Abstract
We observed that glioma cells are differentially sensitive to N-{4-[4-(4′-chloro-biphenyl-2-ylmethyl)-piperazin-1-yl]-benzoyl}-4-(3-dimethylamino-1-phenylsulfanylmethyl-propylamino)-3-nitro-benzenesulfonamide (ABT-737) and administration of ABT-737 at clinically achievable doses failed to induce apoptosis. Although elevated Bcl-2 levels directly correlated with sensitivity to ABT-737, overexpression of Bcl-2 did not influence sensitivity to ABT-737. To understand the molecular basis for variable and relatively modest sensitivity to the Bcl-2 homology domain 3 mimetic drug ABT-737, the abundance of Bcl-2 family members was assayed in a panel of glioma cell lines. Bcl-2 family member proteins, Bcl-xL, Bcl-w, Mcl-1, Bax, Bak, Bid, and Noxa, were found to be expressed ubiquitously at similar levels in all cell lines tested. We then examined the contribution of other apoptosis-resistance pathways to ABT-737 resistance. Bortezomib, an inhibitor of nuclear factor-kappaB (NF-κB), was found to enhance sensitivity of ABT-737 in phosphatase and tensin homolog on chromosome 10 (PTEN)-wild type, but not PTEN-mutated glioma cell lines. We therefore investigated the association between phosphatidylinositol 3-kinase (PI3K)/Akt activation and resistance to the combination of ABT-737 and bortezomib in PTEN-deficient glioma cells. Genetic and pharmacological inhibition of PI3K inhibition sensitized PTEN-deficient glioma cells to bortezomib- and ABT-737-induced apoptosis by increasing cleavage of Bid protein, activation and oligomerization of Bax, and loss of mitochondrial membrane potential. Our data further suggested that PI3K/Akt-dependent protection may occur upstream of the mitochondria. This study demonstrates that interference with multiple apoptosis-resistance signaling nodes, including NF-κB, Akt, and Bcl-2, may be required to induce apoptosis in highly resistant glioma cells, and therapeutic strategies that target the PI3K/Akt pathway may have a selective role for cancers lacking PTEN function.
Introduction
Human malignant gliomas are aggressive tumors that generally respond poorly to current therapy with surgery, radiation, and conventional chemotherapy. The molecular basis of their resistance to apoptosis has not been fully elucidated (Hanahan and Weinberg, 2000, 2011; Omuro et al., 2007). In studies using a large-scale short interfering RNA (siRNA) screening approach to identify critical “nodes” for cell death signaling, we identified several targets, including nuclear factor kappaB (NF-κB) and the proteasome, as well as Akt (Thaker et al., 2009, 2010a,b) and Bcl-2 family members (Kitchens et al., 2011) that, when inhibited, promoted apoptotic signaling in glioma cells. Studies from other groups have also indicated that dysregulation of the NF-κB (Bredel et al., 2011), Bcl-2 (Steinbach and Weller, 2004), and Akt (Knobbe et al., 2002) pathways may be integrally involved in mediating glioma resistance to apoptotic signaling. The recent availability of agents to inhibit these targets suggests the application of apoptosis-promoting strategies for therapy-resistant cancers, such as gliomas.
Members of the Bcl-2 family are critical regulators of apoptosis. Interactions between prosurvival and proapoptotic members may determine cell fate, and the balance of Bcl-2 family proteins may confer constitutive and therapy-inducible resistance in gliomas (Bögler and Weller, 2003). Many cancers, particularly gliomas, are resistant to apoptosis by up-regulation of antiapoptotic Bcl-2 family members. New anticancer therapeutics are being developed to specifically target the prosurvival members of the Bcl-2 family by using small-molecule mimetics of BH3-only proteins. The most prominent among them are the Bcl-2 antagonists N-{4-[4-(4′-chloro-biphenyl-2-ylmethyl)-piperazin-1-yl]-benzoyl}-4-(3-dimethylamino-1-phenylsulfanylmethyl-propylamino)-3-nitro-benzenesulfonamide (ABT-737) and (R)-4-(4-((4′-chloro-4,4-dimethyl-3,4,5,6-tetrahydro-[1,1′-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((4-((4-morpholino-1-(phenylthio)butan-2-yl)amino)-3-((trifluoromethyl)sulfonyl)phenyl)sulfonyl)benzamide (ABT-263). ABT-737 binds specifically and with high affinity to the Bcl-2 family of proteins, including Bcl-2, Bcl-xL, and Bcl-w, but not to Mcl-1. As a single agent, however, ABT-737 induces apoptosis in limited tumor types, such as leukemia and lymphomas (Konopleva et al., 2006; Trudel et al., 2007; Vogler et al., 2008; High et al., 2010; Bodet et al., 2011). The reason for varied sensitivities to ABT-737 is not clear.
Bortezomib, a member of a class of agents inhibiting the 20S proteasome, blocks the elimination of diverse cellular proteins targeted for degradation (Adams, 2004). Among its principal effects are the inhibition of NF-κB. Bortezomib has been approved by the U.S. Food and Drug Administration for multiple myeloma (Kane et al., 2006), mantle cell lymphoma (Kane et al., 2007), and solid tumors (Aghajanian et al., 2002; Papandreou et al., 2004). Bortezomib has shown increased and/or synergistic activity with several novel targeted agents, indicating its potential to substantially enhance the clinical activity of these novel therapies. We have demonstrated that bortezomib dramatically sensitized glioma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (Jane et al., 2011) and vorinostat cytotoxicity (Premkumar et al., 2011), suggesting that this agent may have promise in combination strategies for glioma therapeutics. Other investigators have shown that overexpression of Akt reduced the apoptosis induced by proteasomal inhibitors and TRAIL (Kahana et al., 2011) or histone deacetylase inhibitor (HDACI) (Yu et al., 2008), suggesting that Akt activation may provide a compensatory apoptosis-resistance mechanism in gliomas that may need to be independently inhibited to achieve maximal therapeutic effects.
Akt is commonly activated constitutively in these tumors by virtue of mutations in PTEN, a tumor suppressor gene located on chromosome 10q23 (Li et al., 1997; Wang et al., 1997) that regulates the phosphatidylinositol-3-kinase (PI3K)/Akt pathway (Stambolic et al., 1998). The PTEN gene is frequently deleted or mutated not only in human glioblastoma but also in a wide range of advanced human malignancies, such as prostate, endometrial, breast, lung, kidney, bladder, testis, head, and neck cancers, malignant melanoma, and lymphoma (Li et al., 1997; Steck et al., 1997; Hanahan and Weinberg, 2000, 2011). PTEN inactivation in turn leads to the enhanced phosphorylation and activation of Akt, a cell survival protein kinase that mediates its activity through various downstream effectors, resulting in cell migration and cell cycle progression, in addition to inhibition of apoptosis (Knobbe et al., 2002; Parsons et al., 2008). PTEN mutation and increased Akt/PKB activity have been correlated with poor prognosis in glioma patients (Ermoian et al., 2002).
In view of the potential contributory roles of antiapoptotic proteins, such as Bcl-2, NF-κB, and Akt to glioma apoptosis resistance (Hanahan and Weinberg, 2000, 2011; Knobbe et al., 2002; Omuro et al., 2007; Parsons et al., 2008), we examined the efficacy of ABT-737 and bortezomib in malignant human glioma cell lines as a function of PTEN status and the relationship between inhibition of these targets and apoptosis induction.
Materials and Methods
Cell Lines.
The established malignant glioma cell lines U87 and LN229 were obtained from the American Type Culture Collection (Manassas, VA). LN18, LNZ308, and LNZ428 were provided by Dr. Nicolas de Tribolet (University of Lausanne, Switzerland). U87 cells were cultured in growth medium composed of minimum essential medium; all other lines were cultured in α-minimal essential medium. All growth media contained 10% fetal calf serum, l-glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA) supplemented with sodium pyruvate and nonessential amino acids. LN18, LN229, and LNZ428 are wild type for PTEN. However, LNZ308 and U87 are PTEN-deficient glioma cell lines. PTEN status of these glioma cell lines has been characterized elsewhere (Furnari et al., 1997).
Reagents and Antibodies.
ABT-737, 3-[4-(4-morpholinylpyrido[3′,2′:4,5]furo[3,2-d]pyrimidin-2-yl]phenol (PI-103), and bortezomib were purchased from Chemie Tek (Indianapolis, IN). N-acetyl-l-cysteine (NAC), 2-morpholin-4-yl-8-phenylchromen-4-one (LY294002), and (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester (E64d) were purchased from EMD Chemicals (Gibbstown, NJ). The protein cross-linker dithiobis(succinimidylpropionate) (DSP) was purchased from Thermo Fisher Scientific (Waltham, MA). Caspase inhibitors (Z-VAD-FMK, Z-IETD-FMK, Z-DEVD-FMK, and Z-LEHD-FMK) were purchased from R&D Systems (Minneapolis, MN). The following antibodies from Cell Signaling Technology (Danvers, MA) were used: Bcl-2, Bcl-xL, Mcl-1, Bim, Bak, Bax, A1/Bfl-1, Apaf-1, Bcl-w, Bid, cytochrome c, cleaved poly(ADP)-ribose polymerase (PARP), cleaved caspase-3, cleaved caspase-8, cleaved caspase-9, total Akt, phospho Akt, PTEN, Smac/DIABLO, and β-actin. Noxa and apoptosis-inducing factor (AIF) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Monoclonal anti-Bax was from BD Pharmingen (San Diego, CA).
Cell Proliferation and Cytotoxicity Assay.
