Abstract
G protein-coupled receptors (GPCRs) are integral membrane proteins that change conformation after ligand binding so that they can transduce signals from an extracellular ligand to a variety of intracellular components. The detailed interaction of a molecule with a G protein-coupled receptor is a complicated process that is influenced by the receptor conformation, thermodynamics, and ligand conformation and stereoisomeric configuration. To better understand the molecular interactions of fenoterol analogs with the β2-adrenergic receptor, we developed a new agonist radioligand for binding assays. [3H](R,R′)-methoxyfenoterol was used to probe the binding affinity for a series of fenoterol stereoisomers and derivatives. The results suggest that the radioligand binds with high affinity to an agonist conformation of the receptor, which represents approximately 25% of the total β2-adrenoceptor (AR) population as determined with the antagonist [3H]CGP-12177. The β2-AR agonists tested in this study have considerably higher affinity for the agonist conformation of the receptor, and Ki values determined for fenoterol analogs model much better the cAMP activity of the β2-AR elicited by these ligands. The thermodynamics of binding are also different when interacting with an agonist conformation, being purely entropy-driven for each fenoterol isomer, rather than a mixture of entropy and enthalpy when the fenoterol isomers binding was determined using [3H]CGP-12177. Finally, computational modeling identified the molecular interactions involved in agonist binding and allow for the prediction of additional novel β2-AR agonists. The study underlines the possibility of using defined radioligand structure to probe a specific conformation of such shape-shifting system as the β2-adrenoceptor.
Introduction
The binding affinity of a compound to the β2-adrenoceptor (β2-AR) is routinely determined using competitive binding assays based upon the concentration-dependent displacement of a marker radioligand. Although this technique is used to characterize the binding of β2-AR agonists and antagonists, the most often employed marker ligands are nonselective β-AR antagonists, which often have significant binding affinities to the β1-AR, β2-AR, and β3-AR (Brodde et al., 1983; Staehelin et al., 1983; Toews et al., 1983; Hoffmann et al., 2004; Joseph et al., 2004; Baker, 2005; Nikulin et al., 2006; Perrone et al., 2008). These radioligands include (−)-3-[125I]iodocyanopindolol (Brodde et al., 1983), [3H]4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one (CGP-12177) (Staehelin et al., 1983), [125I]iodopindolol (Toews et al., 1983), and [3H]dihydroalprenolol (Staehelin et al., 1983; Perrone et al., 2008). One of the most widely used markers, [3H]CGP-12177, is a nonconventional antagonist of the β1-AR that binds at two sites on the receptor (Joseph et al., 2004). Thus, it is not clear which site or sites on the β2-AR interact with [3H]CGP-12177 or how these interactions affect the identification and characterization of β2-AR agonists.
The concerns associated with the use of [3H]CGP-12177 as a marker ligand were evident in our recent work on the characterization of the individual stereoisomers of the β2-AR agonist fenoterol (Fen) and a series of Fen analogs (Fig. 1) (Jozwiak et al., 2007, 2010a,b; Toll et al., 2011). During the course of these studies, we determined the binding thermodynamics of the four stereoisomers of Fen, (R,R′)-, (R,S′)-, (S,R′)-, and (S,S′)-Fen to the β2-AR (Jozwiak et al., 2010a). All of these compounds are full agonists of the β2-AR with respect to the stimulation of cAMP accumulation in HEK293 cells stably transfected with β2-AR (HEK-β2-AR), and the binding studies were performed using membranes obtained from these cells. In these studies, [3H]CGP-12177 was used as the marker ligand, and the binding affinities were expressed as Ki CGP values. The results indicated that there were significant stereochemistry-based differences in the binding mechanisms as this process was entropy-driven when (R,R′)- and (R,S′)-Fen were the ligands, whereas the binding of the S,R′- and S,S′-isomers was an enthalpy-driven process. In addition, the calculated Hill coefficients (nH values) also differed as an nH of ∼1 was calculated for the R,R′- and R,S′-isomers and an nH of ∼2 was determined for the S,R′- and S,S′-isomers. These binding studies have also been used to develop comparative molecular field analysis models for the interaction of the Fen analogs, and other agonists and antagonists for the β2-AR, and we have been able to model the differential interactions of the stereoisomers. However, binding studies using the antagonist [3H]CGP-12177 may only explore a portion of the binding interactions.
Fig. 1.
The structures of the compounds used in this study, where only the R,R′ configurations of the stereoisomers are presented.
It is well known that GPCRs, such as the β2-AR, bind ligands in multiple conformations. In particular, antagonists seem to have similar affinities to most or all receptor conformations, whereas an agonist will stabilize the receptor in a conformation for which it has high affinity, but it will bind with low affinity to other “antagonist” conformations (Kent et al., 1980). Therefore, our (and most other) previous studies have primarily examined the interaction of Fen analogs and other agonists with the antagonist conformation. This is evident if one compares the binding affinity of (R,R′)-Fen when competing with [3H]CGP-12177, Ki = 345 nM, versus its potency for stimulation of cAMP accumulation in the same cells, EC50 = 0.30 nM (Jozwiak et al., 2010b).
To examine the binding of Fen analogs to a different conformation of the β2-AR, we synthesized [3H](R,R′)-4-methoxyfenoterol, [3H]MFen (Fig. 1) to use as the marker ligand in receptor binding studies (Kozocas et al., 2010). We have previously characterized MFen as a potent and selective β2-AR agonist that stimulates cAMP accumulation in HEK-β2-AR cells (Toll et al., 2011), induces cardiomyocyte contractility in a mouse cardiomyocyte model (Jozwiak et al., 2010b), and inhibits 1321N1 mitogenesis (Toll et al., 2011). Here we report the initial study of the use of this compound as a marker in the determination of β2-AR agonist binding affinities, examine the thermodynamics of Fen binding to an “agonist” conformation, and we use molecular dynamics calculations to perform in silico docking of Fen analogs to this agonist conformation.
Materials and Methods
Materials.
The Fen analogs used in this study (Fig. 1) were synthesized as described previously (Jozwiak et al., 2007), and the preparation of [3H]MFen (25 Ci/mmol; Fig. 1) has been reported (Kozocas et al., 2010). Dulbecco's modified Eagle's medium was purchased from Lonza Walkersville, Inc. (Walkersville, MD), fetal bovine serum was purchased from Mediatech, Inc. (Manassas, VA), penicillin-streptomycin and G418 (Geneticin) were purchased from Invitrogen (Carlsbad, CA), sodium chloride and calcium chloride were purchased from Mallinckrodt Baker, Inc. (Phillipsburg, NJ) and (rac)-propranolol, (R)-isoproterenol, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol (ICI-118-551), Tris-HCl, Trizma base, potassium chloride, magnesium chloride, and d-(+)-glucose were purchased from Sigma-Aldrich (St. Louis, MO).
