Abstract
To assess how the shift from a healthy diet rich in omega-3 fatty acids to a diet rich in saturated fatty acid affects the substrates for brain plasticity and function, we used pregnant rats fed with omega-3 supplemented diet from their 2nd day of gestation period as well as their male pups for 12 weeks. Afterwards, the animals were randomly assigned to either a group fed on the same diet or a group fed on a high-fat diet (HFD) rich in saturated fats for 3 weeks. We found that the HFD increased vulnerability for anxiety-like behavior, and that these modifications harmonized with changes in the anxiety-related NPY1 receptor and the reduced levels of BDNF, and its signalling receptor pTrkB, as well as the CREB protein. Brain DHA contents were significantly associated with the levels of anxiety-like behavior in these rats.
Anxiety disorders are the most prevalent mental disorders in developed countries. Depression is about to edge out HIV/AIDS as the world's most significant health problem according to the World Health Organization. For Americans born a century ago, the chances of suffering any episode of major depression in the lifetime was only about 1 percent. Today, the lifetime incidence has increased almost 2000 times and is 19.2 percent1. Anxiety disorders, such as post-traumatic stress disorder (PTSD), obsessive-compulsive disorder, panic disorder, social phobia, and generalized anxiety disorder, often accompany depression2. In turn, obesity has become a worldwide epidemic particularly in US, and a major cause for an increased risk of depressive and anxiety disorders3,4,5,6. Increased availability and excessive intake of energy-rich foods generally found in or fast foods is a significant factor contributing to obesity, and has made invasion in most cultures around the world. In spite of its poor health consequences, there is presently little information on how the diet switch to a high-fat diet that contributes to development of obesity heightens the risk for anxiety disorders.
In turn, western diets that are high in saturated fat induce metabolic dysfunction and promote cognitive alterations7,8,9. It is well known that diets rich in saturated fat increase oxidative stress in brain10,11, reduce neurogenesis12,13, enhance neuroinflammation14 and exert anxiety-like behavior15. Further recent evidences suggest that maternal high-fat diet consumption may have profound effects on the offspring's preference and consumption of high-fat high-sugar diets16,17. Contrary to what is known about the HF diet, recent clinical investigations have provided strong evidence that long chain omega-3 polyunsaturated fatty acids (PUFA) possess significant antidepressant activity18. Indeed recent meta-analyses have reported a moderate effect size for omega-3 PUFA in depression comparable to that of conventional antidepressants, and reduced levels of omega-3 PUFA in the blood of patients with depression19,20. Epidemiological observations report an inverse correlation between omega-3 PUFA intake and the development of depression21,22. In addition, a diet that is rich in omega-3 fatty acids is garnering appreciation for supporting cognitive processes in humans23 and for up-regulating genes that are important for maintaining synaptic function and plasticity in rodents24. The strong dichotomy between a HF diet and a PUFA diet implies that a switch from a PUFA diet to a HFD can have dramatic consequences for brain function; however, as far as we know, this question has not been addressed experimentally. Accordingly we have designed studies to determine the effects of this dietary switch on brain function and plasticity, which results are highly relevant for public health based on the increasingly common dietary changes related to industrialization and cultural migrations in our modern society.
We have focused these studies on brain-derived neurotrophic factors (BDNF) because of its described involvement on cognitive function and emotions25,26,27,28,29, and its action supporting mechanisms of synaptic plasticity and neuronal excitability30. Dietary deprivation of omega-3 fatty acids in rodents result in reduced BDNF levels in striatum31 and frontal cortex32 leading to reduced cAMP response element binding protein (CREB) transcription factor activation. On the other hand omega-3 supplementation in adult rats has been shown to increase hippocampal BDNF, and CREB levels which were associated with improved cognition24. Accordingly, we designed this study to assess the effects of this dietary transition on plasticity related molecules in hippocampus & frontal cortex, which in turn may be responsible for underlying behavior alterations.
Results
Metabolic adverse effects of diet transition
The diet transition to HFD in the animals previously on DHA diet (Fig. 1; experimental design) resulted in metabolic adverse effects. In the present study animals subjected to diet transition on a HFD for 3 weeks gain significantly more body weight as compared to their counterparts continued a healthy DHA supplemented diet (p< 0.001; Figure 2A). The animals fed HFD for 3 weeks also showed significantly higher blood glucose, cholesterol, triglycerides (p<0.05) and higher uric acid (p<0.01). The results are shown in Table 1.
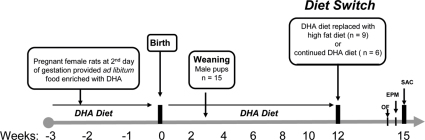
Figure 1. Experimental design.
Two day pregnant female Sprague-Dawley rats were fed an n-3 enriched fatty acid diet (DHA diet). Male pups were subjected to same diet as their dams. On postnatal day 90 the male rats were randomly divided into two subgroups i.e. DHA (n = 6) continued on same n-3 enriched diet and high-fat diet (HFD; n = 9). After 3 weeks of diet transition, rats were tested in an open field. The day after open field, rats were subjected to elevated plus maze test. A day after the last behavioral test the animals were killed by decapitation.
