Abstract
Plasma membrane monoamine transporter (PMAT) is a polyspecific organic cation (OC) transporter that transports a variety of endogenous biogenic amines and xenobiotic cations. Previous radiotracer uptake studies showed that PMAT-mediated OC transport is sensitive to changes in membrane potential and extracellular pH, but the precise role of membrane potential and protons on PMAT-mediated OC transport is unknown. Here, we characterized the electrophysiological properties of PMAT in Xenopus laevis oocytes using a two-microelectrode voltage-clamp approach. PMAT-mediated histamine uptake is associated with inward currents under voltage-clamp conditions, and the currents increased in magnitude as the holding membrane potential became more negative. A similar effect was also observed for another cation, nicotine. Substrate-induced currents were largely independent of Na+ but showed strong dependence on membrane potential and pH of the perfusate. Detailed kinetic analysis of histamine uptake revealed that the energizing effect of membrane potentials on PMAT transport is mainly due to an augmentation of Imax with little effect on K0.5. At most holding membrane potentials, Imax at pH 6.0 is approximately 3- to 4-fold higher than that at pH 7.5, whereas K0.5 is not dependent on pH. Together, these data unequivocally demonstrate PMAT as an electrogenic transporter and establish the physiological inside-negative membrane potential as a driving force for PMAT-mediated OC transport. The important role of membrane potential and pH in modulating the transport activity of PMAT toward OCs suggests that the in vivo activity of PMAT could be regulated by pathophysiological processes that alter physiological pH or membrane potential.
Introduction
Organic cations (OCs) consist of a class of structurally diverse endogenous compounds (e.g., biogenic amines) and xenobiotics (e.g., drugs and environmental toxins) that carry a net positive charge at physiological pH. To eliminate hydrophilic OCs from the body, mammalian cells have evolved complex OC transport systems including the classic organic cation transporters 1 to 3 (OCT1–3) from the solute carrier 22 (SLC22) family and the multidrug and toxin extrusion proteins 1 and 2 from the solute carrier 47 (SLC47) family (Koepsell and Endou, 2004; Wright and Dantzler, 2004; Otsuka et al., 2005; Fujita et al., 2006). Our laboratory recently cloned and characterized a novel polyspecific organic cation transporter, the plasma membrane monoamine transporter (PMAT) (Engel et al., 2004; Engel and Wang, 2005; Ho et al., 2011). By gene ontology, PMAT (SLC29A4) belongs to the equilibrative nucleoside transporter (ENT/SLC29) family and is alternatively named ENT4 (Kong et al., 2004). However, detailed functional characterization work demonstrated that PMAT functions as a polyspecific organic cation transporter and shares large overlapping substrate and inhibitor specificity with the OCTs (Engel et al., 2004; Engel and Wang, 2005; Zhou et al., 2007b; Ho et al., 2011). For example, many well established PMAT substrates, such as MPP+, monoamine neurotransmitters, and metformin, are also classic substrates for the OCTs. Similar to the OCTs, transport mediated by PMAT is membrane potential-sensitive and Na+-independent (Sweet and Pritchard, 1999; Koepsell et al., 2003; Engel et al., 2004; Wright and Dantzler, 2004). In humans and rodents, PMAT mRNA is most strongly expressed in the brain, and transcripts are also found in other organs such as the small intestine, kidney, and heart (Engel et al., 2004; Zhou et al., 2007b; Xia et al., 2009). Many of these tissues also coexpress other OC transporters such as the OCTs.
The functional characteristics and tissue distribution of PMAT suggest that this transporter may play important roles in regulating cellular uptake and organ-specific disposition of endogenous monoamine and xenobiotic OCs in vivo (Dahlin et al., 2007; Xia et al., 2007; Zhou et al., 2007a). We previously localized PMAT to the mucosal epithelial layer in human small intestine and suggested a role for PMAT in the intestinal absorption of metformin and other OC drugs (Zhou et al., 2007b). Our recent study in mouse brain synaptosomes suggested a major role of PMAT in mediating low-affinity, high-capacity (i.e., uptake2) uptake of serotonin and dopamine in the brain (Duan and Wang, 2010). We also showed that PMAT mRNA and protein are highly expressed in choroid plexus, which constitutes the blood-cerebrospinal fluid barrier (Dahlin et al., 2007, 2009). Recent brain microdialysis studies in rats further support a role of PMAT in brain-to-blood transport of OCs such as monoamines and cationic neurotoxins (Okura et al., 2011). Given the biological and pharmacological significance of PMAT, it is important to understand the transport mechanism of PMAT and identify physiological and pathological factors and/or conditions that may exert modulatory roles on PMAT activity toward OCs.
