Abstract
OBJECTIVE:
Latino children of Caribbean descent remain at high risk for poorly controlled asthma. Controller medications improve asthma control; however, medication adherence remains suboptimal, particularly among minorities. This study assessed socioeconomic, family-based, and parent factors in medication adherence among children with asthma from Rhode Island (RI; Latino and non-Latino white [NLW]) and Puerto Rico.
METHODS:
Data collection occurred as part of a multicenter study of asthma disparities. Our sample included children (ages 7–16) prescribed objectively monitored controller medications (n = 277; 80 island Puerto Rico, 114 RI Latino, 83 RI NLW). Parents completed questionnaires regarding family background and beliefs about medications. Families participated in an interview regarding asthma management. Multilevel analyses (maximum likelihood estimates) accounting for children being nested within site and ethnic group assessed the contribution of social context, family, and parent variables to medication adherence.
RESULTS:
Medication adherence differed by ethnic group (F2, 271 = 7.46, P < .01), with NLW families demonstrating the highest levels of adherence. Multilevel models indicated that parental beliefs about medication necessity and family organization regarding medication use were significant predictors of adherence, even for families below the poverty threshold. With family factors in the model, a substantial improvement in model fit occurred (Akaike Information Criterion change of 103.45).
CONCLUSIONS:
Adherence to controller medications was lower among Latino children in our sample. Targeted interventions that capitalize on existing family resources, emphasize structure, and address parental beliefs about the importance of medications may be of benefit to families from different cultural backgrounds.
KEY WORDS: asthma, patient nonadherence, disparities
What’s Known on This Subject:
Asthma disparities exist, with Latino children of Caribbean descent at risk for poor disease control. Controller medications reduce symptoms; however, medication adherence remains suboptimal. Identifying what factors predict poor medication adherence in at-risk groups could identify important treatment targets.
What This Study Adds:
This study is the first to assess objective rates of medication use among children with asthma in Puerto Rico. Findings suggest that interventions incorporating family resources and addressing parental beliefs about medications may be of benefit across cultural groups.
Pediatric asthma is a major health concern in the United States, affecting more than 9 million children.1 Over the past 2 decades, disease prevalence among children has increased ∼4.3% per year, and the financial burden of treating asthma in children has also risen.1 Pediatric asthma is a manageable disease; those with persistent symptoms require the use of daily medications, most commonly inhaled corticosteroids (ICS), to control symptoms and prevent chronic airway inflammation. Adherence is typically measured by parent or child report, or through electronic monitoring devices. Although electronic devices are costly, rates of objectively measured adherence are more accurate because self-report bias is eliminated.2 Among children, adherence to daily asthma medications is generally poor, with nonadherence rates ranging from 34%3 to 71%.4 Poor adherence has implications for declining asthma control; decreases in adherence over time have been associated with increased health care use.3
Ethnic minority children typically experience worse asthma outcomes than non-Latino white (NLW) children.5 Latino children from Caribbean areas, such as Puerto Rico (PR), have particularly high asthma burden.6 The lifetime prevalence rate of asthma in PR children is 26%, as compared with 13% in NLW children. PR children also experience worse asthma morbidity, including asthma attacks and health care use, compared with NLW children. Reports suggest that the prevalence of exacerbations in PR children is 12%, compared with 6% in NLW children.6
The sources of disparities in asthma outcomes are likely multidetermined, and include factors relating to the health care system (eg, access barriers), the individual (eg, genetics, illness beliefs), and the environment (eg, housing).7 Low rates of medication prescription and use may be important modifiable sources of disparity.
Rates of controller medication dispenses for pediatric asthma are particularly low in PR.8 Parents of Latino children with asthma in the mainland United States also report low rates of controller medication use.9 Medication adherence, or actual medication use once obtained, tends to be lower among minority children compared with NLW children.2,10 Medication beliefs may play a role in adherence; many Latino parents have greater concerns regarding controller medication use,11,12 which may influence their acceptance of medications. Few reports have investigated adherence rates specific to Latino children, or factors relating to adherence among this high-risk group.
