Abstract
Catamenial epilepsy is caused by fluctuations in progesterone-derived GABAA receptor-modulating anticonvulsant neurosteroids, such as allopregnanolone, that play a significant role in the pathophysiology of epilepsy. However, there is no specific mouse model of catamenial epilepsy. In this study, we developed and characterized a mouse model of catamenial epilepsy by using the neurosteroid-withdrawal paradigm. It is hypothesized that seizure susceptibility decreases when neurosteroid levels are high (midluteal phase) and increases during their withdrawal (perimenstrual periods) in close association with GABAA receptor plasticity. A chronic seizure condition was created by using the hippocampus kindling model in female mice. Elevated neurosteroid levels were induced by sequential gonadotropin treatment, and withdrawal was induced by the neurosteroid synthesis inhibitor finasteride. Elevated neurosteroid exposure reduced seizure expression in fully kindled mice. Fully kindled mice subjected to neurosteroid withdrawal showed increased generalized seizure frequency and intensity and enhanced seizure susceptibility. They also showed reduced benzodiazepine sensitivity and enhanced neurosteroid potency, similar to the clinical catamenial seizure phenotype. The increased susceptibility to seizures and alterations in antiseizure drug responses are associated with increased abundance of the α4 and δ subunits of GABAA receptors in the hippocampus. These findings demonstrate that endogenous neurosteroids protect against seizure susceptibility and their withdrawal, such as that which occurs during menstruation, leads to exacerbation of seizure activity. This is possibly caused by specific changes in GABAA receptor-subunit plasticity and function, therefore providing a novel mouse model of human perimenstrual catamenial epilepsy that can be used for the investigation of disease mechanisms and new therapeutic approaches.
Introduction
Catamenial epilepsy, the cyclical occurrence of seizure exacerbations during particular phases of the menstrual cycle in women with epilepsy, affects a high proportion of women of reproductive age with drug-refractory epilepsy. The word “catamenial” is derived from the Greek word “katamenios” meaning monthly. Catamenial seizure exacerbations are reported to affect up to 70% of women with epilepsy (Herzog et al., 2004; Bazán et al., 2005; Quigg et al., 2009). There are three forms of catamenial epilepsy: perimenstrual and periovulatory in normal cycles and luteal in inadequate luteal-phase cycles (Herzog et al., 1997, 2011). The most common form is perimenstrual, the cyclical seizure exacerbation during menstrual periods. It is a multifaceted neuroendocrine condition attributed to numerous causes. There is growing evidence from animal experiments suggesting that enhanced seizure susceptibility in perimenstrual catamenial epilepsy is caused by the withdrawal of the progesterone-derived neurosteroids as a result of the fall in progesterone at the time of menstruation (Reddy, 2009; Pack et al., 2011). In women, there is evidence for seizure exacerbation after the inadvertent inhibition of neurosteroid synthesis (Herzog and Frye, 2003).
Neurosteroids are endogenous regulators of seizure susceptibility. The prototype neurosteroid allopregnanolone (AP) is synthesized from progesterone or other intermediate precursors. AP-like neurosteroids are broad-spectrum anticonvulsants and exhibit protective effects against seizures induced by pentylenetetrazol, pilocarpine, 6-Hz stimulation, and kindling (Reddy, 2011). Neurosteroids rapidly alter neuronal excitability through direct interaction with GABAA receptors (Hosie et al., 2007). GABAA receptors are composed of five subunits from several classes (α1–6, β1–4, γ1–3, δ, ε, θ, and ρ1–3). The major isoforms of the pentameric receptor consist of 2α, 2β, and 1γ or δ subunits. Neurosteroids act on all GABAA-receptor isoforms, but cause large effects on extrasynaptic δ-subunit receptors that mediate tonic inhibition (Belelli et al., 2002; Stell et al., 2003). Neurosteroids potentiate both synaptic and extrasynaptic GABAA receptors and thereby play a significant role in the pathophysiology of epilepsy (Reddy, 2011). Ovarian cycle-linked fluctuations in progesterone and neurosteroids have been proposed to affect seizure susceptibility (Reddy et al., 2001; Maguire et al., 2005; Tuveri et al., 2008). Seizures decrease in the midluteal phase when serum progesterone levels are high and increase premenstrually when progesterone levels fall, causing the perimenstrual-type catamenial epilepsy. Progesterone protects against seizures by its conversion to neurosteroids, predominantly AP (Reddy et al., 2004). Consequently, perimenstrual seizure exacerbations may be caused by withdrawal of the antiseizure effects of neurosteroids (Reddy, 2009). Neurosteroid withdrawal is associated with a marked up-regulation of the α4 subunit in the hippocampus (Smith et al., 1998a,b), which is associated with an increase in seizure susceptibility and benzodiazepine resistance (Smith and Gong, 2005; Gangisetty and Reddy, 2010). Thus, neurosteroid withdrawal may be a key triggering factor for catamenial seizure exacerbations.
Based on the neurosteroid-withdrawal approach, we have developed a rat model of perimenstrual catamenial epilepsy (Reddy et al., 2001). Rodents have a 4- to 5- day estrous cycle, and studies of fluctuations in seizure susceptibility in cycling female rats have not led to results that are relevant to the human menstrual cycle. To provide a model that more closely mimics the human situation, a condition of elevated progesterone was created in rats by gonadotropin treatment. This resulted in prolonged high circulating levels of progesterone similar to those that occur in the luteal phase of the menstrual cycle. Then, to simulate the withdrawal of AP that occurs at the time of menstruation, the animals were treated with finasteride 11 days after the initiation of gonadotropin treatment. Withdrawal of neurosteroids had led to decreased seizure threshold and increased seizure activity (Reddy et al., 2001). This paradigm was also verified in female epileptic rats with spontaneous seizures (Reddy and Zeng, 2007; Lawrence et al., 2010). Although rat models are useful for investigating therapies for catamenial epilepsy (Reddy and Rogawski, 2001), there is currently no mouse model that recapitulates neuroendocrine and clinical features of catamenial epilepsy for use in molecular and genetic investigations.
In this study, we developed a mouse model of catamenial epilepsy based on the emerging neuroendocrine mechanisms. The model is based on the premise that seizure susceptibility decreases when neurosteroid levels are high (luteal phase) and increases during their withdrawal (perimenstrual periods) in females in association with specific changes in GABAA receptor subunit plasticity.
Materials and Methods
Animals.
Female adult C57BL6 mice, weighing 25 to 30 g, were used in the study. Mice were housed four to a cage with free access to food and water. The mice were housed in an environmentally controlled animal facility under a 12-h light/dark cycle. The animals were cared for in strict compliance with the guidelines outlined in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). All animal procedures were approved by our university's Institutional Animal Care and Use Committee.
Gonadotropin-Induced Neurosteroid Synthesis and Withdrawal Paradigm.
