Abstract
Hydrogen sulfide (H2S) depresses mitochondrial function and thereby metabolic rates in mice, purportedly resulting in a state of “suspended animation.” Volatile anesthetics also depress mitochondrial function, an effect that may contribute to their anesthetic properties. In this study, we ask whether H2S has general anesthetic properties, and by extension, whether mitochondrial effects underlie the state of anesthesia. We compared loss of righting reflex, electroencephalography, and electromyography in mice exposed to metabolically equipotent concentrations of halothane, isoflurane, sevoflurane, and H2S. We also studied combinations of H2S and anesthetics to assess additivity. Finally, the long-term effects of H2S were assessed by using the Morris water maze behavioral testing 2 to 3 weeks after exposures. Exposure to H2S decreases O2 consumption, CO2 production, and body temperature similarly to that of the general anesthetics, but fails to produce a loss of righting reflex or muscle atonia at metabolically equivalent concentrations. When combined, H2S antagonizes the metabolic effects of isoflurane, but potentiates the isoflurane-induced loss of righting reflex. We found no effect of prior H2S exposure on memory or learning. H2S (250 ppm), not itself lethal, produced delayed lethality when combined with subanesthetic concentrations of isoflurane. H2S cannot be considered a general anesthetic, despite similar metabolic suppression. Metabolic suppression, presumably via mitochondrial actions, is not sufficient to account for the hypnotic or immobilizing components of the anesthetic state. Combinations of H2S and isoflurane can be lethal, suggesting extreme care in the combination of these gases in clinical situations.
Introduction
Hydrogen sulfide (H2S) is a small molecular toxin whose primary mechanism of action is the inhibition of complex IV of the oxidative phosphorylation chain in mitochondria. Inhaled concentrations above 500 ppm are lethal to both humans and laboratory rodents within minutes to hours (Reiffenstein et al., 1992). Lower concentrations attracted clinical interest in 2005, when it was reported that mice breathing H2S at 80 ppm experienced a decrease in CO2 production, O2 consumption, and core body temperature within 5 min of exposure, a state termed “suspended animation” (Blackstone et al., 2005). This effect was reversible and apparently without long-term neurological consequences. Because of its ability to lower metabolism and body temperature, H2S is now being investigated as providing protection against hypoxia and ischemia, for example, in brain (Holzer, 2010) and kidneys and liver (Henderson et al., 2011). Other therapeutic applications stem from H2S's role as a smooth muscle relaxant and neurological signaling molecule (Kimura, 2002; Wang, 2003; Wagner et al., 2009).
Another class of small molecules known to inhibit oxidative phosphorylation via interactions with mitochondria respiratory complexes is the volatile general anesthetics. Halothane, isoflurane, and sevoflurane all inhibit complex I activity in cardiac mitochondria, whereas sevoflurane and isoflurane also inhibit complex V to some extent (Hanley et al., 2002; Bains et al., 2006). Volatile anesthetics bind specifically to many mitochondrial proteins and preferentially accumulate in mitochondria (Eckenhoff and Shuman, 1990). In addition to these metabolic effects, volatile general anesthetics have profound neurological effects such as loss of consciousness and amnesia. Several studies point to a connection between the metabolic and neurological pathways. For example, in Caenorhabditis elegans, a mutation affecting the function of complex I, causes hypersensitivity to halothane, isoflurane, and enflurane as determined by immobility (Morgan and Sedensky, 1995; Falk et al., 2006). Furthermore, children with complex I mitochondriopathies are hypersensitive to sevoflurane as measured by the processed electroencephalogram (bispectral index) (Morgan et al., 2002). It is possible that the high-energy demand of neuronal tissue puts it at risk of dysfunction with even minor metabolic dysfunction, suggesting that a possible cellular and molecular mechanism for volatile anesthetic actions reside in mitochondrial actions.
The primary aim of this study was to determine whether hydrogen sulfide has general anesthetic properties. We used a variety of endpoints to characterize anesthetic properties of both H2S and volatile anesthetics. In addition to careful metabolic measurements, we examined righting reflex, electroencephalography (EEG), and electromyography (EMG), as well as the effects of anesthetic/H2S combinations. These results will establish not only the anesthetic character of H2S and the safety of combination with conventional anesthetics, but also whether mitochondrial dysfunction is an important contributor to the state of general anesthesia.
Materials and Methods
Animals
Animal protocols were approved as required by the University of Pennsylvania Institutional Animal Care and Use Committees, and all mice were treated in strict accordance with institutional and National Institutes of Health guidelines (Institute of Laboratory Animal Resources, 1996). Male C57BL/6 mice, aged 2 to 3 months, were housed in approved University Laboratory Animal Resources facilities on a standard 12-h light/dark cycle with free access to water and rodent chow.