Because parents are largely responsible for asthma management, parental factors may contribute to adherence.7 Among adults, positive beliefs about asthma medications have been associated with increased adherence.13 Parental beliefs about medication efficacy have been associated with parent-reported ICS adherence in children.14,15 Island PR caregivers may encounter additional barriers to filling prescriptions relative to mainland Latino and NLW.16 Parent psychopathology, particularly maternal depression, has also been associated with reported problems with inhaler technique and consistent medication usage,17 as well as greater health care use among children.18,19
Family factors are also relevant; organization surrounding medication routines has been associated with higher medication adherence.20 Similarly, lower levels of family stress have been associated with higher adherence.4 Ethnic group differences in adherence rates may also be related to financial resources. Higher levels of socioeconomic status (SES) have been associated with more optimal adherence among adults,21 and suboptimal adherence has been found among African Americans of low SES.3
We investigated socioeconomic, family-based, and parental predictors of medication adherence among NLW and Latino children in PR and mainland United States. This study is unique in the assessment of objective rates of medication adherence by ethnic background and place of residence, and in the evaluation of predictors of controller medication use in children with persistent asthma. We expected that Latino children, and island PR children in particular, would have lower rates of medication adherence than NLW children. Because of the potential for confounding between social context (eg, poverty) and demographic groupings, we used a multilevel approach, which takes into account that these groupings have specific associated features. We anticipated that family variables would emerge as important predictors in adherence. Last, we expected that maternal psychopathology would be negatively associated with adherence, and that positive parental beliefs about medication would be associated with higher adherence across groups.
Methods
Participants for this report were a subset from a larger study (n = 805)22,23 investigating pediatric asthma disparities between Latino and NLW families. Previous work from this sample has evaluated asthma severity across demographic groups,23 differences in lung function perception between Latino and NLW children,24 and medication beliefs among Latino parents11; however, data regarding medication adherence have not been reported.
Data were collected in both Rhode Island (RI) and PR. Participants were recruited from primary care clinics, cultural events, and educational programs. Child participants had physician-diagnosed asthma confirmed through medical history, physical examination, and spirometry. Children with other chronic conditions that could complicate asthma diagnosis (eg, vocal-cord dysfunction) were excluded. PR families with a child with asthma (aged 7–15) were eligible at the PR site. In RI, caregivers of either Latino (specifically PR or Dominican) or NLW ethnicity were eligible. For the present analysis, we included only children who were prescribed a controller medication that could be monitored objectively, specifically montelukast (oral), fluticasone (metered-dose inhaler [MDI]), and fluticasone/salmeterol combination (diskus). This resulted in an initial sample of 352 families, including 132 island Puerto Rican children, 128 RI Latino children, and 92 NLW children in RI.
Procedures
Data collection occurred in 4 study visits over 3 to 4 months. Visits were typically conducted in the home by trained research assistants. A clinical visit involving spirometry was conducted by medical staff in a hospital setting. Informed consent and demographic information were obtained at the initial visit. Data for the present article were collected at a second visit, typically 1 to 2 weeks later, during which the family was interviewed regarding asthma management, completed surveys, and began the adherence monitoring protocol. Adherence devices were collected 5 to 6 weeks later. Families were paid for participating.
Measures
Social Context Factors
An income-to-needs ratio was calculated for each family by dividing annual income by the poverty threshold for that family size.25 Families for which the ratio was ≤1.0 were coded as below the Poverty Threshold. An index of Financial Strain was also calculated, reflecting families’ ability to pay for rent and other basic necessities. Levels of Neighborhood Disadvantage were assessed with the Neighborhood Unsafety Scale,26 a measure of parents’ perception of neighborhood characteristics over the previous year.
Family Factors
Information regarding family composition (2-parent versus single-parent home) was collected. A clinical rating of the family’s organization and strategies regarding medication adherence was made from a validated, semistructured interview.27 Questions probed topics, such as family barriers to medication use, plans for using medication away from home, and ease of following medication routine.
Individual Parent Factors
Parents completed a self-report survey of parental beliefs about the necessity of asthma medication and concerns about its use.28 Parents completed an inventory of psychiatric symptoms, the Behavior and Symptom Identification Scale (BASIS), which is reliable and valid for Latinos.29 An overall index of psychiatric symptoms was derived.
Adherence Monitoring
Adherence was monitored in 3 ways. The TrackCap Medication Events Monitoring System monitor (AARDEX, Union City, CA), a replacement pill bottle with an electronic cap that records the date and time of opening, was used to measure adherence to oral montelukast. The MDIlog II (Westmed, Englewood, CO), a small electronic device attached to a “sleeve” that fits over the inhaler, was used to assess MDI adherence. Last, the dosage counter on fluticasone/salmeterol combination medication was used. A protocol to document counter readings at the beginning and end of the monitoring period was used, accounting for replacement medications during that period.