Previous radiotracer uptake studies on PMAT after expression in MDCK cells showed that PMAT-mediated OC transport is not dependent on Na+ or Cl− but is sensitive to changes in membrane potential and extracellular pH (Engel et al., 2004; Xia et al., 2007). However, no detailed study has yet been done on the precise transport mechanism and the electrophysiological properties of the transporter. It is not known how the membrane potential regulates the kinetic behavior of PMAT and whether the pH effect is due to direct coupling with proton. Understanding the transport mechanism of PMAT is critical for elucidating the in vivo function and in predicting kinetic behaviors of PMAT under different physiological conditions in various tissues. In this study, we used a two-microelectrode voltage-clamp method (Loo et al., 1993; Kekuda et al., 1998) to analyze the electrophysiological characteristics and transport mechanism of PMAT to gain a better understanding of the physiological and pharmacological roles of this transporter.
Materials and Methods
Materials.
[3H]Histamine was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Unlabeled chemicals, including histamine, MPTP, nicotine, metformin, amantadine, TEA+, and other organic cations were purchased from Sigma-Aldrich (St. Louis, MO).
PMAT Expression in Xenopus laevis Oocytes.
The full-length human PMAT cDNA was previously cloned from a human kidney cDNA library (Engel et al., 2004). The coding sequence of human PMAT was amplified by polymerase chain reaction and inserted into the oocyte expression vector pGH19 (Kekuda et al., 1998). The construct was then linearized with NotI, and the cDNA was transcribed in vitro using T7 RNA polymerase in the presence of ribonuclease inhibitor and RNA cap analog using the mMESSAGE mMACHINE Kit (Ambion, Austin, TX) according to the manufacturer's protocol. Mature (stage V–VI) oocytes from X. laevis (Nasco, Fort Atkinson, WI) were isolated, manually defolliculated, and maintained at 18°C in modified Barth's medium supplemented with 10 mg/l gentamicin as described previously (Kekuda et al., 1998). Oocytes were injected with 50 ng of cRNA. Oocytes injected with water were used as controls. Electrophysiological measurements were performed on days 5 or 6 after cRNA or water injection.
Electrophysiological Measurement.
Electrophysiological studies were performed by the two-microelectrode voltage-clamp method (Loo et al., 1993; Kekuda et al., 1998) to measure steady-state substrate-evoked currents. Oocytes were first superfused with a NaCl- or a N-methyl-d-glucamine (NMDG)-containing buffer (96 mM NaCl or NMDG, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes/Mes/Tris, pH 7.5) followed by the same buffer containing a substrate at the indicated concentrations. To investigate the influence of pH on substrate-evoked currents, perfusion buffers of different pH values were prepared by varying the concentrations of Tris, Hepes, and Mes. Membrane potential was held steady at −50 mV. For studies involving the current-voltage (I-V) relationship, step changes in membrane potential were applied, each for a duration of 100 ms in 20-mV increments between −10 and −150 mV. Kinetic parameters for the saturable transport of histamine were obtained by fitting the data to the Michaelis-Menten equation by nonlinear regression followed by confirmation with linear regression. Data are expressed as the mean ± S.E.M. Where applicable, Student's t test was used to determine the significance of differences between the means from two groups. Data groups are considered significantly different if p < 0.01.
Histamine Uptake in Oocytes.
Uptake of [3H]histamine into oocytes were measured in NaCl or NMDG buffers at pH 6.0 as described previously (Fei et al., 1995). Uptake was determined in groups of 8 to 10 oocytes injected with water or PMAT cRNA at room temperature in a 24-well microtiter plate. The incubation time was 60 min, and the final concentration of histamine was 100 μM. At the end of the incubation, radioactivity associated with individual oocytes was determined. Data are expressed as the mean ± S.E.M. Statistical significance was determined by Student's t test.
Results
Histamine-Induced Currents in PMAT-Expressing Oocytes.