Metabolism
Groups of eight mice were exposed to several concentrations of isoflurane (0.3, 0.5, 0.7, and 0.9%) (Butler Animal Health Supply, Dublin, OH), halothane (0.5, 0.7, and 0.9%) (Sigma-Aldrich, St. Louis, MO), sevoflurane (1.0, 1.5, and 2.0%) (Baxter, McGaw Park, IL), and H2S (80 and 250 ppm) all delivered in air. H2S stock gas of 510 ppm in nitrogen (Airgas Specialty Gases, Riverton, NJ) was mixed with air and O2 calibrated to a final concentration of 21% O2. Anesthetic vapor concentrations were calibrated and monitored with an anesthesia gas monitor (Poet 8500 IQ2; Criticare Systems, Inc., Waukesha, WI) and delivered to four mice at a time in a small metabolic chamber with a volume of 600 ml (Fig. 1). Metabolic effects of H2S and isoflurane in combination were also studied, but only at 80 ppm H2S. The exposure time for each concentration of anesthetic was 15 min, and for each concentration of H2S it was 30 min with 800 ml/min of total gas flow. We chose these exposure times because they resulted in a measurable and stable decrease in CO2 production and were short enough that the mice did not become substantially hypothermic. For example, mice breathing H2S at 80 ppm at 27°C had a significant drop in core body temperature after only 30 min of exposure (Volpato et al., 2008). In a separate cohort of mice, O2 consumption, CO2 production, and the respiratory quotient were also determined. All mice breathed spontaneously without intubation in all experiments. At the end of the exposures, gas delivery and outflow were stopped with valves to create a closed circuit with mixing provided by in-circuit diaphragm pumps. Chamber CO2 (torr) and O2 (%) levels were monitored for 3 min to two-digit accuracy, and both CO2 production and O2 consumption rates were calculated. After measurement, the circuit was again opened, and fresh gas (air, without either anesthetic or H2S) was circulated to allow full recovery. Mice were given a minimum of 1 h/day over 4 days to habituate to the chamber before exposures, at least 1 day to recover before a repeat exposure, and at least 1 week to recover before exposure to a different anesthetic.
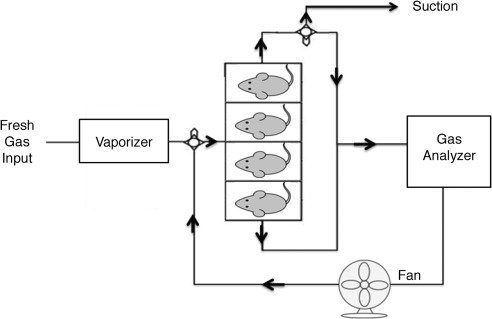
Fig. 1.
Schematic illustration of metabolic exposure chambers. Mice were habituated and exposed to various concentrations of volatile anesthetic or hydrogen sulfide gas for 15 min. Then, to measure CO2 production, the two gas valves (circles), located after the anesthetic vaporizer and at one output of the mouse chambers, isolated the system and allowed continuous measurement of CO2 concentration in the gas analyzer for 3 min, at which time the circuit was again opened to fresh gas.
Loss of Righting Reflex
Loss of righting reflex (LORR) was studied in mice (n = 12) exposed to 80 ppm H2S and 250 ppm H2S in combination with isoflurane. Chamber and gas flow setup has been reported previously (Sun et al., 2006). Temperature was maintained by using a heated water bath to maintain euthermia. A stable concentration of H2S (80 or 250 ppm) was combined with isoflurane, the concentration of which was adjusted in 0.03 to 0.04% increments every 15 min. Mice were flipped onto their backs and given 2 min to regain a prone position. If a mouse was unable to turn prone onto all four paws within 2 min, it was scored as having lost its righting reflex. Tests were videotaped for independent confirmatory scoring by a blinded experimenter. H2S and volatile anesthetics were delivered in 21% O2 to be comparable with the metabolic and EEG/EMG experiments. Volatile anesthetics were delivered in air.
Raw and Processed EEG and EMG Analysis
Implantation of electrodes and physiological analysis was performed according to published methods (Pick et al., 2011). In brief, anesthesia was induced with 2.5% isoflurane, which was then lowered to 1.5 to 2.0% for maintenance during surgeries, titrated to the absence of a response after tail or hindpaw pinch. Suitably anesthetized mice were placed into a stereotactic frame (David Kopf Instruments, Tujunga, CA) and warmed on a heating pad. Using aseptic technique, a lightweight, custom-fabricated socket headpiece (Allied Electronics, Fort Worth, TX) equipped with six 28-gauge Teflon-insulated silver wires (AM Systems, Sequim, WA) was positioned and inserted with two epidural wires for recording EEG placed bilaterally into burr holes drilled at 2 mm lateral to midline/2 mm rostral to bregma and another two epidural EEG wires at 2 mm lateral to midline/3 mm caudal to bregma (corresponding to primary motor and visual cortex, respectively). An additional set of two silver wires was used for EMG electrodes and inserted into the neck muscles. The headpiece was secured to the mouse skull with dental cement (AM Systems). After surgery and for subsequent recordings, mice lived in individual custom-fabricated, cylindrical 4.9-liter exposure chambers (Pick et al., 2011) at 23°C with free access to food and water. Two weeks after surgery, exposures to anesthetics or H2S began. A heated water bath was used to maintain rectal temperature at 34 ± 2°C during exposures. Isoflurane, sevoflurane, halothane, or H2S was delivered similarly to the metabolic studies. Gas flow through each of the eight airtight chambers was 1.25 liters per min. A gas monitor measured anesthetic concentrations of outflow gas. Exposure times were 30 min for each concentration of anesthetic and 1 h for each of the two H2S concentrations. After exposures, mice were given at least 2 days to fully recover before exposure to a different anesthetic. H2S was always given as the final exposure. Prior work has established the lack of acute adaptation or tachyphylaxis to repeated anesthetic exposure (Chalon et al., 1983; Milutinovic et al., 2009).