Figure 2. Effect of diet switch to HFD on body weight gain and anxiety-like behaviors.
(A) Diet switch to a HFD food significantly increased body weight gain as early as second week (p<0.0001) which remains significantly higher at the end of three weeks of HFD (p<0.0001) as compared to the rats on a healthy omega-3 supplemented diet. (B) Open field: significant decrease in the distance travelled in the open field (p<0.001) in rats switched to a HFD was noticed after 3 weeks of diet switch. (C–D) EPM: a non-significant trend toward decrease in percentage open arm entries in the rats subjected to diet switch to a HFD and a significant decrease in percentage time spent in open arm (p<0.05) in rats subjected to diet switch to a HFD was observed. Values are expressed in mean ±SEM. *p<0.05, *** p<0.001 Vs DHA diet.
Table 1. Effects of diet switching to a high fat diet on metabolic syndrome related molecules in blood.
| Diet | Glucose (mg/dL) | Cholestrol (mg/dL) | Triglycerides (mg/dL) | Uric Acid (mg/dL) |
|---|---|---|---|---|
| DHA | 93.83 ± 2.915 | 62.5 ± 3.51 | 193 ± 13.22 | 2.983 ± 0.0654 |
| HFD | 112.3 ± 4.729* | 85.89 ± 6.981* | 370 ± 60.42* | 3.767 ± 0.176** |
Statistically significant changes are represented *p<0.05 and **p<0.01 compared with DHA diet. Data are analyzed by using two-tailed unpaired t-test.
Effects of diet transition to HFD on anxiety-like behavior
Both group of animals either fed a HFD or DHA supplemented diet were tested for anxiety-like behaviors after 3 weeks of diet transition in open field and elevated plus maze. The HFD animals showed a remarkably distinct behavior in open field as characterized by significantly less distance travelled (p<0.001; Figure 2B) compared to DHA fed animals. The animals switched to HFD made significantly less entries to the centre of open field (p<0.001) and spent significantly less time in centre of open field (p<0.001). The animals switched to HFD spent significantly less time in the open arms of elevated plus maze (p<0.05; Figure 2C–D) as compared to DHA fed animals. These results strongly suggest that switching to a saturated HFD increases anxiety-like behavior.
Effects of diet transition to HFD on the levels of fatty acids in brain
To assess the effect of diet transition to HFD, we measured the levels of various fatty acids in brain by using gas chromatography. Detailed composition of fatty acids in the frontal cortex is shown in Table 2. Most importantly, we found significant decrease (13.48±0.11, n = 9, p<0.001, Figure 3A) in the levels of DHA in the animal group fed on high-fat diet as compared to the DHA fed diet counterpart (14.81 ± 0.05, n = 5, p,0.05, Figure 3B, Table 2). We observed a positive correlation of frontal cortex DHA levels with distance travelled in open field (r = 0.6567; p<0.05, Figure 3C). The ratio of n-6/n-3 PUFA also showed a strong negative correlation with distance travelled in open field (r = −0.7746; p<0.01, Figure 3D) suggesting that change in dietary n-3 levels in HFD animals is associated with increased anxiety-like behavior.
Table 2. Effects of diet transition to HFD on the levels of fatty acids in brain.
| Fatty acid | DHA | HFD |
|---|---|---|
| C14:0 | 0.22±0 | 0.25±0.01 |
| C16:0 | 18.4±0.13 | 17.93±0.13 |
| C16:1 | 0.44±0.02 | 0.45±0.01 |
| C18:0 | 19±0.14 | 18.38±0.16 |
| C18:1 | 15.6±0.18 | 15.19±0.17 |
| C18:2n6 (LA) | 0.56±0.01 | 0.84±0.03 |
| C20:0 | 0.61±0.06 | 0.60±0.03 |
| C20:1 | 1.47±0.06 | 1.59±0.03 |
| C20:2 | 0.6±0.06 | 1.05±0.09 |
| C20:3n6 | 0.6±0.05 | 1.26±0.06a |
| C20:4n6 (AA) | 9.36±0.09 | 9.83±0.12 |
| C22:4n6 | 2.68±0.06 | 2.91±0.06 |
| C22:5n6 (DPA) | 0.03±0.01 | 0.24±0.03 |
| C24:0 | 1.61±0.09 | 1.16±0.03 |
| C22:6n3 (DHA) | 14.8±0.05 | 13.48±0.11a |
| Ratio n-6/n-3 PUFA | 0.67±0.01 | 0.81±0.01a |
Each parameter is presented as percentage mean relative to total fatty acids (±SEM) in frontal cortex. Statistically significant changes are represented ap<0.05 compared with DHA diet. Data are analyzed by using two-tailed unpaired t-test.
Figure 3. Effect of diet switch to HFD on brain DHA levels and their association with anxiety-like behavior.
