Abstract
The objective of this study was to quantitatively examine the protein expression of relevant transporters and other proteins in the brain capillary endothelial cells isolated from wild-type mice and P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and P-gp/Bcrp knockout mice. After the isolation of brain capillary endothelial cells, a highly sensitive liquid chromatography-tandem mass spectrometry method with multiple reaction monitoring was used to determine the quantitative expression of membrane transporters at the blood-brain barrier (BBB) of the various mouse genotypes. Quantitative expression of 29 protein molecules, including 12 ATP-binding cassette transporters, 10 solute carrier transporters, five receptors, and two housekeeping proteins, was examined by quantitative proteomics in the four mouse genotypes. There was no significant difference in the expression of P-gp between the wild-type and Bcrp1(−/−) mice. Likewise, Bcrp expression was not significantly different between the wild-type and Mdr1a/b(−/−) mice. There was no significant difference in the expression of any of the measured proteins in the brain capillary endothelial cells across the genotypes, except for the lack of expression of the corresponding protein in the mice that had a genetic deletion of P-gp or Bcrp. In conclusion, using a quantitative proteomic approach, we have shown that there are no changes in the expression of several relevant transporters in brain capillary endothelial cells isolated from single and combination knockout mice. These data suggest that the mechanism behind the functional compensation between P-gp and Bcrp at the BBB is not related to compensatory changes in transporter expression.
Introduction
There have been major advances in our understanding of the impact that drug transporters have on the absorption, distribution, metabolism, and elimination of xenobiotics. Several studies have examined how active transporters influence distribution of drugs to various tissues, especially to sanctuary sites such as the brain. It is well known that the blood-brain barrier (BBB) presents a major obstacle for many compounds to enter into the brain (Pardridge, 2007). Whereas tight junctions are a major aspect of the BBB that minimize paracellular diffusion of drugs, efflux transporters also play an important role in limiting the brain distribution of various drugs. ATP-binding cassette proteins such as P-glycoprotein (P-gp; Abcb1) and breast cancer resistance protein (Bcrp; Abcg2) are two important efflux transporters present at the BBB that have been studied extensively for their role in restricting CNS delivery of drugs (Schinkel and Jonker, 2003; Agarwal et al., 2011a). Numerous studies show that many agents are substrates of these transporters and, as a result, have significantly limited brain distribution (Löscher and Potschka, 2005).
The development of genetically engineered transporter-knockout mice, which are deficient in one or more transporters, has revolutionized brain distribution studies designed to investigate the impact of active efflux transport at the BBB. Given that P-gp and Bcrp are considered to be two major contributors to drug efflux at the BBB, several studies have used mice deficient in one or both of these transporters to study their impact on CNS drug delivery. P-gp knockout [Mdr1a/b(−/−)], Bcrp knockout [Bcrp1(−/−)], and the combined P-gp/Bcrp knockout [Mdr1a/b(−/−)Bcrp1(−/−)] mice have allowed researchers to show that these two transporters significantly limit the distribution of several drugs into the brain (Schinkel, 1998; Lagas et al., 2009; Vlaming et al., 2009).
An interesting finding from brain distribution studies using these mouse models was an unexpected “synergistic” or “cooperative” role of P-gp and Bcrp in the efflux of dual substrates at the BBB. This was first reported by de Vries et al. (2007) and Polli et al. (2009), who showed that P-gp and Bcrp work together to modulate the CNS penetration of topotecan (de Vries et al., 2007) and lapatinib (Polli et al., 2009), respectively. Furthermore, in our previous studies in Bcrp, P-gp, and P-gp/Bcrp knockout mice, we reported similar findings with dasatinib (Chen et al., 2009), gefitinib (Agarwal et al., 2010), and sorafenib (Agarwal et al., 2011b). These results showed that brain penetration of dual substrates increased disproportionally in the absence of both P-gp and Bcrp at the BBB. The studies also showed that absence of Bcrp in the Bcrp1(−/−) mice did not significantly influence the brain distribution of drugs that were shown to be good Bcrp substrates in vitro (Kodaira et al., 2010; Agarwal et al., 2011b).
