Abstract
β-Lactam antibiotics provide the cornerstone of treatment and reduce the rate of decline in lung function in patients with cystic fibrosis, but their use is limited by a high frequency of delayed-type allergic reactions. The objective of this study was to use cloned T-cells expressing a single T-cell receptor from five piperacillin-hypersensitive patients to characterize both the cellular pathophysiology of the reaction and antigen specificity to define the mechanism of activation of T-cells by piperacillin. More than 400 piperacillin-responsive CD4+, CD4+CD8+, or CD8+ T-cell clones were generated from lymphocyte transformation test and ELIspot-positive patients. The T-cell response (proliferation, T helper 2 cytokine secretion, and cytotoxicity) to piperacillin was concentration-dependent and highly specific. Enzyme-linked immunosorbent assay, gel electrophoresis, and mass spectrometry revealed that piperacillin bound exclusively to albumin in T-cell culture. Irreversible piperacillin binding at Lys 190, 195, 199, 432, and 541 on albumin and the stimulation of T-cells depended on incubation time. A synthetic piperacillin albumin conjugate stimulated T-cell receptors via a major histocompatibility complex- and processing-dependent pathway. Flucloxacillin competes for the same Lys residues on albumin as piperacillin, but the resulting conjugate does not stimulate T-cells, indicating that binding of the β-lactam hapten in peptide conjugates confers structural specificity on the activation of the T-cell receptors expressed on drug-specific clones. Collectively, these data describe the cellular processes that underlie the structural specificity of piperacillin antigen binding in hypersensitive patients with cystic fibrosis.
Introduction
β-Lactam hypersensitivity was first reported in patients with cystic fibrosis (CF) in 1970. Several studies have since demonstrated a high prevalence of β-lactam reactions: 26 to 50% compared with 1 to 10% in the general population (Koch et al., 1991; Pleasants et al., 1994; Parmar and Nasser, 2005; Whitaker et al., 2011b). The prevalence of reactions makes it increasingly difficult to treat infective exacerbations effectively because of the restriction in available antibiotics. Reactions are nonimmediate; symptoms include maculopapular rashes, fever, and/or flu-like symptoms (Pleasants et al., 1994; Burrows et al., 2007; Whitaker et al., 2011b). It is noteworthy that we have recently demonstrated that piperacillin selectively stimulates peripheral blood mononuclear cells (PBMC) from hypersensitive patients ex vivo (Whitaker et al., 2011a).
It is well established that β-lactam antibiotics form covalent bonds with lysine residues on protein (Levine and Ovary, 1961; Batchelor et al., 1965; Jenkins et al., 2009; Meng et al., 2011), and drug-protein binding is thought to be an obligatory step in the instigation of an immune response (Brander et al., 1995; Padovan et al., 1997). Consequently, we have recently developed and used mass spectrometry methods to characterize the amino acid residues on albumin modified with piperacillin in patients (Whitaker et al., 2011a). Lysine residues at positions 190, 195, 432, and 541 were preferentially modified with piperacillin, and the albumin conjugate stimulated PBMC isolated from hypersensitive patients. These data are consistent with previous studies using uncharacterized synthetic penicillin-albumin constructs generated under forced chemical conditions and indicate that the formation of drug-protein adducts is an important step in β-lactam antigenicity and ultimately the disease pathogenesis (Brander et al., 1995).
Despite this, PBMC studies provide limited information on mechanisms of antigen presentation and antigen specificity at individual T-cell receptors. Thus, the objectives of the current study were to use T-cells cloned from piperacillin-hypersensitive patients to characterize the cellular pathophysiology of the reaction, investigate the structural specificity of T-cells, and define unequivocally the mechanism of β-lactam presentation to T-cells. Stimulation of CD4+ and CD8+ clones with piperacillin was found to be highly specific in terms of drug structure and dependent on the formation of protein adducts. Piperacillin-modified albumin was the only conjugate detected in cell culture systems; adduct formation was concentration- and time-dependent, and protein processing was a prerequisite for the activation of T-cells. Pretreatment of albumin with flucloxacillin blocked piperacillin albumin binding and the piperacillin-specific T-cell response.
Materials and Methods
Donor Characteristics.
PBMC were isolated from eight piperacillin-hypersensitive patients and five drug-exposed tolerant controls. Table 1 summarizes the patients' demographics and the clinical features of the hypersensitivity reactions. Approval for the study was acquired from the Liverpool and Leeds local research ethics committees; informed written consent was obtained from each donor.
TABLE 1.
Clinical details of patients
| ID | Details of the Reaction | Age/ Sex | Sputum Classification | Time to Reaction | Time Since Reaction | Courses Before Reaction | Other Reactions |
|---|---|---|---|---|---|---|---|
| days | months | ||||||
| Piperacillin-hypersensitive patients | |||||||
| 1 | Flu-like illness | 18/male | Chronic Pseudomonas | 2 | 6 | 3 | Ceftazidime; meropenem; aztreonam |
| 2 | Maculopapular exanthema | 24/male | Chronic Pseudomonas | 11 | 5 | 9 | Sulfamethoxazole |
| 3 | Maculopapular exanthema, fever | 20/male | Chronic Pseudomonas | 9 | 8 | 3 | Ceftazidime; meropenem; aztreonam sulfamethoxazole |
| 4 | Fever, arthralgia | 29/female | Chronic Pseudomonas | 9 | 7 | 7 | Ceftazidime; meropenem |
| 5 | Maculopapular exanthema, fever | 20/male | Burkholderia cepacia | 2 | 2 | 4 | Ceftazidime |
| 6 | Maculopapular exanthema | 27/male | Chronic Pseudomonas | 5 | 3 | 3 | Ceftazidime |
| 7 | Fever, eosinophilia | 30/female | Intermittent Pseudomonas | 7 | 2 | 14 | |
| 8 | Arthralgia | 22/female | Non-Pseudomonas | 5 | 5 | 4 | Ceftazidime; Sulfamethoxazole |
| Piperacillin-tolerant patients | |||||||
| 1 | 25/male | Chronic Pseudomonas | |||||
| 2 | 22/female | Chronic Pseudomonas | |||||
| 3 | 18/female | Non-Pseudomonas | |||||
| 4 | 27/female | Chronic Pseudomonas | Sulfamethoxazole | ||||
| 5 | 26/female | Intermittent Pseudomonas | |||||
Medium for PBMC Culture and T-Cell Cloning.
Culture medium consisted of RPMI 1640 supplemented with pooled heat-inactivated human AB serum (10%, v/v), HEPES (25 mM), l-glutamine (2 mM), transferrin (25 μg/ml), streptomycin (100 μg/ml), and penicillin (100 U/ml).
Generation of Autologous Antigen-Presenting Cells.
Epstein–Barr virus- transformed B-cell lines, used as antigen-presenting cells, were generated with previously described methods by incubating PBMC with supernatant from the Epstein–Barr virus-producing cell line B9-58 (Wu et al., 2007).
Detection of Piperacillin-Specific PBMC Responses.
Proliferation of patients' PBMC (0.15 × 106 per well) against piperacillin (62.5–4000 μM) and tetanus toxoid (5 μg/ml) was measured by using the lymphocyte transformation test (Whitaker et al., 2011a). IFN-γ- and IL-13-secreting PBMC were visualized by using ELIspot by culturing PBMC (0.5 × 106 per well; total volume 200 μl) with piperacillin or phytohemagglutinin (5 μg/ml) for 48 h.
Generation of T-Cell Clones.
