CM, Abendroth C, Creveling CR. (1998) Induction of nuclear catechol-O-methyltransferase by estrogens in hamster kidney: implications for estrogen-induced renal cancer. Carcinogenesis 19:1307–1312 [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Nagata K, Ogino M, Fujita K, Shiraga T, Iwasaki K, Hata T, Yamazoe Y. (1998) Molecular cloning and expression of an amine sulfotransferase cDNA: a new gene family of cytosolic sulfotransferases in mammals. J Biochem 123:479–486 [DOI] [PubMed] [Google Scholar]
- Zhang YK, Yeager RL, Klaassen CD. (2009) Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos 37:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.