These findings have led to several questions about the predictive validity of the transgenic mouse models for use in brain distribution studies. Although some of these questions pertain to the integrity of the BBB in these mice (Agarwal et al., 2010), the major question is whether genetic modification of one transporter causes changes in regulation and/or expression (up or down) of other critical transporter proteins that may influence their functional role in drug disposition to the brain. Certainly, up-regulation of P-gp in the Bcrp1(−/−) mice could, in part, explain why brain penetration of dual substrates is not enhanced in these mice. For example, Cisternino et al. showed that the naturally occurring P-gp mutant CF-1 mice [mdr1a(−/−)] had a 3-fold higher BCRP mRNA expression in their brain capillaries compared with the wild-type mice [mdr1a(+/+)] (Cisternino et al., 2004). Therefore, a detailed and precise study examining the expression of relevant transporters at the BBB is necessary and can provide a more accurate depiction of possible compensatory changes in transporter function that may be related to changes in protein expression in the gene knockout mice.
The objective of the current study was to examine quantitatively the expression of P-gp, Bcrp, and other relevant proteins in the brain capillary endothelial cells of the transgenic mouse models. Using a highly sensitive liquid chromatography-linked tandem mass spectrometry (LC/MS/MS) with multiple reaction monitoring (MRM), we have quantified several proteins in mouse brain capillary endothelial cells. We show that, other than the absence of transporters that were genetically knocked out, there were no differences in expression of other measured transporters between the wild-type and transporter knockout mice. The results conclusively show that there are no compensatory changes in the expression of drug transport proteins at the BBB in the transgenic mice under normal experimental conditions.
Materials and Methods
Animals.
Proteomic analysis was done in male FVB (wild-type), Mdr1a/b(−/−) (P-gp knockout), Bcrp1(−/−) (Bcrp knockout), and Mdr1a/b(−/−)Bcrp1(−/−) (triple knockout) mice of a FVB genetic background (Taconic Farms, Germantown, NY). All animals were 8 to 10 weeks old at the time of brain capillary isolation. Animals were maintained in a 12-h light/dark cycle with an unlimited access to food and water. All studies were carried out in accordance with the guidelines set by the Principles of Laboratory Animal Care (National Institutes of Health, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Capillary Isolation.
Brain capillaries were isolated according to the method of Hartz et al. (2010). Wild-type, Mdr1a/b(−/−), Bcrp1(−/−), and Mdr1a/b(−/−)Bcrp1(−/−) mice were killed (n = 10 each), and whole brains were harvested and pooled according to genotype for capillary isolation. Pooled brain tissue was placed on a Whatman filter paper that was wetted with cold PBS buffer. Superficial meninges were removed using a cotton swab, and the brains were carefully cleaned to remove all visible white matter and homogenized in ice-cold phosphate-buffered saline (PBS) buffer (2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4, 0.9 mM CaCl2, and 0.5 mM MgCl2 supplemented with 5 mM d-glucose and 1 mM sodium pyruvate). The homogenate was mixed with 2 volumes of 32% Ficoll (final concentration, 16%) and centrifuged at 5800g for 20 min at 4°C. The resulting pellet was suspended in PBS buffer containing 1% bovine serum albumin solution and passed over a 0.4-mm glass bead column. Capillaries adhering to the glass beads were collected by gentle agitation using 1% bovine serum albumin. Capillaries were washed three times with PBS buffer, followed by centrifugation at 7000g for 10 min at 4°C to collect the capillary pellet. Capillary pellets were frozen at −80°C until further analysis.
HPLC LC/MS/MS.
Protein expression amounts of the target molecules were simultaneously determined by means of multiplexed MRM analysis as described previously (Kamiie et al., 2008; Uchida et al., 2011). Pellets of isolated brain capillaries from each genotype (50 μg of protein) were solubilized in 500 mM Tris-HCl, pH 8.5, 7 M guanidine hydrochloride, and 10 mM EDTA, and the proteins were S-carbamoylmethylated as described previously (Kamiie et al., 2008). The alkylated proteins were precipitated with a mixture of methanol and chloroform. The precipitates were dissolved in 6 M urea in 100 mM Tris-HCl, pH 8.5, diluted 5-fold with 100 mM Tris-HCl, pH 8.5, and treated with N-tosyl-l-phenylalanine chloromethyl ketone-treated trypsin (Promega, Madison, WI) at an enzyme/substrate ratio of 1:100 at 37°C for 16 h. The tryptic digests were mixed with internal standard peptides and formic acid and centrifuged at 4°C and 17,360g for 5 min. The supernatants were subjected to HPLC-MS/MS analysis. Three independent measurements were done on three separate aliquots from the pooled homogenate from each genotype.