A state of elevated neurosteroid was induced in female mice by a sequential gonadotropin regimen (Brooke et al., 2007). To produce prolonged elevated levels of progesterone and neurosteroids that more closely model the luteal changes in women, mice were treated with pregnant mare's serum gonadotropin (PMSG; 5 IU s.c.) at 3:00 PM followed 46 h later by human chorionic gonadotropin (HCG; 5 IU s.c.) at 1:00 PM. The day of the second gonadotropin injection was considered day 1 of elevated neurosteroids. On the morning of the ninth day, mice were injected with the 5α-reductase and neurosteroid synthesis inhibitor finasteride (50 mg/kg i.p.) to produce an abrupt decline in neurosteroid levels to more closely model perimenstrual changes in women. Animals were tested 24 h after finasteride administration (neurosteroid withdrawal). The control group received saline injections. This protocol is similar to standard progesterone treatment approaches used previously for the induction of neurosteroid withdrawal (Moran and Smith, 1998; Smith et al., 1998a,b) and is also comparable with the pseudopregnancy model in rats (Reddy et al., 2001; Reddy and Rogawski, 2001). Although it is not practical to replicate the actual endocrine milieu of the menstrual cycle in mouse models, this endocrine state may be physiologically similar to the perimenstrual period. We did not use the gonadectomy model because of potential problems of interpretation associated with complete deficiency of ovarian-derived hormones; such animals need hormone replacements that may have variable effects on seizures depending on the age, dose, and duration of treatment (Scharfman et al., 2005).
Determination of Plasma AP Levels.
Animals were anesthetized with isoflurane, and ∼0.5 ml of carotid blood was collected in heparinized tubes. The plasma was separated by centrifugation at 12,000g for 10 min and stored at −20°C in 10-ml glass tubes coated with 7.5% EDTA solution. The concentration of AP was analyzed by liquid chromatography-mass spectrometry as described previously (Reddy et al., 2004). A 0.2-ml plasma sample was added to a tube containing evaporated internal standard. The steroid and internal standard were extracted with 4 ml of hexane. Each sample was analyzed by using the atmospheric-pressure chemical ionization technique under acidic conditions. A standard curve was plotted by using pure AP in methanol mixed with 0.2 ml of blank mouse plasma.
Hippocampus Kindling Model of Epilepsy.
To determine the effect of neurosteroid exposure and withdrawal on seizure susceptibility, we used the hippocampus kindling model, which is a model of human complex partial seizures (Goddard et al., 1969). Kindling is the repetition of stimuli that initially evoke afterdischarges (ADs) but not seizures. A mild focal, nonconvulsant electrical stimulus to the hippocampus on a daily basis leads to the development of a kindled state exhibiting electrographic and behavioral seizures. Once an animal has been kindled, the heightened response to the stimulus is permanent and seizures occur upon stimulation even after several months (Reddy and Mohan, 2011).
Electrode implantation and stimulation procedures for mouse hippocampus kindling were performed as described previously (Gangisetty and Reddy, 2010). Mice were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). A twisted bipolar stainless-steel wire electrode (model MS303/1; Plastic One, Roanoke, VA) was stereotaxically implanted in the right hippocampus (2.9 mm posterior, 3.0 mm lateral, and 3.0 mm below dura) (Franklin and Paxinos, 1997) and anchored with dental acrylic to three jeweler's screws placed in the skull. A period of 7 to 10 days was allowed for recovery. The stimulation paradigm consisted of 1-ms duration, bipolar, square current pulses delivered at 60 Hz for 1 s by using a kindling stimulator (A-M Systems, Carlsborg, WA). The AD threshold (ADT) was determined by stimulation at 5-min intervals beginning with an intensity of 25 μA and increasing in increments of 25 μA until an AD of at least 5 s was obtained. Stimulation on subsequent days used an intensity that was 125% of the threshold value. Seizure activity after each stimulation was rated according to the criterion of Racine (1972) as modified for the mouse: stage 0, no response or behavior arrest; stage 1, chewing or head nodding; stage 2, chewing and head nodding; stage 3, forelimb clonus; stage 4, bilateral forelimb clonus and rearing; and stage 5, falling. The AD was recorded from the hippocampus electrode with a Grass CP511 preamplifier (Astro-Med, West Warwick, RI) and stored in digital form by using Axoscope 8.1 (Molecular Devices, Sunnyvale, CA). AD duration was the total duration of hippocampus electrographic spike activity (amplitude >2× baseline) occurring in a rhythmic pattern at a frequency >1Hz. The day of AD threshold determination was considered day 1 of kindling. Kindling stimulation was delivered daily until stage 5 seizures were elicited on 3 consecutive days. Stimulation was continued on a 5-day-per-week schedule each afternoon. Mice were used for the neurosteroid-withdrawal paradigm when they consistently exhibited stage 5 seizures with stimulation, which is considered the “fully kindled” state.
Test Drug Administration and Stimulation Protocol.
To examine the ability of test drugs to suppress the expression of kindled seizures, fully kindled animals were tested 24 h after the induction of neurosteroid withdrawal. Two different drugs, diazepam and AP, were selected for pharmacological evaluation in neurosteroid-withdrawn animals. Diazepam is a benzodiazepine-site agonist at α1-, α2-, or α3-containing GABAA receptors with potent antiseizure activity, but it is insensitive as an agonist at α4-containing GABAA receptors expressed in the hippocampus (Smith et al., 1998b; Gulinello et al., 2001). The neurosteroid AP binds to all isoforms but has enhanced sensitivity at the δ-subunit-containing GABAA receptors. In the kindling studies, animals were tested for drug sensitivity 24 h after neurosteroid withdrawal. On the day of testing, animals were injected intraperitoneally with diazepam (0.1–1 mg/kg) or AP (1–10 mg/kg) 15 min before kindling stimulations. Control animals were injected similarly with vehicle (15% cyclodextrin). During each stimulation session, the behavioral seizure score and the AD duration were noted.
TaqMan Real-Time PCR Assay.
GABAA receptor subunit mRNA expression was determined by the TaqMan real-time PCR assay as described previously (Gangisetty and Reddy, 2009). Mice were anesthetized with isoflurane, and the hippocampus was rapidly dissected for RNA isolation. The total RNA was extracted from the hippocampus by using a TRIzol reagent, and cDNA was prepared by using the Superscript II first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). The PCR primers and TaqMan probe specific for GABAA-receptor subunits and glyceraldehyde-3-phosphate dehydrogenase genes were designed by using Primer Express software (Applied Biosystems, Foster City, CA). Primer set and probe for the α4 subunit were composed of the following sequences: forward, 5′-AGA-ACT-CAA-AGG-ACG-AGA-AAT-TGT-3′; reverse, 5′-TTC-ACT-TCT-GTA-ACA-GGA-CCC-C-3′; and sequence-specific TaqMan probe: 5′-6-FAM-ACG-CAG-CCT-GTT-GTC-ATA-ACC-ATC-CAG-C-TAMRA-3′. A set of primers and sequence-specific TaqMan probes were designed and optimized for real-time PCR analysis of the δ-subunit gene. TaqMan PCRs were carried out in an AB 7500 fast real-time system (Applied Biosystems). Real-time PCR was performed with TaqMan Universal PCR Master Mix (Applied Biosystems), which contained AmpliTaq Gold DNA Polymerase, AmpErase, uracil-N-glycosylase, deoxynucleoside-5′-triphosphates with 2′-deoxyuridine-5′-triphosphate, and optimized buffer components. Each sample was run in triplicate design, and each 25-μl reaction mixture consisted of 12.5-μl TaqMan Universal PCR Master mix, 400 nM primers, and 300 nM TaqMan probe for the target genes as described previously (Gangisetty and Reddy, 2009). The real-time PCR run consisted first of one cycle of 50°C for 2 min, then one cycle of 95°C for 10 min, 50 cycles of 95°C for 15 s, and 60°C for 1 min. The target input amount for each target gene was normalized to glyceraldehyde-3-phosphate dehydrogenase expression in the same samples to control for loading variability, and then expressed as a percentage of change with respect to mean control values in the same run.