Baseline EEG measurements were obtained by using a low-pass filter with cutoff at 35 Hz and recorded from mice breathing room air for 1 h the day (24 h) before the first anesthetic exposure. EEG signals were imported into Somnologica (Somnologica Studio; Embla, Broomfield, CO) and scored for states of sleep and wakefulness by using the Somnologica Rodent Sleep Scoring Module with the epoch length set to 10 s. Spectral power was also determined by Somnologica using a Fourier analysis on 10-s epochs. Acqknowledge 3.9.1 (Biopac Systems Inc., Golea, CA) was used to rectify the EMG signal and then integrate the amplitude over each 10-s epoch. To promote steady-state conditions, EEG and EMG data collected during only the last 15 min of exposure to the general anesthetics or H2S concentrations were used for analysis.
Immunohistochemistry
A separate group of mice, 8 to 10 weeks old, was exposed to 80 ppm H2S while being either actively warmed in a heated water bath to maintain rectal temperature at 34 ± 2°C (n = 4) or room temperature (n = 4) or exposed to air at room temperature (n = 4) for 6 h, and they were euthanized 18 h later. The brains were perfused with ice-cold saline, postfixed in 4% paraformaldehyde, and embedded in paraffin. Serial coronal 8- to 10-μm sections were cut and stained for caspase-3 immunofluorescence detection. The slides were dewaxed, incubated with blocking buffer, and then incubated at 4°C overnight with antiactivated caspase-3 (Cell Signaling Technology, Danvers, MA) at 1:100. After washing, the sections were incubated in a dark chamber with IgG-fluorescein isothiocyanate (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:200 for 1 h at room temperature followed by counterstain with propidium iodide at 1:3000 for 30 min. The sections were viewed and quantitated with an Olympus (Tokyo, Japan) IX70 microscope equipped with a SensiCam SVGA high-speed cooled digital camera (Cooke Corp., Auburn Hills, MI) and IPLab Suite v3.6 Imaging Processing and Analysis software (Biovision Technologies, Exton, PA). The number of caspase-positive cells in the cortex and adjacent hippocampus were counted in 10 sections per animal, and the density of caspase-3-positive cells for the combined area was determined.
Behavioral Testing
Because of the toxic potential of H2S, we tested for delayed cognitive effects after H2S exposures. Mice, aged 8 to 10 weeks of age, were exposed to 80 ppm H2S while being either actively warmed in a heated water bath to maintain rectal temperature at 34 ± 2°C (n = 8) or room temperature (n = 8) or exposed to air at room temperature (n = 8) for 6 h. Four days after the exposure, behavioral testing began with the Morris water maze. The water in the 1.5-m diameter pool was maintained between 25 and 29°C and opacified with titanium (IV) dioxide throughout all training and testing to obscure the submerged 15-cm-diameter platform. After each trial, the mouse was dried and warmed under a heat lamp before returning to its cage. The time between trials was at least 30 to 45 min to assure euthermia. Mice were tracked with a video camera mounted above the pool that fed to a computer running IMAQ PCI-1407 software (National Instruments, Austin, TX).
Training (Days 4–8 after Exposure).
The mice were first trained to locate the submerged and flagged platform. A white curtain surrounded the pool to eliminate visual cues. Starting points were varied, and mice were allowed 60 s to find the platform. Any animals that did not find the platform in 60 s were gently guided to it and allowed to remain for 10 to 15 s before removal. The training period involved 5 days with four trials per day.
Reference Memory Testing (Days 11–15 after Exposure).
The curtain and platform flag were removed, and fixed external visual cues were placed on the walls around the pool. The platform remained in the same location as for the training, and starting points were varied. Escape latency (time required to find the platform) was recorded, and the mean for each day was calculated for each animal. If an animal did not reach the platform within 60 s, it was gently directed to it, and the escape latency was marked as 60 s.
Probe Test (Day 15 after Exposure).
This task reflects memory retention and was conducted after the last reference memory trial. The platform was removed from the pool, and the swim path data for each mouse were recorded for 60 s. The starting location was always in the opposite quadrant to the target quadrant that previously contained the platform. The percentage of time spent in each quadrant was measured, and the ratios of time spent in the target to opposite quadrants were calculated. Swim speeds were also recorded during the 60-s probe test, and the means were calculated.