The HPLC-MS/MS analysis was performed by coupling an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA) to a triple quadrupole mass spectrometer (API5000 or QTRAP5500; AB SCIEX, Foster City, CA) equipped with Turbo V ion source (AB SCIEX). Samples equivalent to 33.3 μg of protein were injected onto a Waters XBridge BEH130 C18 (1.0 ′ 100 mm, 3.5 μm) column together with 500 fmol of internal standard peptides. Mobile phases A and B consisted of 0.1% formic acid in water and 0.1% formic acid in acetonitrile, respectively. The peptides were separated and eluted from the column at room temperature using a linear gradient with a 120-min run time at a flow rate of 50 μl/min. The sequence was as follows (A/B): 99:1 for 5 min after injection, 50:50 at 55 min, 0:100 at 56 min and up to 58 min, and 99:1 at 60 min and up to 120 min.
The eluted peptides were simultaneously and selectively detected by means of electrospray ionization in a multiplexed MRM mode, which can quantify many molecules simultaneously by using 300 MRM transitions (Q1/Q3) at maximum. The dwell time was 8 ms per MRM transition. Each molecule was monitored with four sets of MRM transitions (Q1/Q3-1, Q1/Q3-2, Q1/Q3-3, Q1/Q3-4) derived from one set of standard and internal standard peptides. P-gp (Abcb1, Mdr1a, Mdr1b), Mrp1 (Abcc1), Mrp2 (Abcc2), Mrp3 (Abcc3), Mrp4 (Abcc4), Mrp5 (Abcc5), Mrp6 (Abcc6), Mrp7 (Abcc7), Bcrp (Abcg2), glucose transporter 1 (Glut1; Slc2a1), large amino acid transporter 1 (Lat1; Slc7a5), 4F2 heavy chain (Slc3a2), monocarboxylate transporter 1 (Mct1; Slc16a1), organic anion-transporting peptide 2 (Oatp2; Slco1a1), Oatp14 (Slco161), organic anion transporter 3 (Oat3; Slc22a8), Na+/K+ ATPase (Atp4a), and γ-GTP were monitored with the peptides and MRM transitions reported by Kamiie et al. (2008). Abca1, Abca2, clusters of differentiation 147 (CD147), equilibrative nucleoside transporter 1 (Ent1; Slc29a1), insulin receptor (Insr), low-density lipoprotein receptor-related protein 1 (Lrp1), and transferrin receptor 1 (Tfr1) were monitored with the peptides and MRM transitions reported by Uchida et al. (2011). MRM transitions and peptide sequences used for monitoring Pmat, rlip76, and claudin-5 have been provided (Supplemental Table 1). Chromatogram ion counts were determined by using the data acquisition procedures in Analyst software version 1.5 (AB SCIEX). Signal peaks with a peak area count of over 5000 detected at the same retention time as an internal standard peptide were defined as positive. When positive peaks were observed in three or four sets of MRM transitions, the molecules were considered to be expressed in brain capillaries, and the protein expression amounts were determined as the average of three or four quantitative values. The limit of quantification was calculated as described previously (Uchida et al., 2011).
Statistical Analysis.
Statistical testing was conducted using the SigmaStat software (Systat Software Inc., San Jose, CA). Protein expression across the four groups was compared using one-way ANOVA at a significance level of 0.05. If the results of the ANOVA showed a statistically significant difference between the group means, pairwise multiple comparisons were done using the Bonferroni post hoc t test. Statistical significance was declared if the resultant p value was less than 0.05.
Results
The quantitative expression of 29 proteins, including 12 ABC transporters, 10 SLC transporters, five receptors, and two housekeeping proteins, was examined by quantitative proteomics in the brain capillary endothelial cells isolated from wild-type, Mdr1a/b(−/−), Bcrp1(−/−), and Mdr1a/b(−/−)Bcrp1(−/−) mice.
ABC Transporters.