Antigen-specific T-cells were enriched by culturing PBMC from five patients with piperacillin (500–2000 μM) for 14 days. IL-2 (60 U/ml) was added to maintain antigen-specific proliferation. T-cells were cloned by serial dilution using established methodology (Wu et al., 2007; Castrejon et al., 2010). To test the specificity of the clones, T-cells (0.5 × 105) were incubated with irradiated antigen-presenting cells (0.1 × 105) and piperacillin. After 48 h, [3H]thymidine (0.5μCi) was added, and 16 h later proliferation was measured by scintillation counting.
Proliferative Response of T-Cell Clones and Cross-Reactivity.
T-cell clones were tested for reactivity against piperacillin (100–4000 μM) and eight related compounds with structural modifications to either the side chain or the β-lactam nucleus, namely, penicillin-G, ampicillin, amoxicillin, carbenicillin, flucloxacillin, cefoperazone, cefalexin, and 7-amino-desacetoxycephalosporanic acid (all 100-4000 μM; see Fig. 1 for drug structures). Proliferation was measured by [3H]thymidine incorporation.
Fig. 1.
Structures of the drugs used in T-cell assays.
The Phenotype and Functionality of T-Cell Clones.
Antigen-specific T-cell clones were characterized in terms of CD phenotype by flow cytometry. To measure immune-mediated killing, [51Cr]-loaded antigen-presenting cells (2.5 × 103) were incubated for 4 h with T-cells at effector/target ratios of 10:1 to 50:1 in the presence or absence of piperacillin. In separate experiments, antigen-presenting cells were pulsed with piperacillin for 16 h before [51Cr] loading. Specific lysis was calculated as 100 × (experimental release − spontaneous release)/(maximal release-spontaneous release). Increased membrane expression of the degranulation marker CD107a after piperacillin exposure was measured by flow cytometry (El-Ghaiesh et al., 2011). Levels of secreted cytokines (IL-4, IL-5, IL-13, IFN-γ, TNF-α, IL-1β, MIP-1β, and IL-10) were measured in the supernatant from piperacillin-stimulated T-cell clones by using a Bio-Plex Pro human cytokine assay kit (Bio-Rad Laboratories, Hercules, CA) on a Bio-Plex Suspension Array System (model Luminex 100).
Characterization of Piperacillin Protein Binding in Culture.
To study piperacillin binding, piperacillin was incubated with antigen-presenting cells and T-cell clones in serum-supplemented culture medium for 1 to 16 h. At each time point, cellular protein was isolated from serum protein by centrifugation. Cells were washed repeatedly to remove unbound piperacillin. Serum was precipitated from culture supernatant by the addition of nine volumes of ice-cold methanol followed by centrifugation at 14,000g and 4°C for 15 min. Aliquots of protein (5 μg) were then separated by electrophoresis on a 10% SDS-polyacrylamide gel and electroblotted onto nitrocellulose membrane. Nonspecific binding was blocked by using Tris/saline/Tween buffer (TST; 150 mM NaCl, 10 mM Tris-HCl, and 0.05% Tween 20, pH 8.0) containing 10% nonfat dry milk for 16 h at 4°C. The blot was incubated with primary antipenicillin antibody (mouse antipenicillin monoclonal antibody; Serotec, Oxford, UK) diluted 1:20,000 in 5% milk/TST for 1 h, followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG antibody (Abcam plc, Cambridge, UK) diluted 1:10,000 in 5% milk/TST for another 1 h. Signal was detected by enhanced chemiluminescence (Western Lightning; PerkinElmer Life and Analytical Sciences, Waltham, MA) using autoradiography film and a GS800 calibrated scanning densitometer (Bio-Rad Laboratories, Hemel Hempstead, UK).
Irreversibly bound piperacillin protein conjugates were also quantified by ELISA with the antipenicillin antibody described above. Results are expressed as Δoptical density (OD) (sample OD − vehicle OD).
To identify the key piperacillin-modified lysine residues in albumin involved in the stimulation of T-cell clones, we used our recently described mass spectrometry methods (Meng et al., 2011; Whitaker et al., 2011b). Before mass spectrometry, all samples were incubated with dithiothreitol (10 mM) at room temperature for 15 min and iodoacetamide (166 mM) for another 15 min at room temperature before again being subjected to methanol precipitation. They were reconstituted in ammonium bicarbonate buffer (50 mM), digested with trypsin overnight at 37°C, and then desalted by using C18 Zip-Tips (Millipore Corporation, Billerica, MA).
Samples were reconstituted in 2% acetonitrile/0.1% formic acid (v/v), and aliquots of 2.4 to 5 pmol were delivered into a QTRAP 5500 hybrid quadrupole-linear ion trap mass spectrometer (ABSciex, Framingham, MA) by automated in-line liquid chromatography (U3000 HPLC System, 5-mm C18 nano-precolumn and 75 μm × 15 cm C18 PepMap column; Dionex, Sunnyvale, CA) via a 10-μm inner diameter PicoTip (New Objective, Woburn, MA). A gradient from 2% acetonitrile/0.1% formic acid (v/v) to 50% acetonitrile/0.1% formic acid (v/v) in 70 min was applied at a flow rate of 280 nl/min. The ionspray potential was set to 2200 to 3500 V, the nebulizer gas was set to 18, and the interface heater was set to 150°C. Multiple reaction monitoring (MRM) transitions specific for drug-modified peptides were selected as follows: the m/z values were calculated for all possible peptides with a missed cleavage at a lysine residue; to these were added the mass of the appropriate hapten (cyclized 517amu, hydrolyzed 535 amu); the parent ion masses were then paired with a fragment mass of 160 ([M+H]+ of cleaved thiazolidine ring present in all of the haptens) and/or a fragment mass of 106 ([M+H]+ of cleaved benzylamine group of hydrolyzed haptens). MRM transitions were acquired at unit resolution in both the Q1 and Q3 quadrupoles to maximize specificity, they were optimized for collision energy and collision cell exit potential, and dwell time was 20 ms. MRM survey scans were used to trigger enhanced product ion tandem mass spectrometry scans of drug-modified peptides, with Q1 set to unit resolution, dynamic fill selected, and dynamic exclusion for 20 s. Total ion counts were determined from a second aliquot of each sample analyzed by conventional liquid chromatography/tandem mass spectrometry and used to normalize sample loading on column. MRM peak areas were determined by MultiQuant 1.2 software (ABSciex). Epitope profiles were constructed by comparing the relative intensity of MRM peaks for each of the modified lysine residues within a sample and normalization of those signals across samples.
Synthesis and Characterization of Piperacillin and Flucloxacillin Albumin Conjugates.
Synthetic drug albumin conjugates were generated for functional studies by incubating piperacillin or flucloxacillin with human serum albumin at a molar ratio of 50:1 for 24 h in phosphate buffer. Furthermore, the effect of preconjugation of albumin with flucloxacillin on the subsequent covalent modification with piperacillin was explored. First, solvent extracted flucloxacillin-modified albumin was incubated with piperacillin at a molar ratio of 1:50 for 24 h in phosphate buffer (referred to as piperacillin/flucloxacillin conjugate; 1). This approach was used to assess the influence of covalently bound flucloxacillin on piperacillin binding. Second, flucloxacillin-modified albumin was primed with flucloxacillin at a molar ratio of 1:50 for 30 min without solvent extraction before further incubation with piperacillin at a molar ratio of 1:50 for 24 h in phosphate buffer (referred to as piperacillin/flucloxacillin conjugate; 2). This approach was used to assess the influence of both covalently and noncovalently bound flucloxacillin on piperacillin binding. Each conjugate was subjected to mass spectrometric analysis before dilution and addition to T-cell assays.