(A) A significant decrease (p<0.01) in brain DHA levels in rats subjected to a diet switch to HFD for 3 weeks as compared to the counterpart rats which were fed omega-3 supplemented diet (B) An increase (p<0.001) in the ratio of omega-6 (arachidonic acid) to omega-3 (DHA) fatty acids in rats switched to HFD was observed. The distance travelled in the open field was directly proportional to brain DHA levels (C; r = 0.5657; p<0.05), while inversely proportional to the ratio of n6/n3 PUFA (D; r = 0.7746; p<0.001); indicating that reduced brain DHA levels may be compensated by a corresponding increase in the brain arachidonic acid. The Values are expressed in mean ±SEM. **p<0.01, *** p<0.001 Vs DHA diet.
Effects of diet transition to HFD on proteins associated with anxiety-like behavior and plasticity
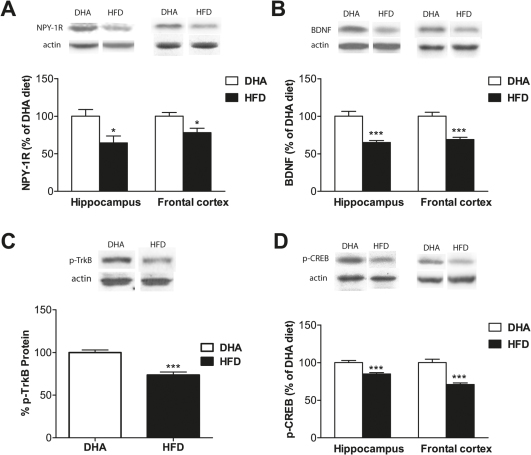
Neuropeptide Y (NPY) in the brain not only regulates the stress-induced activation of the HPA axis, but also mediates the behavioral and autonomic changes associated with stress-related illnesses including anxiety, depression, and cardiovascular disease. The anxiety-reducing effects of NPY and the anxiety-enhancing effects of antagonists of NPY receptors are fairly well-documented, providing strong evidence for NPY's role in modulating anxiety responses. In the present study we assessed the modulating effects of DHA or HFD diet on the levels of NPY1 receptor. We found a significant decrease in frontal cortex (22%, p<0.05; Fig. 4A) and hippocampus (35.5%, p<0.05; Fig. 4A), when rats were fed with HFD as compared to DHA diet rats. The results showed that the percentage levels of BDNF were decreased to 31.1% in the frontal cortex of rats fed on HFD diet (P<0.0001; Fig. 4B), and in hippocampus the decrease observed was 35.5% (p<0.0001; Fig. 4B), as compared to DHA diet. Reports suggest that mice lacking functional full-length TrkB specifically in the newborn neuron population of four to six weeks of age exhibited a markedly enhanced anxiety- like behavior as evidenced by their decreased explorative activity in the open field and elevated plus maze tests33. In our present study, we observed a significant decrease in frontal cortex (26.33%, p<0.05; Fig. 4C), in the levels of phosphorylated TrkB in HFD compared to rats fed on DHA diet. Currently, MAP kinase and PI-3 kinase pathways are two of the best-studied BDNF/TrkB-mediated signalling pathways. Both MAPK and PI-3K signalling pathways lead to the regulation of transcription factor, cyclic AMP response element binding protein (CREB), which has been reported to be a key mediator of cell survival34. We assessed level of pCREB to further elucidate the effects of HFD on BDNF signalling. We found that levels of phosphorylated form of this nuclear factor dramatically decreased in frontal cortex (29.22%, p<0.0001; Fig. 4D), and in hippocampus (15.33%, p<0.05; Fig. 4D) of HFD animals as compared to DHA fed animals.
Figure 4. Effects of diet switch to HFD on plasticity markers.
(A) Levels of neuropeptide Y (NPY) 1 receptor significantly decreased in hippocampus (p<0.05) as well as in frontal cortex (p<0.05). (B) Brain derived neurotrophic factor (BDNF) showed a significant decrease in hippocampus (p<0.001) and frontal cortex (p<0.001). (C) Phosphorylated TrkB (pTrkB) showed significant decrease in hippocampus (p<0.001). (D) A significant decrease in activation of CREB as depicted by levels of pCREB was observed in the hippocampus (p<0.001) and frontal cortex) p<0.001). Representative western blot bands are shown for BDNF, pTrkB, pCREB and actin in hippocampus and frontal cortex. Values are expressed in mean ±SEM. *p<0.05, ***p<0.001 Vs DHA diet.