Table 1 shows the protein levels of various ABC transporters at the BBB in the four mouse genotypes. Among the ABC transporters in the wild-type mice, P-gp (Abcb1) was the most abundant (16.3 ± 0.8 fmol/μg protein), followed by Bcrp, Mrp4, and Abca1. The expression of P-gp was approximately 5-fold greater than that of Bcrp. Protein levels in the Bcrp1(−/−) mice were similar to that in the wild-type mice for all ABC transporters except Bcrp, which was absent. There was no significant difference in the expression of P-gp between the wild-type and Bcrp1(−/−) mice (p > 0.05). Likewise, Bcrp expression was not significantly different between the wild-type and Mdr1a/b(−/−) mice (p > 0.05). These results show that under basal conditions, there are no compensatory changes in expression of P-gp in the Bcrp-deficient mice, and vice versa. Moreover, the expression of all of the ABC transporters did not change in the Mdr1a/b(−/−)Bcrp1(−/−) mice compared with the wild-type mice (p > 0.05), except for P-gp and Bcrp, which were absent. Thus, even in the combined P-gp/Bcrp knockout mice, there are no compensatory changes in protein expression for the transporters we measured.
TABLE 1.
Protein expression levels of membrane proteins in isolated mouse brain capillaries
The quantitative expression level of membrane proteins in brain capillary endothelial cells isolated from wild-type, Mdr1a/b(−/−) (P-gp knockout), Bcrp1(−/−) (Bcrp knockout), and Mdr1a/b(−/−)Bcrp1(−/−) (P-gp/Bcrp combined knockout) mice is shown. Whole tissue lysates of brain capillaries were digested with trypsin as described under Materials and Methods. The protein expression amounts were measured by subjecting the digests to LC/MS/MS with internal standard peptides. The data represent mean ± S.E.M. (three independent measurements were done on three separate aliquots from the pooled capillary homogenate from 10 mice of each genotype). There was no statistically significant difference in expression of all the tested ABC and SLC transporters between the wild-type, P-gp knockout, Bcrp knockout, and P-gp/Bcrp combined knockout mice (p > 0.05).
| Molecular Names | Protein Expression Level |
|||
|---|---|---|---|---|
| Wild Type | Mdr1a/b(−/−) | Bcrp1(−/−) | Mdr1a/b(−/−)Bcrp1(−/−) | |
| fmol/μg protein | ||||
| ABC transporters | ||||
| Abca1 | 0.397 ± 0.065 | 0.499 ± 0.041 | 0.358 ± 0.027 | 0.392 ± 0.056 |
| Abca2 | N.D. | N.D. | N.D. | N.D. |
| Mdr1a (Abcb1a) | 16.3 ± 0.8 | N.D. | 17.0 ± 0.3 | N.D. |
| Mdr1b (Abcb1b) | N.D. | N.D. | N.D. | N.D. |
| Mrp1 (Abcc1) | N.D. | N.D. | N.D. | N.D. |
| Mrp2 (Abcc2) | N.D. | N.D. | N.D. | N.D. |
| Mrp3 (Abcc3) | N.D. | N.D. | N.D. | N.D. |
| Mrp4 (Abcc4) | 2.18 ± 0.13 | 2.31 ± 0.15 | 1.79 ± 0.09 | 1.98 ± 0.10 |
| Mrp5 (Abcc5) | N.D. | N.D. | N.D. | N.D. |
| Mrp6 (Abcc6) | N.D. | N.D. | N.D. | N.D. |
| Bcrp (Abcg2) | 3.53 ± 0.21 | 4.20 ± 0.18 | N.D. | N.D. |
| Mrp7 (Abcc7) | N.D. | N.D. | N.D. | N.D. |
| SLC transporters | ||||
| Glut1 (Slc2a1) | 110 ± 5 | 121 ± 6 | 115 ± 6 | 114 ± 5 |
| Lat1 (Slc7a5) | 1.80 ± 0.32 | 2.03 ± 0.42 | 1.89 ± 0.41 | 1.71 ± 0.45 |
| 4F2 hc (Slc3a2) | 17.1 ± 1.0 | 20.5 ± 0.9 | 17.6 ± 0.7 | 18.6 ± 0.6 |
| Mct1 (Slc16a1) | 25.2 ± 2.5 | 27.8 ± 1.9 | 27.5 ± 1.8 | 23.5 ± 0.7 |
| Oatp2 (Slco1a1) | 1.55 ± 0.31 | 2.01 ± 0.12 | 1.92 ± 0.45 | 0.648 ± 0.155 |