Spatial Working Memory Testing (Days 18–27 after Exposure).
Mice were given 60 s to find the hidden platform in different preset locations with varied starting points. Criterion was defined as locating the platform in less than 20 s for three consecutive trials. Mice were given up to eight trials a day. If criterion was reached, mice would be given a new platform location the next day. If criterion was not reached, the platform location would not change the next day. The number of trials to reach criterion for each platform were recorded over the 10 days of testing.
Statistical Analyses
All statistics were done by using Prism 5.04 software (GraphPad Software, Inc., San Diego, CA). Data were analyzed by using one-way or two-way analysis of variance (ANOVA) with the appropriate Bonferroni or Dunnett's post-tests to the baseline or H2S condition as noted in the figure legends. For righting reflex studies, data were fit to a sigmoidal-dose response variable slope Hill plot. Top and bottom were, respectively, constrained to 0 and 100%. LogEC50 and Hill slopes are reported along with the 95% confidence interval bracketing each. Significant differences were assessed by using a one-way ANOVA with Bonferroni post-testing. In all instances, p values < 0.05 were considered significant.
Results
Metabolism.
The purpose of the metabolic effect comparisons between H2S and volatile general anesthetics was to use a common endpoint to determine equivalent effective dosages for subsequent experiments. As expected, pilot studies determined that changes in oxygen consumption paralleled those of carbon dioxide production. This is best depicted by the respiratory quotient, the ratio of carbon dioxide production to oxygen consumption, which showed no significant differences from baseline (Fig. 2). Consequently, we used the change in carbon dioxide production to define the equivalent levels of metabolic inhibition between different drugs. Figure 3 summarizes the group data for inhaled drug treatment upon CO2 production. ANOVA revealed a significant overall effect of volatile anesthetics and H2S upon metabolism (F15,79 = 36.20; p < 0.0001) (Fig. 3A). Post hoc comparisons, using the Bonferroni's multiple comparison testing, revealed that, similar to the volatile anesthetics, H2S significantly decreased CO2 production compared with baseline wakeful levels. Moreover, the low-concentration (80 ppm) H2S decreased CO2 production in a manner indistinguishable to 0.3 and 0.5% isoflurane, 0.5 and 0.7% halothane, and 1.0 and 1.5% sevoflurane. Likewise, the high-concentration (250 ppm) H2S decreased CO2 production equivalently to 0.7 and 0.9% isoflurane, 0.9% halothane, and 1.5 and 2.0% sevoflurane. Thus, for subsequent LORR, EEG, and EMG studies, we compared H2S at 80 ppm to 0.3% isoflurane, 0.5% halothane, and 1.0% sevoflurane, whereas we compared H2S at 250 ppm to 0.9% isoflurane, 0.9% halothane, and 2.0% sevoflurane.
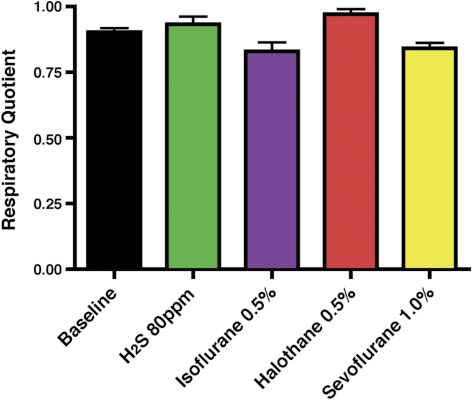
Fig. 2.
The ratio of CO2 production divided by O2 consumption, also known as the respiratory quotient, does not change significantly from baseline (black) with exposure to hydrogen sulfide at 80 ppm (green), 0.5% isoflurane (purple), 0.5% halothane (red), or 1.0% sevoflurane (yellow). This indicates equivalent changes occur in both CO2 production and O2 consumption during inhaled gas exposures.
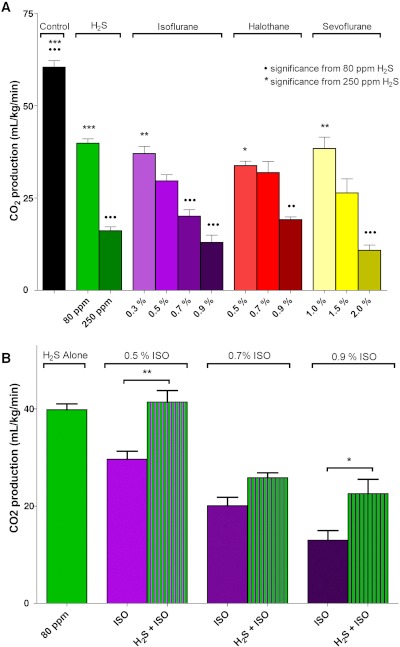
Fig. 3.