Data Analysis.
Group data are expressed as the mean ± S.E.M. Differences in kindling seizure stage between groups were compared with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test. Comparison of means of the AD duration between groups was made with one-way analysis of variance, followed by unpaired two-tailed Student's t test. Comparison of the mean percentage of inhibition of seizure stage and AD duration in fully kindled animals was made by Wilcoxon signed ranks test and paired two-tailed Student's t test, respectively. To construct dose-effect curves, diazepam and AP were tested at several doses spanning the dose producing 50% protection (ED50) in the kindling model. GABAA receptor α4- and δ-subunit expression was analyzed based on the relative quantification approach as described previously (Gangisetty and Reddy, 2009). In all statistical tests, the criterion for statistical significance was p < 0.05.
Drugs.
Gonadotropins (Sigma, St. Louis) were dissolved in saline. Stock solutions of AP (Steraloids Inc., Newport, RI) and other drugs for injection were made in 15% β-cyclodextrin in saline, and additional dilutions were made by using sterile saline. Diazepam solution (Hospira, Lake Forest, IL) was diluted in sterile saline. Drug solutions were administered subcutaneously or intraperitoneally in a volume equaling 1% of the animal's body weight.
Results
Progression of Hippocampus Kindling Development in Female Mice.
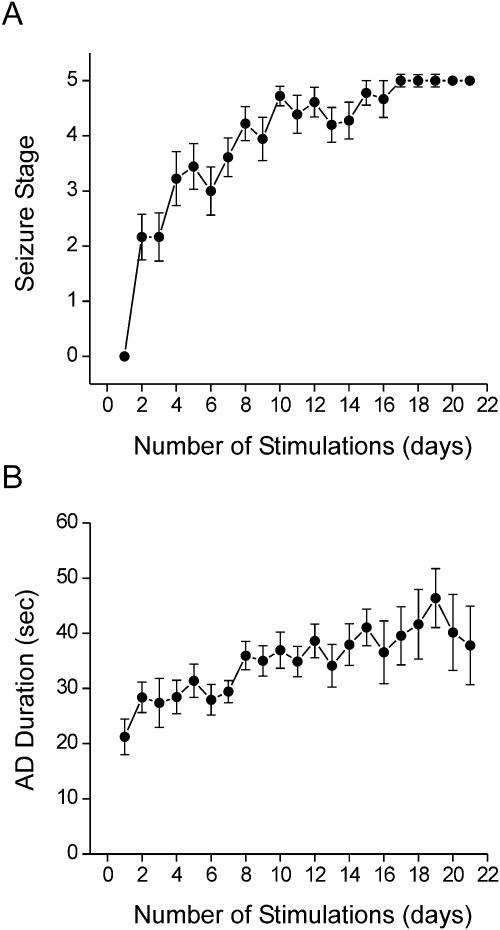
To create a background model for developing catamenial epilepsy in female mice, we used the hippocampus model of complex partial seizures. As shown in Fig. 1, daily kindling stimulation was associated with a steady progression of behavioral seizures (Fig. 1A) and AD duration (Fig. 1B). Mice were subjected to once-daily kindling via an implanted electrode in the dentate gyrus region until they exhibited stage 5 seizures for 3 consecutive days, which is considered the fully kindled state. Mice reached the fully kindled state with consistent stage 5 seizures after ∼14 stimulations (Fig. 1A).
Fig. 1.
Progression of hippocampus kindling development in female mice. A, progression of behavioral seizure stages after each stimulation. B, progression of AD duration after each stimulation. Values represent the mean ± S.E.M. (n = 18 animals per group).
Gonadotropin-Induced Neurosteroid Synthesis and Withdrawal Paradigm.
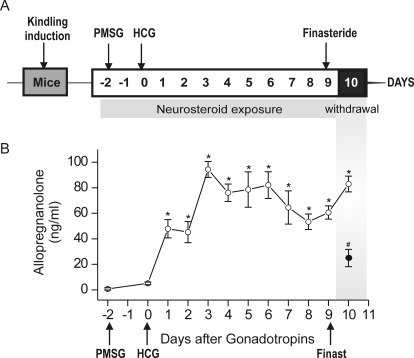
To create a model of the perimenstrual endocrine milieu to simulate menstruation-associated neurosteroid withdrawal, a state of prolonged neurosteroid levels was induced in mice by using a sequential gonadotropin regimen (Fig. 2A). Neurosteroid withdrawal was induced by treatment with finasteride (50 mg/kg i.p.), a 5α-reductase inhibitor that blocks the conversion of progesterone into AP. As shown in Fig. 2B, plasma AP levels, measured by liquid chromatography/tandem mass spectrometry assay, were increased after gonadotropin treatment (mean level, 95 ng/ml on day 3 versus vehicle control, 5 ng/ml). The levels of AP were gradually elevated from the baseline after gonadotropin treatment. Plasma AP was significantly reduced (∼80%) 24 h after finasteride treatment (Fig. 2B). Thus, the elevated plasma AP concentrations after gonadotropin treatment mimic the high levels of neurosteroids during the luteal phase of the menstrual cycle, whereas the finasteride-induced withdrawal is similar to the marked decrease in neurosteroid concentrations that occur before the start of menstruation.
Fig. 2.
Gonadotropin-induced neurosteroid synthesis and withdrawal paradigm. A, experimental protocol for the neurosteroid exposure and withdrawal model in mice. To mimic the prolonged exposure (like that of the luteal phase) followed by withdrawal (like that of menstruation) of progesterone and therefore the “neurosteroid” AP, mice were treated sequentially with gonadotropins. Mice were treated with PMSG (5 IU s.c.) at 3:00 PM followed 46 h later by HCG (5 IU, s.c.) at 1:00 PM. Then, on day 9 they were given finasteride (50 mg/kg i.p.), a 5α-reductase inhibitor that blocks the synthesis of progesterone-derived neurosteroid AP. B, plasma AP levels in mice after treatment with gonadotropins and finasteride (50 mg/kg i.p.) given as outlined in A for the induction of neurosteroid withdrawal. Thus, the dramatic decline in neurosteroid levels 24 h after finasteride (see Fig. 2B) would create a state of acute neurosteroid withdrawal, which is proposed to partly model the neuroendocrine milieu commonly observed around the perimenstrual period. Values represent the mean ± S.E.M. (n = 6 animals per group). *, p < 0.01 gonadotropin versus vehicle; #, p < 0.01 gonadotropin group (○) versus gonadotropin + finasteride group.