Cells (5 × 103/well) were plated in 96-well microtiter plates (Costar; Corning Life Sciences, Cambridge, MA) in 100 μl of growth medium, and after overnight attachment, exposed for 3 days to inhibitors or vehicle (DMSO). After the treatment interval, cells were washed in medium, and the number of viable cells was determined by using a colorimetric cell proliferation assay (CellTiter96 Aqueous Nonradioactive Cell Proliferation Assay; Promega, Madison, WI) as described previously (Jane et al., 2011). Morphological changes in response to inhibitor treatment were evaluated by microscopic inspection and imaging of cells by using an Olympus (Tokyo, Japan) FluoView 1000 microscope. Images were assembled by using Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA).
Clonogenic Growth Assay.
The effect of inhibitor treatment on colony-forming ability was assessed by using a clonogenic assay. A total of 250 cells were plated in six-well trays in growth medium, and after overnight attachment, exposed to inhibitors or vehicle for 1 day. Cells were then washed with inhibitor-free medium, grown for 2 weeks under inhibitor-free conditions, and fixed and stained (Hema 3 Manual Staining Systems; Thermo Fisher Scientific). Plates were then scanned, and images were assembled by using Adobe Photoshop CS2 software.
Annexin V Apoptosis Assay.
Apoptosis was evaluated by using a fluorescein isothiocyanate (FITC)-conjugated annexin V/propidium iodide (PI) assay kit (Invitrogen) based on annexin V binding to phosphatidylserine exposed on the outer leaflet of the plasma membrane lipid bilayer of cells entering the apoptotic pathway as described previously (Jane et al., 2011). In brief, cells were treated with or without inhibitors for the indicated duration, collected by trypsin-EDTA, pelleted by centrifugation (1000 rpm for 5 min), washed in ice-cold phosphate-buffered saline (PBS), and resuspended in the annexin V-FITC and 1 μg/ml propidium iodide reagent in the dark for 15 min before flow cytometric analysis. Labeling was analyzed by flow cytometry with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). A minimum of 20,000 cells per sample was collected.
Subcellular Fractionation.
Cells were treated with or without inhibitors, and cytosolic proteins were fractionated as described previously (Premkumar et al., 2011). In brief, cells were resuspended in a lysis buffer containing 0.025% digitonin, 250 mM sucrose, 20 mM HEPES, pH 7.4, 5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. After 10-min incubation at 4°C, cells were centrifuged (2 min at 13,000g), and the supernatant (cytosolic fraction) was removed and frozen at −80°C for subsequent use.
3′,3′-Dihexyloxacarbo-Cyanine Iodide Labeling and Detection of Mitochondrial Membrane Depolarization.
Mitochondrial membrane depolarization was measured as described previously (Jane et al., 2011; Premkumar et al., 2011). In brief, floating cells were collected, and attached cells were trypsinized and resuspended in PBS. Cells were loaded with 50 nM 3′,3′-dihexyloxacarbo-cyanine iodide (DiOC6; Invitrogen) at 37°C for 15 min. The positively charged DiOC6 accumulates in intact mitochondria, whereas mitochondria with depolarized membranes accumulate less DiOC6. Cells were spun at 3000g, rinsed with PBS twice, and resuspended in 1 ml of PBS. Fluorescence intensity was detected by flow cytometry and analyzed with CellQuest software (BD Biosciences). The percentage of cells with decreased fluorescence was determined.
Western Blotting Analysis.
Western blot analysis was performed as described previously (Jane et al., 2011; Premkumar et al., 2011). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred onto a nylon membrane (Invitrogen). Nonspecific antibody binding was blocked by incubation of the blots with 4% bovine serum albumin in Tris-buffered saline/Tween 20 (0.1%) for 1 h at room temperature. The blots were then probed with appropriate dilutions of primary antibodies as described previously (Jane et al., 2011). Where indicated, the blots were stripped and reprobed with antibodies against β-actin to ensure equal loading and transfer of proteins.
In Vitro Cross-Linking and Analysis of Bax Oligomerization.
Cytosolic and membrane fractions were prepared by selective plasma membrane permeabilization with 0.05% digitonin, followed by membrane solubilization with 1% CHAPS as described elsewhere (Mikhailov et al., 2001). In brief, control and experimental cells in dishes were treated with 0.05% digitonin in isotonic buffer (10 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, and 1 mM EGTA, pH 7.4) containing protease inhibitors [1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 0.8 mM aprotinin, 50 mM bestatin, 15 mM E64d, 20 mM leupeptin, and 10 mM pepstatin A] for 1 to 2 min at room temperature. The permeabilized cells were shifted to 4°C, scraped with a rubber policeman, and collected into centrifuge tubes. The supernatants (digitonin-extracted cytosolic fraction) were routinely collected after centrifugation at 15,000g for 10 min. After centrifugation, the pellet was washed with isotonic buffer and further extracted with ice-cold detergent (1% CHAPS) in isotonic buffer containing protease inhibitors for 60 min at 4°C to release membrane- and organelle-bound proteins including mitochondrial cytochrome c. CHAPS-soluble (membrane fraction) fractions were collected by high-speed (15,000g) centrifugation for 10 min. The protein cross-linker DSP was dissolved in DMSO and prepared just before use. Equal amounts of CHAPS-extracted membrane fraction protein were incubated with 1 mM DSP for 45 min at room temperature and subsequently quenched by adding 20 mM Tris-HCl, pH 7.4. Proteins were resolved by nonreducing SDS-PAGE, and immunoblots were analyzed as described above.
Measurement of Active Bax Staining.
After treatment, cells were detached and fixed in 3.7% prewarmed formaldehyde (Sigma, St. Louis, MO) (15 min, room temperature), and then washed three times (5 min) with PBS buffer. Cells were permeabilized with 0.1% Triton X-100/PBS buffer for 15 min at room temperature before being incubated with blocking solution for 2 h (0.3% bovine serum albumin and 1% goat serum in PBS). Primary antibody was added in the same buffer and incubated overnight at 4°C. Anti-active-human-Bax antibody (6A7; BD Pharmingen) was used at a 1:100 dilution. Fluorescein isothiocyanate-conjugated secondary antibody (Invitrogen) was used at a 1:200 dilution (60 min in the dark, room temperature). Stained cells were analyzed by flow cytometry. The average percentage increase in Bax-activated cells was calculated over the DMSO controls.
Adenovirus Infection.
PTEN wild-type adenovirus (Ad-PTEN-wild type) and Ad-CMV were kindly provided by Dr. Craig Henke (University of Minnesota, Minneapolis, MN) and Dr. Christopher Kontos (Duke University Medical Center, Durham, NC), respectively. Ad-Akt [dominant negative (DN)] was purchased from Vector Biolabs (Philadelphia, PA). Glioma cells were infected with adenovirus vectors at the indicated multiplicity of infection (MOI) for 48 h at 37°C. The medium was changed and treated with inhibitors for the indicated duration. Cells were processed for Western blot or annexin V apoptosis analysis as described above.
Transient Transfection or Stable Cell Line Generation.
Empty vector pcDNA3 was from Dr. Michael Greenberg (Harvard Medical School, Boston, MA). The expression vector pcDNA3-Bcl-2 was obtained from Dr. Stanley Korsmeyer (Dana Farber Cancer Institute, Boston, MA). pcDNA3-Myr-HA-Akt1 was obtained from Dr. William Sellers (Dana Farber Cancer Institute). Glioma cell lines were plated in six-well plates, maintained in complete media, and kept in 37°C in a humidified 5% CO2 incubator. Transfection was performed at 60 to 70% confluence by using FuGENE 6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's recommendations. The total amount of transfected DNA was maintained at 1 μg. After a 48-h transient transfection period, cells were treated with or without inhibitors for an additional 24 h. Stable cell lines were generated after selection with G418 (250 μg/ml) following the method described by the manufacturer (Invitrogen). Cells were processed for Western blot or annexin V apoptosis analysis as described above. Control cells received equal amounts of DMSO.
Statistical Analysis.
Unless otherwise stated, data are expressed as mean ± S.D. The significance of differences between experimental conditions was determined by using a two-tailed Student's t test. Differences were considered significant at p <0.05.
Results
ABT-737 as a Monotherapy Is Ineffective in Human Glioma Cells.
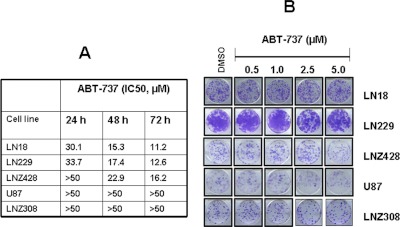
ABT-737 has monotherapeutic toxicity against leukemia, lymphoma, and other malignancies (Konopleva et al., 2006; Tahir et al., 2007; Trudel et al., 2007; Bodet et al., 2011; Song et al., 2011). Established cell lines or primary cells from patients with chronic lymphocytic leukemia (Del Gaizo Moore et al., 2007), acute lymphocytic leukemia (Del Gaizo Moore et al., 2008; High et al., 2010), and B lymphoma (Vogler et al., 2008) have been shown to be extremely sensitive to ABT-737. In this article, we examined the effect of ABT-737 on malignant human glioma cell lines. Cells were treated with increasing concentrations of ABT-737, and cell proliferation was assessed by MTS assay after 24, 48, and 72 h. ABT-737 alone produced minimal effects on cellular proliferation. We observed that glioma cells are differentially sensitive to ABT-737; we found moderately sensitive (72 h, IC50 ∼10–15 μM; LN18, LN229, and LNZ428) and resistant (72 h, IC50 >50 μM; U87 and LNZ308) glioma cell lines (Fig. 1A). Microscopic analysis after 24 h revealed that ABT-737 (10 μM) caused cell rounding, reduced cell size, and blebbing in the ABT-737-sensitive but not in the resistant cell lines (data not shown). We further examined whether ABT-737-treated cells exhibited delayed onset cell death by assessing colony-forming activity. Cells were incubated with either medium or ABT-737 (0–10 μM) for 24 h. After 1 day, inhibitor was removed, and then cells were cultured in inhibitor-free medium for 14 additional days. As shown in Fig. 1B, no significant difference in colony-forming ability was observed between untreated and ABT-737-treated cells, suggesting the limited independent activity of ABT-737 in these tumors when administered within the clinically achievable range.