Membrane Binding Studies.
HEK-β2-AR (provided by Dr. Brian Kobilka, Stanford University Medical Center, Palo Alto, CA) were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 0.05% penicillin-streptomycin with 400 μg/ml G418. The cells were scraped from the 150 × 25-mm plates and centrifuged at 500g for 5 min. The pellet was washed twice by homogenization in 50 mM Tris-HCl, pH 7.7, and centrifuged at 27,000g for 10 min. The pellet was resuspended in 15 mM Tris-HCl, pH 7.4, containing 120 mM sodium chloride, 5.4 mM potassium chloride, 1.8 mM calcium chloride, 0.8 mM magnesium chloride, and 5 mM glucose. The binding assays contained 3.9 nM [3H]MFen and 60 μg of cell membranes, in a volume of 1.0 ml. The mixture was incubated at 25°C for 2 h and filtered over glass fiber filters soaked in 0.05% polyethylenimine. Nonspecific binding was determined using 10 μM (rac)-propranolol. The reaction was terminated by filtration using a Tomtec 96 harvester (Tomtec, Orange, CT) through glass fiber filters. Bound radioactivity was counted on a Wallac β-plate liquid scintillation counter (PerkinElmer Life and Analytical Sciences, Waltham, MA) and expressed in counts per minute. Saturation experiments were conducted using concentrations ranging from 0.2 to 20 nM [3H]MFen.
Docking Simulations.
The 2RH1 and 3POG molecular models (from the PDB) of the β2-AR were used in the simulated docking studies. Ligand molecules were prepared using HyperChem (ver. 6.03; HyperCube Inc., Gainesville, FL) software using Model Build procedure. Molegro Virtual Docker (MVD; ver. 2010.4.0.0) software was employed for docking simulations. The MolDock SE search algorithm was used, and the number of searching runs was set to 100. The following parameters were set during docking simulation: population size, 50; maximum iteration, 1500; energy threshold, 100.00; and max steps, 300. The estimation of ligand-protein interactions was described by the MVD implemented scoring functions: MolDock Score, Rerank Score, Hbond Score, Similarity Score, and Docking Score.
Statistical Analysis.
Results were analyzed by nonlinear regression analysis using the program Graphpad/Prism (ISI, San Diego, CA). For competition experiments, IC50 values and Hill coefficients (nH) were determined using at least six concentrations of each Fen analog. The Ki values were calculated by the method of Cheng and Prusoff (1973).
Results
Characterization of [3H]MFen Binding to Membranes from HEK-β2-AR Cells.
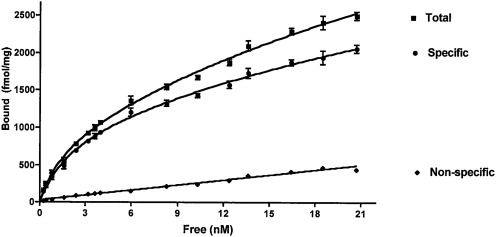
Saturation analysis of [3H]MFen binding to membranes from HEK-β2-AR cells indicated a single binding component with a Kd MFen of 4.88 ± 0.41 nM and a Bmax of 2136 ± 114 fmol/mg protein. Nonspecific binding represented less than 20% of total binding (Fig. 2). The binding was inhibited by the nonselective β-AR antagonist (rac)-propranolol and the selective β2-AR antagonist ICI 118-551, indicating that [3H]MFen specifically bound to the β2-AR. The calculated Kd MFen value was ∼100-fold lower than the previously reported β2-AR affinity of MFen determined using the same cellular membranes and [3H]CGP-12177 as the marker ligand (Ki CGP = 473 nM; Table 1) (Jozwiak et al., 2007). The calculated Bmax value was also lower than the previously reported value, 8901 ± 1161 fmol/mg protein, also determined using [3H]CGP-12177 as the marker ligand (Jozwiak et al., 2007). These results suggest that [3H]MFen binds with high affinity to an agonist conformation of the receptor and that, under these conditions, the agonist conformation represents only approximately 25% of the total β2-AR receptor population.
Fig. 2.
Saturation binding of [3H]MFen to membranes obtained from HEK-β2-AR cells, showing nonspecific, specific, and total binding. Nonspecific binding was determined in the presence of 10 μM propranolol. Data shown are from a single experiment conducted in triplicate. This experiment was repeated two additional times with similar results.
TABLE 1.
The β2-AR binding affinities determined using either [3H]MFen (Ki MFen) or [3H]CGP-12177 (Ki CGP) as the marker ligand and membranes obtained from HEK-β2-AR cells
The induced cAMP accumulation in HEK-β2-AR cells are presented as EC50 values. The Ki values were determined at 25°C and are presented as ± S.E.M. with n ≥ 3. See Materials and Methods for experimental details.