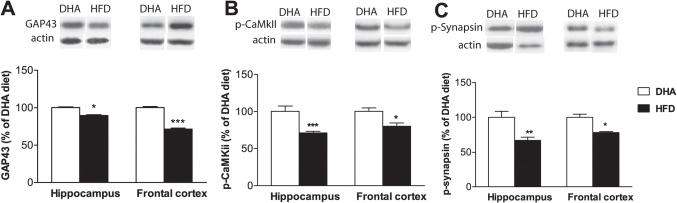
The levels of GAP-43 protein significantly declined in frontal cortex (28.56%; p<0.001; Fig. 5A) and in hippocampus (10.39%; p<0.05; Fig. 5A) of HFD animals, as compared to DHA fed animals. We observed reduced activation of CaMKII levels as suggested by reduced levels of p-CaMKII in frontal cortex (19.5%; p<0.05; Fig. 5B) and in hippocampus (29.11%; p<0.001; Fig. 5B) after 3 weeks of HFD. After 3 weeks of HFD the levels of synaptic plasticity marker protein p-synapsin declined significantly in frontal cortex (22.06%; p<0.001; Fig. 5C) and in hippocampus (32.96%; p<0.01; Fig. 5C) as compared to DHA fed animals.
Figure 5. Effects of diet switch to HFD on plasticity markers.
(A) A significant reduction in the levels of GAP-43 was observed in hippocampus (p<0.05) and frontal cortex (p<0.001). (B) A significant reduction in the levels of phospho-CaMKii was observed in hippocampus (p<0.001) and frontal cortex (p<0.05). (C) A significant reduction in the levels of phospho-synapsin (p-syn) was observed in hippocampus (p<0.01) and frontal cortex (p<0.05). Values are expressed in mean ±SEM. *p<0.05, ***p<0.001 Vs DHA diet.
HFD induced alterations in BDNF signalling & plasticity related proteins are associated with behavioral deficits
We observed that the BDNF levels in hippocampus (Fig 6A; r = 7787; p<0.001) and frontal cortex (Fig 6B; r = 0.6089; p<0.05) are strongly correlated to the outcomes in open field such as distance travelled. The number of open arm entries made in elevated plus maze are positively correlated with the levels of hippocampal p-CREB (r = 0.6189; p<0.05). The distance travelled in open field is positively correlated with the pCREB protein levels in frontal cortex (r = 0.6908; p<0.001)
Figure 6. Association of plasticity markers with anxiety-like behavior.
(A–B) The distance travelled in open field was found to be positively associated with the levels of BDNF in hippocampus (r = 0.7787; p<0.001) and frontal cortex (r = 0.6089; p<0.05). (C–D) The number of open arm entries made in elevated plus maze was positively correlated with the levels of hippocampal p-CREB (r = 0.6189; p<0.05). The distance travelled in open field was positively correlated with the levels of p-CREB in frontal cortex (r = 0.6908); p<0.001). Values are expressed in mean ±SEM. *p<0.05, ***p<0.001 Vs DHA diet.
Discussion
The purpose of the present study is to understand how changes in dietary habits e.g. transition from a healthy diet rich in omega-3 fatty acids to a HFD food diet deficient in omega-3 but rich in saturated fatty acids, leads to vulnerability for psychiatric disorders. Here we show that consumption of a HFD for 3 weeks is enough to induce maladaptation in anxiety-like behavior, and molecular systems associated with these behaviors. We found that the HFD reduced brain DHA contents, and that reduced levels of DHA were associated with increase in anxiety-like behaviors in these rats. These data emphasize the detrimental effects of the HFD on brain function and behavior that are particularly manifested from switching from a healthy diet. The results of this study have important implications for public health, in terms of the risk imposed by poor dietary practices on mood disorders.
The rationale for the present study stemmed from our recent findings that animals fed on a diet deficient in omega-3-fatty acids during gestation, prenatal and postnatal growth periods were more prone to anxiety-like behavior as compared to animals fed on DHA supplemented diet35. Given the positive effects of the DHA diet, it was reasonable to assume that switching to an unhealthy diet could have detrimental results for brain function. We found that 3 weeks of HFD increased the vulnerability for anxiety-like behaviors. A recent study reported the ability of high-fat diet to exacerbate the depressive-like behavior in a rat model of genetic depression36. The anxiogenic effects of high-fat diet in the current study may be mediated by the pro-inflammatory signalling induced by the high-fat diet consumption. There is substantial evidence that rodent diet-induced obesity model involves an inflammatory reaction in key hypothalamic areas critical for regulating food intake37. A recent study has reported that hypothalamic inflammation was evident just after 1 to 3 days after onset of high-fat diet consumption prior to any substantial weight gain14. In order to evaluate the effects of the HFD on the body, we measured several metabolic markers in blood and found elevations in glucose, cholesterol, triglycerides, and uric acid. This implies that the HFD influences several parameters associated with obesity, in conjunction with its effects on the brain. The effects of the HFD highly contrast with the known roles of essential omega-3 polyunsaturated fatty acids on body and brain. Omega-3 fatty acids are crucial for brain function during development and adulthood, and their deficiency is considered risk factors for anxiety-like behavior in various animal models38,39.