Determination of the Pathways of Piperacillin Presentation to T-Cell Clones and the Relationship between the Kinetics of Piperacillin Albumin Binding and the Stimulation of T-Cell Clones.
To evaluate mechanisms of piperacillin-specific T-cell activation we implemented the following approach. First, antigen-presenting cells were pulsed with piperacillin in cell culture medium for 1, 4, and 16 h. After repeated washing to remove unbound drug, the pulsed antigen-presenting cells were irradiated and incubated with T-cell clones for 72 h. Second, T-cell clones were cultured with irradiated antigen-presenting cells and the synthetic piperacillin/flucloxacillin albumin conjugates described above. Third, T-cell clones were cultured with the piperacillin albumin conjugate in the absence of antigen-presenting cells in the presence of glutaraldehyde-fixed antigen-presenting cells (fixation blocks antigen processing; Zanni et al., 1998) and anti-MHC class I and/or anti-MHC class II blocking antibodies. Finally, T-cell clones were cultured with a piperacillin albumin conjugate and the kinetics of CD3 internalization (a sensitive measure of T-cell activation; Zanni et al., 1998) was measured by flow cytometry.
Statistical Analysis.
The Mann–Whitney test was used for comparison of control and test values.
Results
Stimulation of PBMC from Piperacillin-Hypersensitive Patients.
PBMC from hypersensitive patients were stimulated to proliferate with piperacillin in a concentration-dependent manner and secrete the cytokines IFN-γ/IL-13 (Table 2). Piperacillin-specific proliferative responses and cytokine secretion were not detected with PBMC from drug-exposed tolerant controls.
TABLE 2.
Lymphocyte transformation test and PBMC ELIspot
| ID | Lymphocyte Transformation Test |
Piperacillin ELIspot |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Piperacillin |
Tetanus Toxoid | IFN-γ, 2 mM | IL-13, 2 mM | |||||||
| 0.06 mM | 0.13 mM | 0.25 mM | 0.5 mM | 1 mM | 2 mM | 4 mM | |||||
| cpm | |||||||||||
| Piperacillin-hypersensitive patients | |||||||||||
| 1 | 2957 | <2a | <2 | 2.3 | 2.8 | 8.3 | 6.3 | 7.0 | 23.4 | +++b | ++ |
| 2 | 804 | 2.1 | 2.8 | 6.5 | 5.9 | 5.7 | 4.4 | 1.8 | 24.0 | ++ | ++ |
| 3 | 2138 | <2 | <2 | <2 | <2 | 4.8 | 3.1 | 2.6 | 22.1 | +++ | ++ |
| 4 | 1787 | <2 | 2.4 | 3.6 | 6.9 | 6.7 | 6.8 | 4.5 | 13 | + | + |
| 5 | 1685 | 30.0 | 39.8 | 47.2 | 62.5 | 60.0 | 55.0 | 41.1 | 35.4 | +++ | +++ |
| 6 | 1436 | 5.6 | 11.5 | 16.0 | 20.1 | 31.4 | 26.7 | 28.1 | 16.0 | ++ | + |
| 7 | 1222 | <2 | <2 | <2 | 2.8 | 3.7 | 6.3 | 6.6 | 27.3 | ++ | − |
| 8 | 1017 | <2 | <2 | <2 | 2.5 | 7.7 | 5.9 | 10.4 | 47 | ++ | − |
| Piperacillin-tolerant patients | |||||||||||
| 1 | 542 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | 21.8 | − | − |
| 2 | 301 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | 11.1 | − | − |
| 3 | 980 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | 8.9 | − | − |
| 4 | 163 | <2 | <2 | <2 | 2.2 | <2 | <2 | <2 | 3.2 | − | − |
| 5 | 233 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | 28.1 | − | − |
Data presented as stimulation index (cpm in test incubations/cpm in control incubations).
Spot-forming cells after piperacillin (2 mM) treatment, with control subtracted: −, <10; +, 10–30; ++, 30–100; +++, >100.
Specificity of Piperacillin-Responsive CD4+, CD8+, and CD4+CD8+ T-Cell Clones.
A total of 1420 T-cell clones were generated from the piperacillin-hypersensitive patients. Of these, 414 CD4+, CD8+, and CD4+CD8+ clones were identified as piperacillin-responsive (Table 3). Clones expressing high levels of CD8+ were isolated from all but one of the hypersensitive patients.
TABLE 3.
Origin, phenotype and specificity of T-cell clones from hypersensitive patients
| ID | Clones Tested | Specific Clones | Proliferation Control Drug at 2 mM) | Phenotype |
|||
|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD4 + CD8+ | |||||
| n | cpm | % | |||||
| 1 | 736 | 152 | 3954.5 ± 3721.9 | 12596.6 ± 11915.2 | 66 | 22 | 12 |
| 2 | 209 | 120 | 5712.4 ± 5514.3 | 25709 ± 22145.9 | 75 | 13 | 12 |
| 3 | 187 | 30 | 3232.7 ± 2566.2 | 10608.4 ± 10950.6 | 55 | 45 | 0 |
| 4 | 96 | 12 | 17416.8 ± 2440.7 | 47903.7 ± 28946.3 | N.A. | N.A. | N.A. |
| 5 | 192 | 100 | 1361.1 ± 995.3 | 15526.9 ± 16868 | 100 | 0 | 0 |
N.A., not analyzed.
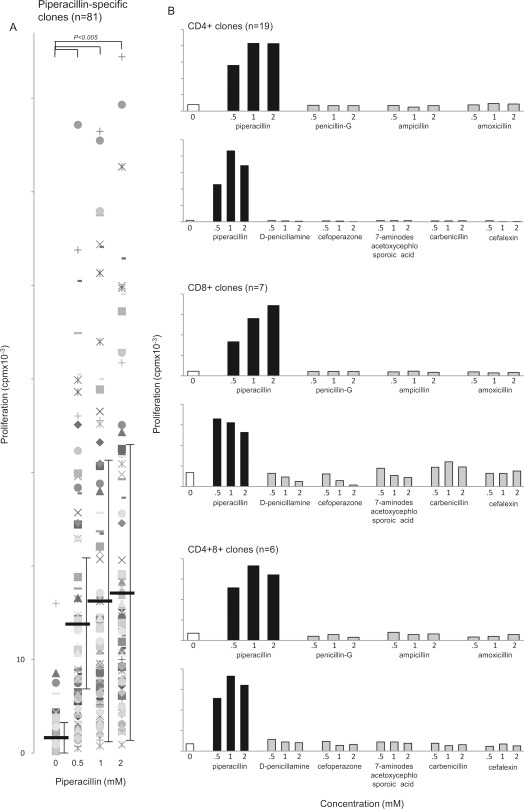
Eighty one well-growing clones expressing CD4+ and/or CD8+ receptors and displaying wide-ranging proliferative responses with piperacillin (Fig. 2A) were expanded further and used in the functional studies described below.
Fig. 2.
Stimulation of CD4+, CD8+, and CD4+CD8+ piperacillin-responsive T-cell clones from hypersensitive patients with piperacillin and related structures. A, concentration-dependent piperacillin-specific proliferation of 81 T-cell clones. Each data point represents mean [3H]thymidine incorporation of replicate cultures. Black bars show the mean cpm associated with each treatment group. B, CD4+, CD8+, and CD4+CD8+ clones were stimulated with piperacillin, but not related drug structures. Representative CD4+, CD8+, and CD4+CD8+ clones are shown. Results are presented as mean [3H]thymidine incorporation at each drug concentration. Differences in replicate cultures at each antigen concentration were less than 15%. Numbers in parentheses show the number of clones tested that displayed a similar cross-reactivity profile.