| Compound | Receptor Binding |
Ki CGP/KiM Fen | cAMP Stimulation EC50 | |||
|---|---|---|---|---|---|---|
| [3H]CGP-12177a |
[3H]MFen |
|||||
| Ki | nH | Ki | nH | |||
| nM | nM | nM | ||||
| (R)-Isoproterenol | 192 ± 24 | 0.85 ± 0.1 | 2.44 ± 0.28 | 0.78 ± 0.1 | 79 | 0.2 |
| Propranolol | 0.46 ± 0.06 | 1.24 ± 0.1 | 3.69 ± 1.36 | 1.88 ± 0.3 | 0.1 | N.A. |
| ICI-118,551 | 0.60 ± 0.3 | 1.34 ± 0.4 | 2.52 ± 0.29 | 2.01 ± 0.2 | 0.2 | N.A. |
| (R,R′)-Fen | 345 ± 33.8 | 0.92 ± 0.1 | 4.00 ± 0.75 | 0.76 ± 0.1 | 86 | 0.3 |
| (R,S′)-Fen | 3695 ± 245 | 0.81 ± 0.1 | 183 ± 30.0 | 0.97 ± 0.1 | 20 | 4.7 |
| (S,R′)-Fen | 10,330 ± 1405 | 1.02 ± 0.1 | 1827 ± 117 | 0.92 ± 0.1 | 6 | 8.5 |
| (S,S′)-Fen | 27,749 ± 6816 | N.D. | 3370 ± 210 | 1.34 ± 0.2 | 8 | 580.2 |
| (R,R′)-MFen | 473 ± 35 | 0.98 ± 0.1 | 4.09 ± 0.55 | 0.80 ± 0.1 | 116 | 0.3 |
| (R,S′)-MFen | 1929 ± 135 | 1.01 ± 0.1 | 26.1 ± 2.44 | 1.00 ± 0.1 | 74 | 2 |
| (S,R′)-MFen | 5268 ± 508 | 1.28 ± 0.1 | 91.3 ± 32.01 | 0.86 ± 0.1 | 58 | 7.2 |
| (S,S′)-MFen | 5880 ± 2722 | 2.30 ± 0.3 | 2870 ± 234 | 1.68 ± 0.5 | 6 | 33.2 |
| (R,R′)-PhFen | 864 ± 248 | 0.97 ± 0.02 | 26.6 ± 1.49 | 0.79 ± 0.1 | 70 | N.D. |
| (R,R′)-1-NapFen | 41 ± 38 | 1.06 ± 0.2 | 3.66 ± 0.42 | 0.88 ± 0.2 | 66 | 12.5 |
| (R,S′)-1-NapFen | 341 ± 32 | 0.93 ± 0.01 | 3.67 ± 1.32 | 0.86 ± 0.1 | 93 | 2.7 |
| (S,R′)-1-NapFen | 1783 ± 208 | 1.06 ± 0.1 | 57.6 ± 4.73 | 0.91 ± 0.04 | 31 | 66.7 |
| (S,S′)-1-NapFen | 2535 ± 295 | 1.12 ± 0.1 | 615 ± 85.2 | 0.99 ± 0.04 | 4 | 29.7 |
| (R,R′)-2-NapFen | 404 ± 97 | 0.97 ± 0.04 | 4.52 ± 1.14 | 0.83 ± 0.1 | 89 | 0.4 |
| (R,S′)-2-NapFen | 509 ± 5 | 1.06 ± 0.1 | 134 ± 10.7 | 1.04 ± 0.2 | 4 | 7.6 |
| (R,R′)-NH2Fen | 2933 ± 238 | 1.01 ± 0.1 | 42.8 ± 11.3 | 0.83 ± 0.05 | 69 | 2.42 |
| (R,S′)-NH2Fen | 7937 ± 561 | 1.07 ± 0.03 | 187 ± 42.6 | 0.91 ± 0.03 | 42 | N.D. |
| (S,R′)-NH-2Fen | 23,125 ± 2093 | N.D. | 463 ± 103 | 1.00 ± 0.1 | 50 | N.D. |
| (R,R′)-EtFen | 1273 ± 81 | 1.01 ± 0.01 | 39.1 ± 5.38 | 0.93 ± 0.1 | 33 | 2.8 |
| (R,S′)-EtFen | 5758 ± 833 | 2.07 ± 0.4 | 294 ± 45.1 | 0.87 ± 0.2 | 20 | 16.6 |
| (R,R′)-MNF | 277 ± 11 | 1.07 ± 0.09 | 13.3 ± 2.72 | 0.86 ± 0.1 | 21 | 3.9 |
| (R,S′)-MNF | 317 ± 6 | 1.06 ± 0.02 | 12.7 ± 1.83 | 0.90 ± 0.2 | 25 | 4 |
nH, Hill coefficient; N.D., not determined (for binding experiments this was because binding affinities were too low to obtain Hill coefficients); N.A., not applicable; PhFen, phenylfenoterol; NapFen, naphthylfenoterol; NH2-Fen, aminofenoterol; EtFen, ethylfenoterol; MNF, methoxynaphthylfenoterol.
Data obtained from Jozwiak et al. (2010a,b) and Toll et al. (2011).
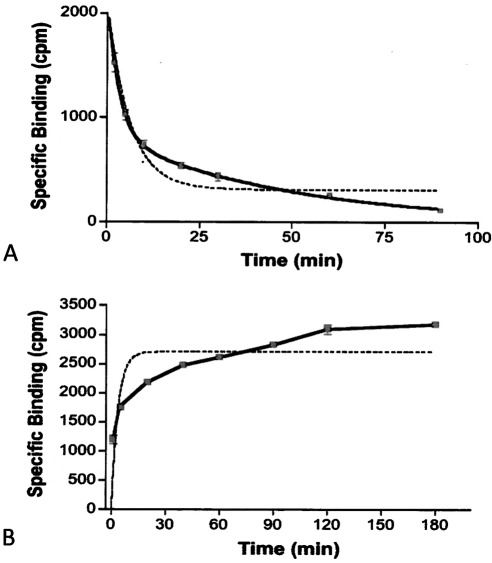
Unlike saturation analysis, the kinetic analysis of [3H]MFen binding suggested that there was more than one binding conformation or component. The analysis of the relationship between specific binding and time revealed that the data fit better to a two-component model of binding than to a single-component model (Fig. 3), making it impossible to calculate definitive kinetic binding constants. Nonlinear regression analysis of the data indicated that the association rate seems to be biphasic, with kon of 1.21 min−1 for approximately 35% of the sites and 0.016 min−1 for the remaining 65%. The same results were obtained from the analysis of the koff data: approximately 35% of the receptors have a koff of 0.018 min−1 and 65% have a koff of 0.20 min−1. The data support the supposition that [3H]MFen binds to at least two sites on or conformations of the β2-AR, although this cannot be readily detected by saturation analysis.
Fig. 3.
Dissociation (A) and association (B) kinetics of [3H]MFen binding to membranes obtained from HEK-β2-AR cells. Solid lines represent a two-site model, and dashed lines represent best fit to a one-site binding model.
Determination of β2-AR Binding Affinities by Using [3H]MFen as the Marker Ligand.
[3H]MFen was used as the marker ligand in the determination of the β2-AR binding affinities (Ki MFen) of 22 fenoterol analogs, isoproterenol, propranolol, and ICI-118-551, and the data are presented in Table 1. In these experiments, a low concentration (3.9 nM) of [3H]MFen was used; consequently, greater than 75% of the binding represents the high-affinity binding conformation. The Ki MFen value observed for the nonselective β-AR antagonist (rac)-propranolol (3.69 nM) was nearly 10-fold higher than the Ki CGP value (0.46 nM) determined using [3H]CGP-12177, as was the calculated affinity of the selective β2-AR antagonist ICI 118−551 (Ki MFen, 2.52 nM; Ki CGP = 0.60 nM; Table 1). The opposite result was obtained with the β2-AR agonist isoproterenol: the Ki MFen was 79-fold lower than the Ki CGP, 2.44 and 192 nM, respectively, and more in line with the EC50 value that induced cAMP accumulation in HEK-β2-AR cells (0.20 nM; Table 1). The calculated Hill coefficients for the agonist compounds were slightly less than 1.0, suggesting that there is some heterogeneity of binding, consistent with the saturation and kinetic experiments. It is noteworthy that the Hill coefficients for the antagonists (rac)-propranolol and ICI 118-551 were 2.10 and 1.84, respectively, suggesting that some degree of positive cooperativity of binding may exist.