To further elucidate possible differential effects of the diet across brain regions associated with anxiety-like behavior, we centred our studies in the frontal cortex and hippocampus. The human orbitofrontal cortex receives reciprocal connections from the hippocampus, nucleus accumbens, and hypothalamus40 and is thought to play a significant role in hedonic and emotional processes implicated in the psychiatric disorders. The frontal cortex, together with hippocampus, amygdala and hypothalamus, are limbic regions forming part of well-defined anxiety and fear-related circuits in the forebrain owing to the fact that all these limbic regions play an important role in mood disorders, it is significant that dietary fatty acids manipulation showed to affect the hippocampus and frontal cortex. Accordingly, we assessed neuropeptide Y (NPY) based on its role both anxiety and depression like behavior, particularly in the frontal cortex and limbic regions41. It has been suggested that NPY produces an anxiolytic effects via NPY 1-type receptors (NPY-1R)42. The anxiety-reducing effects of NPY and the anxiety-enhancing effects of antagonists of NPY receptors are fairly well-documented, providing strong evidence for NPY's role in modulating anxiety responses. Our results showed that n-3 deficiency decreased the levels of NPY-1R in the frontal cortex, hypothalamus and hippocampus, in agreement with the anxiolytic involvement of NPY-1R. In addition, these findings suggest that a radical shift in dietary omega-3 fatty acids intake to HFD can hinder the animal's natural ability to face challenges further in their life and leads to more anxiety-like behavior.
Our present study shows that HFD significantly reduced the levels of BDNF in frontal cortex and hippocampus. BDNF has been associated with the action of treatments for anxiety43. We have also observed that levels of BDNF in frontal cortex and hippocampus showed positive significant association with the distance travelled in open field task indicating reductions in BDNF may be responsible for the observed anxiety-like behaviour after diet transition to HFD. Previously it has been reported that changes in BDNF signalling in different areas of the adult brain may be implicated in the pathophysiology of psychiatric disorders, such as depression44,45,46. Not only this, manipulations of the early environment can affect the expression of neurotrophins both during development and adulthood47,48,49. BDNF binds with high affinity to the tropomyosin-related kinase B transmembrane receptor, (TrkB) resulting in BDNF signalling. Deficiency in TrkB activation has been linked to psychiatric illness in humans45,50. Furthermore a very recent report showed that an 11 base pair deletion in the TrkB promoter could have effects on the anxiety related traits in human51.
Our current results show a reduction in the activation of BDNF receptor TrkB in the hippocampus in rats fed on HFD diet. These results hold well with previous findings that mice lacking functional full-length TrkB signalling, specifically in the newborn neuron population, exhibit a markedly enhanced anxiety-like behavior52. The fact that DHA is a structural component of the plasma membrane important for membrane fluidity and function of transmembrane receptors, suggests that DHA regulates the function of TrkB receptors.
The transcription factor, cyclic AMP-dependent response element binding protein (CREB), regulates the expression of many genes, including BDNF53,54 and NPY-155. It has been shown that decreases in CREB phosphorylation and NPY expression in the central amygdala might be associated with anxiety-like behaviors in models of ethanol withdrawal in rats56. In our studies, we showed reduction in the activation of CREB with HFD in hippocampus and frontal cortex and the levels of p-CREB showed significant positive association with measures of anxiety-like behaviour. CREB has been implicated in the pathophysiology of depression as well as of bipolar disorder. Further a marked reduction in the levels of phospho-CaMKII was also observed in hippocampus and frontal cortex after diet transition to HFD. Accordingly, the ability of the HFD to reduce CREB phosphorylation, in conjunction with BDNF receptor activation, could be related to elevated risk for anxiety-like behavior.
Adoption of a HFD is an increasingly common event observed in the modern society which is tightly related to today's obesogenic environment where high calorie food is readily available. According to our results, the switch from a healthy n-3 PUFA diet to the HFD may be responsible for increased vulnerability to mood disorders, in addition to metabolic dysfunction. The transition to HFD reduced markers of synaptic plasticity such as GAP43 and phopsho-synapsin in the frontal cortex and hippocampus. There is moderate amount of information available on the beneficial metabolic effects of DHA supplementation but there is lack of knowledge for the effects of opposite diet switch from HFD to DHA enriched diet. Our data emphasize the importance of maintaining a healthy diet in order to support substrates that determine the balance between brain health and disease. Although our behavioral assessment was focused on anxiety-like behaviors, it is important to consider that there is strong association and comorbidity between anxiety and depressive disorders57. Further studies are necessary to assess the association between diet and depression in mechanistic studies in animals.
Methods
Experimental design
Female Sprague–Dawley rats were obtained on the 2nd day of pregnancy from Charles River (Portage, MI) weighing between 280 and 300 g were housed in cages and maintained in environmentally controlled rooms (22–24°C) with a 12-h light/dark cycle. Pregnant females were fed an n-3 enriched fatty acid diet (DHA diet). Rats were maintained on this diet through gestation and lactation, and their pups were weaned to the same diet as their dams. Male pups were subjected to same diet as their dams for 12 weeks (Fig 1). The custom diet used was based on the composition of the American Institute of Nutrition diet and prepared commercially (Dyets, Bethlehem, PA) as previously described58. However, several substitutions were made to produce an n-3 fatty acid enriched diet and this was achieved by adding a small amount of flaxseed oil and docosahexaenoic acid (Nordic Naturals, Inc. Watsonville, CA, USA) to the n-3 diet. These fats supply LNA and DHA, respectively, as their principal component. The total fat content in diet was 10 g/100 g of diet, and the amount of n-3 fatty acids in the n-3 diet was 3.8% of total fatty acids.