Adherence was measured for a minimum of 28 days. Adherence data were inspected and cleaned according to standard procedures.10 Seventy-five cases were eliminated from the adherence analysis. Protocol difficulties resulting in unusable or missing data included prescription issues (eg, discontinuation; 6.5%), device damage/loss (5.4%), missing documentation (6.5%), and use of another bottle/MDI (2.8%). This resulted in 277 usable cases for the final sample. The PR site encountered greater device loss and malfunction (χ2 = 41.21, P < .001). The final sample had a higher proportion of severe persistent cases than the original sample (χ2 = 11.61, P < .003), but did not differ in terms of child age or sex, or poverty threshold. Monitoring period length was similar across devices: montelukast (45.1 days), fluticasone (47.5 days), dosage counter (48.5 days).
Adherence estimates were calculated as total doses per day/prescribed doses per day, for MDIlog II and TrackCap devices. Daily adherence was capped at 100% of prescribed doses to ensure that participants could not “make up” missed doses on the following day. Dosage counter adherence was calculated based on total doses taken/prescribed doses over the monitoring period. Given that participants could be prescribed more than 1 monitored medication, a categorical variable was constructed to represent each medication combination encountered in this study, namely montelukast alone (61.7%), fluticasone alone (7.9%), fluticasone/salmeterol combination (8.7%), fluticasone and montelukast (8.3%), and fluticasone/salmeterol combination with montelukast (13.4%). For participants on 2 monitored medications, adherence estimates were averaged.
Statistical Analyses
Probit transformations to normalize proportional data were applied before analysis. Preliminary analyses evaluated overall differences in adherence by medication monitoring type (F4, 271 = 3.02, P < .05) and child age (r = −0.24, P < .001). Therefore, all analyses adjusted for these variables. Multilevel analyses (maximum likelihood estimates) were estimated by using SPSS (SPSS, Inc, Chicago, IL) mixed model procedure. Clustering of individuals within site and cultural group (island PR, RI Latino, RI NLW) violates the assumption of independence; the multiple levels represented individuals nested within site/ethnicity group. We used the Akaike Information Criterion (AIC) to compare the goodness of fit of our models; smaller values indicate better fit.30 Analyses evaluated the contribution of our predictor variables, social context (poverty status, financial strain, neighborhood safety), family factors (1 vs 2 parents, family organization), and individual parent variables (medication beliefs, psychological functioning), on our outcome variable, medication adherence, in a sequential manner. Specifically, Model 0 (baseline model) evaluated the contribution of demographic group (PR, RI Latino, RI NLW) to adherence. In Model A, the effects of child age and monitoring type were assessed. Models B through D evaluated the variable sets associated with social context variables, family effects, and individual parent effects. Sequencing the models in this manner allowed for evaluation of the influence of key predictor sets while controlling for the influence of potential confounds. We estimated power based on nested analysis of variance models. Power was found to be 0.50 for medium effects.
Results
Participant characteristics are presented in Table 1. Of Latino parents in RI, 46.9% (n = 60) were Puerto Rican, and 53.1% (n = 68) were Dominican. We combined these 2 subgroups given their high degree of demographic similarity documented previously in this sample.24 The proportion of families below the federally defined guidelines for poverty differed among the 3 groups (χ2 = 43.7, P < .001). Among the RI groups, 57.0% of the Latino families were below the poverty threshold, and 14.2% of the NLW families met this criterion. In PR, 49.2% of the families were below the poverty threshold.
TABLE 1.
Demographics of Children and Caregivers
| Child ethnicity/site, n (%) | |
|---|---|
| RI Latino | 128 (36) |
| RI NLW | 92 (26) |
| Island PR | 132 (38) |
| Child age, M (SD) | 10.3 (2.4) |
| Child gender, female, n (%) | 149 (42.2) |
| Asthma severity, n (%) | |
| Mild persistent | 106 (30.1) |
| Moderate persistent | 131 (37.2) |
| Severe persistent | 115 (32.7) |
| Caregiver identity, n (%) | |
| Biological mother | 335 (95.3) |
| Biological father | 12 (3.3) |
| Other female relative | 5 (1.4) |
| Two-parent home, n (%) | 166 (47.2) |
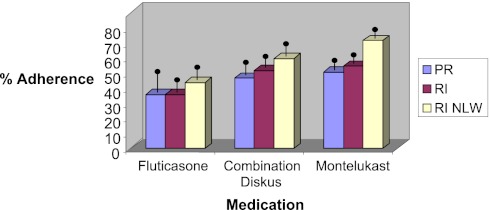
Medication adherence differed by ethnic group (F2, 271 = 7.46, P < .01, with NLW families demonstrating the highest levels. Overall means by demographic group and medication are presented in Fig 1. Social context, family, and individual parent variables evaluated in the multilevel analyses are presented in Table 2 and described in the following sections.