The data obtained with the 22 fenoterol analogs tested in this study are consistent with the supposition that Ki MFen values better reflect the β2-AR agonist properties than Ki CGP values. When [3H]MFen was the marker ligand, the apparent affinities uniformly increased relative to the Ki CGP values for each of the compounds tested (Table 1; competition curves are shown in Supplemental Fig. S1). The changes ranged from a 116-fold decrease in Ki (increase in affinity) for (R,R′)-MFen to a 4-fold decrease when (S,S′)-1-naphthylfenoterol and (R,S′)-2-naphthylfenoterol were studied. Nevertheless, there was a good correlation between the Ki values using the two radioligands, with a square of the correlation coefficient of R2 = 0.7899 (see Supplemental Fig. S2). In general, the magnitude of the change was dependent upon the configuration at the β-OH carbon, with an R-configuration producing a greater enhancement in the binding affinity when [3H]MFen was the marker ligand.
When the Ki values were compared with the corresponding EC50 values determined for the stimulation of cAMP accumulation in HEK-β2-AR cells, the magnitude of the Ki MFen values were more reflective of this activity than the Ki CGP values (Table 1). For each compound, the binding affinity using [3H]MFen was closer to the EC50 value for cAMP accumulation that was the Ki for [3H]CGP-12177 binding. In addition, the pKi of [3H]MFen is significantly better correlated with pEC50 of cAMP than the pKi of [3H]CGP-12177 (correlation coefficients of 0.5532 versus 0.4143, respectively) (Supplemental Fig. S2). These results suggest that the high-affinity site probed by [3H]MFen is associated with the cAMP activity of the β2-AR and that all of the agonists used in this study bind to this site to a greater extent than to a lower affinity site probed by [3H]CGP-12177.
Effect of GTP on β2-AR Binding.
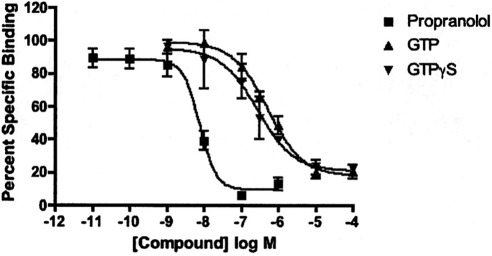
Experiments were conducted to determine how GTP and GTPγS affect binding of [3H]MFen and compare that with their effect on the binding of the antagonist [3H]CGP-12177. GTP and its nonhydrolyzable analog GTPγS both dose-dependently reduced binding of [3H]MFen, although the potency of GTP and particularly GTPγS was lower than what might be expected from literature values (Fig. 4). It would seem that this is not competitive antagonism but reflects a GTP-induced decrease in agonist affinity. It is noteworthy that GTP and GTPγS did not have much effect on [3H]CGP-12177 binding. As expected, [3H]CGP-12177 binding remained high in the presence of GTP. We were surprised, however, that GTP did not induce a decrease in affinity of (R,R′)-fenoterol and slightly increased the affinity of (R,R′)-methoxyfenoterol (Table 2).
Fig. 4.
Effect of GTP and GTPγS on [3H]MFen binding. Binding was conducted to HEK-β2-AR cell membranes as described under Materials and Methods in the presence of various concentrations of GTP, GTPγS, and propranolol as a standard. Values shown are average ± S.D. of three experiments conducted in triplicate.
TABLE 2.
Effect of GTP on agonist inhibition of [3H]CGP-12177 binding
Binding was conducted as described under Materials and Methods in the presence and absence of 10 μM GTP.
| Compound | −GTP | +GTP |
|---|---|---|
| Propranolol | 2.27 ± 0.78 | 2.03 ± 1.26 |
| (R,R′)-Fen | 560 ± 116 | 553 ± 151 |
| (R,R′)-MFen | 411 ± 229 | 220 ± 111 |
Thermodynamic Studies.
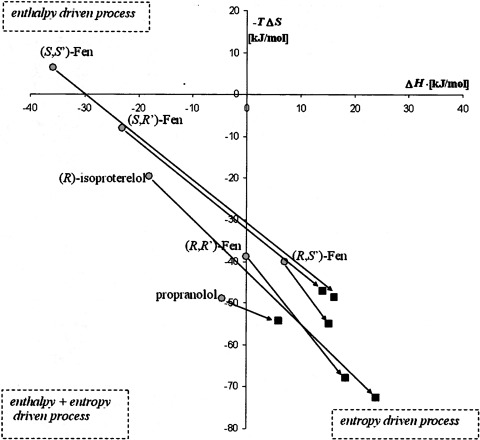
In a previous study, the effect of temperature on the binding of (R,R′)-, (R,S′)-, (S,R′)-, and (S,S′)-Fen along with propranolol and isoproterenol was determined using [3H]CGP-12177 as the radioligand, and the data were subjected to van't Hoff analysis (Jozwiak et al., 2010a). In the current study, the temperature dependence of the Ki MFen values of the same test compounds was determined at 4, 25, and 37°C (Table 3). The three temperature points were used to construct van't Hoff plots (1n(1/Ki) versus 1/T), which were further employed to calculate enthalpic and entropic contribution to the free energy change of binding by linear regression of the equation 1n(1/Ki) = ΔS°/R − ΔH°/R 1/T × ΔH° [where S is the absolute entropy, R is the gas constant, H is enthalpy, T is absolute temperature, G is Gibbs free energy, and ° indicates standard conditions for temperature and pressure (∼298.15 K and ∼101.325 Pa)]. ΔS° and ΔG° values calculated using [3H]MFen affinity data are presented in Table 4. The calculations indicate that the binding of all of the test compounds was purely entropy driven: ΔH° > 0 and −TΔS° < 0. These results are different from those of the previous study using [3H]CGP-12177 as the marker ligand, in which the binding of (S,S′)-Fen was an enthalpy-driven process (ΔH° = −35.8 kJ/mol; −TΔS° = +6.3 kJ/mol) and the binding of (S,R′)-Fen, isoproterenol, and propranolol were combined enthalpy/entropy-driven processes (Table 3) (Jozwiak et al., 2010a). This observation was confirmed using the approach developed by Borea et al. (2000) (Merighi et al., 2010), in which the data obtained in this study and the data previously obtained using [3H]CGP-12177 as the radioligand (Jozwiak et al., 2010a) were placed in a scatter plot of −TΔS° versus ΔH°. As seen in Fig. 5, all of the data from the current study was located within the quadrant associated with an entropy-driven process, which is in contrast to the data obtained using [3H]CGP-12177, in which the data span quadrants.