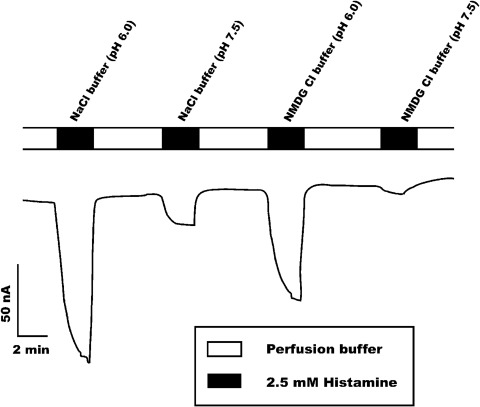
To determine whether PMAT is an electrogenic transporter, we measured substrate-induced steady-state currents using a two-microelectrode, voltage-clamp method. Histamine was used as the substrate because previous radiotracer uptake studies showed that this biogenic amine is transported by PMAT with the highest maximal velocity (Engel and Wang, 2005). Electrophysiological measurements were conducted at pH 6.0 and 7.5 in Na+-containing or Na+-free buffer. As shown in Fig. 1, perfusion of the oocytes with 2.5 mM histamine at pH 6.0 induced inwardly directed currents (∼110 nA). The currents were relatively independent of Na+. In contrast, when the pH of the perfusion buffer was changed from 6.0 to 7.5, a substantial reduction in histamine-induced current was observed in both Na+ and Na+-free buffers. There were no detectable currents when water-injected oocytes were exposed to histamine under the same experimental conditions (data not shown). These data demonstrated unequivocally that transport of histamine by PMAT is associated with net transfer of positive charges into the oocytes, demonstrating the electrogenic nature of the transport process. In addition to histamine, several compounds known to interact with PMAT also induced inward currents in PMAT-expressing oocytes (Table 1). These include metformin and TEA+, which have been previously shown to be transported by PMAT in radiotracer uptake assays (Engel and Wang, 2005; Zhou et al., 2007b). The ability of MPTP, nicotine, and amantadine to evoke inward currents in PMAT-expressing oocytes suggests that these organic cations are also PMAT substrates. For all compounds, the induced currents were pH-sensitive, and the magnitudes were much greater at pH 6.0 than at pH 7.5 (Table 1).
Fig. 1.
Inward currents induced by 2.5 mM histamine at pH 6 and 7.5 in the presence (NaCl) or absence (NMDG chloride) of Na+. There were no detectable currents when water-injected oocytes were exposed to histamine under similar experimental conditions.
TABLE 1.
Compounds that induced inward currents in PMAT-expressing oocytes
Data represent means ± S.D. for two to three independent experiments. Perfusion buffer: NaCl buffer; substrate concentration: 2.5 mM.
| Compounds | Current |
|
|---|---|---|
| pH 6.0 | pH 7.5 | |
| nA | ||
| Histamine | −110 ± 10.3 | −30.5 ± 9.19 |
| MPTP | −62.7 ± 8.26 | −5.67 ± 3.53 |
| Nicotine | −75.7 ± 14.3 | −6.30 ± 1.24 |
| Metformin | −38.0 ± 2.92 | −2.02 ± 0.50 |
| Amantadine | −21.2 ± 4.73 | −7.26 ± 0.74 |
| TEA+ | −11.7 ± 1.00 | −0.627 ± 1.79 |
Influence of pH on Histamine-Induced Currents.
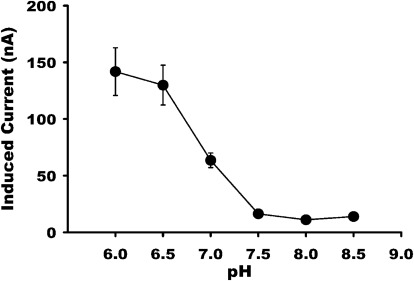
To quantitatively evaluate the effect of extracellular pH on PMAT-mediated organic cation transport, histamine-induced currents were measured in PMAT-expressing oocytes in a Na+-containing buffer at pH values ranging from 6.0 to 8.5. Membrane potential was held constant at −50 mV. Histamine-induced currents exhibited great sensitivity to the pH of the perfusate, and the currents increased progressively with decreasing extracellular pH (Fig. 2). These data demonstrated that PMAT-associated currents are stimulated by an inwardly directed proton gradient under voltage-clamp conditions.
Fig. 2.
Influence of pH on histamine-induced currents in the presence of Na+. PMAT-expressing oocytes were exposed to histamine (2.5 mM) in a NaCl-containing buffer at different pH. The data show that histamine-induced currents increased when the pH was made acidic.
Current-Voltage Relationship.