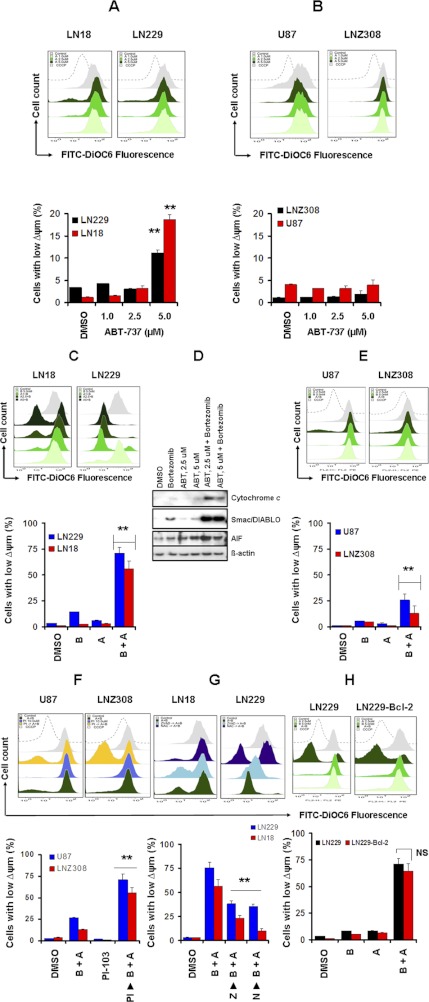
Fig. 1.
ABT-737 as a monotherapy is ineffective in human glioma cells. A, LN18, LN229, LNZ428, U87, and LNZ308 cells were exposed to the indicated concentrations of ABT-737 for 24, 48, and 72 h. The relationship between ABT-737 and cell numbers was assessed semiquantitatively by spectrophotometric measurement of MTS bioreduction in five established malignant human glioma cell lines. Control cells were treated with equivalent concentrations of vehicle (DMSO). B, LN18, LN229, LNZ428, U87, and LNZ308 cells were treated with the indicated concentrations of ABT-737 for 24 h. Control cells received DMSO. Clonogenic assay was performed as described under Materials and Methods.
Overexpression of Bcl-2 in Glioma Does Not Result in Enhanced Protection to ABT-737.
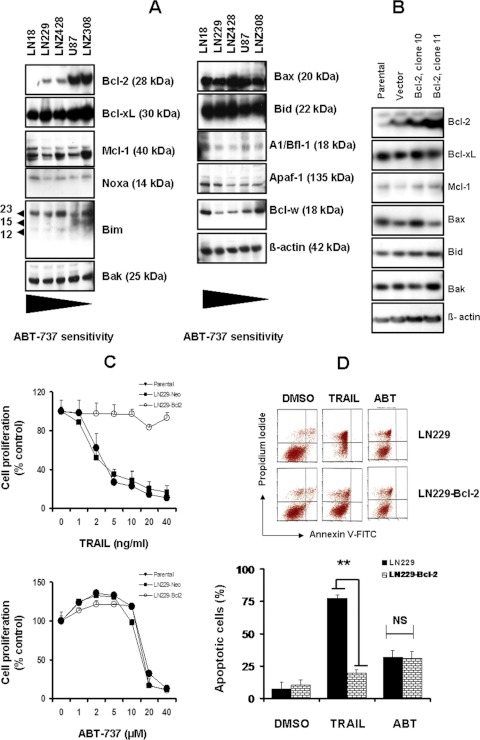
Recent studies have shown that high Bcl-2 or low Mcl-1 expression levels correlate with increased sensitivity to ABT-737 in different cancers (van Delft et al., 2006; Lin et al., 2007; Tahir et al., 2007). Because ABT-737 targets the antiapoptotic Bcl-2 family proteins (Bcl-2, Bcl-xL, and Bcl-w), thereby sequestering proapoptotic BH3 domain proteins, promoting Bax and Bak oligomerization, and ultimately programming the cell death of malignant cells (Oltersdorf et al., 2005), we studied the expression profile of Bcl-2 family members. As shown in Fig. 2A, Bcl-2 was expressed at variable levels with the highest levels detected in U87 and LNZ308 cells (ABT-737-resistant cell lines), and the lowest levels were in LN18, LN229, and LNZ428 cells (moderately sensitive to ABT-737). However, all cell lines ubiquitously expressed Bcl-xL, Bcl-w, and Mcl-1. For proapoptotic proteins, Bax, Bak, Bid, and Noxa was expressed ubiquitously at similar levels in all cell lines tested.
Fig. 2.
Overexpression of Bcl-2 in glioma does not result in enhanced protection to ABT-737. A, five established human glioma cells were seeded at 60% confluence and allowed to attach overnight. Cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. β-Actin served as loading control. B, Western blots of representative stable clones derived from the LN229 cell line transfected with a Bcl-2 expression vector (clones 10 and 11), the empty vector (vector), or the parental cell LN229 line (parental). Overexpression of Bcl-2 did not result in changes in expression of other Bcl-2 family members. C, MTS proliferation assays of stably expressing LN229-Bcl-2 cell lines were conducted to assess their dose response to TRAIL (top) or ABT-737 (bottom). Control cells received DMSO. Points represent the mean of three experiments ± S.D. Overexpression of Bcl-2 almost completely inhibited TRAIL-induced effects on cell numbers; whereas there was no indication that enhanced Bcl-2 expression in turn protected glioma cells against ABT-737. D, vector control (LN229) or Bcl-2 stably expressing transfectants (LN229-Bcl-2) cells were seeded at 60% confluence, allowed to attach overnight, and treated with ABT-737 (10 μM) or TRAIL (10 ng/ml) for 24 h. Control cells received equivalent amounts of DMSO. Apoptosis was assessed by annexin V-FITC and PI staining and FACS analysis as described under Materials and Methods. The representative histogram (top) and the bar chart (bottom; indicating apoptotic cells) show data from three independent experiments. **, p < 0.005. NS, not significant.
To determine the role of Bcl-2 in ABT-737-induced apoptosis, ABT-737-sensitive LN229 cells were stably transfected with the human Bcl-2 cDNA or vector alone (pcDNA3). G418-resistant clones found to overexpress Bcl-2 proteins were selected and used for subsequent experiments (clones 10 and 11). Overexpression of Bcl-2 did not result in changes in expression of other Bcl-2 family members (Fig. 2B). Bcl-2-overexpressing cells were incubated with increasing concentrations of TRAIL or ABT-737, and cell proliferation (after 72 h) was assessed by MTS cell proliferation assay. As shown in Fig. 2C, overexpression of Bcl-2 almost completely inhibited TRAIL-induced cell killing (Fig. 2C, top); whereas there was no indication that enhanced Bcl-2 expression in turn influenced glioma cells response to ABT-737 (Fig. 2C, bottom). Annexin V/PI dye binding assay revealed that treatment with TRAIL resulted in 78% cell death in LN229 cells expressing control vector compared with 20% cell death in LN229 cells stably expressing Bcl-2 (Fig. 2D). However, there was no indication that enhanced Bcl-2 expression in turn protected cells from ABT-737 toxicity, suggesting that high levels of Bcl-2 expression did not play a key role in mediating the resistance to ABT-737 in malignant human glioma cell lines.
Cotreatment of Bortezomib and ABT-737 Induces Apoptotic Cell Death.
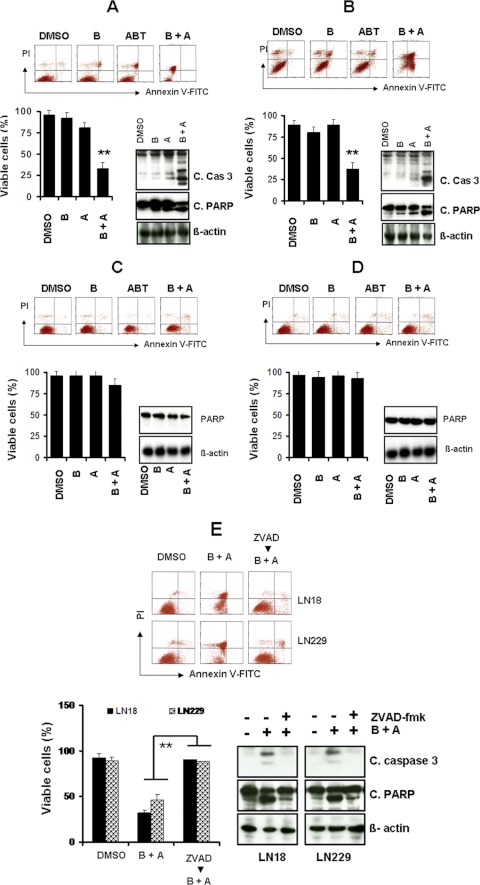
In our recent studies, we demonstrated that bortezomib exhibited significant activity against proliferation in glioma cells and sensitized highly resistant glioma cells to TRAIL (Jane et al., 2011) or HDACI-induced cytotoxicity (Premkumar et al., 2011). Because several members of the Bcl-2 family (Yu et al., 2008; Premkumar et al., 2011), Akt (Yu et al., 2006) and NF-κB (Jane et al., 2011), are known targets of bortezomib in glioma, we investigated the combination of bortezomib and ABT-737 to assess sensitivity in vitro. The combination of ABT-737 and bortezomib strongly induced apoptosis and activated caspase-3 and PARP, and to a lesser extent, caspase-8 and caspase-9 (data not shown), relative to untreated control cells in ABT-737-sensitive LN229 (Fig. 3A) and LN18 (Fig. 3B) cell lines. In contrast, cotreatment of bortezomib and ABT-737 failed to induce apoptosis in U87 (Fig. 3C) and LNZ308 (Fig. 3D) cell lines. Annexin V/PI apoptosis analysis further demonstrated that U87 and LNZ308 cells showed no sensitivity to ABT-737 (0–10 μM) and minimal or no response in combination with bortezomib (10 μM ABT-737 + 5 nM bortezomib) (data not shown).