TABLE 3.
The influence of temperature on the binding to the β2-AR of the antagonist propranolol and the agonists isoproterenol, (R,R′)-Fen, (R,S′)-Fen, (S,R′)-Fen, and (S,S′)-Fen using [3H](R,R′)-MFen as the maker ligand, where n = 3
All values are Ki except for (R,R′)-MFen, which are Kd values derived from saturation analysis. These Kd values were used to calculate Ki for the remaining compounds, as described under Materials and Methods. See Materials and Methods for experimental procedures.
| Compound |
Ki |
||
|---|---|---|---|
| 4°C | 25°C | 37°C | |
| nM | |||
| (R,R′)-MFen | 5.68 ± 1.35 | 4.88 ± 0.41 | 3.66 ± 0.82 |
| Propranolol | 4.74 ± 1.94 | 3.69 ± 1.36 | 3.66 ± 0.66 |
| Isoproterenol | 4.40 ± 0.59 | 2.44 ± 0.28 | 1.89 ± 0.53 |
| (R,R′)-Fen | 7.97 ± 3.79 | 4.00 ± 0.75 | 2.59 ± 0.20 |
| (R,S′)-Fen | 187.40 ± 35 | 183 ± 30 | 83.00 ± 3.22 |
| (S,R′)-Fen | 3338 ± 764 | 1827 ± 117 | 1798.50 ± 198 |
| (S,S′)-Fen | 3800 ± 482 | 3370 ± 210 | 1639 ± 340 |
TABLE 4.
Thermodynamic parameters of binding to the β2-AR, using [3H]MFen as the marker ligand compared with the previously reported parameters obtained using [3H]CGP-12177 as the marker ligand (Jozwiak et al., 2010a)
Distance parameter is the Euclidean distance describing a shift of the (ΔH°; −TΔS°) point, determined in the [3H]MFen experiment with respect to the [3H]CGP-12177-derived point, as illustrated in Fig. 5.
| ΔH° |
−TΔS° |
ΔG° |
Distance | ||||
|---|---|---|---|---|---|---|---|
| MFen | CGP | MFen | CGP | MFen | CGP | ||
| kJ/mol | kJ/mol | kJ/mol | |||||
| Propranolol | +5.98 ± 0.04 | −4.5 | −54.32 ± 0.04 | −48.95 | −48.3 ± 0.06 | −53.5 | 11.8 |
| (R)-Isoproterenol | +18.4 ± 0.13 | −18.03 | −67.9 ± 0.13 | −19.5 | −49.5 ± 0.2 | −37.6 | 60.6 |
| (R,R′)-Fen | +24.1 ± 0.52 | 0 | −72.6 ± 1.4 | −38.8 | −48.5 ± 1.5 | −38.8 | 41.5 |
| (R,S′)-Fen | +15.3 ± 3.0 | +7.1 | −54.9 ± 1.2 | −40.0 | −39.6 ± 3.2 | −31.1 | 17.0 |
| (S,R′)-Fen | +18.4 ± 0.13 | −23.0 | −47.0 ± 1.1 | −8.1 | −32.7 ± 1.6 | −31.1 | 53.9 |
| (S,S′)-Fen | +16.2 ± 2.2 | −35.8 | −48.5 ± 2.2 | +6.3 | −32.2 ± 3.1 | −29.5 | 75.6 |
Fig. 5.
Scatter plot of −TΔS° versus ΔH° values for the compounds used in the thermodynamic studies. ○, values previously determined using [3H]CGP-12177 as the marker ligand (Jozwiak et al., 2010b); ■, values determined in this study using [3H]MFen as the marker ligand.
Simulated Docking Studies.
The hypothesis that [3H]CGP-12177 and [3H]MFen can be used to probe different conformations of the β2-AR was tested using simulated receptor-ligand docking studies. Two molecular models of the β2-AR binding site have been reported and were used in the studies: 1) the PDB code 2RH1 model {β2-AR-In}, derived using β2-AR cocrystalized with (S)-carazolol, which is regarded as an inactive form of the receptor (Rasmussen et al., 2007); and 2) the PDB code 3POG model {β2-AR-Ac} obtained from β2-AR cocrystalized with the agonist 5-hydroxy-8-{2-[2-(2-methylphenyl)-1,1-dimethyl-ethylamino]-1-hydroxyethyl}-4H-benzo[1,4]oxazin-3-one (BI-167,107) and the NB90 nanobody, which is regarded as an active form of the receptor (Rasmussen et al., 2011).
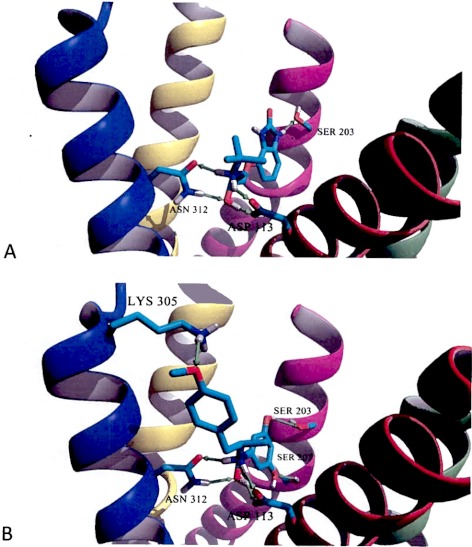
The lowest energy poses obtained in docking simulations of (S)-CGP-12177 were obtained with the β2-AR-In model (Fig. 6A). In these simulations, the position of (S)-CGP-12177 shares a number of similarities with the position (S)-carazolol cocrystalized in the binding site of the β2-AR-In model. As depicted in Fig. 6A, there is a network of four hydrogen bonds formed between the amino and the β-hydroxy moieties of (S)-CGP-12177 and two protein residues, Asp113 of TM3 and Asn312 of TM7. In addition (S)-CGP-12177 forms a hydrogen bond with Ser203 of TM5. A similar interaction was originally observed between (S)-carazolol and the β2-AR in the crystal model in PDB code 2RH1. Thus, docking of (S)-CGP-12177 reveals the mode of binding conserved for other antagonists or inverse agonists such as (S)-carazolol or ICI-118-551 interacting with the receptor.
Fig. 6.