Elevated Endogenous Neurosteroids Decrease Seizure Expression in Fully Kindled Mice.
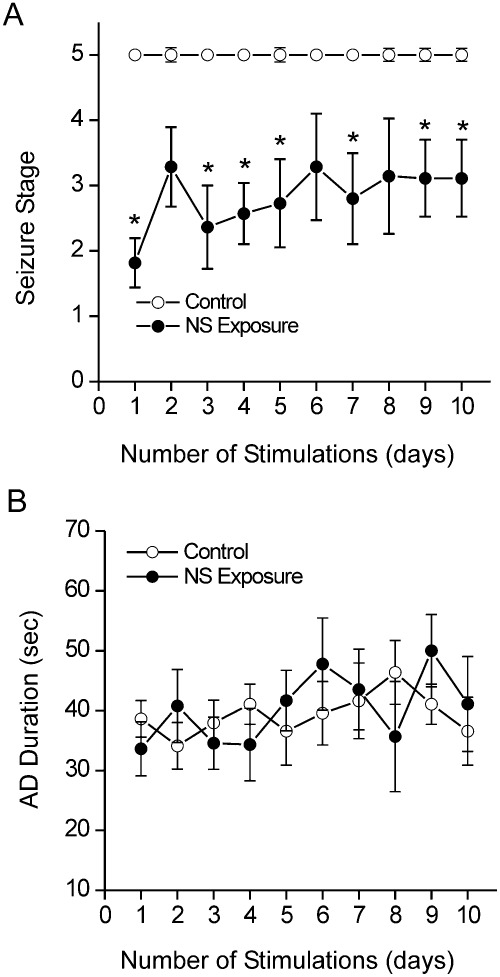
To determine the effect of elevated neurosteroid level on kindled seizures, fully kindled mice were given gonadotropin treatment to boost endogenous AP levels (Fig. 2A), then the animals were subjected to once-daily stimulation sessions, and seizures were measured as electrographic AD and behavioral seizures. As shown in Fig. 3, the severity of generalized (stage 4/5) seizures was markedly reduced during the period of gonadotropin-induced elevation in neurosteroids, indicating endogenous neurosteroids reduce or check seizure susceptibility.
Fig. 3.
Effect of elevated neurosteroids (NS) on seizure expression in fully kindled mice. A, behavioral seizure stage during the 10-day period associated with elevated neurosteroids. *, p < 0.05 versus control group. B, AD duration during the study period. Values represent the mean ± S.E.M. (n = 9–15 animals per group).
Neurosteroid Withdrawal Increases Seizure Susceptibility in Fully Kindled Mice.
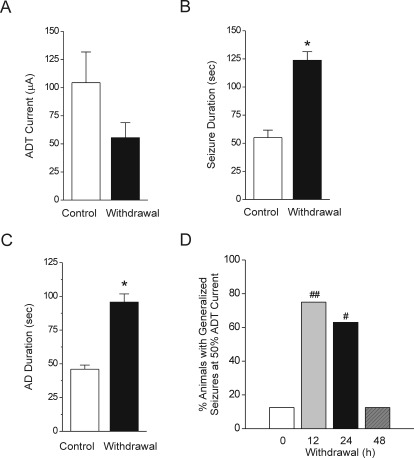
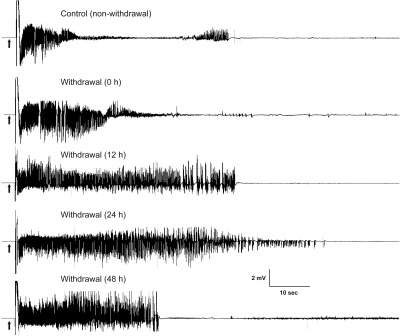
To determine whether neurosteroid withdrawal is associated with heightened seizure susceptibility, we analyzed the stimulation-evoked seizure activity in animals undergoing neurosteroid withdrawal. Fully kindled mice were subjected to the neurosteroid-withdrawal protocol as described in Fig. 2B. Four parameters were assessed as indices of seizure propensity: 1) ADT current for generalized seizures, 2) stimulation-induced electrographic ADT duration, 3) behavioral seizure intensity measured as per the Racine scale, and 4) duration of generalized seizures. Consistent with heightened excitability, there was a marked decrease in the ADT current to induce generalized seizures at 24 h after neurosteroid withdrawal (mean ADT value, 105 and 60 μA for control and withdrawal, respectively) (Fig. 4A). The mean duration of the individual generalized seizures was longer in withdrawal than in control animals (Fig. 4B). The total duration of AD was significantly higher in withdrawal animals (Fig. 4C). The number of animals exhibiting generalized seizures at 50% ADT current was significantly higher after neurosteroid withdrawal than in the control group (Fig. 4D). This response was significantly higher 12 and 24 h after neurosteroid withdrawal and returned to control level by 48 h after withdrawal (Fig. 4D), indicating a transitory period for seizure exacerbation after neurosteroid withdrawal. The electrographic events are illustrated in Fig. 5. Neurosteroid-withdrawn animals showed continuous bursts of spikes that progressively increased in amplitude and duration, indicating heightened epileptiform activity (Fig. 5). The electrographic AD duration was increased markedly 12 h after withdrawal, reached maximal level 24 h after withdrawal, and declined to almost control level by 48 h after withdrawal (Fig. 5). Finasteride did not cause such seizure exacerbations in fully kindled control (nonwithdrawing) animals, indicating the specificity of neurosteroid withdrawal on the exacerbation of seizure activity in fully kindled mice.
Fig. 4.
Increased seizure activity during neurosteroid withdrawal in fully kindled mice. Neurosteroid-withdrawn animals exhibited seizure exacerbation as evident by a markedly decreased ADT current to induce generalized seizures (A), increased behavioral seizure duration (B), increased AD duration (C), and time course of the percentage of animals exhibiting generalized seizures (stage 4/5) at 50% of regular ADT current (D). Values represent the mean ± S.E.M. (n = 6–10 animals per group). *, p < 0.05 versus control group; #, p < 0.05 and ##, p < 0.01 versus control (nonwithdrawn) group.
Fig. 5.
Neurosteroid withdrawal induced exacerbation of electrographic seizure activity in fully kindled mice. Representative traces illustrate time-dependent exacerbation of electrographic seizure activity in a fully kindled mouse during the neurosteroid-withdrawal period. Traces show depth recordings from a right hippocampus stimulating/recording electrode. Arrows indicate onset of the 1-s kindling stimulus, which is followed by the stimulus artifact. Control trace was obtained without neurosteroid withdrawal.