Fig. 3.
Cotreatment of bortezomib and ABT-737 induces apoptotic cell death. A to D, LN18 (A), LN229 (B), U87 (C), and LNZ308 (D) cells were seeded at 60% confluence, allowed to attach overnight, and treated with 5 nM bortezomib (B), 2.5 μM ABT-737 (A), or a combination of both (B + A). Control cells received an equivalent amount of DMSO. Apoptosis was assessed by annexin V-FITC and PI staining and FACS analysis as described under Materials and Methods. The representative histogram (top) and the bar chart (bottom left; indicating apoptotic cells) show data from three independent experiments. Asterisks indicate significant differences in relation to single agent alone (**, p < 0.005). In parallel, cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies (bottom right). β-Actin served as loading control. E, LN18 and LN229 cells were pretreated with 25 μM Z-VAD-FMK (pan caspase inhibitor) for 2 h followed by the combination of bortezomib (5 nM) plus ABT-737 (2.5 μM; B + A) for 24 h. Control cells received an equivalent amount of DMSO. Apoptosis (top; representative annexin V binding histogram; bottom left, bar chart representing three independent experiments) was analyzed by flow cytometry as described under Materials and Methods. **, P < 0.005, values considered statistically significant. In parallel, cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies (bottom right). β-Actin served as loading control.
To demonstrate that apoptosis induced by ABT-737 and bortezomib is caspase-dependent; we pretreated cells with Z-VAD-FMK and observed the combination of bortezomib and ABT-737-induced caspase-3 and PARP activation. As shown in Fig. 3E, the addition of the broad-range caspase inhibitor Z-VAD-FMK completely abolished ABT-737 + bortezomib-induced PARP activation in the LN18 and LN229 cell lines. Annexin V apoptosis assay (Fig. 3E) clearly demonstrated that ABT + bortezomib-induced cell death was inhibited by Z-VAD-FMK. Taken together, these experiments demonstrate that the combination of ABT-737 and bortezomib induces apoptosis in a subset of malignant human glioma cell lines, although others remain resistant.
The High Levels of Akt in PTEN-Deficient U87 and LNZ308 Cell Lines Mediate the Reduced Response to ABT-737 and Bortezomib-Induced Apoptosis.
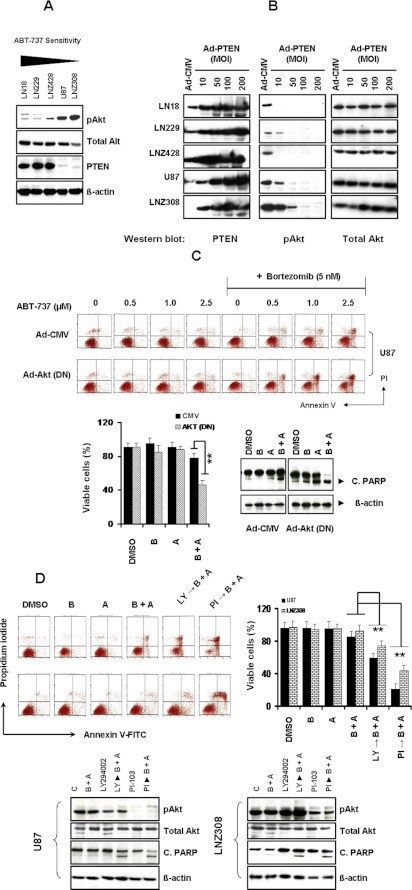
Because Akt is a central regulator of many intracellular processes implicated in glioma tumor progression and PTEN, the negative regulator of Akt, is functionally inactivated in a significant proportion of advanced human gliomas (Chakravarti et al., 2004; Parsons et al., 2008), we explored whether increased Akt activity may be involved in ABT-737 + bortezomib resistance. To test the hypothesis that response to ABT-737 and bortezomib is associated with PTEN status, first we explored the relationship between PTEN and the activation status of Akt by Western blot analysis using phospho-active Akt antibody, which recognizes the phosphorylation of Ser473 of Akt, and PTEN antibody, respectively. Western blot analysis revealed that LN18, LN229, and LNZ428 (PTEN-proficient) cells express PTEN protein, whereas PTEN-mutated U87 and LNZ308 cells do not express this protein (Fig. 4A). PTEN-expressing cells possess lower levels of activated (phosphorylated) Akt compared with PTEN-deficient cells. No change in total Akt and β-actin levels was observed (Fig. 4A).
Fig. 4.
The high levels of Akt in PTEN-deficient U87 and LNZ308 cell line mediate the reduced response to ABT-737 and bortezomib-induced apoptosis. A, five established human glioma cells were seeded at 60% confluence and allowed to attach overnight. Cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. β-Actin served as loading control. B, logarithmically growing LN18, LN229, LNZ428, U87, and LNZ308 cells were infected with Ad-PTEN at the indicated MOI or empty vector (Ad-CMV, 200 MOI per cell). Forty eight hours after infection, cells were lysed, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. C, U87 cells were infected with 50 MOI of empty vector (Ad-CMV) or Ad-Akt-dominant negative (Ad-Akt DN). Forty eight hours after infection, cells were incubated in the presence of bortezomib (5 nM) and ABT-737 (indicated concentration) for 24 h. Control cells received DMSO (vehicle). At the end of the incubation period, the viable cell numbers were determined by flow cytometric analysis (top). In the histogram (bottom left), the mean number of apoptotic cells acquired from three independent experiments is shown. B, 5 nM bortezomib; A, 2.5 μM ABT-737; B + A, combination of bortezomib (5 nM) and ABT-737 (2.5 μM). The values represent the mean ± S.D. **, P < 0.005 values considered statistically significant. In parallel experiments (bottom right) cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. B, 5 nM bortezomib; A, 2.5 μM ABT-737; B + A, combination of bortezomib (5 nM) and ABT-737 (2.5 μM). β-Actin served as loading control. D, U87 and LNZ308 cells were seeded at 60% confluence and allowed to attach overnight. The next day, cells were pretreated with 25 μM LY294002 or 5 μM PI-103 (PI3K/Akt inhibitor) for 2 h followed by the inhibitors bortezomib (5 nM) or ABT-737 (2.5 μM) or a combination of both (B + A) for 24 h. Control cells received DMSO (vehicle). At the end of the incubation period, the viable cell numbers were determined by flow cytometric analysis (top left). The bar chart (top right) represents the mean number of apoptotic cells acquired from three independent experiments. The values represent the mean ± S.D. **, P < 0.005 values considered statistically significant. In parallel experiments (bottom) cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. B, 5 nM bortezomib; A, 2.5 μM ABT-737; B + A, combination of bortezomib (5 nM) and ABT-737 (2.5 μM). β-Actin served as loading control.
To test whether Akt inhibition restores bortezomib and ABT-737 sensitivity, we used pharmacologic and genetic tools to perturb these pathways. PTEN-deficient U87 and LNZ308 cells were infected with adenovirus-expressing empty vector (Ad-CMV) or Ad-PTEN. As shown in Fig. 4B, the initial adenoviral infection and immunoblotting experiments revealed a dose-dependent increase of PTEN activation in glioma cells without altering total Akt protein level. As expected, expression of wild-type PTEN efficiently led to the dephosphorylation of Akt/PKB kinase (Fig. 4B), a downstream target of the PI3K-Akt pathway that is dephosphorylated and inactivated by PTEN (Stambolic et al., 1998), indicating that the increased Akt expression in the PTEN-mutated cell lines directly resulted from reduced PTEN expression and restoration of PTEN expression reversed this effect.
To determine whether directly inhibiting Akt could restore bortezomib and ABT-737 sensitivity, U87 cells were infected with adenovirus-expressing dominant negative Akt (Ad-Akt-DN) or empty vector (Ad-CMV). Forty eight hours after infection, cells were incubated with or without bortezomib and ABT-737 for an additional 24 h, and apoptosis was determined by fluorescence-activated cell sorting (FACS) and Western blot analysis. Cotreatment with bortezomib and ABT-737 in cells infected with DN Akt resulted in a significant increase in apoptosis (Fig. 4C, top). Akt (DN)-infected U87 cells were more sensitive to bortezomib + ABT-737 (55% apoptosis compared with 22% in vector control; Fig. 4C, bottom left). This was further confirmed by Western blot analysis (combination of bortezomib and ABT-737 resulted in pronounced activation of PARP; Fig. 4C, bottom right), suggesting that the inhibition of the PI3K/Akt signaling pathway was critical to the combined effect of bortezomib and ABT-737 in PTEN-deficient glioma cell lines. A similar result was obtained when cells were infected with PTEN adenovirus (data not shown). To validate these results, U87 and LNZ308 cells were pretreated with the pharmacological inhibitor of PI3K/Akt, LY294002, or PI-103 in complete medium 2 h before treatment with bortezomib + ABT-737 for 24 h. Cells were processed for annexin V apoptosis by FACS analysis. Figure 4D revealed that pretreatment with LY-294002 or PI-103 resulted in 40 and 80% cell death compared with ABT-737 + bortezomib resulting in 15% cell death in U87 cells (Fig. 4D). These data clearly suggest that elevated expression of phosphorylated Akt caused by the loss of PTEN function may play an important role in ABT-737 and bortezomib resistance seen in malignant human glioma cell lines.