Molecular models of (S)-CGP-12177 interacting with an inactive model of β2-AR (PDB code 2RH1, β2-AR-In) (A) and (R,R′)-MFen interacting with an active model of β2-AR (PDB code 3POG, β2-AR-Ac) obtained during docking simulations (B). For clarity of both figures, TM1, TM2, and extracellular loop 2 were hidden, and the remaining transmembrane segments are color coded as follows: TM3, red; TM4, green; TM5, magenta; TM6, yellow; and TM7, blue. Only the residues forming hydrogen bonds (shown as green arrows) with a ligand molecule are shown explicitly. All aliphatic hydrogen atoms are hidden.
The inward shift of the TM5 in the ligand binding domain of the β2-AR-Ac model results in a condition such that the (R,R′)-MFen molecule can achieve a similar network of four hydrogen bonds between amino and β-hydroxy moieties of the ligand and Asp113 and Asn312 residues, as depicted in Fig. 6B. In addition, Ser203 and Ser207, of TM5, interact with two meta-hydroxyl moieties of MFen, and Lys305 of TM7 interacts with the 4′-methoxy moiety of the ligand. The latter interaction for compounds such as formoterol and fenoterol (having para-hydroxy moiety at the aminoalkyl tail) was recently proposed as an important factor in disrupting the ionic lock switch between Lys305 and Asp192 of extracellular loop 2 occurring in the inactive state and postulated to break during the activation of the receptor (Bokoch et al., 2010). Thus the docking simulations support the assumption that binding of (R,R′)-MFen should stabilize the active form of the receptor.
The binding of the marker ligands to the β2-AR-Ac and β2-AR-In models was also examined using the scoring functions, MolDockScore values, generated by MVD software, which energetically characterizes the simulated ligand-receptor complexes. The MolDockScore value calculated for the (R,R′)-MFen–β2-AR-Ac complex, shown in Fig. 6B, was significantly lower than the analogous value calculated for (R,R′)-MFen–β2-AR-In complex, −136.98 kJ/mol versus −132.53 kJ/mol, respectively. It is noteworthy that the difference in MolDockScore values was very small in docking simulations between antagonist (S)-CGP-12177 and β2-AR-In (Fig. 6A), compared with β2-AR-Ac models, −116.60 kJ/mol and −115.82 kJ/mol, respectively. This result is consistent with the observation that antagonists bind with roughly equal affinity to agonist and antagonist conformations of GPCRs and suggests that the binding of (S)-CGP-12177 to the inactive conformation is slightly more favorable than its interaction with active conformation.
Discussion
The thermodynamics of the binding of agonists and antagonists to β-ARs have been described as fundamentally different processes in which the binding of an agonist is enthalpy-driven, whereas the binding of an antagonist is entropy-driven (Weiland et al., 1979; Contreras et al., 1986; Miklavc et al., 1990). This observation was generalized as the principle of “thermodynamic agonist-antagonist discrimination” (Borea et al., 2000). We have recently reported the results of a study of the binding thermodynamics of (R,R′)-Fen, (S,S′)-Fen, (R,S′)-Fen, and (S,R′)-Fen to the β2-AR (Jozwiak et al., 2010a). In this study, the binding affinities were determined at five different temperatures using [3H]CGP-12177 as the marker ligand and cellular membranes obtained from HEK-β2-AR cells. The data indicated that the binding of (S,S′)- and (S,R′)-Fen were predominantly enthalpy-driven processes, whereas the binding of (R,R′)- and (R,S′)-Fen were entropy-driven. Because all of the Fen stereoisomers were full β2-AR agonists in the HEK-β2-AR cells, the results were inconsistent with the principle of “thermodynamic agonist-antagonist discrimination.” In the discussion of this inconsistency, we suggested that the results of our study might reflect the fact that the β2-AR exists in an inactive (R) conformation and one or more ligand-specific active conformations (R*n) (Seifert and Dove, 2009) and that displacement binding studies using [3H]CGP-12117, a high-affinity neutral antagonist (Baker et al., 2008), may reflect the relative affinity of the Fen stereoisomers for the inactive receptor state. We also suggested that a potential approach to clarifying these interactions was to conduct the displacement binding studies with the β2-AR agonist [3H](R,R′)-MFen, and this article reports the results of such studies.
The data from the current study indicate that the binding properties of [3H]MFen are what one would expect for a high-affinity β2-AR agonist, and similar to those described previously for [3H]formoterol and earlier studies with [3H]hydroxybenzylisoproterenol (Lefkowitz and Williams, 1977; Mak et al., 1994). [3H]MFen has high affinity for β2-AR with a Kd of 4.88 nM, and binding is decreased by the presence of GTP analogs. In addition, saturation and kinetic analyses indicate that the binding occurs at a single high-affinity binding site, or perhaps two independent but high-affinity sites, that seems to differ from the site probed by [3H]CGP-12177.
Whereas the data from the saturation analysis indicated that [3H]MFen binds to a single high-affinity conformation of the receptor, the results from association and dissociation experiments suggested binding to two conformations of the receptor, as both curves fit two-site models better than single-site models. In addition, low Hill coefficients for the agonist inhibition of [3H]MFen binding also are consistent with two potential binding sites or conformations. These results suggest the potential of two independent high-affinity receptor conformations, because kinetic experiments, but not equilibrium experiments, such as saturation, can identify different binding components with roughly equal affinity but different kinetics. These results are consistent with biophysical experiments that demonstrated two kinetically distinguishable conformational states after agonist binding (Swaminath et al., 2004).
In the analysis of the [3H]MFen saturation binding studies with the membranes from the HEK-β2-AR, the maximum binding capacity, Bmax value, was 2136 fmol/mg protein. This was significantly lower than the Bmax value calculated for [3H]CGP-12177 binding to the same membranes, 8901 fmol/mg protein (Jozwiak et al., 2007). The most reasonable explanation for this observation is that in the HEK-β2-AR cell line, the majority of the β2-AR receptors reside in a conformation that has low affinity for [3H]MFen. Therefore, the data suggest that, in these saturation binding studies, [3H]MFen probes a subset of the conformations probed by [3H]CGP-12177. In the binding experiments, the low affinity binding is absent. This is consistent with the results from the docking studies in which the difference in MolDockScore values was very small in docking simulations between (S)-CGP-12177 and either β2-AR-In (Fig. 6A), or β2-AR-Ac models (−116.60 kJ/mol and −115.82 kJ/mol, respectively), whereas the difference for MFen was relatively much larger (−136.98 kJ/mol versus −132.53 kJ/mol). Because of the high dissociation rate of agonists from low-affinity sites, presumably, bound radioactivity dissociates during the washing in a filtration assay; accordingly, the 3H-labeled agonist does not seem to easily recognize a low-affinity binding site. This is consistent with the Hill coefficients close to but slightly less than 1.0 for agonists inhibiting [3H]MFen binding. It is noteworthy that agonists also have high Hill coefficients when inhibiting [3H]CGP-12177 (see Supplemental Table T1). Furthermore, GTP and GTPγS, which stabilize a low-affinity agonist conformation, have very little effect on [3H]CGP-12177 binding or on the ability of the fenoterol analogs to compete with [3H]CGP-12177 binding. Together, these data indicate that [3H]CGP-12177 binds preferably to and stabilizes the low-affinity agonist conformation, without much overlap with the high-affinity agonist conformation, the one to which [3H]MFen presumably binds.