| Oatp14 (Slco161) | 1.70 ± 0.1 | 1.84 ± 0.09 | 1.68 ± 0.08 | 1.57 ± 0.17 |
| Oat3 (Slc22a8) | 1.74 ± 0.18 | 1.75 ± 0.08 | 1.75 ± 0.27 | 1.47 ± 0.29 |
| Ent1 (Slc29a1) | 1.18 ± 0.40 | 0.887 ± 0.151 | 1.15 ± 0.33 | 1.06 ± 0.30 |
| Pmat | N.D. | N.D. | N.D. | N.D. |
| Rlip76 | N.D. | N.D. | N.D. | N.D. |
| Receptors | ||||
| Insr | 0.797 ± 0.096 | 0.911 ± 0.039a | 0.837 ± 0.083a | 0.482 ± 0.019 |
| Lrp1 | 1.02 ± 0.20a | 0.883 ± 0.008 | 1.01 ± 0.06a | 0.400 ± 0.001 |
| Tfr1 | 5.16 ± 0.44 | 5.49 ± 0.42 | 4.91 ± 0.34 | 3.98 ± 0.40 |
| Claudin-5 | 8.05 ± 1.73 | 9.16 ± 1.21 | 8.96 ± 1.15 | 6.40 ± 1.70 |
| Cd147 | 21.3 ± 0.80 | 22.9 ± 0.80 | 23.3 ± 1.40 | 18.4 ± 1.60 |
| Housekeeping genes | ||||
| Na+/K+ ATPase | 39.1 ± 1.4 | 41.0 ± 1.2 | 41.8 ± 1.2 | 44.5 ± 1.8 |
| γ-gtp | 3.25 ± 0.30 | 3.34 ± 0.38 | 3.10 ± 0.26 | 3.16 ± 0.24 |
N.D., not detected.
Among the receptors that were studied, Insr and Lrp1 expression was trended lower in the P-gp/Bcrp combined knockout mice (p < 0.05 compared with P-gp/Bcrp combined knockout mice).
We quantified the expression of Abca2, Mdr1b, Mrp1, Mrp2, Mrp3, Mrp5, Mrp6, and Mrp7 to check the possibility whether there are any compensatory changes in regulation of other ABC transporters in these mice. We found that all of these transporters were below detection limits in all four mouse genotypes (Table 2).
TABLE 2.
Molecules that were not detected in mouse brain capillaries and their limit of quantification
P-gp was not detected in P-gp and P-gp/Bcrp knockout mice, and Bcrp was not detected in Bcrp and P-gp/Bcrp knockout mice. The other molecules were not detected in any samples. The limit of quantification was calculated as described previously (Uchida et al., 2011). In brief, the limit of quantification was defined as the protein expression level that would give a peak area count of 5000 in the chromatogram when the brain capillary sample was measured in LC/MS/MS.
| Molecular Names | Limit of Quantification |
|---|---|
| fmol/μg protein | |
| Abca2 | 0.178 |
| Mdr1a (Abcb1a) | 0.135 |
| Mdr1b (Abcb1b) | 0.0810 |
| Mrp1 (Abcc1) | 0.111 |
| Mrp2 (Abcc2) | 3.28 |
| Mrp3 (Abcc3) | 0.0570 |
| Mrp5 (Abcc5) | 0.136 |
| Mrp6 (Abcc6) | 0.118 |
| Bcrp (Abcg2) | 0.0390 |
| Mrp7 (Abcc7) | 0.253 |
| Pmat | 0.0467 |
| Rlip76 | 0.195 |
SLC Transporters.
Among the SLC superfamily (Table 1), Glut1 was expressed at the highest level in all four mouse genotypes, followed by Mct1, 4F2 heavy chain (Slc3a2), Lat1, Oat3, Oatp14, Oatp2, and Ent1. The expression of Pmat and Rlip76 was below detection limits (Table 2). The expression of all of the SLC transporters was not significantly different between the four mouse genotypes (p > 0.05).
Other Proteins.
Among the receptors measured (Table 1), CD147 was most abundant among all the four mouse genotypes, followed by claudin-5, Tfr1, Lrp1, and Insr. Expression of Insr was trended lower in the Mdr1a/b(−/−)Bcrp1(−/−) mice compared with wild-type (p = 0.06), Mdr1a/b(−/−) (p = 0.01), and Bcrp1(−/−) (p = 0.03) mice. Likewise, expression of Lrp1 showed a similar trend in the Mdr1a/b(−/−)Bcrp1(−/−) mice compared with wild-type (p = 0.01), Mdr1a/b(−/−) (p = 0.06), and Bcrp1(−/−) (p = 0.02) mice. The protein expression of endothelial marker γ-GTP and the membrane marker Na+/K+-ATPase (Table 1) was uniform across the four mouse genotypes.