Cotreatment of ABT-737 and Bortezomib Potentiates Cytochrome c Release from Mitochondria and Induces Mitochondrial Transmembrane Potential Decrease.
Because bortezomib induces mitochondrial membrane potential (Δψm) dissipation as a mechanism for promoting apoptosis (Jane et al., 2011; Premkumar et al., 2011), and mitochondrial changes are necessary for the activation of downstream caspases (Nencioni et al., 2005), we compared the effect of bortezomib and ABT-737 on Δψm in PTEN-intact (LN18 and LN229) and PTEN-deficient (U87 and LNZ308) cell lines by using DiOC6 (Petit et al., 1990). The mitochondrial uncoupler carbonylcyanide m-chlorophenylhydrazone (CCCP) was used as a positive control for Δψm disruption. As shown in Fig. 5A, ABT-737 treatment (0–2.5 μM for 24 h) had no effect on Δψm as measured by flow cytometry. However, treatment of 5.0 μM ABT-737 significantly increased the LN229 (11%) and LN18 (19%) cell population with low Δψm (Fig. 5A). However, no significant change in Δψm (Fig. 5B) was seen in the U87 or LNZ308 cell lines. Then we investigated the combined effect of bortezomib and ABT-737. The number of LN18 and LN229 cells with Δψm increased to 60 and 72%, respectively (from ∼ 7% in single-agent treatment) after ABT-737 and bortezomib treatment (Fig. 5C). Because disruption of Δψm may cause the release of cytochrome c and other mitochondrial apoptogenic proteins, we examined the release of cytochrome c, Smac/DIABLO, and apoptosis-inducing factor to the cytosol after inhibitor treatment, alone or in combination in LN229 cells. As shown in Fig. 5D, the signal of cytochrome c and Smac/DIABLO was barely detectable in the cytosol when the cells were incubated with bortezomib or ABT-737 alone, but was clearly detected with the combination of bortezomib and ABT-737. Under the same conditions, we also detected the release of apoptosis-inducing factor (AIF), another apoptotic regulator, with a similar pattern to that of cytochrome c release. However, the percentage of cells with low Δψm increased modestly in U87 and LNZ308 cells (26 and 13% respectively) after ABT-737 + bortezomib treatment (Fig. 5E).
Fig. 5.
Cotreatment with ABT-737 and bortezomib potentiates cytochrome c release from mitochondria and induces mitochondrial transmembrane potential decrease. A and B, LN18 and LN229 (A) or U87 and LNZ308 (B) cells were treated with ABT-737 at the indicated concentrations for 24 h. Losses of mitochondrial membrane potential were determined by DiOC6 staining and flow cytometry. Untreated control cells received equivalent amounts of DMSO. Carbonylcyanide m-chlorophenylhydrazone (50 μM) served as positive control. Histogram (top) and the bar chart (bottom) represent the mean number of Δψm cells acquired from three independent experiments. Increasing the dose of ABT-737 resulted in a statistically significant loss of mitochondrial membrane potential in LN18 and LN229 but not in U87 and LNZ308. The values represent the mean ± S.D. Asterisks indicate significant differences in relation to control (**, p < 0.005). C, LN18 and LN229 cells were treated with 1 or 2.5 μM ABT-737 in combination with or without bortezomib (5 nM) for 24 h. Control cells received equivalent amounts of DMSO. Loss of mitochondrial membrane potential was determined by DiOC6 staining and flow cytometry as described under Materials and Methods. Histogram (top) and bar chart (bottom) represent the mean number of Δψm cells acquired from three independent experiments. Asterisks indicate significant differences in relation to single agent alone (**, p < 0.005). D, LN229 cells were incubated with inhibitors at the indicated concentrations of ABT-737 with or without bortezomib (5 nM) for 24 h. Control cells received equal amounts of DMSO. Cytosolic extracts and the digitonin-insoluble pellets were prepared as described under Materials and Methods. Equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. E, U87 and LNZ308 cells were treated with 2.5 μM ABT-737 in combination with or without bortezomib (5 nM) for 24 h. Control cells received equivalent amounts of DMSO. Loss of mitochondrial membrane potential was determined by DiOC6 staining and flow cytometry as described under Materials and Methods. Histogram (top) and the bar chart (bottom) represent the mean number of Δψm cells acquired from three independent experiments. Asterisks indicate significant differences in relation to single agent alone (**, p < 0.005). F, U87 and LNZ308 cells were seeded at 60% confluence and allowed to attach overnight. The next day, cells were pretreated with 5 μM PI-103 (PI3K/Akt inhibitor) for 2 h followed by the inhibitors bortezomib (5 nM) or ABT-737 (2.5 μM), or the combination of both (B + A) for 24 h. Control cells received equivalent amounts of DMSO. Loss of mitochondrial membrane potential was determined by DiOC6 staining and flow cytometry as described under Materials and Methods. Histogram (top) and the bar chart (bottom) represent the mean number of Δψm cells acquired from three independent experiments. PI3/Akt inhibition enhanced bortezomib + ABT-737-induced loss of mitochondrial membrane potential (the values represent the mean ± S.D; **, p < 0.005). G, LN18 and LN229 cells were pretreated with 25 μM Z-VAD-FMK (Z, pan caspase inhibitor) or 5 mM NAC (N, ROS scavenger) for 2 h followed by the combination of bortezomib (5 nM) and ABT-737 (2.5 μM, B + A) for 24 h. Control cells received equivalent amounts of DMSO. Loss of mitochondrial membrane potential was determined by DiOC6 staining and flow cytometry as described under Materials and Methods. Histogram (top) and the bar chart (bottom) represent the mean number of Δψm cells acquired from three independent experiments. Caspase inhibitor and ROS scavenger partially inhibit bortezomib + ABT-737-induced loss of mitochondrial membrane potential (the values represent the mean ± S.D; **, p < 0.005). H, vector control (LN229) or Bcl-2 stably expressing transfectants (LN229-Bcl-2) cells were seeded at 60% confluence and allowed to attach overnight. Cells were treated with 2.5 μM ABT-737 in combination with or without bortezomib (5 nM) for 24 h. Control cells received equivalent amounts of DMSO. Loss of mitochondrial membrane potential was determined by DiOC6 staining and flow cytometry as described under Materials and Methods. Histogram (top) and the bar chart (bottom) represent the mean number of Δψm cells acquired from three independent experiments. The values represent the mean ± S.D. NS, not significant.
To validate the above findings that the elevated expression of PI3K/Akt signaling inhibits ABT-737- and bortezomib-induced apoptosis by maintaining the integrity of mitochondria in PTEN-deficient cell lines, we examined whether the inhibition of PI3K/Akt signals would alter mitochondrial transmembrane potential. U87 and LNZ308 cells were pretreated with the Akt inhibitor PI-103 2 h before bortezomib + ABT-737 treatment for 24 h. As shown in Fig. 5F, treatment of U87 and LNZ308 cell lines with PI-103 led to a significant loss of Δψm as indicated by an increase in the percentage of cells showing Δψm from 26% in ABT-737 + bortezomib-treated U87 cells to 71% in cells pretreated with PI-103, suggesting that the PI3K/Akt kinase may lie upstream of mitochondria.
To determine whether the loss of mitochondrial potential in LN229 cells was a direct result of caspase activation, we examined the effect of Z-VAD-FMK on ABT-737- and bortezomib-induced dissipation of Δψm. Preincubation of LN229 cells with the pan caspase inhibitor (Z-VAD-FMK) or the ROS scavenger (NAC) prevented the loss of Δψm (reduced to 23 or 47%, respectively), suggesting that cotreatment with bortezomib + ABT-737 promoted Δψm, leading to apoptotic death (Fig. 5G) via the caspase-dependent pathway. Then we examined whether overexpression of Bcl-2 protected LN229 cells from the loss of Δψm. As shown in Fig. 5H, we found that there was no significant change in Δψm from the stably transfected cells and the vector-transfected cells. Taken together, these data suggest that the phosphorylation level of Akt, a key antiapoptotic protein (and PTEN status), plays an important role in maintaining mitochondrial membrane integrity, thus determining the sensitivity of glioma cells to ABT-737 and bortezomib treatment.
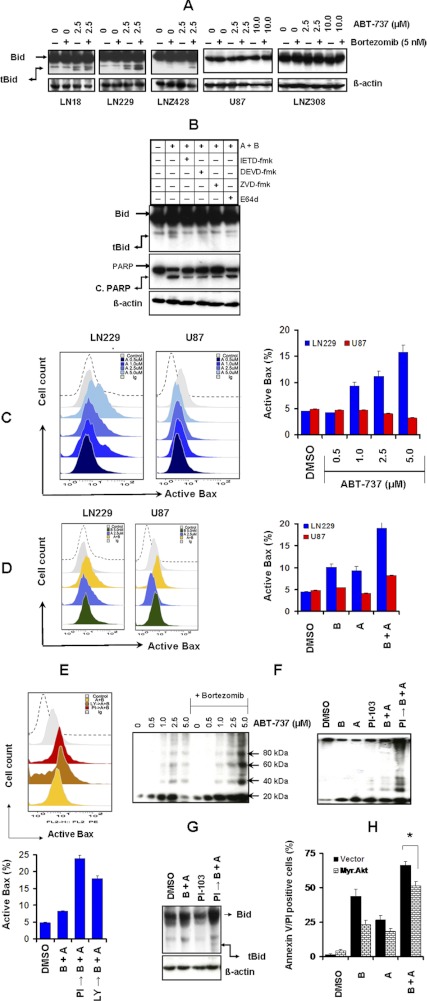
Activation of Bid and Bax Contributes to Bortezomib-Mediated Sensitization to ABT-737.