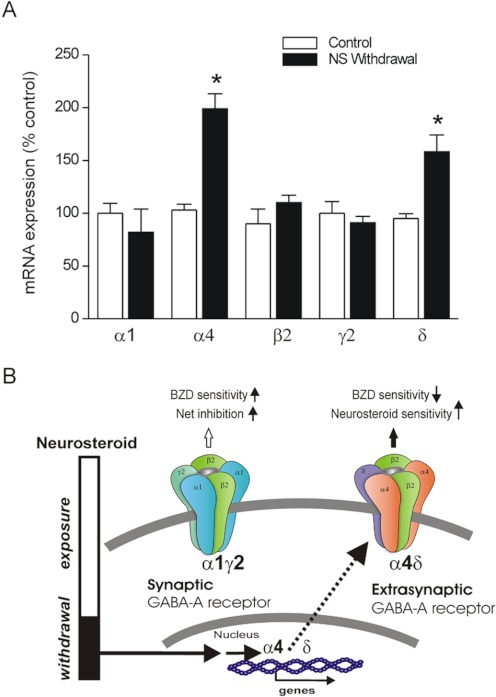
Neurosteroid Withdrawal Alters GABAA Receptor Subunit Plasticity.
To investigate the potential molecular mechanisms underlying the seizure exacerbations, we determined the changes in GABAA receptor-subunit mRNA expression during neurosteroid withdrawal in the hippocampus, which has previously been shown to exhibit neurosteroid-dependent plasticity (Smith et al., 1998b; Maguire and Mody, 2007; Gangisetty and Reddy, 2010). Twenty-four hours after neurosteroid withdrawal, the levels of α4 subunit were significantly increased compared with its expression in the control group (Fig. 6A). The abundance of δ-subunit mRNA in the hippocampus was also significantly increased in withdrawn animals (Fig. 6A). In contrast, no changes in levels of β2- and γ2-subunit expression were observed 24 h after neurosteroid withdrawal (Fig. 6A). Overall, these findings indicate a marked increase in the expression of α4- and δ-subunit expression in the hippocampus during neurosteroid withdrawal in the mouse catamenial paradigm (Fig. 6B). The localization of GABAA receptor subunits is not known with the TaqMan PCR technique. Although dentate gyrus normally has high expression of α4 and δ subunits, they are also expressed in CA1 hippocampus in response to fluctuations in neurosteroids (Smith et al., 1998a).
Fig. 6.
Neurosteroid withdrawal causes up-regulation of GABAA receptor α4- and δ-subunit expression in the hippocampus. A, TaqMan real-time PCR analysis of GABAA receptor subunit mRNA expression in the hippocampus in mice. The subunit expression was quantified in the hippocampus samples collected from mice following the neurosteroid (NS)-withdrawal protocol as described in Fig. 2A. Total RNA was extracted from the hippocampus, and cDNA was prepared for TaqMan PCR analysis. Values represent the mean ± S.E.M. (n = 6–8 animals per group). *, p < 0.01 versus control group. B, a working model indicating alterations in pharmacology caused by neurosteroid withdrawal-induced changes in extrasynaptic GABAA receptor plasticity. Neurosteroid-withdrawal increases in the α4 subunit may underlie the increased excitability caused by reduced net inhibition and benzodiazepine (BZD) insensitivity, whereas up-regulation of extrasynaptic δ subunit-containing receptors may promote neurosteroid sensitivity.
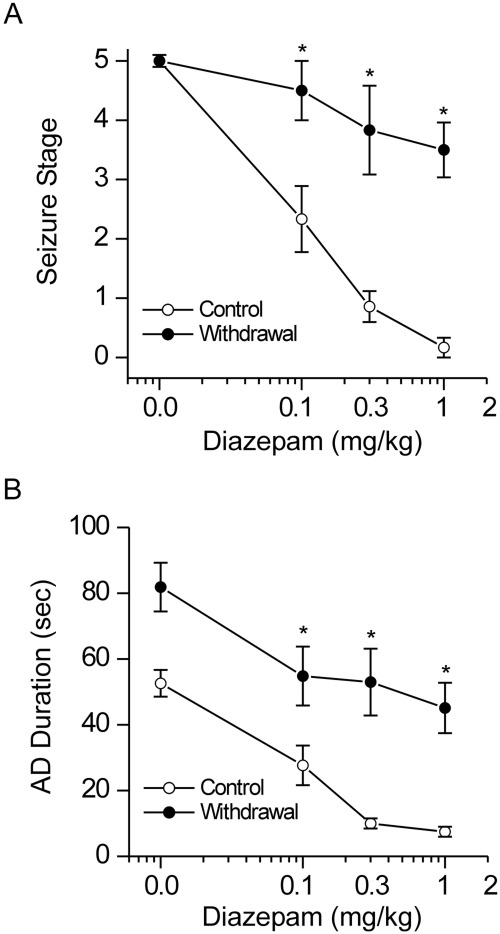
Neurosteroid Withdrawal Induces Diazepam Insensitivity in Fully Kindled Mice.
To further investigate whether neurosteroid withdrawal is associated with alterations in the antiseizure profile of benzodiazepines, diazepam was characterized pharmacologically in fully kindled mice at 24 h after neurosteroid withdrawal (Fig. 7). Diazepam produced a dose-dependent suppression of behavioral seizure activity (Fig. 7A) and AD duration with significant effects at 0.1, 0.3, and 1 mg/kg in control (nonwithdrawal) animals (Fig. 7B), confirming diazepam protection against hippocampus kindling-induced seizures. In contrast, mice undergoing neurosteroid withdrawal had significantly decreased seizure protection by diazepam (Fig. 7). At a dose of 1 mg/kg, diazepam produced an average of 95 and 30% decrease in seizure expression in control and withdrawal groups, respectively. Taken together, these results are consistent with the notion that neurosteroid withdrawal causes relative insensitivity to diazepam caused by increased α4-containing GABAA receptor expression in the hippocampus.
Fig. 7.
Neurosteroid withdrawal reduces the antiseizure sensitivity of diazepam in fully kindled mice. Dose-response curves for diazepam (0.1–1 mg/kg i.p.)-induced suppression of behavioral seizures (A) and AD duration (B) in control and neurosteroid-withdrawn mice are shown. Diazepam is a benzodiazepine-site agonist at α1/2-containing, but not α4-containing, GABAA receptors. The diazepam insensitivity in mice undergoing neurosteroid withdrawal was consistent with increased α4-containing GABAA receptor abundance in vivo. Vehicle or diazepam was injected intraperitoneally 15 min before kindling stimulations. Values represent the mean ± S.E.M. (n = 6–10 animals per group). *, p < 0.05 versus diazepam-treated control (nonwithdrawn) group.
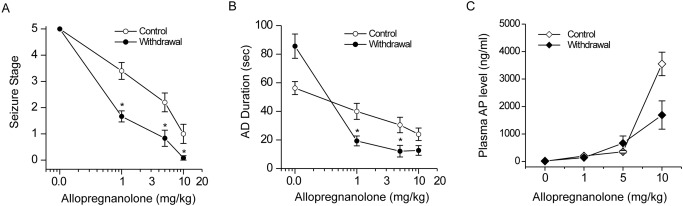
Neurosteroid Withdrawal Confers Enhanced AP Sensitivity in Fully Kindled Mice.