The response of clones to piperacillin was found to be highly specific in terms of drug structure. All 32 clones tested proliferated in the presence of piperacillin, but not eight structurally related compounds (Fig. 2B).
Cytokine Secretion by T-Cell Clones from Piperacillin-Hypersensitive Patients.
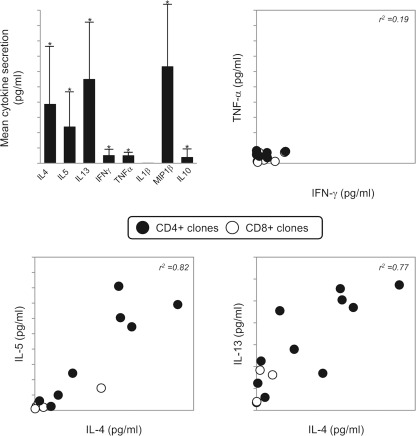
Th2 cytokines were secreted by the majority of CD4+ and CD8+ piperacillin-stimulated clones. Furthermore, a positive correlation was observed between the levels of IL-4, IL-5, and IL-13 secreted from individual clones. Antigen stimulation was also associated with the secretion of low levels of IFN-γ, TNF-α, and IL-10 from a limited number of clones (Fig. 3).
Fig. 3.
Cytokine secretion from piperacillin-stimulated CD4+ and CD8+ T-cell clones. Top left, mean ± SD levels of cytokine secretion from 14 CD4+ or CD8+ clones stimulated with piperacillin. Top right and bottom, comparison of IFN-γ, TNF-α, IL-4, IL-5, and IL-13 secretion by individual clones [values in the absence of antigen (less than 10 pg/ml, with the exception of IL-13 at 50 pg/ml) subtracted]. *, P < 0.05 when cytokine secretion with piperacillin-stimulated and unstimulated cells were compared.
The chemokine MIP-1β was detected in high levels after stimulation of clones with piperacillin (Fig. 3).
The Piperacillin-Specific T-Cell Response Depends on Incubation Time.
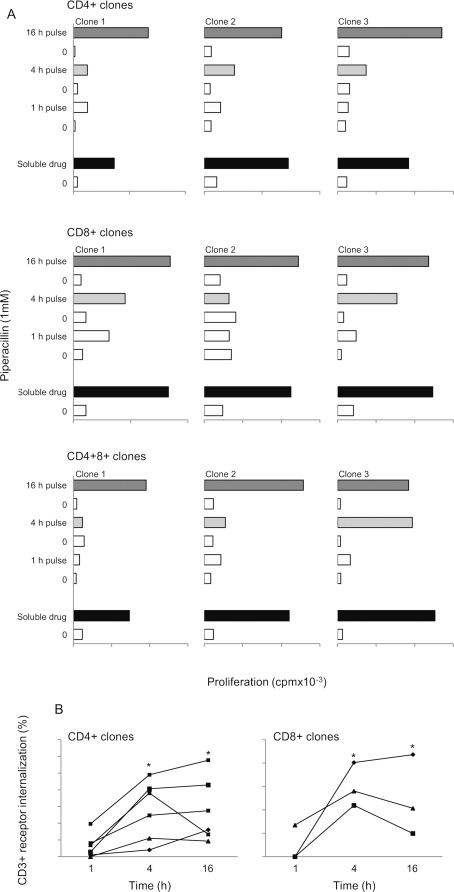
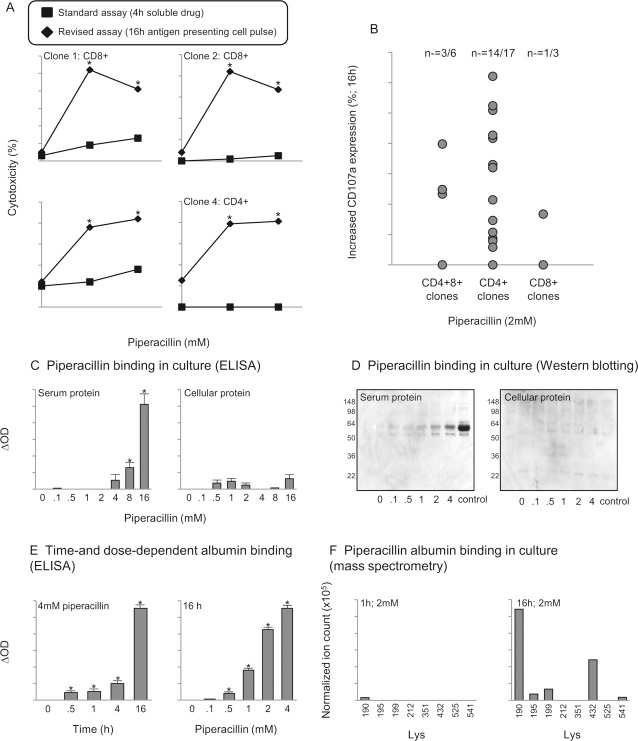
To investigate the role of adduct formation in the presentation of piperacillin to T-cells, antigen-presenting cells were pulsed with piperacillin for 1, 4, and 16 h before washing and exposure to clones. Antigen-presenting cells pulsed with piperacillin for 1 h did not stimulate a proliferative response. A 4-h pulse stimulated a proliferative response with certain clones, whereas a 16-h pulse stimulated all clones to proliferate and the strength of the response was similar to that seen with the soluble drug (Fig. 4A). Measurement of T-cell receptor internalization, a sensitive marker of T-cell activation (Zanni et al., 1998), confirmed that a 4-to 16-h culture period was required to stimulate piperacillin-responsive clones (Fig. 4B).
Fig. 4.
A, piperacillin causes a time-dependent stimulation of CD4+, CD8+, and CD4+CD8+ T-cell clones. Three representative CD4+, CD8+, and CD4+CD8+ clones are shown. Each bar shows proliferative response in the presence and absence of piperacillin-pulsed antigen-presenting cells. Antigen-presenting cells were cultured with piperacillin for the indicated times. Before coculture with clones, the antigen-presenting cells were washed and irradiated. The data show the mean of replicate wells. Coefficient of variation was consistently less than 15%. B, decreased T-cell receptor expression 4 to 16 after piperacillin stimulation of CD4+ (left; n = 6) and CD8+ (right; n = 3) clones. CD4+ and CD8+ clones were cultured with antigen-presenting cells and piperacillin for the indicated times. Cells were then washed, labeled with an anti-CD3+ antibody, and analyzed by flow cytometry. Data show the mean percentage decrease in CD3+ expression when piperacillin and unstimulated cells were compared. Coefficient of variation was consistently less than 15%. *, P < 0.05 when piperacillin-stimulated and unstimulated cells were compared. Different clones were used in A and B,.
With this basic understanding of the piperacillin-specific T-cell response the traditional 4-h [51Cr] release (cytotoxicity) assay that yielded negative results with more than 20 piperacillin-specific clones was modified. Specifically, antigen-presenting cells were pulsed with piperacillin for 16 h before [51Cr] loading. Using this revised protocol, piperacillin-specific T-cell cytolysis was observed with CD4+ and CD8+ clones (Fig. 5A). Furthermore, piperacillin treatment was associated with an increase in membrane expression of the degranulation marker CD107a on CD4+, CD8+, and CD4+CD8+ clones (Fig. 5B).
Fig. 5.