Discussion
The present study is the first to comprehensively determine the quantitative expression of membrane transporters and receptors in brain capillary endothelial cells isolated from single knockout [Mdr1a/b(−/−), Bcrp1(−/−)] and combination knockout [Mdr1a/b(−/−)Bcrp1(−/−)] mice of a FVB genetic background. This study shows that the expression of relevant ABC and SLC transporters, and many other surface receptors at the BBB, are not changed when P-gp, Bcrp, or both have been genetically deleted in the gene knockout mice.
The development of transporter knockout mice has afforded researchers a powerful tool to study the impact of efflux transporters on brain distribution of drugs (Lagas et al., 2009). Brain distribution studies using these mice have shown that many drugs have limited CNS distribution due to P-gp- and/or Bcrp-mediated efflux (Lee et al., 2005; de Vries et al., 2007; Polli et al., 2009; Agarwal et al., 2010, 2011b; Kodaira et al., 2010; Tang et al., 2012). However, the finding that brain distribution of several dual P-gp and Bcrp substrates increased remarkably in mice deficient in both P-gp and Bcrp was unexpected, because there was no dramatic increase in their brain penetration in the single P-gp- or Bcrp-deficient mice. This has now been reported for several drugs that are substrates for both P-gp and Bcrp (de Vries et al., 2007; Polli et al., 2009; Agarwal et al., 2010; Kodaira et al., 2010). Whereas studies have tried to explain this cooperation of P-gp and Bcrp at the BBB using simple quantitative models with several assumptions (Kodaira et al., 2010), the exact mechanism of this synergistic/cooperative effect is still unclear.
There are several underlying assumptions in the interpretation of brain distribution data from transporter knockout mice. The first assumption is that there is no change in the expression of other transporters at the BBB in these mice. Compensatory up- or down-regulation of efflux and influx transporters could explain some findings in the P-gp/Bcrp knockout mice. This idea has been suggested for other mouse model strains by Cisternino et al. (2004), who reported ∼3 times more Bcrp mRNA in the brain microvessels from P-gp mutant CF-1 mice compared with wild-type CF-1 mice. However, the study did not show whether these changes in mRNA translated into altered expression of the protein. In contrast, de Vries et al. (2007) reported no difference in the protein expression of Bcrp in P-gp-deficient and wild-type mice of FVB genetic background using Western blots. Another assumption is that the integrity of BBB tight junctions is similar between the wild-type and transgenic mice. Disruption of these tight junctions can alter paracellular transport of drugs into and out of the brain, thereby influencing an alternative pathway of drug distribution to the brain. Therefore, this study was undertaken to investigate these assumptions in an attempt to explain how the observed synergistic or cooperative effect of P-gp and Bcrp could be related to changes in expression of transporters at the BBB.
The first significant finding was that there was no difference in the expression of P-gp between the wild-type and Bcrp1(−/−) mice. Likewise, Bcrp expression was not different between the wild-type and Mdr1a/b(−/−) mice. Our results show that distinctive adaptive expression of these transporters did not occur in the capillary endothelial cells of single knockout mice. The finding that brain distribution does not increase in the single knockout mice is, therefore, not because P-gp or Bcrp is up-regulated and thereby overexpressed in these mice, offering increased compensatory efflux of the substrate drugs at the BBB. Furthermore, we show that expression of P-gp is ∼5-fold higher than Bcrp in wild-type mice (Table 1). In our previous study, we evaluated how greater expression of P-gp at the BBB might be the reason behind it being the dominant transporter at the BBB (Agarwal et al., 2011b). Higher expression of P-gp at the BBB may also explain why brain penetration of dual substrates with similar affinities for P-gp and Bcrp does not increase when Bcrp is absent in the Bcrp1(−/−) mice or is pharmacologically inhibited in the wild-type mice. Expression of P-gp alone may be sufficient to prevent dual substrate drugs from entering the brain.
Altered expression of other transporters that may have overlapping substrates can confound the interpretation of the in vivo functional relevance of changes in brain distribution due to the deletion of P-gp or Bcrp. The current study addressed this by determining the expression of other ABC transporters that can influx or efflux drugs at the BBB. Other than P-gp and Bcrp, the most studied drug efflux transporters belong to the MRP family (Dallas et al., 2006). Our results show that the expression level of Mrp4 was approximately ∼7.5-fold less than P-gp and 1.5-fold less than Bcrp expression. Moreover, the expression of Mrp4 was not significantly different between the four mouse genotypes. The other transporters in the MRP family, such as Mrp1, Mrp2, Mrp3, Mrp5, Mrp6, and Mrp7, were under the limit of quantification (Tables 1 and 2). Although this does not guarantee that these transporters are not expressed at the BBB in these mice, it does indicate that even if these MRPs are present, they are at levels that are significantly less than P-gp and Bcrp. The unchanged expression of quantifiable MRPs in the four genotypes, especially the Mdr1a/b(−/−)Bcrp1(−/−) mice, confirms that they are not related to the greater than proportional increase in brain penetration of dual substrates in these mice.