Bcl-2 family member Bid, a substrate of caspase-8, can move to the mitochondrial membrane and contributes to the opening of the permeability transition pore when Bid is cleaved by caspase (Zamzami et al., 2000). Because a decrease in Δψm and release of cytochrome c from the mitochondria have been shown to be initiated by the translocation of a 15-kDa truncated Bid (tBid) from the cytosol to the mitochondria (Yin, 2000), we investigated the effect of bortezomib and ABT-737 on this process in glioma cell lines. As shown in Fig. 6A, treatments with these inhibitors individually caused no effect on Bid truncation. However, the combination of ABT-737 with bortezomib treatment significantly increased the cleaved forms of Bid in LN18, LN229, and LNZ428 (PTEN-intact) cell lines (Fig. 6A). In contrast, no Bid cleavage was evident in U87 and LNZ308 (PTEN-deficient) cell lines (Fig. 6A). For example, U87 and LNZ308 cells showed no sensitivity to ABT-737 (0–10 μM) and no response (appearance of tBid fragment) in combination with bortezomib (10 μM ABT-737 + 5 nM bortezomib).
Fig. 6.
Activation of Bid and Bax contributes to bortezomib-mediated sensitization to ABT-737. A, LN18, LN229, LNZ428, U87, and LNZ308 cells were seeded at 60% confluence, allowed to attach overnight, and treated with 5 nM bortezomib (B) or ABT-737 (indicated concentrations) or a combination of both. Control cells received equivalent amount of DMSO. Cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. β-Actin served as loading control. B, LN229 cells were pretreated with inhibitors of either caspase-8 (Z-IETD-FMK), caspase-3 (Z-DEVD-FMK), pan-caspase (Z-VAD-FMK), or calpain (E64d) at 25 μM for 2 h before the addition of bortezomib + ABT-737. Cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. β-Actin served as loading control. C, Bax conformational changes in LN229 or U87 cells treated with the indicated concentrations of ABT-737 are presented. After treatment, cells were fixed with formaldehyde and then incubated with 6A7 monoclonal anti-Bax antibody. After incubation with FITC-conjugated secondary antibody for 2 h, the signals of activated conformation of Bax proteins were measured by flow cytometry (representative histograms, left; bar chart, right). Data are representative of three independent experiments. D, LN229 and U87 cells were treated with 2.5 μM ABT-737 in combination with or without bortezomib (5 nM) for 24 h. Control cells received equivalent amounts of DMSO. Bax conformational changes were measured as described under Materials and Methods by flow cytometry (representative histogram, left; bar chart, right). Data are representative of three independent experiments. E, U87 cells were pretreated with 25 μM LY294002 (LY → B + A) or 5 μM PI-103 (PI → B + A) for 2 h before the addition of bortezomib + ABT-737 (B + A). Control cells received equivalent amounts of DMSO. Bax conformational changes were measured as described under Materials and Methods by flow cytometry (representative histogram, top; bar chart, bottom). Data are representative of three independent experiments. F, LN229 cells (left) were treated with ABT-737 (indicated concentrations) in combination with or without bortezomib (5 nM) for 24 h. Control cells received equivalent amounts of DMSO. U87 cells (right) were pretreated with 5 μM PI-103 (PI → B + A) for 2 h before the addition of 5 nM bortezomib (B) or 2.5 μM ABT-737 (A) or combination of both (B + A). Control cells received equivalent amounts of DMSO. Membrane fractions were obtained as described under Materials and Methods, and proportional amounts corresponding to total protein were analyzed for Bax oligomerization by Western blotting under nonreducing conditions. Slow-moving Bax oligomers in DSP cross-linked cells were derived from Bax monomers, and the molecular masses of oligomers containing Bax were calculated by plotting their migrations against migrations of molecular mass standards. G, U87 cells were pretreated with 5 μM PI-103 (PI → B + A) for 2 h before the addition of both bortezomib (5 nM) and 2.5 μM ABT-737 (B + A). Control cells received equivalent amounts of DMSO. Cell extracts were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. β-Actin served as loading control. H, LN229 cells were transiently transfected with pcDNA 3 (vector) or Myr. Akt1. Forty eight hours after transfection, cells were treated with bortezomib (B, 5 nM), ABT-737 (A, 2.5 μM), or a combination of both (B + A). Apoptosis was determined by FACS analysis. Data represent mean ± S.D of three independent experiments carried out in triplicate. *, p < 0.005.
Because caspase-8 (Gross et al., 1999), caspase-3 (Slee et al., 2000), and calpain (Mandic et al., 2002) are the best characterized proteases responsible for the cleavage of Bid, we incubated LN229 cells with caspase-8 (Z-IETD-FMK), caspase-3 (Z-DEVD-FMK), pan-caspase (Z-VAD-FMK), or calpain (E64d) inhibitors at 25 μM for 2 h before the addition of bortezomib and ABT-737. As shown in Fig. 6B, pretreatment of cells with caspase-8, caspase-3, and pan caspase inhibitors completely prevented inhibitor-induced generation of tBid, suggesting that caspase-8 and caspase-3 are indispensable for bortezomib- and ABT-737-induced cleavage of Bid to tBid, accompanied by reduction in the levels of active PARP. However, blockade of calpain activity with E64d had no effect on the proteolytic processing of Bid and PARP cleavage after inhibitor treatment.
Because tBid is thought to occur via its stimulating effect on the conformational change of Bax, leading to trigger the release of cytochrome c (Esposti, 2002), thus facilitating formation of the Bax/Bak complex (Tagscherer et al., 2008) that initiates mitochondrial membrane permeabilization and apoptosis (Desagher et al., 1999), we examined the effect of ABT-737 and bortezomib on Bax activation. Cells were treated with or without bortezomib and ABT-737 for 24 h. Bax activation is a highly regulated, multistep process involving mitochondrial translocation and oligomerization, and this process ultimately leads to mitochondrial dysfunction and apoptosis (Annis et al., 2005). To examine the effect of bortezomib and ABT-737, we analyzed activity-related conformational changes of Bax by flow cytometric analysis with antibodies recognizing N-terminal epitopes of Bax (anti-bax, 6A7). When LN229 cells were exposed to ABT-737 (1–5 μM) alone, a clear dose-dependent (15.8% in cells exposed to 5 μM) increase in Bax conformational change was observed (Fig. 6C). On the other hand, bortezomib (5 nM) alone minimally induced Bax conformational change. It is noteworthy that cells coexposed to bortezomib and ABT-737 displayed a further increase in Bax conformational change compared with cells treated with ABT-737 alone (Fig. 6D). Parallel studies were then done in ABT-737-resistant U87 cells to assess Bax conformational change. In contrast to results involving LN229, ABT-737 by itself had no effect on Bax activation (3.21% in cells exposed to 5 μM; Fig. 6C), whereas bortezomib + ABT-737 modestly induced Bax activation (approximately 8%, Fig. 6D). Then we examined whether inhibition of PI3K/Akt signals would alter Bax activation in U87 cells. Cells were pretreated with the Akt inhibitor PI-103 or LY294002 2 h before bortezomib + ABT-737 treatment for 24 h. For example, as shown in Fig. 6E, treatment of U87 cells with Akt inhibitor led to a significant increase in Bax activation (pretreatment with PI-103 led to 23.9% and LY294002 led to 17.7% from 8.25%), suggesting that that PI3K/Akt kinase may play a vital role in Bax activation.
Upon the induction of apoptosis, Bax migrates to the mitochondria, where it is integrated into the outer membrane as a monomer. Bax monomers then oligomerize and may form large pores in the outer mitochondrial membrane (Annis et al., 2005). Because cleaved Bid migrates to mitochondria where it induces permeabilization of the outer mitochondrial membrane that depends on the proapoptotic proteins Bax and/or Bak oligomerization (Desagher et al., 1999; Korsmeyer et al., 2000; Billen et al., 2008), we sought to determine whether bortezomib and ABT-737 might cause Bax oligomerization in PTEN-intact LN229 cells. Membrane fractionation was performed followed by chemical cross-linking with disuccinimidyl suberate as described under Materials and Methods. Western blot analysis of cross-linked proteins showed that Bax oligomerized into dimers and higher in multiples of ∼20-kDa monomers (Fig. 6F, left), suggesting that these represented newly formed complexes of Bax.
Then we tested the involvement of the PI3K/Akt pathways on Bax activation. PTEN-deficient U87 cells were pretreated with PI-103 (10 μM) for 2 h before challenging them with ABT-737 and bortezomib for 24 h. The combination of bortezomib + ABT-737 induced a minimal activation of Bax oligomerization. As expected, pretreatment with PI-103 evoked robust Bax oligomerization (Fig. 6F, right) and generation of tBid fragments (Fig. 6G). We next tried to determine whether overexpression of a constitutively active (myristolated) Akt1 could protect PTEN-intact LN229 cells from bortezomib + ABT-737-induced apoptosis. Cells were transfected with either the empty vector or the Myr.Akt 1 construct, and apoptosis was assessed by annexin V FACS. As shown in Fig. 6H, cotreatment of ABT-737 and bortezomib induced significant levels of apoptosis in vector-transfected LN229 cells (∼68%). However, the apoptotic potential of ABT-737 + bortezomib was reduced to 50% in cells transfected with Myr.Akt 1, strongly suggesting the involvement of the Akt pathway.