FIGURE 1.
Medication adherence rates. All means are adjusted for child age. Means are presented by medication for ease of interpretation; however, direct comparisons by medication and ethnic group are not presented given the heterogeneity of medication combinations across children.
TABLE 2.
Multilevel Predictors of Child Medication Adherence
| Model 0 | Model A | Model B | Model C | Model D | |
|---|---|---|---|---|---|
| Intercept, β [95% CI] | 5.18 [4.75, 5.61]a | 6.49 [5.81, 7.17]a | 7.01 [6.20, 7.82]a | 5.69 [4.62, 6.76]a | 4.82 [3.54, 6.10]a |
| Covariates | |||||
| Demographic group | 0.04 [0.005, 0.33] | 0.05 [0.006, 0.34] | 0.01 [0.001, 0.42] | 0.02 [0.001, 0.47] | 0.003 [0.00, >1.0] |
| Child age | –0.10 [–0.15, –0.05]a | –0.10 [–0.15, –0.05]a | –0.07 [ –0.12, –0.02]a | –0.09 [–0.14, –0.03]a | |
| Monitoring type | |||||
| Montelukast only | –0.19 [–0.55, 0.17] | –0.24 [–0.60, 0.12] | –0.19 [–0.56, 0.17] | –0.18 [–0.54, 0.19] | |
| Fluticasone only | –0.93 [ –1.45, –0.41]a | –0.98 [–1.51, –0.46]a | −1.16 [–1.68, –0.63]a | −1.2 [–1.73, –0.68]a | |
| Fluticasone/salmeterol combination | –0.44 [ –0.94, 0.07] | –0.51 [–1.02, 0.01] | –0.50 [–1.04, 0.05] | –0.47 [–1.0, 0.06] | |
| Fluticasone and montelukast | –0.30 [ –0.81, 0.21] | –0.33 [ –0.84, 0.19] | –0.34 [–0.85, 0.16] | –0.41 [ –0.92, 0.09] | |
| Fluticasone/salmeterol combination with montelukast (Reference group) | 0 | 0 | 0 | 0 | |
| Hypothesized predictors | |||||
| Social context factors | |||||
| Financial strain | –0.20 [ –0.51, 0.12] | –0.03 [–0.37, 0.31] | –0.02 [–0.37, 0.34] | ||
| Neighborhood safety | –0.09 [–0.28, 0.09] | –0.05 [–0.24, 0.15] | –0.06 [–0.25, 0.13] | ||
| Poverty threshold | –0.12 [–0.39, 0.14] | –0.11 [–0.40, 0.18] | –0.17 [–0.45, 0.12] | ||
| Family factors | |||||
| One versus two parents | 0.20 [ –0.09, 0.50] | 0.19 [–0.10, 0.48] | |||
| Organization | 0.11 [ 0.04, 0.18]a | 0.10 [0.03, 0.18]a | |||
| Individual parent factors | |||||
| Medication necessity | 0.29 [0.11, 0.47]a | ||||
| Medication concerns | –0.05 [–0.21, 0.11] | ||||
| Psychological functioning | 0.10 [–0.22, 0.41] | ||||
| AIC | 812.29 | 792.52 | 779.78 | 676.33 | 658.49 |
| Comparison with Model 0 | 19.77 | 32.51 | 135.96 | 153.80 |
P < .01.
Ethnic Group (Model 0)
The unconditional means model (nested within site/ethnicity grouping) provided the basis for subsequent testing of more complex models. The AIC was 812.92.
Demographic, Age, and Monitoring-Type Factors (Model A)
Younger child age was associated with higher medication adherence. Adherence was also associated with monitoring type. The highest adherence rates were found in children prescribed montelukast alone; the lowest adherence rates were found among children prescribed fluticasone alone. As monitoring type is a categorical value, the medication assignment of montelukast and combination fluticasone/salmeterol was used as a reference value. Model A also demonstrated an improvement in fit over Model 0 (AIC change = 19.77).
Covariates (Demographic, Age, Monitoring Type) and Social Context Factors (Model B)
In the next step, factors related to the family’s social context (poverty status, financial strain, neighborhood safety) were added to the model. Child age and monitoring type remained as predictors of medication adherence. Poverty threshold, financial strain, and neighborhood safety were not significant predictors in the model. The improvement in fit between Models A (AIC = 792.52) and B (AIC = 779.78) was 12.74.
Covariates, Social Context, and Family Factors (Model C)
A substantial improvement in fit was evidenced by the addition of family factors to the model (AIC change from Model B to C [779.78 – 676.33] = 103.45). Of the family factors, higher interview-based ratings of family organization regarding adherence were associated with greater medication adherence. Child age and monitoring type continued to be significant predictors of medication adherence with family factors in the model.