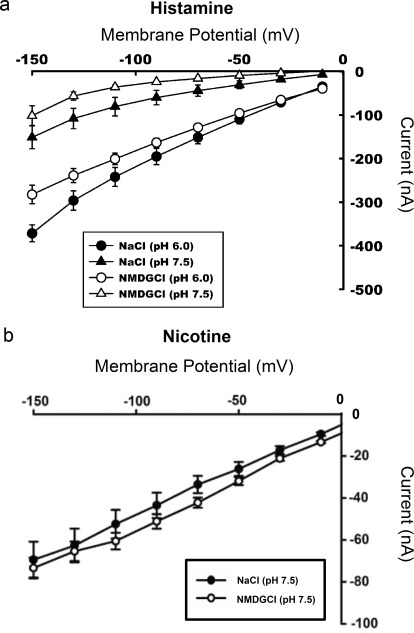
To determine the effect of membrane potential on substrate-induced currents, inward currents induced by 2.5 mM histamine were monitored in PMAT-expressing oocytes at different testing membrane potentials. At both pH 6.0 and pH 7.5, the current increased in magnitude as the membrane became more hyperpolarized, demonstrating that PMAT is activated by an inside-negative membrane potential (Fig. 3a). As seen previously, an inwardly directed H+ gradient greatly enhanced the currents at a fixed membrane potential regardless of the presence or absence of Na+. In contrast, at a fixed membrane potential under the same extracellular pH (7.5 or 6.0), there was minimal difference in the magnitude of substrate-evoked currents whether the oocytes were perfused with a Na+-containing buffer or a Na+-free buffer (p = 0.04 at pH 6.0 at −150 mV; p > 0.05 for all other points) (Fig. 3a). To demonstrate that the influence of membrane potential is not substrate-specific, we also examined the I-V relationship of nicotine, an OC that evoked inward currents in PMAT-expressing oocytes (Table 1) at pH 7.5. Similar to histamine, nicotine-induced currents also increased progressively as the holding membrane potential became more negative (Fig. 3b). Na+, on the other hand, had no significant effect on nicotine-induced currents at all membrane potentials tested.
Fig. 3.
a, I-V relationship for histamine-induced currents at different experimental conditions. Inward currents induced by 2.5 mM histamine were monitored in PMAT-expressing oocytes at different testing membrane potentials. The data show that H+ enhanced the currents, both in the presence (NaCl) and absence (NMDG chloride) of Na+, and that hyperpolarization also enhanced the currents, indicating the role of membrane potential in the energization of the transporter. b, I-V relationship for nicotine-induced currents in Na+-containing or Na+-free perfusates at pH 7.5. Inward currents induced by 2.5 mM nicotine were monitored in PMAT-expressing oocytes at different testing membrane potentials. The data show that hyperpolarization enhanced the currents.
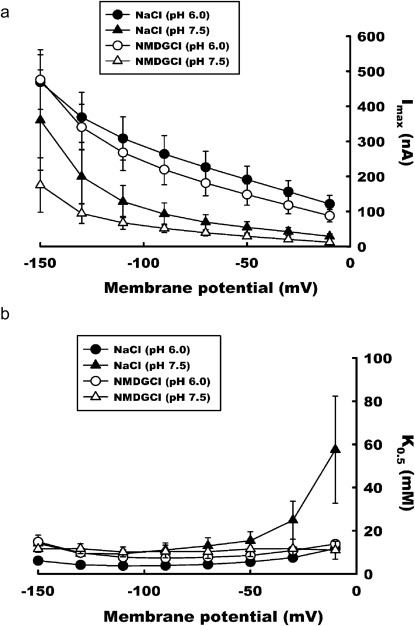
Influence of Membrane Potential on Imax and K0.5.
PMAT-expressing oocytes were exposed to increasing concentrations of histamine at various testing membrane potentials over the range of −10 to −150 mV. The substrate-dependent currents were subjected to kinetic analysis according to the Michaelis-Menten equation, and the kinetic parameters, Imax and K0.5, were calculated. The data show that hyperpolarization increases the magnitude of the maximal currents induced by histamine. This effect is apparent at pH 7.5 and 6, both in the presence and absence of Na+ (Fig. 4a). Although the absence of Na+ produced a small reduction in Imax at a fixed membrane potential and pH, the difference was mostly statistically insignificant (p = 0.04 at pH 7.5 and −10 mV; p > 0.05 for all other points) (Fig. 4a). On the other hand, the data showed that the apparent affinity (K0.5) was largely independent of membrane potential, and the values averaged around 10 mM under most testing membrane potentials at both pH 7.5 and pH 6.0 (Fig. 4b). The currents measured at membrane potential −30 or −10 mV were very small, which introduced significant variability in the estimation of K0.5. No statistical difference was found for the K0.5 values at pH 7.5 in the presence of Na+ from other K0.5 values measured at either −30 or −10 mV (p > 0.05).