Discussion
Multiple factors contribute to the development of drug resistance of cancer cells, including reduction of intracellular drug accumulation, increase in DNA damage repair, constitutive activation of PI3K/Akt signaling, activation of the Ras and mitogen-activated protein kinase pathways, and dysfunction of the tumor suppressor genes. In this article, we have shown for the first time that elevated expression of Akt is a crucial mediator of apoptosis sensitivity in response to ABT-737 and bortezomib, inhibitors of Bcl-2 and NF-κB, respectively, in glioma cells. This calls attention to the need to target multiple apoptosis-promoting mediators in these treatment-resistant tumors. Our study showed that, unlike hematologic malignancies (Konopleva et al., 2006; Vogler et al., 2008; High et al., 2010; Bodet et al., 2011), glioma cells are relatively resistant to ABT-737 in that a relatively high dose of ABT-737, well above the clinically achievable range, was required to suppress cell proliferation and induce apoptosis in vitro, even among the more sensitive cell lines. Our analysis of a panel of glioma cell lines showed no correlation between Bcl-2 family protein expression level and response to ABT-737. This lack of correlation of sensitivity with Mcl-1 or other Bcl-2 family protein expression has also been shown in other cancers (Hauck et al., 2009). We demonstrated that enforced Bcl-2 overexpression does not influence ABT-737-induced inhibition of cell proliferation or induction of apoptosis in glioma cell lines, which are already fairly resistant to this agent, but does induce resistance to TRAIL-mediated apoptosis. These findings are consistent with data from others who showed that Bcl-2, when ectopically overexpressed, could not block ABT-737-dependent apoptosis of small-cell lung cancer cells (Hauck et al., 2009).
Recently, we have shown that the proteasome inhibitor bortezomib synergizes with TRAIL (Jane et al., 2011) and HDACI (Premkumar et al., 2011) in glioma cells. Bortezomib treatment causes multiple changes in the cell, including inhibition of NF-κB, a transcription factor that is activated and contributes to the survival of glioma cells. In addition, bortezomib induces the generation of ROS and mitochondrial membrane dysfunction and alters the balance between proapoptotic and antiapoptotic Bcl-2 family members. Hence, we sought to determine whether direct targeting of Bcl-xL and Bcl-2 using a highly specific small-molecule inhibitor (ABT-737) would result in synergism with bortezomib, given that NF-κB and Bcl family members were noted in our previous studies to be key apoptosis-resistance nodes in glioma cells. Indeed, the combination of ABT-737 with bortezomib resulted in synergistic induction of glioma cell death, as measured by annexin V, clonogenic survival, and Western blot analysis in glioma cell lines with intact PTEN expression. The combination of drugs strongly induced mitochondrial membrane depolarization, as shown by flow cytometry with DiOC6 dye and subsequent potent induction of apoptosis. The combination of bortezomib and ABT-737 induced the loss of Δψm and the release of cytochrome c to the cytoplasm, indicating that this drug combination acts via the mitochondrial death pathway. In addition, the observation that caspase-8 was activated by these inhibitors, and that ABT-737 and bortezomib-induced cleavage of Bid into tBid was inhibited by a caspase-8 inhibitor, indicate the involvement of the extrinsic apoptotic pathway as well. Furthermore, apoptosis induced by the combination of ABT-737 and bortezomib occurred via caspase-dependent pathways as indicated by the fact that the pan-caspase inhibitor reduced the effect of ABT-737 and bortezomib on apoptosis. The mitochondrial apoptotic pathway is controlled by a balance between the proapoptotic protein members (i.e., the multidomain proapoptotic Bax, Bak, and BH3-only proapoptotic Bid, Bim, Bad, Bik, Noxa, Puma, Bmf, and Hrk) and antiapoptotic protein members (i.e., the multidomain antiapoptotic Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and Bfl/A1) of the Bcl-2 family. Those multidomain Bcl-2 proteins are functionally regulated by the BH3-only proteins. Bid is localized in the cytosolic fraction of cells as an inactive precursor and tBid, the active form of Bid, is generated upon proteolytic cleavage by caspases (Gross et al., 1999; Slee et al., 2000). Experiments with caspase inhibitors indicated that in this system caspase-3 and caspase-8 mediates Bid cleavage.
Our studies clearly demonstrated that although PTEN-intact glioma cell lines were sensitive to the combination of ABT-737 and bortezomib, PTEN-deficient cell lines, such as U87 and LNZ308, were not. PTEN is a well studied tumor suppressor gene that is mutated or deleted in various cancers including glioma (Parsons et al., 2008). But not until more recently was PTEN, or the lack of it, implicated in the resistance of cancer cells to conventional radiation therapy and chemotherapy (Li et al., 1997; Hanahan and Weinberg, 2000; Knobbe et al., 2002; Omuro et al., 2007; Parsons et al., 2008; Hanahan and Weinberg, 2011). Akt is a potent antiapoptosis molecule common to both the PTEN and Bcl-2 pathways (Pugazhenthi et al., 2000). In this study, we clearly demonstrate that Akt1 is a crucial mediator of bortezomib and ABT-737 resistance in PTEN-deficient glioma cells. Such a conclusion is based on several findings. The phosphorylation levels of Akt kinase were up-regulated in U87 and LNZ308 cell lines. Preincubation of U87 and LNZ308 cells with LY294002 or PI-103 (PI3K/Akt inhibitors) down-regulated Akt1 phosphorylation and restored sensitivity to bortezomib and ABT-737 by inducing Δψm and Bax activation. Similar results were observed when PTEN-deficient U87 and LNZ308 cells were infected with Ad-Akt 1 (DN) or Ad-PTEN and challenged with bortezomib + ABT-737.
The initiating stimulus leading to the change in Bax conformation caused by bortezomib and ABT-737 is as yet undetermined. The conformational change and translocation of Bax may also be regulated by the PI3K/Akt pathway, with active PI3K capable of maintaining a cytosolic Bax distribution (Yamaguchi and Wang, 2001). The activation of Bax, including Bax conformational changes and oligomerization, seems to play a crucial role in the initiation of bortezomib- and ABT-737-induced apoptosis. Our group has demonstrated that the activation of Bax, including Bax conformational changes and oligomerization, seems to play a crucial role in the initiation of apoptosis after other signaling-targeted therapies in gliomas. This effect could be counteracted by overexpression of constitutively active Akt, suggesting the critical involvement of Akt signaling pathways involved in Bax activation and apoptosis in glioma (Premkumar et al., 2010). A previous study proposed active Akt to affect the conformation of Bax and thus inhibit Bax oligomerization in the mitochondrial outer membrane (Yamaguchi and Wang, 2001). Another report presented evidence that Bax initially inserts into the mitochondrial outer membrane as a monomer and then undergoes a concerted conformational change and homo-oligomerization to form pores (Annis et al., 2005). Further studies need to be done to elucidate the precise mechanism by which elevated expression of Akt attenuated apoptotic signaling, which could include directly impeding Bax oligomerization or other actions that reduce mitochondrial dysfunction caused by bortezomib and ABT-737 in glioma.
Thus, bortezomib and ABT-737 may be a promising combination for the treatment of patients with PTEN-intact gliomas. However, glioma cells lacking PTEN contain high levels of activated Akt and probably would be resistant to such therapy. In such cases, down-regulation of Akt makes these cells sensitive to bortezomib and ABT-737. Our studies also demonstrate that down-regulation of Akt1 phosphorylation (by pharmacological or genetic manipulation) enhanced sensitivity to the inhibitors, strongly supporting the hypothesis that up-regulation of Akt1 is of primary importance for the bortezomib + ABT-737 resistance observed in PTEN-deficient glioma cells. Our data suggest that PI3K/Akt-dependent protection may occur upstream of mitochondria; thus, therapeutic strategies that target the PI3K/Akt pathway may be useful for cancers lacking PTEN function.
Acknowledgments
We thank Robert Lacomy and Alexis Styche for FACS analysis.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant P01NS40923] (to I.F.P.); and The Walter L. Copeland Fund of The Pittsburgh Foundation (D.R.P.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- NF-κB
- nuclear factor-kappaB
- Ad
- adenovirus
- BH3
- Bcl-2 homology domain 3
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- CMV
- cytomegalovirus
- DiOC6
- 3′,3′-dihexyloxacarbo-cyanine iodide
- DMSO
- dimethyl sulfoxide
- DN
- dominant negative
- DSP
- dithiobis(succinimidylpropionate)
- E64d
- (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester
- MTS
- 3-[4,5-dimethylthiazol- 2yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H, tetrazolium
- FACS
- fluorescence-activated cell sorting
- FITC
- fluorescein isothiocyanate
- HDACI
- histone deacetylase inhibitor
- MOI
- multiplicity of infection
- NAC
- N-acetyl-l-cysteine
- PBS
- phosphate-buffered saline
- PAGE
- polyacrylamide gel electrophoresis
- PARP
- poly(ADP)-ribose polymerase
- PI
- propidium iodide
- PI3K
- phosphatidylinositol 3-kinase
- PTEN
- phosphatase and tensin homolog on chromosome 10
- ROS
- reactive oxygen species
- Smac/DIABLO
- second mitochondria-derived activator of caspases/direct inhibitor of apoptosis binding protein with low PI
- t-Bid
- truncated Bid
- TRAIL
- tumor necrosis factor-related apoptosis-inducing ligand
- ABT-737
- N-{4-[4-(4′-chloro-biphenyl-2-ylmethyl)-piperazin-1-yl]-benzoyl}-4-(3-dimethylamino-1-phenylsulfanylmethyl-propylamino)-3-nitro-benzenesulfonamide
- ABT-263
- (R)-4-(4-((4′-chloro-4,4-dimethyl-3,4,5,6-tetrahydro-[1,1′-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((4-((4-morpholino-1-(phenylthio)butan-2-yl)amino)-3-((trifluoromethyl)sulfonyl)phenyl)sulfonyl)benzamide
- PI-103
- 3-[4-(4-morpholinylpyrido[3′,2′:4,5]furo[3,2-d]pyrimidin-2-yl]phenol
- LY294002
- 2-morpholin-4-yl-8-phenylchromen-4-one
- Δψm
- mitochondrial membrane potential.