The assumption that [3H]MFen binds to a high-affinity conformation of the β2-AR is supported by the comparative Ki values determined using [3H]MFen and [3H]CGP-12177 as the marker ligands. For the Fen analogs and (R)-isoproterenol, which are all full β2-AR agonists in the HEK-β2-AR cell line, each of the Ki MFen values were considerably lower (i.e., higher affinity) than the corresponding Ki CGP values (Table 1). The magnitude of the increase in binding affinity when using [3H]MFen was, to a great extent, dependent upon the configuration at the β-OH carbon, with an R configuration producing a greater enhancement in the binding affinity when [3H]MFen was the marker ligand. (R,R′)-Fen and (R,S′)-Fen greatly prefer binding to the high-affinity state probed by [3H]MFen, in that the Ki CGP/Ki MFen ratios are 86- and 20-fold, respectively. (S,R′)-Fen and (S,S′)-Fen have little preference between the two states, as seen in the Ki CGP/Ki MFen ratios of 6- and 8-fold, respectively. The opposite effect was observed with the two β-AR antagonists used in this study, propranolol and ICI-118-551, because the Ki MFen values were 8- and 4-fold higher than the corresponding Ki CGP values (Table 1). The apparent decrease in binding affinity when using [3H]MFen suggests that the antagonists have a lower but still significant affinity for the high-affinity agonist conformation of the receptor. For each of the agonists, the Ki MFen values were consistent with ligand potency, and there was considerably better correlation between binding affinity and functional activity, measured as EC50 values for stimulation of cAMP accumulation.
The data from the thermodynamic studies also support the hypothesis that the binding studies using [3H]MFen reflect ligand binding to a subset of the conformations probed by [3H]CGP-12177. The results indicate that the binding to the conformation probed by [3H]MFen was entropy-driven for all of the competing ligands used in the thermodynamic section of the study, including the antagonists (Table 3). In our previous studies using [3H]CGP-12177, the binding of (S,S′)-fenoterol to the β2-AR was found to be a purely enthalpy-driven process, the binding of (S,R′)-fenoterol, (R)-isoproterenol, and (rac)-propranolol was enthalpy/entropy-driven, and the binding of (R,S′)-fenoterol and (R,R′)-fenoterol was purely entropy-driven (Table 3; Fig. 5). (Jozwiak et al., 2010a). In all, these results suggest that the thermodynamic properties obtained using [3H]CGP-12177 represent the sum total of multiple factors, including unequal distributions of high- and low-affinity receptor conformations leading to a mixture of enthalpy- and entropy-driven processes.
The hypothesis that [3H]MFen can be used to explore an active, high-affinity conformation of the β2-AR was tested using simulated receptor-ligand docking studies employing the PDB code 2RH1 model {β2-AR-In}, regarded as an inactive form of the receptor (Rasmussen et al., 2007) and the PDB code 3POG model {β2-AR-Ac}, which is regarded as an active form of the receptor (Rasmussen et al., 2011). The docking of [3H]CGP-12177 and [3H]MFen in the β2-AR-In and β2-AR-Ac models confirmed a conserved binding mode proposed earlier for this group of molecules (Weis and Kobilka, 2008), with amino and β-hydroxy groups of both ligands trapped in the network of cross-interactions with Asp113 and Asn312 residues. Small differences in topological organization of ligand binding sites in the β2-AR-In and β2-AR-Ac models allows both MFen and CGP-12177 to adopt positions in which their aromatic ring systems may exercise optimized interactions with Ser203 [(S)-CGP-12177 and (R,R′)-MFen] and Ser207 [(R,R′)-MFen] of TM5 (Fig. 6). The comparison of the MolDockScore functions generated during docking simulations suggest that (R,R′)-MFen should preferentially bind to the β2-AR-Ac model, whereas (S)-CGP-12177 would bind to both the β2-AR-In and β2-AR-Ac models, with a slight preference for the inactive conformation of the receptor. This is consistent with the relative Ki values obtained in the saturation binding studies using the two probes.
In conclusion, the results of this study indicate that [3H]MFen can be used as a probe in the determination of binding affinities to the β2-AR. The data also indicate that this compound binds to a high-affinity active conformation of the receptor and allows for the characterization of selective β2-AR agonists. [3H]MFen may also be useful in the experimental verification of predictions made using the β2-AR-Ac model and for QSAR studies aimed at the development of highly selective and active β2-AR agonists.
Supplementary Material
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This research was supported in part by the Intramural Research Program of the National Institutes of Health National Institute on Aging; by the National Institutes of Health National Institute on Aging [Contract N01-AG31009]; and by the Foundation for Polish Science (TEAM Programme). The article was developed using the equipment purchased within the Project “The equipment of innovative laboratories doing research on new medicines used in the therapy of civilization and neoplastic diseases” within the Operational Program Development of Eastern Poland 2007–2013, Priority Axis I Modern Economy, Operations I.3 Innovation Promotion.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- β2-AR
- β2-adrenoceptor
- CGP-12177
- 4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one
- GPCR
- G protein-coupled receptor
- Fen
- fenoterol
- HEK
- human embryonic kidney
- HEK-β2-AR
- HEK293 cells stably transfected with β2-AR
- MFen
- (R,R′)-4-methoxyfenoterol
- ICI 118-551
- (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol
- PDB
- Protein Data Bank
- MVD
- Molegro Virtual Docker
- GTPγS
- guanosine 5′-O-(3-thio)triphosphate
- BI-167,107
- 5-hydroxy-8-{2-[2-(2-methylphenyl)-1,1-dimethyl-ethylamino]-1-hydroxyethyl}-4H-benzo[1,4]oxazin-3-one
- TM
- transmembrane.
Authorship Contributions
Participated in research design: Toll, Jozwiak, and Wainer.
Conducted experiments: Pajak, Plazinska, and Jimenez.