Rate of CO2 production in mice after breathing 15 min of H2S or volatile general anesthetics. A, each gas significantly decreased CO2 production, in a concentration-dependent manner, compared with control. ••, p < 0.01 and •••, p < 0.001 significance from H2S at 80 ppm. ★, p < 0.05, ★★, p < 0.01, and ★★★, p < 0.001 significance from H2S at 250 ppm. In terms of CO2 production, H2S at 80 ppm was essentially equivalent to 0.3% isoflurane, 0.5% halothane, and 1.0% sevoflurane; and H2S at 250 ppm was equivalent to 0.9% isoflurane, 0.9% halothane, and 2.0% sevoflurane. B, the addition of H2S at 80 ppm reduced (antagonized) the metabolic effect of each concentration of isoflurane (Iso). ★, p < 0.05; ★★, p < 0.01. Statistical analyses for both metabolic studies were performed by using one-way ANOVA with Bonferroni multiple comparison test.
The addition of H2S at 80 ppm to 0.3, 0.7, or 0.9% isoflurane was antagonistic. One-way ANOVA again revealed a significant overall effect of inhaled drug treatments upon metabolism (F6,25 = 30.59; p < 0.0001) (Fig. 3B). Post hoc Bonferroni's multiple comparison testing confirmed that the combined CO2 production was less depressed than with isoflurane alone at each concentration. This suggests that different mechanisms underlie the metabolic inhibition from the two gases. It is important to note that the higher concentration of H2S (250 ppm) when combined with even low concentrations of isoflurane was lethal to all exposed animals. Lethality occurred after emergence from the sedative effects, and the cause of death remained unclear.
Loss of Righting Reflex.
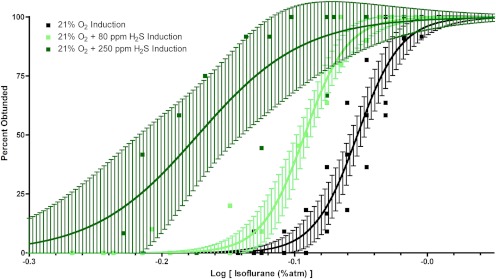
Righting reflex is a protective response to a non-noxious stimulus. The absence of a righting reflex is a commonly used surrogate used to indicate the hypnotic component of general anesthesia in which rodents lose perceptive awareness of the proprioceptive cues accompanying the vulnerable supine position. Using the loss of the righting reflex as one marker of anesthetic-induced hypnosis, we found that H2S at 80 or 250 ppm by itself was capable of abolishing the righting reflex. However, when combined with isoflurane, H2S significantly altered the isoflurane dose-response curve for both logEC50 (F2,56 = 80.0; p < 0.0001) and the Hill slope (F2,56 = 3.6; p = 0.034) (Fig. 4; Table 1). Furthermore, H2S at 80 ppm significantly left-shifted isoflurane-induced loss of righting by approximately 10%, indicating synergy. When combined with H2S at 250 ppm, the concentration effect curve was left-shifted even more, approaching 25%. As noted in the metabolism discussion above, while mice remained alive during the titration of 250 ppm H2S plus isoflurane to a peak isoflurane dose of 0.84%, as evidenced by visual observation of cardiorespiratory function, death ensued in all mice exposed to 250 ppm H2S and isoflurane in 21% oxygen within 4 h of discontinuing the combination mixture of these gases. No deaths were observed in the mice exposed to 80 ppm H2S and isoflurane in 21% oxygen or isoflurane alone in 21% oxygen during or after the righting reflex studies.
Fig. 4.
Isoflurane-induced loss of righting reflex dose-response curves with 95% confidence limits bracketing the best-fit results for isoflurane (black), isoflurane + 80 ppm H2S (light green), and isoflurane + 250 ppm H2S (dark green). Sigmoidal dose-response curves show highly significant left shifts of isoflurane-induced hypnosis by H2S at both 80 and 250 ppm.
TABLE 1.
Effects of H2S on isoflurane-induced loss of righting reflex
| Isoflurane in 21% Oxygen | Isoflurane + 80 ppm H2S in 21% Oxygen | Isoflurane + 250 ppm H2S in 21% Oxygen | |
|---|---|---|---|
| EC50, %atm | 0.89 | 0.81*** | 0.67*** |
| 95% confidence interval for EC50, %atm | 0.87–0.90 | 0.80–0.82 | 0.62–0.72 |
| Hill slope | 26 | 27 | 11* |
| 95% confidence interval for Hill slope | 18–35 | 19–35 | 2–20 |
, P < 0.05;
, P < 0.001 with respect to isoflurane alone.
EEG.