In addition, we investigated the efficacy of the prototype neurosteroid AP in mice 24 h after neurosteroid withdrawal (Fig. 8). Control and neurosteroid-withdrawn mice were tested in the kindling model with three doses of AP (1, 5, and 10 mg/kg i.p.). At these doses, AP exerted dose-dependent suppression of the behavioral seizures (Fig. 8A) and AD duration (Fig. 8B) in control (nonwithdrawal) mice. In contrast, after neurosteroid withdrawal, AP produced greater suppression of behavioral seizures (Fig. 8A) and AD duration (Fig. 8B) with significant effects at 1 and 5 mg/kg compared with the control group. Moreover, plasma levels of AP achieved at various doses of AP treatment were similar between control and withdrawn groups, especially without significant drug accumulation in withdrawn animals (Fig. 8C), indicating that AP sensitivity was not caused by pharmacokinetic factors. The synthetic neurosteroid ganaxolone (1, 3, and 10 mg/kg) also produced enhanced (∼60%) efficacy in fully kindled neurosteroid-withdrawn animals (data not shown), confirming the enhanced sensitivity to neurosteroids in the neurosteroid-withdrawal model of catamenial epilepsy (Reddy and Rogawski, 2001).
Fig. 8.
Neurosteroid withdrawal confers enhanced antiseizure effects to AP in fully kindled mice. Dose-response curves for AP (1–10 mg/kg, i.p.) inhibition of behavioral seizures (A), AD duration (B), and plasma AP levels (C) in control and neurosteroid-withdrawn mice are shown. AP is a neurosteroid that acts on all GABAA receptor isoforms, but has greater sensitivity at δ-containing GABAA receptors. The AP's enhanced antiseizure efficacy in mice undergoing neurosteroid withdrawal was consistent with increased δ-containing GABAA receptor abundance in vivo. Despite similar AP levels in control and withdrawal animals, AP had enhanced antiseizure effect in mice undergoing neurosteroid withdrawal that was not attributed to drug accumulation. Vehicle or AP was injected intraperitoneally 15 min before kindling stimulations or plasma sample collection. Values represent the mean ± S.E.M. (n = 6–8 animals per group). *, p < 0.05 versus AP-treated control (nonwithdrawn) group.
Discussion
The principal findings of the study are that acute inhibition of neurosteroid synthesis or neurosteroid withdrawal in female mice caused exacerbation of seizure activity as evident by an increase in kindling seizure severity, AD duration, and generalized seizure duration. The AD threshold for evoking generalized seizures was markedly decreased 24 h after withdrawal, suggesting vulnerability to seizures. Neurosteroid withdrawal was associated with increased GABAA receptor α4- and δ-subunit expression, reduced antiseizure sensitivity to diazepam, and enhanced antiseizure sensitivity to AP. This potential pathophysiological profile is consistent with clinical catamenial seizure features (Fig. 6B). Taken together, these results suggest that endogenous neurosteroids protect against seizures and their withdrawal, such as that which occurs during menstruation, which may exacerbate seizure activity, providing a novel mouse model of catamenial epilepsy that can be used for the investigation of disease mechanisms and evaluation of novel therapeutic approaches. Our data on δ- and α4-subunit expression provide a potential molecular mechanism for the enhanced seizure susceptibility and drug sensitivity in this epilepsy model.
Alterations in ovarian hormones and modification of GABAergic inhibition are an intensely investigated hypothesis guiding research into pathophysiological mechanisms underlying catamenial epilepsy (Scharfman and MacLusky, 2006; Tuveri et al., 2008; Reddy, 2009). The main concern is the lack of a suitable mouse model to investigate the pathophysiological mechanisms of catamenial seizure exacerbations. Using the neurosteroid-withdrawal paradigm, we developed a rat model and successfully used it for pharmacological testing of agents to inhibit catamenial seizures (Reddy et al., 2001; Reddy and Rogawski, 2001). Catamenial seizures are observed in women with pre-existing epilepsy. Therefore, animal models should mimic this key criterion. Kindling provides a suitable background seizure model for developing a perimenstrual catamenial epilepsy model in female mice. Hippocampus kindling is a widely used model of human complex partial seizures. Unlike the pilocarpine-induced epilepsy model, the kindling model does not result in neuronal loss and maintains reproductive function in female animals. During the menstrual cycle, circulating progesterone levels are low in the follicular phase, but rise in the midluteal phase for approximately 10 to 11 days before declining in the late luteal phase. Circulating AP levels parallel those of its parent progesterone (Tuveri et al., 2008). Although the dynamics of brain AP during the menstrual cycle have not been studied, it is likely that local synthesis of GABAA receptor-modulating neurosteroids occur in regions relevant to epilepsy such as the hippocampus and amygdala (Reddy, 2011). The results from the present study demonstrate a striking increase in seizure activity after neurosteroid withdrawal in the kindling model. The seizure exacerbation was maximal 12 to 24 h after withdrawal and declined to control value at 48 h after withdrawal onset. Thus, neurosteroid withdrawal-associated seizure exacerbation may represent a transient surrogate marker for perimenstrual catamenial epilepsy.
The gonadotropin-induced endocrine state is more physiologically similar to the hormonal milieu of the menstrual cycle than the paradigms that use exogenous progesterone or AP treatment (Shen et al., 2005; Gangisetty and Reddy, 2010). Seizure exacerbation observed in the present study is caused by reduced neurosteroid levels in the brain. Finasteride blocks the synthesis of neurosteroids such as AP and related 5α-reduced pregnane analogs that modulate GABAA receptor function (Mukai et al., 2008). Consistent with the time course for finasteride inhibition of neurosteroid synthesis, we noted a latent period (12–24 h) between finasteride administration and the onset of seizure propensity. Moreover, the seizure susceptibility returned to baseline within 48 h, probably reflecting the time to regenerate 5α-reductase enzyme activity in the brain. Our measurements of plasma AP levels are consistent with the enzyme inhibition effect of finasteride. We have previously published similar results obtained after neurosteroid withdrawal in the pseudopregnancy model (Reddy et al., 2001) and a transitory increase in the frequency of spontaneous seizures in epileptic rats (Reddy and Zeng, 2007). Such heightened seizure susceptibility is consistent with previous reports in related models of neurosteroid withdrawal (Moran and Smith, 1998; Smith et al., 1998a,b). Furthermore, seizure exacerbation as a result of finasteride inhibition of progesterone metabolism has been observed in women with epilepsy (Herzog and Frye, 2003). Patients treated with finasteride had significantly reduced levels of neurosteroids (Dusková et al., 2009). These findings suggest that endogenous neurosteroids in the brain play a key role in controlling seizure propensity, most likely because of their ability to enhance synaptic and tonic inhibition in the hippocampus. It is suggested that the inhibition of neurosteroids may accelerate the putative mechanisms that promote epileptic seizures (see Fig. 6B).