The organic anion-transporting peptides represent a superfamily of solute carriers that are involved in transport of various organic compounds, including bile acids, thyroid hormones, anionic peptides, and other xenobiotics (Hagenbuch and Gui, 2008). The expression of organic anion-transporting peptides was similar between the four mouse genotypes in this study (Table 1). Likewise, the expression levels of the large neutral amino acid transporter (System Lat1), an amino acid transporter that facilitates the brain uptake of endogenous amino acids such as l-leucine and l-phenylalanine (Uchino et al., 2002), and the glucose transporter (Glut1), which provides glucose to brain, were no different between the four mouse genotypes. Furthermore, the expression of relevant receptors, including Tfr1 (mediates delivery of iron to the brain), Lrp1 (mediates cell uptake of a wide variety of ligands, including α2-macroglobulin, aprotinin, lactoferrin, and tissue plasminogen activator), and Insr (insulin transport), were essentially uniform across the mouse genotypes except in the Mdr1a/b(−/−)Bcrp1(−/−) mice, where expression of Lrp1 and Insr was trended lower compared with the other genotype (Table 1). These results show that expression of all these transporters and most receptors was consistent and conserved across the four genotypes.
The other question behind the “more than additive” effect on the transport of dual substrates in triple knockout mice was related to the intactness of the BBB tight junctions in these mice. To address this question from a proteomic perspective, we quantified the expression of an important tight junction protein, claudin-5, in all four mouse genotypes. The results show that the expression of claudin-5 was not different between the wild-type and genetic knockout mice, suggesting the presence of an intact BBB in these mice. Moreover, we showed this functionally in our previous study by determining initial brain spaces of the vascular markers sucrose and inulin (Agarwal et al., 2010).
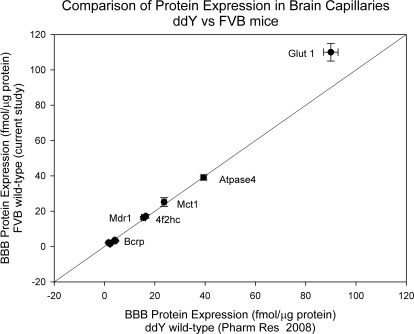
Kamiie et al. (2008) previously reported expression levels of various transporters at the BBB of the ddY mouse strain. We compared the expression of some of the transporters previously determined in ddY mice with that determined in the current study in the FVB wild-type mice. Interestingly, there was no difference in the transporter expression between the FVB mice and ddY mice (Fig. 1). This indicates that expression of all the measured transporters was conserved between the two strains. The results also show the robustness of our LC/MS/MS method, which over two different studies over a significant time interval, with different strains, showed consistent results (Fig. 1).
Fig. 1.
Comparison of absolute protein expression levels of transporters in isolated brain capillary endothelial cells between ddY wild-type mice and FVB wild-type mice. Protein expression levels in ddY mice reported by Kamiie et al. (2008) were compared with protein expression in FVB mice determined in this study. The data suggest that there was no difference in the transporter expression between the FVB mice and ddY mice.
Together, these results show that the expression of all ABC and SLC transporters evaluated was similar between the wild-type and Pgp, Bcrp, and P-gp/Bcrp knockout mice. The integrity of the tight junctions at the BBB is also likely to be similar across the four mouse genotypes, as suggested by the similar levels of claudin-5. Therefore, it is clear that the necessarily frequent assumptions made in the interpretation of brain distribution data are valid, i.e., there are no significant compensatory alterations in the expression of other transporters at the BBB, and the physical barrier presented by the tight junctions is also intact.