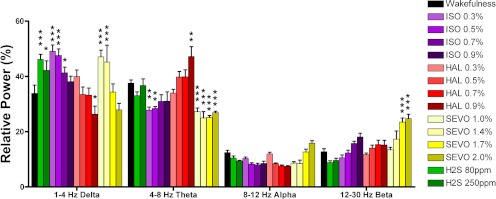
Raw and processed electroencephalography is often used to, respectively, provide qualitative and quantitative assessments of the hypnotic effects of anesthetic drugs on arousal. We performed EEG analysis to compare the effect of H2S with the volatile general anesthetics by using a second objective measure of anesthetic hypnosis. EEG is less dependent on the motor system than the righting reflex, because the latter measure will be affected both by drugs that impair proprioception and muscle tone. Spectral analysis run on the EEG at baseline and during treatment with volatile anesthetics or hydrogen sulfide revealed a significant main effect on relative power of frequency (F3,296 = 73.2; p < 0.0001) and a significant interaction between frequency and inhaled drug exposure (F42,296 = 14.9; p < 0.0001) (Fig. 5). Relative to baseline wakefulness, isoflurane, sevoflurane, and hydrogen sulfide (both 80 and 250 ppm) significantly slowed the EEG, increasing relative δ power (1–4 Hz). Full decomposition of the power spectrum is shown in Supplemental Fig. 1. Burst suppression was observed in mice at the highest dose of isoflurane but not with H2S or any other anesthetic.
Fig. 5.
Electroencephalogram slowing by H2S exposure. Relative spectral power across δ (1–4 Hz), θ (4–8 Hz), α (8–12 Hz), and β (12–30 Hz) bins. Compared with wakefulness (black bars), H2S (green bars), isoflurane (ISO; purple bars), and sevoflurane (SEVO; yellow bars) all induce significant increases in δ power. Two-way ANOVA with Bonferroni post-tests: ★, p < 0.05; ★★, p < 0.01; ★★★, p < 0.001, significance compared with wakefulness (oriented vertically).
EMG.
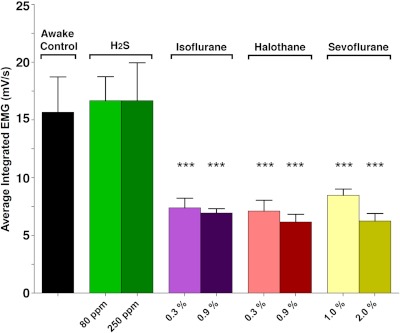
One-way ANOVA demonstrates a significant overall effect of inhaled drug treatment on muscle tone (F14,100 = 8.4; p < 0.0001) (Fig. 6). Post hoc Dunnett's multiple comparison testing revealed that compared with baseline wakefulness all volatile anesthetics significantly decreased integrated EMG. Conversely during H2S exposure, integrated EMG was indistinguishable from that of a waking mouse. These recordings show a clear difference between H2S and general anesthesia. The mice exposed to anesthetics demonstrate a large loss in EMG activity, whereas those exposed to H2S did not. Thus despite finding limited or no spontaneous physical activity detectable by either telemetry or visual monitoring while exposed to 80 ppm H2S (Volpato et al., 2008), nucal EMG tone remains preserved.
Fig. 6.
Average integrated electromyogram of mice exposed to H2S and general anesthetics. Motor tone in mice is not different from controls in mice exposed to either concentration of H2S but significantly lower in mice exposed to any concentration of any anesthetic. Only the highest and lowest dosages of each anesthetic are shown. One-way ANOVA with Dunnett's post-test: F = 8.36; ★★★, p < 0.01, significance from wakefulness.
Immunohistochemistry.
Caspase-3 activation was examined in the cortex and hippocampus of mice 18 h after a 6-h exposure to 80 ppm H2S, with or without warming, or air. No significant differences were found in the density of caspase-3-positive cells (one-way ANOVA; p = 0.4095; F = 0.9877) (data not shown).
Cognitive effects of H2S Exposure.
Behavioral testing using the Morris water maze, performed on mice 11 to 27 days after a single 6-h, 80 ppm H2S exposure with or without warming, revealed no significant differences between H2S-exposed and air-exposed controls for either reference memory (repeated-measures two-way ANOVA; Finteraction = 0.5274; Ftreatment = 0.1230), probe trial (one-way ANOVA; p = 0.7364; F = 0.3108), or spatial working memory (one-way ANOVA; p = 0.9304; F = 0.0724) (data not shown), indicating an absence of delayed cognitive effects caused by 80 ppm H2S, which is consistent with the caspase-3 results.
Discussion
The underlying hypothesis prompting these studies was that the state of suspended animation produced in mice by exposure to H2S (Blackstone et al., 2005) was akin to one of general anesthesia. Using metabolic inhibition to define equipotency, we demonstrate that H2S lacks two key properties that characterize anesthetic drugs: hypnosis and muscle relaxation. The absence of an LORR response, when combined with the distinct EMG character of H2S exposures compared with three general volatile anesthetics, makes it clear that H2S cannot be termed a complete general anesthetic, despite very similar metabolic suppression effects.