Fig. 4.
Influence of membrane potential on Imax (a) and K0.5 (b) for histamine-induced currents. PMAT-expressing oocytes were exposed to increasing concentrations of histamine at varying testing membrane potentials over the range of −10 to −150 mV. The substrate-dependent currents were subjected to kinetic analysis according to the Michaelis-Menten equation, and the kinetic parameters, Imax and K0.5, were calculated. The data show that hyperpolarization increases the magnitude of the maximal currents induced by histamine. This effect is apparent at pH 7.5 and 6, both in the presence (NaCl) and absence (NMDG chloride) of Na+. The data also show that hyperpolarization has very little effect on the affinity of the transporter for histamine.
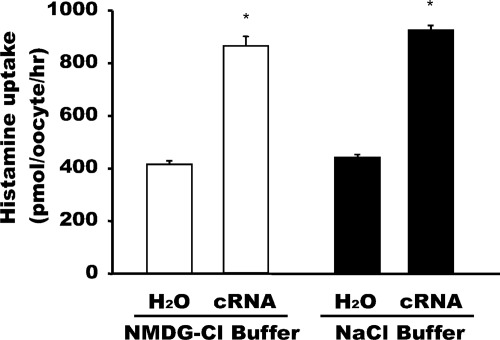
Histamine Uptake in PMAT-Expressing Oocytes.
Uptake of radiolabeled histamine (100 μM) was measured in PMAT cRNA-injected and water-injected oocytes at pH 6 in the presence or absence of Na+. The data show that expression of PMAT induced transport of histamine by ∼2-fold. PMAT-mediated [3H]histamine is Na+-independent (Fig. 5).
Fig. 5.
Uptake of radiolabeled histamine in PMAT-expressing oocytes. Uptake of radiolabeled histamine (100 μM) was measured in water-injected and PMAT cRNA-injected oocytes at pH 6 in the presence (NaCl) or absence (NMDG chloride) of Na+. *, significantly (p < 0.0001) different from water-injected oocytes.
Discussion
PMAT is a polyspecific OC transporter recently implicated in the disposition of organic cations in the intestine and brain (Zhou et al., 2007b; Okura et al., 2011). The goal of this study was to elucidate the electrophysiological characteristics of PMAT, focusing on the role of membrane potential and physiological ion gradients on PMAT-mediated OC transport.
Consistent with previous findings from radiotracer uptake assays, PMAT-associated currents are strongly dependent on membrane potential and extracellular pH (Figs. 1–4). In most animal cells, the resting membrane potential ranges from −60 to −90 mV. This inside-negative potential is used by OCT1–3 in the SLC22 family to drive OC uptake (Busch et al., 1996; Gorboulev et al., 1997). The electrophysiological properties of the OCTs have been well characterized, and all three major OCT isoforms, OCT1–3, have been shown to function as electrogenic transporters (Gorboulev et al., 1997; Kekuda et al., 1998; Wu et al., 1998; Budiman et al., 2000; Dresser et al., 2000). Different from the OCTs, PMAT (also termed ENT4) belongs to the SLC29 family that encodes the classic electroneutral equilibrative nucleoside transporters 1 and 2 (ENT1/2). Our results clearly showed that PMAT is an electrogenic transporter and the physiological inside-negative membrane potential serves as a driving force for PMAT-mediated OC transport (Figs. 1 and 3). Current-voltage relationship studies further showed that membrane hyperpolarization stimulates PMAT-mediated histamine and nicotine uptake, whereas depolarization suppresses transport activity (Fig. 3). These results are consistent with our previous radiotracer uptake assays in PMAT-expressing MDCK cells in which we observed that membrane depolarizing reagents such as potassium and barium significantly reduced PMAT-mediated uptake of OCs such as MPP+ and metformin (Engel et al., 2004; Zhou et al., 2007b). Together, these data clearly established the important role of membrane potential in modulating the transport activity of PMAT toward structurally distinct OCs and suggest that the in vivo activity of PMAT could be regulated by physiological and pathological processes that induce changes in membrane potential.