Authorship Contributions
Participated in research design: Premkumar, Jane, and Pollack.
Conducted experiments: Premkumar, Jane, DiDomenico, Vukmer, and Agostino.
Performed data analysis: Premkumar, Jane, DiDomenico, and Pollack.
Wrote or contributed to the writing of the manuscript: Premkumar and Pollack.
References
- Adams J. (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4:349–360 [DOI] [PubMed] [Google Scholar]
- Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, et al. (2002) A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 8:2505–2511 [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. (2005) Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J 24:2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen LP, Shamas-Din A, Andrews DW. (2008) Bid: a Bax-like BH3 protein. Oncogene 27 (Suppl 1):S93–S104 [DOI] [PubMed] [Google Scholar]
- Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maïga S, Avet-Loiseau H, Bataille R, Moreau P, Le Gouill S, et al. (2011) ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood 118:3901–3910 [DOI] [PubMed] [Google Scholar]
- Bögler O, Weller M. (2003) International Hermelin Brain Tumor Center Symposium on Apoptosis. Cell Death Differ 10:1112–1115 [DOI] [PubMed] [Google Scholar]
- Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, Yu IL, Carro MS, Dai F, Tagge MJ, et al. (2011) NFKBIA deletion in glioblastomas. N Engl J Med 364:627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS. (2004) The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol 22:1926–1933 [DOI] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. (2007) Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 117:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. (2008) BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood 111:2300–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 144:891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermoian RP, Furniss CS, Lamborn KR, Basila D, Berger MS, Gottschalk AR, Nicholas MK, Stokoe D, Haas-Kogan DA. (2002) Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res 8:1100–1106 [PubMed] [Google Scholar]
- Esposti MD. (2002) The roles of Bid. Apoptosis 7:433–440 [DOI] [PubMed] [Google Scholar]
- Furnari FB, Lin H, Huang HS, Cavenee WK. (1997) Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci U S A 94:12479–12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem 274:1156–1163 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. (2000) The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. (2011) Hallmarks of cancer: the next generation. Cell 144:646–674 [DOI] [PubMed] [Google Scholar]
- Hauck P, Chao BH, Litz J, Krystal GW. (2009) Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT-737. Mol Cancer Ther 8:883–892 [DOI] [PubMed] [Google Scholar]
- High LM, Szymanska B, Wilczynska-Kalak U, Barber N, O'Brien R, Khaw SL, Vikstrom IB, Roberts AW, Lock RB. (2010) The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol 77:483–494 [DOI] [PubMed] [Google Scholar]
- Jane EP, Premkumar DR, Pollack IF. (2011) Bortezomib sensitizes malignant human glioma cells to TRAIL, mediated by inhibition of the NF-κB signaling pathway. Mol Cancer Ther 10:198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana S, Finniss S, Cazacu S, Xiang C, Lee HK, Brodie S, Goldstein RS, Roitman V, Slavin S, Mikkelsen T, et al. (2011) Proteasome inhibitors sensitize glioma cells and glioma stem cells to TRAIL-induced apoptosis by PKCε-dependent downregulation of AKT and XIAP expressions. Cell Signal 23:1348–1357 [DOI] [PubMed] [Google Scholar]
- Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. (2007) Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res 13:5291–5294 [DOI] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Sridhara R, Pazdur R. (2006) United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res 12:2955–2960 [DOI] [PubMed] [Google Scholar]
- Kitchens CA, McDonald PR, Shun TY, Pollack IF, Lazo JS. (2011) Identification of chemosensitivity nodes for vinblastine through small interfering RNA high-throughput screens. J Pharmacol Exp Ther 339:851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobbe CB, Merlo A, Reifenberger G. (2002) Pten signaling in gliomas. Neuro Oncol 4:196–211 [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al. (2006) Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10:375–388 [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. (2000) Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7:1166–1173 [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947 [DOI] [PubMed] [Google Scholar]
- Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, Fesik SW, Shen Y. (2007) ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene 26:3972–3979 [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC. (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol 22:3003–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P. (2001) Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 276:18361–18374 [DOI] [PubMed] [Google Scholar]
- Nencioni A, Wille L, Dal Bello G, Boy D, Cirmena G, Wesselborg S, Belka C, Brossart P, Patrone F, Ballestrero A. (2005) Cooperative cytotoxicity of proteasome inhibitors and tumor necrosis factor-related apoptosis-inducing ligand in chemoresistant Bcl-2-overexpressing cells. Clin Cancer Res 11:4259–4265 [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677–681 [DOI] [PubMed] [Google Scholar]
- Omuro AM, Faivre S, Raymond E. (2007) Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther 6:1909–1919 [DOI] [PubMed] [Google Scholar]
- Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, Pien CS, Millikan RE, Tu SM, Pagliaro L, et al. (2004) Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 22:2108–2121 [DOI] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit PX, O'Connor JE, Grunwald D, Brown SC. (1990) Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem 194:389–397 [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Jane EP, Agostino NR, Didomenico JD, Pollack IF. (2011) Bortezomib-induced sensitization of malignant human glioma cells to vorinostat-induced apoptosis depends on reactive oxygen species production, mitochondrial dysfunction, Noxa upregulation, Mcl-1 cleavage, and DNA damage. Mol Carcinog http://dx.doi.org/10.1002/mc.21835 [DOI] [PMC free article] [PubMed]
- Premkumar DR, Jane EP, Pollack IF. (2010) Co-administration of NVP-AEW541 and dasatinib induces mitochondrial-mediated apoptosis through Bax activation in malignant human glioma cell lines. Int J Oncol 37:633–643 [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275:10761–10766 [DOI] [PubMed] [Google Scholar]
- Slee EA, Keogh SA, Martin SJ. (2000) Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ 7:556–565 [DOI] [PubMed] [Google Scholar]
- Song JH, Kandasamy K, Zemskova M, Lin YW, Kraft AS. (2011) The BH3 mimetic ABT-737 induces cancer cell senescence. Cancer Res 71:506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. (1998) Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29–39 [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. (1997) Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15:356–362 [DOI] [PubMed] [Google Scholar]
- Steinbach JP, Weller M. (2004) Apoptosis in gliomas: molecular mechanisms and therapeutic implications. J Neurooncol 70:245–254 [DOI] [PubMed] [Google Scholar]
- Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Böck BC, Macher-Goeppinger S, Radlwimmer B, Wiestler OD, Herold-Mende C, et al. (2008) Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene 27:6646–6656 [DOI] [PubMed] [Google Scholar]
- Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW, et al. (2007) Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res 67:1176–1183 [DOI] [PubMed] [Google Scholar]
- Thaker NG, McDonald PR, Zhang F, Kitchens CA, Shun TY, Pollack IF, Lazo JS. (2010a) Designing, optimizing, and implementing high-throughput siRNA genomic screening with glioma cells for the discovery of survival genes and novel drug targets. J Neurosci Methods 185:204–212 [DOI] [PubMed] [Google Scholar]
- Thaker NG, Zhang F, McDonald PR, Shun TY, Lazo JS, Pollack IF. (2010b) Functional genomic analysis of glioblastoma multiforme through short interfering RNA screening: a paradigm for therapeutic development. Neurosurg Focus 28:E4. [DOI] [PubMed] [Google Scholar]
- Thaker NG, Zhang F, McDonald PR, Shun TY, Lewen MD, Pollack IF, Lazo JS. (2009) Identification of survival genes in human glioblastoma cells by small interfering RNA screening. Mol Pharmacol 76:1246–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel S, Stewart AK, Li Z, Shu Y, Liang SB, Trieu Y, Reece D, Paterson J, Wang D, Wen XY. (2007) The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res 13:621–629 [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M, Dinsdale D, Sun XM, Young KW, Butterworth M, Nicotera P, Dyer MJ, Cohen GM. (2008) A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ 15:820–830 [DOI] [PubMed] [Google Scholar]
- Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R. (1997) Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res 57:4183–4186 [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG. (2001) The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20:7779–7786 [DOI] [PubMed] [Google Scholar]
- Yin XM. (2000) Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res 10:161–167 [DOI] [PubMed] [Google Scholar]
- Yu C, Friday BB, Lai JP, Yang L, Sarkaria J, Kay NE, Carter CA, Roberts LR, Kaufmann SH, Adjei AA. (2006) Cytotoxic synergy between the multikinase inhibitor sorafenib and the proteasome inhibitor bortezomib in vitro: induction of apoptosis through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer Ther 5:2378–2387 [DOI] [PubMed] [Google Scholar]
- Yu C, Friday BB, Yang L, Atadja P, Wigle D, Sarkaria J, Adjei AA. (2008) Mitochondrial Bax translocation partially mediates synergistic cytotoxicity between histone deacetylase inhibitors and proteasome inhibitors in glioma cells. Neuro Oncol 10:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N, El Hamel C, Maisse C, Brenner C, Muñoz-Pinedo C, Belzacq AS, Costantini P, Vieira H, Loeffler M, Molle G, et al. (2000) Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene 19:6342–6350 [DOI] [PubMed] [Google Scholar]