Contributed new reagents or analytic tools: Kozocas, Tanga, and Bupp.
Performed data analysis: Toll, Jozwiak, and Jimenez.
Wrote or contributed to the writing of the manuscript: Toll, Jozwiak, and Wainer.
References
- Baker JG. (2005) The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 144:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Proudman RG, Hawley NC, Fischer PM, Hill SJ. (2008) Role of key transmembrane residues in agonist and antagonist actions at the two conformations of the human beta1-adrenoceptor. Mol Pharmacol 74:1246–1260 [DOI] [PubMed] [Google Scholar]
- Bokoch MP, Zou Y, Rasmussen SG, Liu CW, Nygaard R, Rosenbaum DM, Fung JJ, Choi HJ, Thian FS, Kobilka TS, et al. (2010) Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature 463:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borea PA, Dalpiaz A, Varani K, Gilli P, Gilli G. (2000) Can thermodynamic measurements of receptor binding yield information on drug affinity and efficacy? Biochem Pharmacol 60:1549–1556 [DOI] [PubMed] [Google Scholar]
- Brodde OE, Karad K, Zerkowski HR, Rohm N, Reidemeister JC. (1983) Coexistence of beta 1- and beta 2-adrenoceptors in human right atrium. Direct identification by (±)-[125I]iodocyanopindolol binding. Circ Res 53:752–758 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Contreras ML, Wolfe BB, Molinoff PB. (1986) Thermodynamic properties of agonist interactions with the beta adrenergic receptor-coupled adenylate cyclase system. I. High- and low-affinity states of agonist binding to membrane-bound beta adrenergic receptors. J Pharmacol Exp Ther 237:154–164 [PubMed] [Google Scholar]
- Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. (2004) Comparative pharmacology of human beta-adrenergic receptor subtypes–characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 369:151–159 [DOI] [PubMed] [Google Scholar]
- Joseph SS, Lynham JA, Colledge WH, Kaumann AJ. (2004) Binding of (−)-[3H]-CGP12177 at two sites in recombinant human beta 1-adrenoceptors and interaction with beta-blockers. Naunyn Schmiedebergs Arch Pharmacol 369:525–532 [DOI] [PubMed] [Google Scholar]
- Jozwiak K, Khalid C, Tanga MJ, Berzetei-Gurske I, Jimenez L, Kozocas JA, Woo A, Zhu W, Xiao RP, Abernethy DR, et al. (2007) Comparative molecular field analysis of the binding of the stereoisomers of fenoterol and fenoterol derivatives to the beta2 adrenergic receptor. J Med Chem 50:2903–2915 [DOI] [PubMed] [Google Scholar]
- Jozwiak K, Toll L, Jimenez L, Woo AY, Xiao RP, Wainer IW. (2010a) The effect of stereochemistry on the thermodynamic characteristics of the binding of fenoterol stereoisomers to the beta(2)-adrenoceptor. Biochem Pharmacol 79:1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak K, Woo AY, Tanga MJ, Toll L, Jimenez L, Kozocas JA, Plazinska A, Xiao RP, Wainer IW. (2010b) Comparative molecular field analysis of fenoterol derivatives: A platform towards highly selective and effective beta(2)-adrenergic receptor agonists. Bioorg Med Chem 18:728–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RS, De Lean A, Lefkowitz RJ. (1980) A quantitative analysis of beta-adrenergic receptor interactions: resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol Pharmacol 17:14–23 [PubMed] [Google Scholar]
- Kozocas JA, Bupp JE, Tanga MJ, Pluhar JT, Wainer IW. (2010) Synthesis of tritium labeled (R,R)-4-methoxyfenoterol. J Labelled Comp Radiopharm 53:68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Williams LT. (1977) Catecholamine binding to the beta-adrenergic receptor. Proc Natl Acad Sci USA 74:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JC, Grandordy B, Barnes PJ. (1994) High affinity [3H]formoterol binding sites in lung: characterization and autoradiographic mapping. Eur J Pharmacol 269:35–41 [DOI] [PubMed] [Google Scholar]
- Merighi S, Simioni C, Gessi S, Varani K, Borea PA. (2010) Binding thermodynamics at the human cannabinoid CB1 and CB2 receptors. Biochem Pharmacol 79:471–477 [DOI] [PubMed] [Google Scholar]
- Miklavc A, Kocjan D, Mavri J, Koller J, Hadzi D. (1990) On the fundamental difference in the thermodynamics of agonist and antagonist interactions with beta-adrenergic receptors and the mechanism of entropy-driven binding. Biochem Pharmacol 40:663–669 [DOI] [PubMed] [Google Scholar]
- Nikulin VI, Rakov IM, De Los Angeles JE, Mehta RC, Boyd LY, Feller DR, Miller DD. (2006) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline-6,7-diols as novel affinity and photoaffinity probes for beta-adrenoceptor subtypes. Bioorg Med Chem 14:1684–1697 [DOI] [PubMed] [Google Scholar]
- Perrone MG, Santandrea E, Bleve L, Vitale P, Colabufo NA, Jockers R, Milazzo FM, Sciarroni AF, Scilimati A. (2008) Stereospecific synthesis and bio-activity of novel beta(3)-adrenoceptor agonists and inverse agonists. Bioorg Med Chem 16:2473–2488 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. (2011) Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature 469:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, et al. (2007) Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Seifert R, Dove S. (2009) Functional selectivity of GPCR ligand stereoisomers: new pharmacological opportunities. Mol Pharmacol 75:13–18 [DOI] [PubMed] [Google Scholar]
- Staehelin M, Simons P, Jaeggi K, Wigger N. (1983) CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem 258:3496–3502 [PubMed] [Google Scholar]
- Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. (2004) Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem 279:686–691 [DOI] [PubMed] [Google Scholar]
- Toews ML, Harden TK, Perkins JP. (1983) High-affinity binding of agonists to beta-adrenergic receptors on intact cells. Proc Natl Acad Sci USA 80:3553–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Jimenez L, Waleh N, Jozwiak K, Woo AY, Xiao RP, Bernier M, Wainer IW. (2011) {Beta}2-adrenergic receptor agonists inhibit the proliferation of 1321N1 astrocytoma cells. J Pharmacol Exp Ther 336:524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland GA, Minneman KP, Molinoff PB. (1979) Fundamental difference between the molecular interactions of agonists and antagonists with the beta-adrenergic receptor. Nature 281:114–117 [DOI] [PubMed] [Google Scholar]
- Weis WI, Kobilka BK. (2008) Structural insights into G-protein-coupled receptor activation. Curr Opin Struct Biol 18:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.