Consistent with our previous results from radiotracer uptake assays in PMAT-expressing cell lines, Na+ has no major effect on substrate-evoked currents in oocytes injected with PMAT cRNA. Although removal of Na+ in the perfusion buffer appeared to have produced an effect in the histamine chart recording shown in Fig. 1, it should be noted that this study was performed while different perfusates were switched within a relatively short time interval. In more quantitative analysis of histamine currents under stable perfusion conditions (Figs. 3a and 4), we found no statistically significant difference between the Na+-containing and Na+-free groups. Moreover, no Na+ effect was observed with nicotine-evoked currents (Fig. 3b). Overall, our data demonstrated an insignificant effect for this ion on substrate-evoked currents, which is consistent with our uptake data showing no effect of Na+ on PMAT-mediated histamine uptake (Fig. 5).
To further elucidate the influence of membrane potential on PMAT transport kinetics, apparent binding affinity (K0.5) and maximal velocity (Imax) were measured at incremental testing membrane potentials (Fig. 4). The K0.5 and Imax values under different holding membrane voltages were analyzed and compared. The results revealed that the stimulatory effect of negative membrane potentials on PMAT occurs mainly through augmentation of Imax without affecting apparent substrate-binding affinity (Fig. 4). The K0.5 value for histamine averaged approximately 10 mM (Fig. 4b), which is in excellent correlation with the Km value (10.5 mM) previously determined by [3H]histamine uptake in MDCK cells (Engel and Wang, 2005). Because Imax is determined by the transporter turnover rate (kcat) and the number of transporter molecules (Etotal) on the plasma membrane, the stimulatory effect of membrane potential on Imax must result from a change in kcat or Etotal or both. Because changes in the magnitude of substrate-induced currents were observed instantly as step changes in membrane potential were applied on a time scale of 100 ms (Fig. 3), the stimulatory effect of membrane potential on Imax is most likely due to a direct effect of kcat because it takes time to increase the density of the transporter protein on the plasma membrane through protein synthesis and/or recruitment.
Protons are profoundly involved in membrane transport of OCs. Protons can be directly coupled to drive OC transport in the case of multidrug and toxin extrusion proteins 1 and 2, which are H+/OC antiporters that use an inwardly directed proton gradient to drive OC efflux (Otsuka et al., 2005; Masuda et al., 2006; Terada et al., 2006). As an alternative, protons can exert a direct effect on protein activity or influence the OC transport process through indirect effects such as alteration of membrane potential and/or degree of ionization of the substrate. In mammalian cells, the activities of OCT1–3 have been shown to be sensitive to extracellular pH (Urakami et al., 1998; Wu et al., 1998; Sweet and Pritchard, 1999). However, detailed electrophysiological measurements in OCT2- and OCT3-expressing oocytes showed that under voltage-clamp conditions, substrate-induced currents are not influenced by pH (Okuda et al., 1996; Gorboulev et al., 1997; Kekuda et al., 1998). These results suggest that the pH effect on the OCTs observed in mammalian cells is probably due to an indirect effect of proton on membrane potential (Kekuda et al., 1998). In contrast to the OCTs, whose activities decrease at acidic pH (Gründemann et al., 1997; Kekuda et al., 1998; Urakami et al., 1998; Wu et al., 1998), radiotracer uptake studies demonstrated a pronounced stimulatory effect of acidic pH on PMAT-mediated OC uptake (Xia et al., 2007; Zhou et al., 2007b). Here, we observed that histamine-induced currents in PMAT-expressing oocytes were also highly dependent on extracellular pH and much greater in perfusate buffered with acidic pH (Figs. 1–4). Because the electrophysiological measurements were conducted under voltage-clamp conditions, it is unlikely that the influence of pH on substrate-induced currents occurred through a nonspecific effect on membrane potential. In addition, the primary amine moiety in histamine has a pKa of 9.8. At physiological pH (e.g., 7.4), histamine is almost completely protonated to a singly charged cation. Thus, the enhanced histamine uptake at acidic pH (pH 