Selective, time-dependent piperacillin modification of lysine residues in albumin is involved in the cytotoxic T-cell response. A, cytolytic activity of four CD4+ or CD8+ T-cell clones exposed to soluble drug or antigen-presenting cells pulsed with piperacillin for 16 h. Cytotoxicity is measured as the percentage of 51Cr released from loaded antigen-presenting cells after the cells were incubated for 4 h. Data show the mean 51Cr released from replicate wells. *, P < 0.05 when piperacillin-stimulated and unstimulated cells were compared. B, increased CD107a expression on T-cells clones after piperacillin stimulation. Cells were labeled with anti-CD107 antibody and analyzed by flow cytometry; a minimum of 30,000 cells were acquired by using forward scatter/side scatter characteristics. Results show the increase in CD107 expression when piperacillin-stimulated and unstimulated clones were compared. C and D, ELISA (C0 and Western blots (D) of culture medium and cell lysates from T-cell cultures exposed to piperacillin. The blots were probed with an antibenzylpenicillin antibody revealing concentration-dependent covalent modification of a single protein in the medium at a molecular mass of 64 kDa. Medium from cells cultured with benzylpenicillin was used as a control on the Western blot. F, ELISA analysis of the time- and concentration-dependent binding of piperacillin to albumin. F, mass spectrometry revealed that albumin was the primary target for piperacillin modification in culture medium: the epitope profile shows the lysine residues of albumin modified with piperacillin haptens after 1 and 16 h in culture.
Characterization of Time- and Concentration-Dependent Formation of Piperacillin Adducts in Culture and Identification of the Key Piperacillin-Modified Lysine Residues on Albumin.
Piperacillin bound exclusively to serum protein in a concentration-dependent manner (Fig. 5C). Western blotting revealed albumin as the primary target for the drug (Fig. 5D); the binding interaction depended on drug concentration and incubation time (Fig. 5E). Mass spectrometric analysis of piperacillin-albumin conjugates identified a hapten of the predicted mass of 517amu, which was formed from direct addition of piperacillin and a second hapten of mass 535 amu formed through hydrolysis of the 2,3-dioxopiperazine ring (results not shown) (Whitaker et al., 2011a). After incubating piperacillin with cells in complete culture medium for 16 h, the time associated with a piperacillin-specific T-cell response, piperacillin haptens were detected at five lysine residues (positions Lys 190, 195, 199, 432, and 541; Fig. 5F) on albumin. A lower level of albumin binding and number of lysine residues modified was detected when culture supernatant was analyzed after 1 h (Fig. 5F).
Stimulation of T-Cell Clones with a Synthetic Piperacillin Albumin Conjugate and Characterization of Mechanisms of Antigen Presentation.
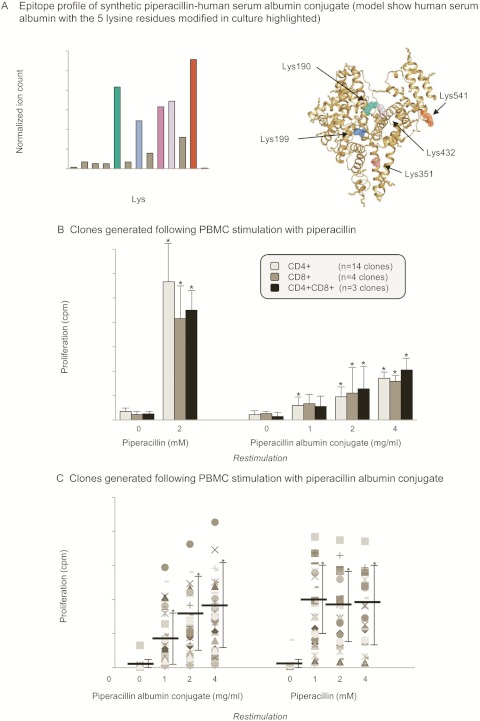
A synthetic piperacillin-albumin conjugate was generated under physiological conditions by culturing human serum albumin with piperacillin (molar ratio 1:50) for 24 h in phosphate buffer. At this molar ratio, 13 Lys residues were modified, including the same five sites observed in culture supernatant (Fig. 6A). The piperacillin albumin conjugate was found to stimulate piperacillin-responsive CD4+, CD8+, and CD4+CD8+ clones in a concentration-dependent fashion (Fig. 6B).
Fig. 6.
Stimulation of T-cell clones with a synthetic piperacillin albumin conjugate. A, epitope profile of the piperacillin-albumin conjugate. Lys residues modified in cell culture supernatant are highlighted in different colors. B, concentration-dependent proliferation of CD4+, CD8+, and CD4+CD8+ T-cell clones with the synthetic albumin conjugate. Clones were generated after PBMC stimulation with piperacillin. The cells were incubated for 2 days, and 0.5 μCi [3H]thymidine was added for the last 16 h of culture. The data show the mean ± S.D. of the indicated number of clones. *, p < 0.05 when drug antigen-treated wells were compared with control wells. C, comparison of piperacillin- and piperacillin-albumin conjugate-specific proliferation of T-cell clones. Clones were generated after PBMC stimulation with the piperacillin albumin conjugate. The cells were incubated for 2 days, and 0.5 μCi [3H]thymidine was added for the last 16 h of culture. The data show the mean ± S.D. of the indicated number of clones. *, p < 0.05 when drug antigen-treated wells were compared with control wells.
In subsequent experiments, T-cell clones were generated from two patients (patients 1 and 2; Table 1) by first stimulating PBMC with the piperacillin albumin conjugate, not the parent drug. Forty two clones (24 from patient 1 and 18 from patient 2) proliferated in the presence of piperacillin-modified albumin. All of the clones were additionally stimulated with the parent drug (Fig. 6C), which forms a conjugate with albumin in culture.
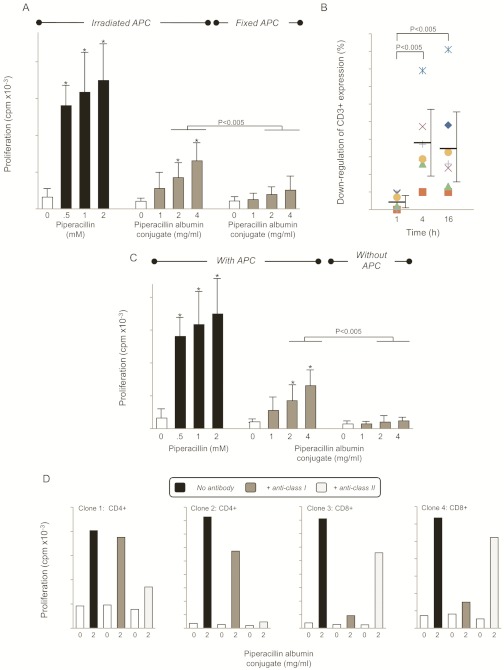
The albumin conjugate stimulated T-cells via a classic hapten mechanism involving antigen-presenting cell processing and the liberation of peptides that associate with MHC molecules before presentation. This conclusion is based on the following experimental observations. First, the concentration-dependent proliferative response observed when clones were stimulated with the piperacillin albumin conjugate was blocked by fixation of antigen-presenting cells with glutaraldehyde (Fig. 7A), which inhibits processing. Second, the proliferative response of clones stimulated with piperacillin-conjugated albumin coincided with the internalization of T-cell receptors after 4 to 16 h, the time required for processing (Fig. 7B). Third, omission of antigen-presenting cells from the proliferation assay completely abrogated the response of clones to the piperacillin albumin conjugate (Fig. 7C). Finally, the proliferative response of CD8+ and CD4+ clones stimulated with the piperacillin albumin conjugate was blocked with anti-MHC class I and II antibodies, respectively (Fig. 7D).