The molecular basis for the enhanced seizure susceptibility in perimenstrual catamenial epilepsy is not completely understood, but may be caused by specific alterations in GABAergic inhibition in the hippocampus. The GABAA receptor composition undergoes dynamic plasticity in response to physiological signals, the hormonal (neurosteroid) milieu, and exogenously administered agents such as benzodiazepines or ethanol (Smith et al., 1998a, 2007; Reddy, 2009). The precise changes in brain GABAA receptor subunit composition occurring during the human menstrual cycle or in animal models of catamenial epilepsy have not been determined. There is strong evidence that ovarian cycle-linked fluctuations in neurosteroids modulate GABAA receptor plasticity (Maguire et al., 2005; Maguire and Mody, 2007). Our observation that neurosteroid withdrawal increases α4-subunit expression in the hippocampus is consistent with the established premise that prolonged exposure to progesterone followed by withdrawal in female rats causes up-regulation of the α4 subunit (Smith et al., 1998a; Gulinello et al., 2001). Similar increases in α4-subunit levels have been observed previously in response to withdrawal from AP (Smith et al., 1998b; Follesa et al., 2000) and synthetic neurosteroids (Mascia et al., 2002). The α4 subunit can coassemble with γ2 to form synaptic GABAA receptors. The key consequence of the incorporation of the normally low-abundance α4 subunit into synaptic GABAA receptors is that currents generated by these receptors have accelerated decay kinetics, so that there is less total charge transfer, which probably results in reduced net synaptic inhibition and a state of hyperexcitability (Smith and Gong, 2005). The increase in α4 when coassembled with either γ2 or δ subunits in the hippocampus may result in benzodiazepine-insensitive receptors (Maguire et al., 2005; Shen et al., 2005). Therefore, when neurosteroids are withdrawn at the time of menstruation, the α4 subunit is up-regulated, and synaptic inhibition is diminished, resulting in enhanced excitability, which, among other effects, causes a predisposition to catamenial seizures.
We observed that neurosteroid-withdrawn mice were strikingly less sensitive to the antiseizure effects of diazepam in the kindling model. These findings are highly consistent with the enhanced abundance of the α4 subunit in vivo in the hippocampus. GABAA receptors containing the α1/2/3/5 subunits in combination with any of the β subunits and the γ2 subunit are most prevalent in the brain (Möhler et al., 2002). These receptors are sensitive to benzodiazepine modulation. Moreover, receptors containing the α4 subunit are highly expressed in the dentate gyrus, but they are benzodiazepine insensitive (Whiting et al., 2000; Möhler et al., 2002). This mechanism may underlie diazepam's reduced protection (insensitivity) against seizures during neurosteroid withdrawal. Similar increases in the α4 subunit and its pharmacological properties have been described after withdrawal from progesterone or neurosteroids (Smith et al., 1998a,b).
In the present study, we found increased expression of the δ subunit in the hippocampus by following the neurosteroid-withdrawal paradigm. The δ subunit preferentially coassembles with the α4 subunit to form perisynaptic/extrasynaptic GABAA receptors (Sur et al., 1999). The δ-containing receptors are expressed predominantly in the dentate gyrus, the gateway that controls hippocampus excitability. Tonic inhibition in dentate gyrus neurons is mediated mainly by δ subunit-containing receptors (Stell et al., 2003). Furthermore, both δ and α4 subunits are increased in the hippocampus of neurosteroid-withdrawn animals. Therefore, the compensatory changes such as increased expression of α4δ subunit-containing receptors seem to preserve GABAergic inhibition in the dentate gyrus around the perimenstrual period associated with heightened excitability. These changes could lead to alterations in tonic inhibition and neurosteroid sensitivity.
In this study, we found that the neurosteroids AP and ganaxolone caused increased anticonvulsant effects in the mouse catamenial epilepsy model. The molecular mechanisms underlying enhanced neurosteroid sensitivity in the catamenial model remain unclear. Our Taqman PCR studies have revealed increased expression of δ subunits in the hippocampus of withdrawing animals. This may be associated with enhanced neurosteroid sensitivity because the δ subunit-containing GABAA receptors have higher neurosteroid sensitivity (Mihalek et al., 1999; Wohlfarth et al., 2002) and are major contributors of tonic inhibition. Thus, neurosteroid withdrawal confers increased sensitivity to neurosteroids, possibly because of increased δ-containing GABAA receptor expression in the hippocampus. Clearly more needs to be learned about the regulation of δ-subunit expression to gain additional insights into tonic inhibition in the catamenial epilepsy model. Nevertheless, enhanced neurosteroid sensitivity in catamenial epilepsy has important therapeutic implications. Synthetic neurosteroids that augment tonic inhibition may provide a rational treatment strategy for controlling catamenial seizures at low doses that do not cause significant GABAergic side effects. Such neurosteroid replacement therapy may prevent seizure occurrence in women with epilepsy (Reddy and Rogawski, 2009).
More importantly, our data on the up-regulation of hippocampal α4 and δ subunits offer a molecular mechanism for the enhanced seizure susceptibility and drug sensitivity in this epilepsy model (Fig. 6B). The enhanced seizure susceptibility subsequent to neurosteroid withdrawal may be related to increased expression of α4-containing synaptic GABAA receptors that exhibit faster decay kinetics and confer benzodiazepine insensitivity. The enhanced potency of neurosteroids may be caused by a relative increase after neurosteroid withdrawal in the expression of neurosteroid-sensitive δ-containing extrasynaptic GABAA receptors. The α4 subunits, which are increased during withdrawal, could coexpress with either or both δ and γ2. Although the increase in neurosteroid sensitivity may be caused by the δ subunit, there is no reason to believe that this is mutually exclusive, because 1) the increase in δ is less than the increase in α4, and although γ2 is unchanged, α1 shows a slight decrease in expression; 2) flumazenil, a benzodiazepine antagonist that acts as an agonist at α4βγ2 (Wafford et al., 1996), produced a greater antiseizure effect during withdrawal (Gangisetty and Reddy, 2010); 3) α4βγ2 receptors exhibit faster kinetics, which probably results in reduced inhibition and increases in the seizure state; and 4) suppression of the α4 subunit prevents the withdrawal-induced increase in seizures (Smith et al., 1998a). Thus, it is likely that seizure exacerbation is not only caused by the relative lack of AP during withdrawal, but could also be caused by increases in α4βγ2 receptors.
In the present study, increased antiseizure activity of AP and ganaxolone is consistent with up-regulation of δ subunit-containing GABAA receptors. The δ subunit increases may be transitory during withdrawal and followed by reduced expression with chronic exposures, as in pregnancy (Maguire and Mody, 2008) or in the prolonged luteal phase of the menstrual cycle. This change is believed to be a compensatory mechanism, which would avoid excessive sedation caused by high neurosteroid levels acting on δ-subunit receptors. At the time of neurosteroid withdrawal, δ expression rapidly recovers. If recovery is not sufficiently fast, however, there could be an enhancement of excitability caused by a reduction in tonic inhibition in the relative absence of neurosteroids.
There are a number of reports on epilepsy-associated plasticity in subunit expression and GABAergic inhibition (Joshi et al., 2011). There is evidence showing that epilepsy models result in reduced expression of the δ subunit in the dentate gyrus (Zhang et al., 2007), although in a mouse model of temporal lobe epilepsy, α4 and γ2 were increased (Peng et al., 2004). A marked decrease in neurosteroid sensitivity was observed in epileptic rats (Mtchedlishvili et al., 2001; Sun et al., 2007). High levels of δ expression, in contrast, would be expected to attenuate the seizure state. Thus, the relevance of increased δ-subunit expression in the catamenial model is unclear. It is suggested that these receptors are merely returning to their normal level of expression, whereas the increase in α4βγ2 would result in neuronal excitability. Further studies with more selective compounds that enhance tonic currents such as gaboxadol or 4-chloro-N-2-(2-thienyl)imidazo[1,2-a]yridine-3-yl-benzamide (DS2) (Wafford et al., 2009) may verify other possible mechanisms.