6.0–7.0) is unlikely because of an increase in the charged cation form of substrate. Moreover, we previously observed that PMAT-mediated transport of a permanently charged cation, MPP+, is also stimulated by acidic pH in a similar way (Xia et al., 2007), further excluding the possibility that acidic pH might indirectly increase transport rate through enhancing substrate protonization. Detailed kinetic analysis further revealed that at all holding membrane potentials, Imax at pH 6.0 is approximately 3- to 4-fold higher than at pH 7.5 whereas K0.5 is not dependant on pH (Fig. 4). These electrophysiology results are consistent with our previous radiotracer uptake studies in MDCK cells in which we observed that Vmax of PMAT-mediated MPP+ uptake was much greater at acidic pH, whereas Km was independent of pH (Xia et al., 2007). Taken together, our data strongly suggest that proton exerts a direct effect on PMAT and the stimulatory effect of acidic pH on PMAT-mediated OC transport is due to an augmentation of the turnover rate. Molecularly, this proton effect may occur via coupling of OC with proton cotransport or through a proton-induced change in protein folding and/or ionization state, resulting in a change in intrinsic catalytic activity. Our previous uptake study in MDCK cells showed that at acidic conditions, proton ionophores drastically reduced PMAT-mediated MPP+ uptake, providing evidence of transporter coupling with a proton gradient. To further delineate the molecular mechanism, we attempted to simultaneously measure [3H]histamine uptake and histamine-induced currents to obtain a charge-to-substrate transfer ratio for histamine. However, the uptake of [3H]histamine was low in PMAT-expressing oocytes and only showed a 2-fold increase over that in water-injected oocytes after a 60-min incubation at pH 6.0. The resting membrane potential in healthy defolliculated Xenopus oocytes ranges from −40 to −60 mV (Dascal et al., 1984; Dascal, 2001). On the basis of the I-V relationship shown in Fig. 3a, this potential range only moderately stimulates PMAT-mediated histamine transport. Because of the low histamine uptake, the charge-to-substrate transfer ratio cannot be accurately determined for PMAT, and thus we are unable to definitively prove a proton-coupling mechanism for PMAT-mediated transport.
In summary, this is the first study using an electrophysiological approach to define the transport mechanism and the driving force for PMAT. We unequivocally demonstrated that PMAT is an electrogenic transporter and established the physiological inside-negative membrane potential as a driving force for PMAT-mediated OC transport. We also determined how membrane potential and pH quantitatively influence the transport kinetics of PMAT. Elucidating the roles of membrane potential and proton on PMAT-mediated OC transport is critical for understanding its in vivo function in OC disposition. For example, in tissues with an acidic environment such as the lumen of the gastrointestinal tract, PMAT may play a more important role than the OCTs in OC drug absorption as acidic pH stimulates PMAT activity but suppresses the activities of the OCTs. In addition, several physiological and pathophysiological conditions (e.g., membrane excitation, ischemia, and hypoxia) are known to alter the physiological pH or membrane potential. These conditions could modulate the activities of PMAT and affect PMAT-mediated processes such as neurotransmitter uptake and disposition of OC drugs and toxins.
This work was supported by the National Institutes of Health National Institute of General Medicine Sciences [Grant GM066233].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- OC
- organic cation
- OCT
- organic cation transporter
- SLC
- solute carrier family
- PMAT
- plasma membrane monoamine transporter
- ENT
- equilibrative nucleoside transporter
- MPP+
- 1-methyl-4-phenylpyridinium
- MDCK
- Madin-Darby canine kidney
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- TEA+
- tetraethyl ammonium
- NMDG
- N-methyl-d-glucamine
- Mes
- 4-morpholineethanesulfonic acid
- I-V
- current-voltage.
Authorship Contributions
Participated in research design: Itagaki, Ganapathy, and Wang.
Conducted experiments: Itagaki, Zhou, and Babu.
Performed data analysis: Itagaki, Ganapathy, Ho, and Wang.
Wrote or contributed to the writing of the manuscript: Itagaki, Ganapathy, Ho, and Wang.