Fig. 7.
The piperacillin albumin conjugate stimulates T-cells via a processing-dependent pathway. A, fixation of antigen-presenting cells blocks the proliferative response. Irradiated or glutaraldehyde-fixed antigen-presenting cells were cocultured with CD4+ and CD8+ T-cell clones in the presence of the piperacillin albumin conjugate for 2 days, and 0.5 μCi [3H]thymidine was added for the last 16 h of culture. The data show the mean ± S.D. *, p < 0.05 when drug antigen-treated wells were compared with control wells. B, T-cell receptor internalization after exposure to piperacillin-conjugated albumin occurs after 4 to 16 h, the time needed for protein processing. Clones were cultured with antigen-presenting cells and the piperacillin albumin conjugate for the indicated times. Cells were then washed, labeled with an anti-CD3+ antibody, and analyzed by flow cytometry. Data shows the mean percentage decrease in CD3+ expression when piperacillin and unstimulated cells were compared. C, exposure of clones to piperacillin-conjugated albumin in the absence of antigen-presenting cells did not stimulate a response. CD4+ and CD8+ T-cell clones were cultured with the piperacillin albumin conjugate for 2 days in the presence or absence of irradiated antigen-presenting cells, and 0.5 μCi [3H]thymidine was added for the last 16 h of culture. The data show the mean ± S.D. *, p < 0.05 when drug antigen-treated wells were compared with control wells. D, stimulation of CD4+ and CD8+ clones with piperacillin-conjugated albumin is MHC-dependent. Clones were cocultured with antigen-presenting cells and piperacillin in the presence or absence of anticlass I/II blocking antibodies for 2 days, and 0.5 μCi [3H]thymidine was added for the last 16 h of culture. Data are from four representative clones. Coefficient of variation was consistently less than 15%.
Flucloxacillin Saturates Drug Binding Sites on Albumin, Blocks Piperacillin Albumin Binding, and Dampens the Piperacillin-Specific T-Cell Response.
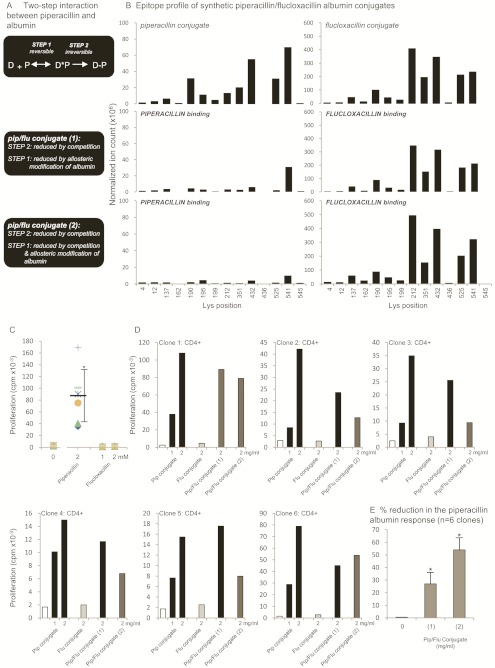
Figure 8A shows the hypothesized two-step binding interaction between β-lactam antibiotics and albumin. Step 1 depicts the noncovalent interaction between the drug and Sudlow sites on albumin. Step 2 depicts the covalent binding interaction between the drug and specific lysine residues.
Fig. 8.
A, proposed two-step interaction between piperacillin (D) and albumin (P). B, mass spectrometric characterization of the key lysine residues in albumin modified with piperacillin and/or flucloxacillin. The epitope profile shows the lysine residues of albumin modified with piperacillin and/or flucloxacillin haptens. C, stimulation of CD4+ clones with piperacillin and flucloxacillin. Clones were cultured with the drugs for 2 days in the presence of irradiated antigen-presenting cells, and 0.5 μCi [3H]thymidine was added for the last 16 h of culture. The data show the response of the six clones used in D. D, piperacillin and/or flucloxacillin albumin conjugates. Clones were cultured with the drug albumin conjugates for 2 days in the presence of irradiated antigen-presenting cells, and 0.5 μCi [3H]thymidine was added for the last 16 h of culture. The data show the mean response of the six clones individually. Coefficient of variation was consistently less than 15%. E, decrease in the piperacillin-conjugated albumin-specific T-cell response with piperacillin/flucloxacillin albumin conjugates. The proliferative response of the six clones used in D was compared when stimulated with the piperacillin albumin conjugate (2 mg/ml) and the pip/flu conjugates (1; 2 mg/ml) and (2; 2 mg/ml). The data show the percentage reduction in the proliferative response.
Irreversibly bound piperacillin and flucloxacillin haptens were detected on 13 lysine residues when the drugs were incubated separately with albumin at a drug/protein ratio of 50:1. The epitope profile of flucloxacillin binding largely mirrored the profile seen with piperacillin; however, the flucloxacillin hapten was detected at 6-fold higher levels (Fig. 8B), disregarding the potential differences in ionization efficiency of the peptides modified with different drugs. It is noteworthy that piperacillin-responsive clones were not activated with the flucloxacillin albumin conjugate.
To investigate whether competition for reversible and/or irreversible β-lactam binding sites on albumin reduces piperacillin binding and the piperacillin-specific T-cell response, two piperacillin conjugates were generated by pretreating albumin with flucloxacillin. Flucloxacillin was selected as a competing drug hapten because it has previously been shown to react readily with human serum albumin (Jenkins et al., 2009). The level of flucloxacillin binding and the sites of modification on these conjugates were comparable with the flucloxacillin conjugate described above [cumulative ion count for all flucloxacillin modified Lys: flucloxacillin conjugate 1.64 × 109; piperacillin/flucloxacillin-conjugate (1) 1.42 × 109; piperacillin/flucloxacillin conjugate (2) 1.83 × 109; Fig. 8B].
The presence of covalently bound flucloxacillin (conjugate 1) reduced piperacillin binding by 71.3%, and the proliferative response of clones was 27% lower than that observed with the piperacillin conjugate. Piperacillin/flucloxacillin conjugate (2) was generated to assess the effect of covalently bound flucloxacillin and noncovalent flucloxacillin occupancy of drug albumin binding sites on piperacillin binding. The cumulative ion count for piperacillin-modified Lys residues in piperacillin/flucloxacillin conjugate (2) was reduced by 90% compared with the piperacillin conjugate, and this translated into a 54% reduction in the proliferative response of T-cell clones (Fig. 8, C–E).
Discussion
T-lymphocytes are thought to be involved in the pathogenesis of allergic drug reactions, causing tissue damage indirectly through the action of cytokines or directly by the secretion of cytolytic molecules (e.g., perforin, granulysin, Fas ligand) (Phillips et al., 2011; Pichler et al., 2011; Romano et al., 2011). To stimulate T-cells, the culprit drug must act as an antigen and in some way ligate specific T-cell receptors. Hapten dogma, originating from the studies of Landsteiner and Jacobs in the 1930s relating skin sensitization potential to protein reactivity, states that a drug must bind irreversibly to self-protein to break immune tolerance (Landsteiner and Jacobs, 1935). T-cells might then be stimulated by peptides liberated from the modified protein after processing. This basic concept has been challenged by the influential studies of Pichler and coworkers (Schnyder et al., 1997, 2000; Castrejon et al., 2010; Keller et al., 2010). Using T-cells isolated from hypersensitive patients they found that many characteristics of the drug-specific T-cell response in vitro could be explained much more accurately through a direct, readily reversible interaction between the parent drug, MHC molecules, and specific T-cell receptors. These findings emphasize the necessity to define further the chemical basis of drug hypersensitivity. Thus, in this study we have used samples from a clinically important patient cohort, CF patients with nonimmediate β-lactam hypersensitivity, to define precisely pathways of drug-specific T-cell activation.