The inhibition of oxidative phosphorylation in mitochondria by both volatile general anesthetics and H2S is presumably responsible for the large observed decrease in O2 consumption, CO2 production, and the associated temperature decline. However, it is possible that the decrease in oxygen consumption is the result of decreased activity and use of energy as would be seen in hypothermia. The fact that metabolically equivalent concentrations of H2S do not produce an anesthetic state serves as a strong argument that nonspecific inhibition of metabolism (presumably at mitochondria) by these gases is not a sufficient underlying mechanism to produce the anesthetic state. The hypothesis that anesthetic-induced mitochondrial inhibition might be the primary mechanism through which anesthetics act was most recently formulated from studies in C. elegans (Kayser et al., 2004). Our data with hydrogen sulfide exposures in mice are fully congruent with the rigorous subsequent studies of the mitochondrial respiratory chain in C. elegans, which indicate that anesthetic hypersensitivity occurs only with dysfunction in complex I and not with genetic mutations that alter activity in complexes II or III (Kayser et al., 2004; Falk et al., 2006). However, genetic mutations that affect complex IV activity also alter the activity of complex I (Suthammarak et al., 2009), so the effects of isolated inhibition of complex IV on anesthetic sensitivity were unresolved before this study. Our study strongly indicates that H2S lacks the anesthetic properties of hypnosis and immobility. However, our observation that H2S modestly increased the potency of isoflurane for LORR is an argument for at least a contribution of mitochondrial actions to the hypnotic state. Additional support for a potentiating hypnotic effect comes from the significant EEG slowing observed upon administration of H2S, which resembled subhypnotic doses of both isoflurane and sevoflurane. However, it should be noted that EEG slowing is not an absolute feature of the anesthetic state and occurs during hypothermia as well as cerebral hypoxia. The fact that H2S partially reversed the CO2 production-depressant effects of isoflurane (at three different isoflurane concentrations) suggests that any synergy for its hypnotic effect is produced by mitochondria-independent pathways. Taken together, these observations are inconsistent with the mitochondria being either a sufficient, or perhaps, important, on-pathway target of general anesthetics. However, they do not rule out unrecognized effects of anesthetic interactions with complex I.
By itself, H2S does not induce loss of righting reflex at any sublethal concentration. When combined with isoflurane, however, the EC50 concentration of isoflurane is significantly decreased. This is consistent with prior work where suppression of respiratory complex I activity via mutation dramatically increased the anesthetic sensitivity in both animals and humans (Morgan et al., 2002; Kayser et al., 2004; Sun et al., 2006; Volpato et al., 2008). However, as mentioned above, the antagonism between H2S and isoflurane on CO2 production make it unlikely that LORR synergy is produced via mitochondrial pathways. Like many small gases, it seems plausible that H2S will bind and have functional effects on an array of targets, some of which may contribute to the loss of perceptual awareness that is reported by righting reflex. In addition, it is becoming increasingly clear that H2S is an endogenous signaling molecule (Dominy and Stipanuk, 2004), which, depending on pathway effects, might easily be expected to contribute to generalized central nervous system depression. It is noteworthy, however, that H2S at 250 ppm causes no significant further depression of the EEG over that observed with 80 ppm. This is in contrast to CO2 production or the situation (both EEG and CO2 production) when the volatile anesthetics are increased by smaller increments. Thus, as is the case for anesthetics in general, the mechanism underlying H2S and anesthetic LORR synergy remains unclear.
Likewise, the mechanism for metabolic antagonism between H2S and isoflurane is uncertain. H2S is thought to specifically inhibit complex IV in the respiratory chain, whereas isoflurane has been shown to be more specific for complexes I and V (Hanley et al., 2002; Bains et al., 2006). It is possible that the different sites of inhibition within the complex array of interacting subunits might actually oppose each other's actions via allosteric communication. Because these are whole-body measurements, it is also possible that this antagonism occurs via extra-mitochondrial sites or actions.
A 6-h exposure to H2S at 80 ppm produced no cognitive impairments in the ensuing days to weeks, in contrast to the reported situation with isoflurane (Culley et al., 2004; Bianchi et al., 2008). Furthermore, when administered alone, H2S at 250 ppm was not lethal to mice, although others have reported mortality with prolonged exposures above 160 ppm (Volpato et al., 2008). Previous studies in mice and piglets that combined anesthetics and H2S did not report any lethality although those studies used lower concentrations of H2S and provided mechanical ventilation to their animals (Li et al., 2008; Baumgart et al., 2010). In this study, we found that the combination of 250 ppm H2S and isoflurane was lethal to mice, but not during the acute exposure. Death only occurred after emergence as defined by a return of righting reflex and was noted in 21/21 mice, minutes to hours after cessation of the isoflurane plus H2S mixture. This suggests that energy production capability is still low at a time of rapidly increasing energy demands because of the emergence and need to re-establish thermal equilibrium. It is important to consider the potential lethal interaction between high doses of H2S and volatile general anesthetics especially because H2S has been proposed to be useful in clinical situations that may also require anesthesia.
In summary, these studies clearly establish the lack of general anesthetic character for the gas hydrogen sulfide and further indicate that the mechanism of general anesthesia is unlikely to reside in its ability to nonspecifically depress mitochondrial activity or depress metabolism. Furthermore, this work suggests extreme caution in the combination of H2S and general anesthetics.
Supplementary Material
Acknowledgments
We thank Feras Mardini, Yi Sun, Angelina Sylvestro, and members of the Departments of Anesthesiology and Critical Care and Chemistry at the University of Pennsylvania for assistance.