Diet transition
A total of 15 male rats were randomly selected for this study with a constraint that at least 2 rats from each litter were selected (Fig 1). On postnatal day (PND) 90 the male rats were randomly divided into two subgroups i.e. DHA (n = 6) continued on same n-3 enriched diet and high-fat diet (HFD; n = 9) provided with a custom diet high in saturated fatty acids that closely resembles to western diet (D12079B, Research Diets, NJ USA). This HFD has 21% total fat but saturated fatty acid make 62.4% of this total fat. After 3 weeks of diet transition, the rats were subjected to a series of behavioral tests. A day after the last behavioral test the animals were killed by decapitation and the blood sample and fresh tissues including frontal cortex and hippocampus were dissected, frozen in dry ice and stored at −70°C until use for biochemical analyses for both groups which are abbreviated throughout in this study as: DHA enriched diet (DHA) and high-fat diet (HFD). Experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the University of California at Los Angeles Chancellor's Animal Research Committee. The suffering and number of animals used were minimized.
Open Field
After 3 weeks of diet transition, rats were tested in an open field. The open field consisted of 1.2-m-diameter circular tank with 60 cm walls. An inner circle, 80 cm in diameter was marked on the tank floor to serve as a central arena. Test began when each rat was placed in the middle of the central arena and allowed to explore the field for 10 min. The rat behavior was recorded by an overhead camera. Measurement included time spent in central arena, number of entries into central arena and the distance the rat travel using AnyMazeTM video tracking software (San Diego instruments, San Diego, CA).
Elevated plus maze
The day after OF, rats were subjected to elevated plus maze (EPM) test. Briefly, the EPM apparatus made of laminated wood consisted of 2 opposing open arms (10 × 50 cm) and 2 opposing closed arms (10 × 50 cm with 30 cm high walls). The maze was placed 60 cm above the floor. White curtains surrounded the maze and behavior was recorded by an overhead video camera. Each rat was placed in the middle of the maze facing the open arm that faced away from the experimenter, and a video camera recorded over a period of 5 min the time spend in each of the arms and the number of entries to each arm. A closed arm entry was counted when the rat placed all four paws in a closed arm. An open arm entry was recorded when the rat placed all four paws in an open arm and/or when the rat's hind-limbs were placed in the central area of the maze and both fore-limbs in an open arm while the head is protruding into the open arm. The ratio of open and closed entries to total arm entries was calculate to account for differences in general motor activity in the maze.
Fatty acid analysis
Fatty acid profiles were determined by using gas chromatography. The system consisted of model 5890A gas chromatograph (Hewlett Packard) and a model 7673A automatic, sampler and controller (Hewlett Packard). An Omegawax 250 column (30 m, 0.25-mm internal diameter, 0.25-μm film thickness; Sigma-aldrich) was used, with helium as the carrier gas. GC oven temperature was initially held at 50°C for 2 min and raised with a gradient of 2°Cmin−1 until 220°C and held for 30 min. The injector and detector were maintained at 250°C and 260°C, respectively. Tissues from middle cortex were grounded to powder under liquid nitrogen and subjected to extraction of total lipids. Fatty acid methylation was done by heating at 100°C for 1 hr with 14% boron tri-fluoride–methanol reagent. A 1 μl sample of Fatty acid methyl esters (FAME) was injected in split injection mode with a 100:1 split ratio. Peaks of resolved fatty acid methyl esters were identified and quantified by comparison with standards (Supelco 37-component FAME Mix).
Western blot
Frontal cortex and hippocampal tissues were homogenized in a lysis buffer using published protocol59. Levels of brain-derived neurotrophic factor (BDNF), Neuropeptide Y (NPY) 1, Phospho tyrosine kinase B (pTrkB), phospho cyclic AMP-response element binding protein (pCREB), p-synapsin, GAP-43 were analyzed by Western blot. Briefly, protein samples were separated by electrophoresis on a 10% (12.5 % for BDNF) polyacrylamide gel and electrotransferred to a PVDF or nitrocellulose membrane (Millipore, Bedford, MA). Non-specific binding sites were blocked in TBS 5% low-fat milk and 0.1% Tween-20 or 2% BSA. Membranes were rinsed in buffer (0.1% Tween-20 in TBS) and then incubated with anti-actin or anti-BDNF, pTrkB, (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-pCREB (Ser133), anti-CREB, anti p-synapsin and anti-GAP-43 (1:1000; Millipore, Bedford, MA), NPY-1R (1:500; Alpha Diagnostics Intl.Inc. San Antonio, Texas) followed by anti-rabbit or anti goat or anti-mouse IgG horseradish peroxidase-conjugate (1:200,000; Santa Cruz Biotechnology). After rinsing with buffer, the immunocomplexes were visualized by chemiluminescence using the ECL plus kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) for NPY1R, pTrkB, pCREB SuperSignal West femto kit (Thermo Scientific , Rockford, IL) for BDNF. Respective protein size was compared by using Bench mark pre-stained protein ladder (Invitogen Technology, Carlsbad, CA). The film signals were digitally scanned and then quantified using ImajeJ software. Specific Protein sizes were chosen and quantified as β-actin (42 kDa), NPY-1 (39-42 kDa), BDNF (14 kDa), pTrkB (145 kDa), pCREB (43 kDa). Actin was used as an internal control for Western blot such that data were standardized according to actin values. All samples of DHA and HFD groups for a particular brain area were run in the same gel and quantified accordingly but a representative band from each group was shown in the figures.