In conclusion, these findings demonstrate that endogenous neurosteroids protect against seizure susceptibility and their withdrawal, such as that which occurs during menstruation, leads to the exacerbation of seizure activity. This is possibly caused by specific changes in GABAA receptor subunit plasticity and function in female mice, providing a novel mouse model that has features of human catamenial epilepsy. The mouse model is helpful for investigating disease mechanisms and developing novel treatments for catamenial epilepsy.
Acknowledgments
The authors acknowledge the support of the Texas A&M Health Science Center Women's Health in Neuroscience (WHIN) program.
This research was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS051398]. J.G. received support from the Texas A&M Health Science Center College of Medicine Summer Research Program.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- AP
- allopregnanolone
- AD
- afterdischarge
- ADT
- AD threshold
- HCG
- human chorionic gonadotropin
- PMSG
- pregnant mare's serum gonadotropin
- PCR
- polymerase chain reaction
- DS2
- 4-chloro-N-2-(2-thienyl)imidazo[1,2-a]yridine-3-yl benzamide.
Authorship Contributions
Participated in research design: Reddy.
Conducted experiments: Reddy, Gould, and Gangisetty.
Performed data analysis: Reddy.
Wrote or contributed to the writing of the manuscript: Reddy.
References
- Bazán AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. (2005) Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arq Neuropsiquiatr 63:751–756 [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. (2002) The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43:651–661 [DOI] [PubMed] [Google Scholar]
- Brooke DA, Orsi NM, Ainscough JF, Holwell SE, Markham AF, Coletta PL. (2007) Human menopausal and pregnant mare serum gonadotrophins in murine superovulation regimens for transgenic applications. Theriogenology 67:1409–1413 [DOI] [PubMed] [Google Scholar]
- Dusková M, Hill M, Hanus M, Matousková M, Stárka L. (2009) Finasteride treatment and neuroactive steroid formation. Prague Med Rep 110:222–230 [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. (2000) Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABAA receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol 57:1262–1270 [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. (1997) The Mouse Brain in Stereotaxic Coordinates, 1st ed, Academic Press, New York [Google Scholar]
- Gangisetty O, Reddy DS. (2009) The optimization of TaqMan real-time RT-PCR assay for transcriptional profiling of GABA-A receptor subunit plasticity. J Neurosci Methods 181:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. (2010) Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience 170:865–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25:295–330 [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. (2001) Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res 910:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR, Liporace JD, Kalayjian LA, Heck CN, Krauss GL, Dworetzky BA, Pennell PB, and Progesterone Trial Study Group (2011) Variation of seizure frequency with ovulatory status of menstrual cycles. Epilepsia 52:1843–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. (2003) Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol 53:390–391 [DOI] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. (2004) Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol 56:431–434 [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. (1997) Three patterns of catamenial epilepsy. Epilepsia 38:1082–1088 [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. (2007) Neurosteroid binding sites on GABAA receptors. Pharmacol Ther 116:7–19 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Joshi S, Rajasekaran K, Kapur J. (2011) GABAergic transmission in temporal lobe epilepsy: the role of neurosteroids. Exp Neurol http://dx.doi.org/10.1016/j.expneurol.2011.10.028 [DOI] [PMC free article] [PubMed]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. (2010) Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol 67:689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. (2007) Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to the ovarian cycle and stress. J Neurosci 27:2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. (2008) GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron 59:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. (2005) Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8:797–804 [DOI] [PubMed] [Google Scholar]
- Mascia MP, Biggio F, Mancuso L, Cabras S, Cocco PL, Gorini G, Manca A, Marra C, Purdy RH, Follesa P, et al. (2002) Changes in GABAA receptor gene expression induced by withdrawal of, but not by long-term exposure to, ganaxolone in cultured rat cerebellar granule cells. J Pharmacol Exp Ther 303:1014–1020 [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, et al. (1999) Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ-subunit knockout mice. Proc Natl Acad Sci U S A 96:12905–12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8 [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith SS. (1998) Progesterone withdrawal I: pro-convulsant effects. Brain Res 807:84–90 [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. (2001) Diminished allopregnanolone enhancement of GABAA receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol 537:453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y, Higashi T, Nagura Y, Shimada K. (2008) Studies on neurosteroids XXV. Influence of a 5α-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol Pharm Bull 31:1646–1650 [DOI] [PubMed] [Google Scholar]
- Pack AM, Reddy DS, Duncan S, Herzog A. (2011) Neuroendocrinological aspects of epilepsy: important issues and trends in future research. Epilepsy Behav 22:94–102 [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. (2004) Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci 24:8629–8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG, and NIH Progesterone Trial Study Group (2009) Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology 73:223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294 [DOI] [PubMed] [Google Scholar]
- Reddy DS. (2009) The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res 85:1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. (2011) Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol 2:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. (2004) Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther 310:230–239 [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. (2001) Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia 42:328–336 [DOI] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. (2011) Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci 31:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2001) Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42:337–344 [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2009) Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics 6:392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Zeng YC. (2007) Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J 21:885.1417197387 [Google Scholar]
- Scharfman HE, Goodman JH, Rigoulot MA, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ. (2005) Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol 196:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. (2006) The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia 47:1423–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. (2005) Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology 49:573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH. (2005) Neurosteroid administration and withdrawal alter GABAA receptor kinetics in CA1 hippocampus of female rats. J Physiol 564:421–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. (1998a) GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392:926–930 [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. (1998b) Withdrawal from 3α-OH-5α -pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4-subunit in association with increased anxiety. J Neurosci 18:5275–5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. (2007) Neurosteroid regulation of GABAA receptors: Focus on the α4 and δ subunits. Pharmacol Ther 116:58–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A 100:14439–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. (2007) Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4-subunit of GABAA receptors in an animal model of epilepsy. J Neurosci 27:12641–12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. (1999) Preferential coassembly of α4 and γ subunits of the γ-aminobutyric acid A receptor in rat thalamus. Mol Pharmacol 56:110–115 [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, et al. (2008) Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia 49:1221–1229 [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. (1996) Functional characterization of human γ-aminobutyric acid A receptors containing the α4 subunit. Mol Pharmacol 50:670–678 [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. (2009) Novel compounds selectively enhance δ subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology 56:182–189 [DOI] [PubMed] [Google Scholar]
- Whiting P, Wafford KA, McKernan RM. (2000) Pharmacologic subtypes of GABAA receptors based on subunit composition, in GABA in the Nervous System: The View at Fifty Years (Martin DL, Olsen RW. eds) pp 113–126, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. (2002) Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci 22:1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. (2007) Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci 27:7520–7531 [DOI] [PMC free article] [PubMed] [Google Scholar]