Piperacillin activation of CD4+ and CD8+ clones was associated with the secretion of high levels of the Th2 cytokines IL-4, IL-5, and IL-13 (Fig. 3), and a strong positive correlation was found to exist between the levels of Th2 cytokines secreted by individual clones. The CCR5 ligand MIP-1β was also secreted from piperacillin-stimulated clones; however, the role of this chemokine in piperacillin reactions remains unclear. Low levels of IFN-γ and TNF-α were secreted, which presumably relates to the positive IFN-γ ELIspot detected with PBMC. The outgrowth of piperacillin-responsive CD4+, CD8+, and CD4+CD8+ clones is consistent with other forms of drug hypersensitivity in patients without CF (Wu et al., 2007). CD4+, CD8+, and CD4+CD8+ clones displayed cytolytic activity, indicating that they might participate in the disease pathogenesis. Collectively, these data describe a heterogeneous population of drug-specific T-cells in hypersensitive patients with CF; their synergistic actions probably result in the classic (maculopapular exanthma, fever) and unusual features (flu-like illness, arthralgia, headache) of the hypersensitivity reaction seen in this patient group. To develop an improved understanding of how piperacillin reactions develop in patients with CF and the role of CD4+, CD8+, and CD4+CD8+ clones in the disease pathogenesis, we are in the process of establishing a prospective study to relate humoral and cellular immune responses to clinical outcome by using samples obtained before a reaction, in the acute phase, and longitudinally as the allergic patient recovers. This form of investigation will allow the investigation of drug antigenicity, allergenicity, and tolerance with respect to patient phenotype, genotype, and clinical outcome.
β-Lactam antibiotics are known to be targeted by specific Lys residues on protein (Levine and Ovary, 1961; Batchelor et al., 1965; Meng et al., 2011). Nucleophilic attack leads to ring opening, binding of the penicilloyl group, and ultimately formation of a stable adduct. We found that clones were stimulated to proliferate with piperacillin, but not other β-lactam-containing antibiotics. This indicates that the penicilloyl core structure alone does not deliver specific antigenic signals to activate T-cells. To investigate the fine specificity of the piperacillin-specific T-cell response, clones were cultured with cefoperazone, which contains a side chain closely related in structure to piperacillin, whereas the thiazolidine ring is replaced by a six-membered ring. Once more, clones were activated with piperacillin, but not with cefoperazone, demonstrating that piperacillin-responsive T-cells are highly drug-specific and recognize the penicilloyl structure and the specific side chain of piperacillin.
A combination of immunochemical and mass spectrometry methods were used to show that piperacillin binds exclusively to serum protein. Using therapeutic and T-cell stimulatory (0.5–2 mM) concentrations of piperacillin, haptenic structures were detected on five of the available lysine residues (Lys 190, 195, 199, 432, and 541) on albumin after 16 h. In contrast, after a shorter 1-h incubation, piperacillin modification was detected only at one site, and the level of modification was low. These data coincided with the need to pulse antigen-presenting cells with piperacillin for 4 to 16 h to stimulate a T-cell proliferative response, internalization of specific T-cell receptors, and cytotoxicity against autologous target cells and highlight that a threshold level of piperacillin albumin binding must be reached for the activation of T-cells.
Characterization of the piperacillin antigen formed in situ with cell samples from hypersensitive patients provided the framework and unique gateway to explore mechanisms of β-lactam presentation to T-cells. A synthetic albumin conjugate modified with piperacillin haptens at the five lysine residues modified in culture was shown to stimulate clones in an MHC- and concentration-dependent fashion. To confirm that clones responsive against piperacillin-modified albumin circulate in peripheral blood of hypersensitive patients, a panel of more than 40 clones was generated from two patients after culturing PBMC with the synthetic conjugate. These clones were found to display 100% cross-reactivity with the parent drug, which as described above forms an albumin conjugate in culture. To stimulate clones, generated from patients after culturing PBMC with piperacillin or the synthetic conjugate, with the synthetic albumin conjugate there was an absolute requirement for antigen-presenting cells and the antigen-presenting cells' processing machinery. Thus, piperacillin preferentially modifies albumin, and processing of piperacillin albumin conjugates liberates peptide antigens for T-cells.
Several drug classes, including the β-lactam antibiotics, bind noncovalently at specific sites on albumin (referred to as Sudlow sites I and II; Sudlow et al., 1975). We hypothesize that this noncovalent interaction is a major factor in the selective, highly restricted covalent binding of β-lactam antibiotics to specific lysine residues. In the next part of our investigation, flucloxacillin was used to explore whether competition for noncovalent and covalent binding sites on albumin reduces piperacillin binding and the antigen-specific T-cell response. It is noteworthy that albumin noncovalently binds up to 95% of circulating flucloxacillin in recipients (Røder et al., 1995), and flucloxacillin has been shown to covalently modify lysine residues on albumin (Jenkins et al., 2009). Flucloxacillin was found to compete for the same Lys residues on albumin as piperacillin but the resulting conjugate did not stimulate T-cells, indicating that binding of the β-lactam hapten in peptide conjugates confers structural specificity on the activation of the T-cell receptors expressed on drug-specific clones. Culturing piperacillin and albumin in the presence of covalently and noncovalently bound flucloxacillin reduced the levels of piperacillin binding and the piperacillin-specific proliferative response of T-cell clones. These results suggest that flucloxacillin occupation of albumin binding sites directly blocks piperacillin binding at specific lysine residues and allosterically regulates the affinity of albumin for piperacillin. Similar results have been described with warfarin; noncovalent occupation of Sudlow site I was shown to reduce the affinity of albumin for heme by one order of magnitude because of a conformational shift in the protein (Fanali et al., 2007; Ascenzi and Fasano, 2010; Ascenzi et al., 2010). Thus, there may be some merit in studying the clinical relevance of administering combinations of β-lactam antibiotic in patient groups where a high prevalence of hypersensitivity reactions is seen.
To conclude, we have used clinically important samples and expertise in drug bioanalysis and cellular immunology to further our basic understanding of the nature of the T-cell response in piperacillin-hypersensitive patients with CF. Collectively, our data describe the cellular processes that underlie the structural specificity of piperacillin antigen binding.
Acknowledgments
We thank the patients and volunteers for their generous blood donations.
This work was funded by the Wellcome Trust [Grant 078598/Z/05/Z] as part of the Centre for Drug Safety Science supported by the Medical Research Council [Grant G0700654]. S.E.-G. and A.E. are Ph.D. students funded by the Egyptian government. M.M.M. is a Ph.D. student funded by the Saudi Arabian government.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- CF
- cystic fibrosis
- PBMC
- peripheral blood mononuclear cells
- IFN
- interferon
- IL
- interleukin
- TNF
- tumor necrosis factor
- MIP
- macrophage inflammatory protein
- ELISA
- enzyme-linked immunosorbent assay
- TST
- Tris/saline/Tween
- OD
- optical density
- MRM
- multiple reaction monitoring
- amu
- atomic mass units: MHC, major histocompatibility complex
- Th2
- T helper 2.