References
- Budiman T, Bamberg E, Koepsell H, Nagel G. (2000) Mechanism of electrogenic cation transport by the cloned organic cation transporter 2 from rat. J Biol Chem 275:29413–29420 [DOI] [PubMed] [Google Scholar]
- Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H. (1996) Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J Biol Chem 271:32599–32604 [DOI] [PubMed] [Google Scholar]
- Dahlin A, Royall J, Hohmann JG, Wang J. (2009) Expression profiling of the solute carrier gene family in the mouse brain. J Pharmacol Exp Ther 329:558–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. (2007) Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience 146:1193–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. (2001) Voltage clamp recordings from Xenopus oocytes. Curr Protoc Neurosci Chapter 6: Unit 6.12 [DOI] [PubMed] [Google Scholar]
- Dascal N, Landau EM, Lass Y. (1984) Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3′,5′-cyclic monophosphate. J Physiol 352:551–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser MJ, Gray AT, Giacomini KM. (2000) Kinetic and selectivity differences between rodent, rabbit, and human organic cation transporters (OCT1). J Pharmacol Exp Ther 292:1146–1152 [PubMed] [Google Scholar]
- Duan H, Wang J. (2010) Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 335:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Wang J. (2005) Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol 68:1397–1407 [DOI] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. (2004) Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem 279:50042–50049 [DOI] [PubMed] [Google Scholar]
- Fei YJ, Prasad PD, Leibach FH, Ganapathy V. (1995) The amino acid transport system y+L induced in Xenopus laevis oocytes by human choriocarcinoma cell (JAR) mRNA is functionally related to the heavy chain of the 4F2 cell surface antigen. Biochemistry 34:8744–8751 [DOI] [PubMed] [Google Scholar]
- Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. (2006) Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci 95:25–36 [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. (1997) Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 16:871–881 [DOI] [PubMed] [Google Scholar]
- Gründemann D, Babin-Ebell J, Martel F, Ording N, Schmidt A, Schömig E. (1997) Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. J Biol Chem 272:10408–10413 [DOI] [PubMed] [Google Scholar]
- Ho HT, Pan Y, Cui Z, Duan H, Swaan PW, Wang J. (2011) Molecular analysis and structure-activity relationship modeling of the substrate/inhibitor interaction site of plasma membrane monoamine transporter. J Pharmacol Exp Ther 339:376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V. (1998) Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem 273:15971–15979 [DOI] [PubMed] [Google Scholar]
- Koepsell H, Endou H. (2004) The SLC22 drug transporter family. Pflugers Arch 447:666–676 [DOI] [PubMed] [Google Scholar]
- Koepsell H, Schmitt BM, Gorboulev V. (2003) Organic cation transporters. Rev Physiol Biochem Pharmacol 150:36–90 [DOI] [PubMed] [Google Scholar]
- Kong W, Engel K, Wang J. (2004) Mammalian nucleoside transporters. Curr Drug Metab 5:63–84 [DOI] [PubMed] [Google Scholar]
- Loo DD, Hazama A, Supplisson S, Turk E, Wright EM. (1993) Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci USA 90:5767–5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, Ogawa O, Inui K. (2006) Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol 17:2127–2135 [DOI] [PubMed] [Google Scholar]
- Okuda M, Saito H, Urakami Y, Takano M, Inui K. (1996) cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun 224:500–507 [DOI] [PubMed] [Google Scholar]
- Okura T, Kato S, Takano Y, Sato T, Yamashita A, Morimoto R, Ohtsuki S, Terasaki T, Deguchi Y. (2011) Functional characterization of rat plasma membrane monoamine transporter in the blood-brain and blood-cerebrospinal fluid barriers. J Pharm Sci 100:3924–3938 [DOI] [PubMed] [Google Scholar]
- Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. (2005) A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci USA 102:17923–17928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DH, Pritchard JB. (1999) rOCT2 is a basolateral potential-driven carrier, not an organic cation/proton exchanger. Am J Physiol 277:F890–F898 [DOI] [PubMed] [Google Scholar]
- Terada T, Masuda S, Asaka J, Tsuda M, Katsura T, Inui K. (2006) Molecular cloning, functional characterization and tissue distribution of rat H+/organic cation antiporter MATE1. Pharm Res 23:1696–1701 [DOI] [PubMed] [Google Scholar]
- Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. (1998) Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther 287:800–805 [PubMed] [Google Scholar]
- Wright SH, Dantzler WH. (2004) Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84:987–1049 [DOI] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. (1998) Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem 273:32776–32786 [DOI] [PubMed] [Google Scholar]
- Xia L, Engel K, Zhou M, Wang J. (2007) Membrane localization and pH-dependent transport of a newly cloned organic cation transporter (PMAT) in kidney cells. Am J Physiol Renal Physiol 292:F682–F690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zhou M, Kalhorn TF, Ho HT, Wang J. (2009) Podocyte-specific expression of organic cation transporter PMAT: implication in puromycin aminonucleoside nephrotoxicity. Am J Physiol Renal Physiol 296:F1307–F1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Engel K, Wang J. (2007a) Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem Pharmacol 73:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Xia L, Wang J. (2007b) Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos 35:1956–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]