Statistical analysis
Data are presented as means and their standard errors. Data were analyzed using statistics software Graph pad 5 and unpaired two-tailed t test was applied for the comparison between two groups. Criterion for significance was set to p≤ 0.05 in all comparisons.
Author Contributions
Conceived and designed the experiments: SS and FGP. Performed the experiments: SS and ZY. Analyzed the data: SS. Contributed reagents/materials/analysis tools: SS and FGP. Wrote the manuscript: SS and FGP. All authors reviewed the manuscript.
Acknowledgments
This work was supported by National Institutes of Health Grants NS50465 and NS56413.
References
- Bromet E. et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med 9, 90 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier D. A., Rae D. S., Narrow W. E., Kaelber C. T. & Schatzberg A. F. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry Suppl 24–28 (1998). [PubMed] [Google Scholar]
- Dong C., Sanchez L. E. & Price R. A. Relationship of obesity to depression: a family-based study. Int J Obes 28, 790–795 (2004). [DOI] [PubMed] [Google Scholar]
- Gariepy G., Nitka D. & Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes 34, 407–419 (2010). [DOI] [PubMed] [Google Scholar]
- Roberts R. E., Deleger S., Strawbridge W. J. & Kaplan G. A. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes 27, 514–521 (2003). [DOI] [PubMed] [Google Scholar]
- Simon G. E. et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen psy 63, 824–830 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and cognitive impairment: a view through the prism of epidemiology. Arch Neurol 62, 1043–1044 (2005). [DOI] [PubMed] [Google Scholar]
- Kanoski S. E. & Davidson T. L. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol & Behav 103, 59–68 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. et al. Diet-induced metabolic disturbances as modulators of brain homeostasis. (BBA) - Mol Basis Dis 1792, 417–422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan A. M., Cutler R. G., Button C., Telljohann R. & Mattson M. P. Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. J Neurochem 118, 611–615 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Ying Z. & Gomez-Pinilla F. Oxidative stress modulates Sir2α in rat hippocampus and cerebral cortex. Eur J Neurosci 23, 2573–2580 (2006). [DOI] [PubMed] [Google Scholar]
- Lindqvist A. et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 13, 1385–1388 (2006). [DOI] [PubMed] [Google Scholar]
- Park H. R. et al. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett 482, 235–239 (2010). [DOI] [PubMed] [Google Scholar]
- Thaler J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122, 153–162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza C. G. et al. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci 81, 198–203 (2007). [DOI] [PubMed] [Google Scholar]
- Chang G.-Q., Gaysinskaya V., Karatayev O. & Leibowitz S. F. Maternal High-Fat Diet and Fetal Programming: Increased Proliferation of Hypothalamic Peptide-Producing Neurons That Increase Risk for Overeating and Obesity. J Neurosci 28, 12107–12119 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloso L. A. Maternal Consumption of High-Fat Diet Disturbs Hypothalamic Neuronal Function in the Offspring: Implications for the Genesis of Obesity. Endocrinology 153, 543–545 (2012). [DOI] [PubMed] [Google Scholar]
- Ross B. M. Omega-3 polyunsaturated fatty acids and anxiety disorders. Prosta Leuko Ess Fat Acid 81, 309–312. [DOI] [PubMed] [Google Scholar]
- Freeman M. P. et al. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand 113, 31–35 (2006). [DOI] [PubMed] [Google Scholar]
- Ross B. M. Omega-3 fatty acid deficiency in major depressive disorder is caused by the interaction between diet and a genetically determined abnormality in phospholipid metabolism. Med Hypoth 68, 515–524 (2007). [DOI] [PubMed] [Google Scholar]
- Suzuki S. et al. Daily omega-3 fatty acid intake and depression in Japanese patients with newly diagnosed lung cancer. Br J Cancer 90, 787–793 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen A. et al. Fish Consumption and Depressive Symptoms in the General Population in Finland. Psychiatr Serv 52, 529–531 (2001). [DOI] [PubMed] [Google Scholar]
- McCann J. C. & Ames B. N. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 82, 281–295 (2005). [DOI] [PubMed] [Google Scholar]
- Wu A., Ying Z. & Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 155, 751–759 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll S. D., Ip N. Y., Lindsay R. M. & Wiegand S. J. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res 812, 200–208 (1998). [DOI] [PubMed] [Google Scholar]
- Hall J., Thomas K. L. & Everitt B. J. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci 3, 533–535 (2000). [DOI] [PubMed] [Google Scholar]
- Linnarsson S., Bjorklund A. & Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci 9, 2581–2587 (1997). [DOI] [PubMed] [Google Scholar]