In conclusion, we have shown by using a quantitative proteomics approach that there are no changes in BBB expression of several “relevant” transporters and receptors in the single and combined knockout mice. This finding clearly suggests that the observed cooperation/synergy in these mice is not a result of altered or “compensatory” transporter expression at the BBB. Thus, the current study serves as a validation of the P-gp/Bcrp knockout mouse model in future studies investigating the brain distribution of drugs in these mice. Additional research will be needed to determine the exact quantitative nature of the functional compensation between P-gp and Bcrp at the BBB. Nevertheless, the current study is important to understand the impact and relevance of such functional relationships at the human BBB, particularly since the relative expression of some transporters (e.g., Bcrp and P-gp) is different at the human BBB compared with the mouse BBB (Uchida et al., 2011).
Supplementary Material
Acknowledgments
We thank Bjoern Bauer and Anika Hartz (University of Minnesota, Duluth, MN) for help and support in development of the brain capillary isolation method.
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA138437] (W.F.E.); and a grant from the Children's Cancer Research Fund at the University of Minnesota (to W.F.E.). S.A. was supported by the Doctoral Dissertation Fellowship from the University of Minnesota. R.S. was supported by the Ronald J. Sawchuk Fellowship. This study was also supported in part by a Grant-in-Aid for Scientific Research (S) [KAKENHI: 18109002] from the Japan Society for the Promotion of Science and by grants from the Research Seeds Quest Program and the Development of Creative Technology Seeds Supporting Program for Creating University Ventures from the Japan Science and Technology Agency.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- BBB
- blood-brain-barrier
- P-gp
- P-glycoprotein
- Bcrp
- breast cancer resistance protein
- Mdr1
- gene encoding the murine P-glycoprotein
- Bcrp1
- gene encoding the murine breast cancer resistance protein
- ABC
- ATP-binding cassette
- FVB
- Friend leukemia virus strain B
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MRP
- multidrug resistance-associated protein
- MRM
- multiple reaction monitoring
- SLC
- solute carrier
- PBS
- phosphate-buffered saline
- HPLC
- high-performance liquid chromatography.
Authorship Contributions
Participated in research design: Agarwal, Uchida, Mittapalli, Sane, Terasaki, and Elmquist.
Conducted experiments: Agarwal, Uchida, and Sane.
Contributed new reagents or analytic tools: Uchida and Terasaki.
Performed data analysis: Agarwal, Uchida, Mittapalli, Terasaki, and Elmquist.
Wrote or contributed to the writing of the manuscript: Agarwal, Uchida, Mittapalli, Terasaki, and Elmquist.
References
- Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. (2010) Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther 334:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Hartz AM, Elmquist WF, Bauer B. (2011a) Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des 17:2793–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Ohlfest JR, Elmquist WF. (2011b) The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther 336:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. (2009) P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther 330:956–963 [DOI] [PubMed] [Google Scholar]
- Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. (2004) Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res 64:3296–3301 [DOI] [PubMed] [Google Scholar]
- Dallas S, Miller DS, Bendayan R. (2006) Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev 58:140–161 [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. (2007) P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13:6440–6449 [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38:778–801 [DOI] [PubMed] [Google Scholar]
- Hartz AM, Mahringer A, Miller DS, Bauer B. (2010) 17-β-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab 30:1742–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, Sekine Y, Uchida Y, Ito S, Terasaki T. (2008) Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res 25:1469–1483 [DOI] [PubMed] [Google Scholar]
- Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. (2010) Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther 333:788–796 [DOI] [PubMed] [Google Scholar]
- Lagas JS, Vlaming ML, Schinkel AH. (2009) Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol Interv 9:136–145 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. (2005) Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther 312:44–52 [DOI] [PubMed] [Google Scholar]
- Löscher W, Potschka H. (2005) Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2:86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. (2007) Blood-brain barrier delivery. Drug Discov Today 12:54–61 [DOI] [PubMed] [Google Scholar]
- Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. (2009) An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos 37:439–442 [DOI] [PubMed] [Google Scholar]
- Schinkel AH. (1998) Pharmacological insights from P-glycoprotein knockout mice. Int J Clin Pharmacol Ther 36:9–13 [PubMed] [Google Scholar]
- Schinkel AH, Jonker JW. (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55:3–29 [DOI] [PubMed] [Google Scholar]
- Tang SC, Lagas JS, Lankheet NA, Poller B, Hillebrand MJ, Rosing H, Beijnen JH, Schinkel AH. (2012) Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer 130:223–233 [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. (2011) Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem 117:333–345 [DOI] [PubMed] [Google Scholar]
- Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, Anders MW, Endou H. (2002) Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol 61:729–737 [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. (2009) Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev 61:14–25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.