This research was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants GM077357, GM088156] (to M.B.K.); the Mary Elizabeth Groff Foundation (M.B.K.); and the Austin Lamont Endowment (R.G.E.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- H2S
- hydrogen sulfide
- EEG
- electroencephalography
- EMG
- electromyography
- LORR
- loss of righting reflex
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Li, M.F. Eckenhoff, R.G. Eckenhoff, and Kelz.
Conducted experiments: Li, McKinstry, and Caltagarone.
Performed data analysis: Li, McKinstry, Moore, M.F. Eckenhoff, R.G. Eckenhoff, and Kelz.
Wrote or contributed to the writing of the manuscript: Li, M.F. Eckenhoff, R.G. Eckenhoff, and Kelz.
References
- Bains R, Moe MC, Larsen GA, Berg-Johnsen J, Vinje ML. (2006) Volatile anaesthetics depolarize neural mitochondria by inhibition of the electron transport chain. Acta Anaesthesiol Scand 50:572–579 [DOI] [PubMed] [Google Scholar]
- Baumgart K, Wagner F, Gröger M, Weber S, Barth E, Vogt JA, Wachter U, Huber-Lang M, Knöferl MW, Albuszies G, et al. (2010) Cardiac and metabolic effects of hypothermia and inhaled hydrogen sulfide in anesthetized and ventilated mice. Crit Care Med 38:588–595 [DOI] [PubMed] [Google Scholar]
- Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. (2008) Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging 29:1002–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone E, Morrison M, Roth MB. (2005) H2S induces a suspended animation-like state in mice. Science 308:518. [DOI] [PubMed] [Google Scholar]
- Chalon J, Tang CK, Roberts C, Walpert L, Hoffman C, Ramanathan S, Turndorf H. (1983) Murine auto- and cross-tolerance to volatile anaesthetics. Can Anaesth Soc J 30:230–234 [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. (2004) Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology 100:309–314 [DOI] [PubMed] [Google Scholar]
- Dominy JE, Stipanuk MH. (2004) New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr Rev 62:348–353 [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Shuman H. (1990) Subcellular distribution of an inhalational anesthetic in situ. Proc Natl Acad Sci U S A 87:454–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MJ, Kayser EB, Morgan PG, Sedensky MM. (2006) Mitochondrial complex I function modulates volatile anesthetic sensitivity in C. elegans. Curr Biol 16:1641–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley PJ, Ray J, Brandt U, Daut J. (2002) Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol 544:687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson PW, Jimenez N, Ruffino J, Sohn AM, Weinstein AL, Krijgh DD, Reiffel AJ, Spector JA. (2011) Therapeutic delivery of hydrogen sulfide for salvage of ischemic skeletal muscle after the onset of critical ischemia. J Vasc Surg 53:785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer M. (2010) Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 363:1256–1264 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Kayser EB, Morgan PG, Sedensky MM. (2004) Mitochondrial complex I function affects halothane sensitivity in Caenorhabditis elegans. Anesthesiology 101:365–372 [DOI] [PubMed] [Google Scholar]
- Kimura H. (2002) Hydrogen sulfide as a neuromodulator. Mol Neurobiol 26:13–19 [DOI] [PubMed] [Google Scholar]
- Li J, Zhang G, Cai S, Redington AN. (2008) Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets. Pediatr Crit Care Med 9:110–112 [DOI] [PubMed] [Google Scholar]
- Milutinovic PS, Zhao J, Sonner JM. (2009) Tolerance to isoflurane does not occur in developing Xenopus laevis tadpoles. Anesth Analg 108:176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PG, Hoppel CL, Sedensky MM. (2002) Mitochondrial defects and anesthetic sensitivity. Anesthesiology 96:1268–1270 [DOI] [PubMed] [Google Scholar]
- Morgan PG, Sedensky MM. (1995) Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol Clin Exp Res 19:1423–1429 [DOI] [PubMed] [Google Scholar]
- Pick J, Chen Y, Moore JT, Sun Y, Wyner AJ, Friedman EB, Kelz MB. (2011) Rapid eye movement sleep debt accrues in mice exposed to volatile anesthetics. Anesthesiology 115:702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiffenstein RJ, Hulbert WC, Roth SH. (1992) Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32:109–134 [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen J, Pruckmayr G, Baumgardner JE, Eckmann DM, Eckenhoff RG, Kelz MB. (2006) High throughput modular chambers for rapid evaluation of anesthetic sensitivity. BMC Anesthesiol 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthammarak W, Yang YY, Morgan PG, Sedensky MM. (2009) Complex I function is defective in complex IV-deficient Caenorhabditis elegans. J Biol Chem 284:6425–6435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, Zapol WM. (2008) Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology 108:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F, Asfar P, Calzia E, Radermacher P, Szabó C. (2009) Bench-to-bedside review: Hydrogen sulfide–the third gaseous transmitter: applications for critical care. Crit Care 13:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. (2003) The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal 5:493–501 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.