- Ying S. W. et al. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 22, 1532–1540 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. et al. Diet-induced metabolic disturbances as modulators of brain homeostasis. Biochim Biophys Acta 1792, 417–422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic J. N. et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci U S A 93, 3679–3683 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa D., Yasui Y., Yamada K., Ohara N. & Okuyama H. Regional differences of the mouse brain in response to an [alpha]-linolenic acid-restricted diet: Neurotrophin content and protein kinase activity. Life Sciences 87, 490–494 (2010). [DOI] [PubMed] [Google Scholar]
- Rao J. S. et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry 12, 36–46 (2007). [DOI] [PubMed] [Google Scholar]
- Bergami M., Berninger B. & Canossa M. Conditional deletion of TrkB alters adult hippocampal neurogenesis and anxiety-related behavior. Commun Integr Biol 2, 14–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A. & Reichardt L. F. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11, 272–280 (2001). [DOI] [PubMed] [Google Scholar]
- Bhatia H. S. et al. Omega-3 Fatty Acid Deficiency during Brain Maturation Reduces Neuronal and Behavioral Plasticity in Adulthood. PLoS One 6, e28451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abildgaard A. et al. A high-fat diet exacerbates depressive-like behavior in the Flinders Sensitive Line (FSL) rat, a genetic model of depression. Psychoneuroendocrinology 36, 623–633 (2011). [DOI] [PubMed] [Google Scholar]
- Lumeng C. N. & Saltiel A. R. Inflammatory links between obesity and metabolic disease. J Clin Invest 121, 2111–2117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances H. et al. Effect of dietary alpha-linolenic acid deficiency on habituation. Life Sci 58, 1805–1816 (1996). [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Greiner R. S. & Salem N. Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem 75, 2563–2573 (2000). [DOI] [PubMed] [Google Scholar]
- Kringelbach M. L. & Rolls E. T. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 72, 341–372 (2004). [DOI] [PubMed] [Google Scholar]
- Redrobe J. P., Dumont Y. & Quirion R. Neuropeptide Y (NPY) and depression: from animal studies to the human condition. Life Sci 71, 2921–2937 (2002). [DOI] [PubMed] [Google Scholar]
- Nakajima M. et al. Neuropeptide Y produces anxiety via Y2-type receptors. Peptides 19, 359–363 (1998). [DOI] [PubMed] [Google Scholar]
- Duman R. S., Heninger G. R. & Nestler E. J. A molecular and cellular theory of depression. Arch Gen psy 54, 597–606 (1997). [DOI] [PubMed] [Google Scholar]
- Berton O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006). [DOI] [PubMed] [Google Scholar]
- Duman R. S. & Monteggia L. M. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59, 1116–1127 (2006). [DOI] [PubMed] [Google Scholar]
- Sen S., Duman R. & Sanacora G. in. Biol Psychiatry. 64, 527–532 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F., Berry A. & Alleva E. Early disruption of the mother-infant relationship: effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev 27, 73–82 (2003). [DOI] [PubMed] [Google Scholar]
- Roceri M. et al. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psych 55, 708–714 (2004). [DOI] [PubMed] [Google Scholar]
- Branchi I. & Alleva E. Communal nesting, an early social enrichment, increases the adult anxiety-like response and shapes the role of social context in modulating the emotional behavior. Behav Brain Res 172, 299–306 (2006). [DOI] [PubMed] [Google Scholar]
- Duman R. S., Heninger G. R. & Nestler E. J. A molecular and cellular theory of depression. Arch Gen Psych 54, 597–606 (1997). [DOI] [PubMed] [Google Scholar]
- Ernst C. et al. in Biol Psychiatry 69, 604–607 (2011 Society of Biological Psychiatry. Published by Elsevier Inc., 2011).
- Bergami M. et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A 105, 15570–15575 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M., Nestler E. J. & Duman R. S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16, 2365–2372 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S. et al. CREB: a major mediator of neuronal neurotrophin responses. Neuron 19, 1031–1047 (1997). [DOI] [PubMed] [Google Scholar]
- Pandey S. C. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci 24, 456–460 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang H. & Pandey S. C. Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides 24, 1397–1402 (2003). [DOI] [PubMed] [Google Scholar]
- Devane C. L., Chiao E., Franklin M. & Kruep E. J. Anxiety disorders in the 21st century: status, challenges, opportunities, and comorbidity with depression. Am J Manag Care 11, S344–353 (2005). [PubMed] [Google Scholar]
- Greiner R. S., Catalan J. N., Moriguchi T. & Salem N. Jr Docosapentaenoic acid does not completely replace DHA in n-3 FA-deficient rats during early development. Lipids 38, 431–435 (2003). [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. & Ying Z. Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience 168, 130–137 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]