Authorship Contributions
Participated in research design: Whitaker, Peckham, French, Pirmohamed, Park, and Naisbitt.
Conducted experiments: El-Ghaiesh, Monshi, Whitaker, Jenkins, Meng, Farrell, Elsheikh, and Peckham.
Performed data analysis: El-Ghaiesh, Monshi, Jenkins, and Naisbitt.
Wrote or contributed to the writing of the manuscript: El-Ghaiesh, Whitaker, Jenkins, Meng, Peckham, French, Pirmohamed, Park, and Naisbitt.
References
- Ascenzi P, Bolli A, Gullotta F, Fanali G, Fasano M. (2010) Drug binding to Sudlow's site I impairs allosterically human serum heme-albumin-catalyzed peroxynitrite detoxification. IUBMB Life 62:776–780 [DOI] [PubMed] [Google Scholar]
- Ascenzi P, Fasano M. (2010) Allostery in a monomeric protein: the case of human serum albumin. Biophys Chem 148:16–22 [DOI] [PubMed] [Google Scholar]
- Batchelor FR, Dewdney JM, Gazzard D. (1965) Penicillin allergy: the formation of the penicilloyl determinant. Nature 206:362–364 [DOI] [PubMed] [Google Scholar]
- Brander C, Mauri-Hellweg D, Bettens F, Rolli H, Goldman M, Pichler WJ. (1995) Heterogeneous T cell responses to β-lactam-modified self-structures are observed in penicillin-allergic individuals. J Immunol 155:2670–2678 [PubMed] [Google Scholar]
- Burrows JA, Nissen LM, Kirkpatrick CM, Bell SC. (2007) β-Lactam allergy in adults with cystic fibrosis. J Cyst Fibros 6:297–303 [DOI] [PubMed] [Google Scholar]
- Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, Naisbitt DJ. (2010) Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol 125:411–418.e4 [DOI] [PubMed] [Google Scholar]
- El-Ghaiesh S, Sanderson JP, Farrell J, Lavergne SN, Syn WK, Pirmohamed M, Park BK, Naisbitt DJ. (2011) Characterization of drug-specific lymphocyte responses in a patient with drug-induced liver injury. J Allergy Clin Immunol 128:680–683 [DOI] [PubMed] [Google Scholar]
- Fanali G, Ascenzi P, Fasano M. (2007) Effect of prototypic drugs ibuprofen and warfarin on global chaotropic unfolding of human serum heme-albumin: a fast-field-cycling 1H-NMR relaxometric study. Biophys Chem 129:29–35 [DOI] [PubMed] [Google Scholar]
- Jenkins RE, Meng X, Elliott VL, Kitteringham NR, Pirmohamed M, Park BK. (2009) Characterisation of flucloxacillin and 5-hydroxymethyl flucloxacillin haptenated HSA in vitro and in vivo. Proteomics Clin Appl 3:720–729 [DOI] [PubMed] [Google Scholar]
- Keller M, Lerch M, Britschgi M, Tâche V, Gerber BO, Lüthi M, Lochmatter P, Kanny G, Bircher AJ, Christiansen C, et al. (2010) Processing-dependent and -independent pathways for recognition of iodinated contrast media by specific human T cells. Clin Exp Allergy 40:257–268 [DOI] [PubMed] [Google Scholar]
- Koch C, Hjelt K, Pedersen SS, Jensen ET, Jensen T, Lanng S, Valerius NH, Pederson M, Hoiby N. (1991) Retrospective clinical study of hypersensitivity reactions to aztreonam and six other β-lactam antibiotics in cystic fibrosis patients receiving multiple treatment courses. Rev Infect Dis 13 (Suppl 7):S608–S611 [DOI] [PubMed] [Google Scholar]
- Landsteiner K, Jacobs J. (1935) Studies on the sensitization of animals with simple chemical compounds. J Exp Med 61:643–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BB, Ovary Z. (1961) Studies on the mechanism of the formation of the penicillin antigen. III. The N-(d-α-benzylpenicilloyl) group as an antigenic determinant responsible for hypersensitivity to penicillin G. J Exp Med 114:875–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Jenkins RE, Berry NG, Maggs JL, Farrell J, Lane CS, Stachulski AV, French NS, Naisbitt DJ, Pirmohamed M, et al. (2011) Direct evidence for the formation of diastereoisomeric benzylpenicilloyl haptens from benzylpenicillin and benzylpenicillenic acid in patients. J Pharmacol Exp Ther 338:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan E, Bauer T, Tongio MM, Kalbacher H, Weltzien HU. (1997) Penicilloyl peptides are recognized as T cell antigenic determinants in penicillin allergy. Eur J Immunol 27:1303–1307 [DOI] [PubMed] [Google Scholar]
- Parmar JS, Nasser S. (2005) Antibiotic allergy in cystic fibrosis. Thorax 60:517–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EJ, Chung WH, Mockenhaupt M, Roujeau JC, Mallal SA. (2011) Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol 127:S60–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler WJ, Naisbitt DJ, Park BK. (2011) Immune pathomechanism of drug hypersensitivity reactions. J Allergy Clin Immunol 127:S74–S81 [DOI] [PubMed] [Google Scholar]
- Pleasants RA, Walker TR, Samuelson WM. (1994) Allergic reactions to parenteral β-lactam antibiotics in patients with cystic fibrosis. Chest 106:1124–1128 [DOI] [PubMed] [Google Scholar]
- Røder BL, Frimodt-Møller N, Espersen F, Rasmussen SN. (1995) Dicloxacillin and flucloxacillin: pharmacokinetics, protein binding and serum bactericidal titers in healthy subjects after oral administration. Infection 23:107–112 [DOI] [PubMed] [Google Scholar]
- Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. (2011) Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol 127:S67–S73 [DOI] [PubMed] [Google Scholar]
- Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. (2000) Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J Immunol 164:6647–6654 [DOI] [PubMed] [Google Scholar]
- Schnyder B, Mauri-Hellweg D, Zanni M, Bettens F, Pichler WJ. (1997) Direct, MHC-dependent presentation of the drug sulfamethoxazole to human αβ T cell clones. J Clin Invest 100:136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow G, Birkett DJ, Wade DN. (1975) Spectroscopic techniques in the study of protein binding. A fluorescence technique for the evaluation of the albumin binding and displacement of warfarin and warfarin-alcohol. Clin Exp Pharmacol Physiol 2:129–140 [DOI] [PubMed] [Google Scholar]
- Whitaker P, Meng X, Lavergne SN, El-Ghaiesh S, Monshi M, Earnshaw C, Peckham D, Gooi J, Conway S, Pirmohamed M, et al. (2011a) Mass spectrometric characterization of circulating and functional antigens derived from piperacillin in patients with cystic fibrosis. J Immunol 187:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker P, Shaw N, Gooi J, Etherington C, Conway S, Peckham D. (2011b) Rapid desensitization for non-immediate reactions in patients with cystic fibrosis. J Cyst Fibros 10:282–285 [DOI] [PubMed] [Google Scholar]
- Wu Y, Farrell J, Pirmohamed M, Park BK, Naisbitt DJ. (2007) Generation and characterization of antigen-specific CD4+, CD8+, and CD4+CD8+ T-cell clones from patients with carbamazepine hypersensitivity. J Allergy Clin Immunol 119:973–981 [DOI] [PubMed] [Google Scholar]
- Zanni MP, von Greyerz S, Schnyder B, Brander KA, Frutig K, Hari Y, Valitutti S, Pichler WJ. (1998) HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human αβ T lymphocytes. J Clin Invest 102:1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]