Covariates, Social Context, Family Factors, and Individual Parent Factors (Model D)
In the final step, individual parent factors (medication beliefs, psychological functioning) were added to the model. Belief in medication necessity was associated with higher adherence; medication concerns and parental psychopathology were not associated with adherence. Child age, monitoring type, and family organization regarding adherence remained significant in the final model. An AIC change of 17.84, from 676.33 (Model C) to 658.49 (Model D), was observed.
Discussion
Despite the efficacy of anti-inflammatory medications to improve disease control, medication adherence remains poor, particularly for ethnic minority children. Our study measured rates of objective medication use among Latino children from PR, where asthma prevalence and morbidity are particularly high,6 and compared these rates to those from Latino children and NLW children from a mainland sample. Data reflect suboptimal adherence to controller medications, and particularly low rates among Latino children in both sites. In our RI sample, where barriers to medication use are reduced because of high rates of health care coverage,31 Latino children still had low adherence. Interestingly, our low proportion of reported ICS prescriptions suggests potential undertreatment of asthma. This may be because of low prescription rates on the part of the providers, families’ hesitation to fill prescriptions, or both. In PR, the combination of overall low prescription rates of controller medications,8 and, when prescribed, low rates of adherence to these medications, is likely to yield suboptimal treatment of persistent asthma.
We evaluated the contributions of contextual, family-level, and individual parent variables to consistent medication use in our sample. The multilevel regression approach accounted for the fact that individuals are nested within specific cultural groups and associated health care delivery contexts. Within these models, SES factors were not associated with adherence, likely because of the differences between our demographic groups on these variables, and limited variability within the RI Latino sample. Of note, our poverty-level thresholds for families living on the US mainland were applied to our PR sample, and may not account for potential regional economic differences.
Our models indicated, across both cultures, that family organization regarding medication use was associated with higher adherence. These findings are consistent with those of Fiese and colleagues,20 who found that family routines around medications were associated with higher adherence. Results suggest that family-based interventions emphasizing structure and organizational strategies regarding medications may benefit families from diverse cultural backgrounds.
Parental belief regarding medication necessity was also associated with adherence. The importance of addressing culturally derived beliefs regarding medication use has been stressed32; however, it is unclear how frequently practitioners implement these recommendations. Our results suggest that addressing cultural beliefs about controller medications may be necessary to promote effective use among Latinos. Specifically, health care providers could engage parents in a brief discussion regarding their beliefs and concerns about medication use, and provide them with information about medication benefits. Culturally tailored approaches that address parental concerns have been shown to be effective for Latino families of children with asthma.33
Our study has a number of limitations. As with many studies investigating objective medication adherence, our sample was largely derived from clinical recruitment, and may not be fully representative of the demographic groups under study. Our sample does not include children who were prescribed medication that was never filled; as a result, our adherence rates may be overestimates. Use of objective monitoring devices to evaluate medication adherence is a strength of our approach because it eliminates self-report bias; however, even objective devices have limitations. Medication Events Monitoring System devices, such as the TrackCap, do not measure actual ingestion of the medication, and even devices that do measure ingestion do not ensure appropriate technique. Our final adherence estimates were based on data from only 79% of our participants initially assigned devices; however, this rate of missing data is not unusual for a community-based, low-income sample (eg,34 77%). Our study is not powered to detect small effects; therefore, nonsignificant findings should be interpreted with caution.
We focused on a number of key predictors of adherence; however, there are likely many others that affect medication use. Future work should examine novel strategies to support families to maintain consistent adherence, and address potential systemic and cultural barriers to medication adherence.
Conclusions
Our data have important clinical implications given the central role of medications in decreasing asthma morbidity. We focused on a group of children at particular risk for asthma burden, and examined predictors of adherence that may be modifiable through intervention. Our low rates of medication adherence indicate that health care providers should assume that many families will have difficulties in using controller medications as consistently as directed. Targeted interventions should focus on 2 important areas. First, interventions should capitalize on existing family structure, working to embed a child’s daily medication usage into existing routines. Second, intervention approaches should address parental beliefs about the importance of medications. Focusing on these issues has the potential to improve medication adherence in children with asthma from a range of different cultural backgrounds.
Glossary
- AIC
Akaike Information Criterion
- ICS
inhaled corticosteroids
- MDI
metered-dose inhaler
- NLW
non-Latino white
- PR
Puerto Rico
- RI
Rhode Island
- SES
socioeconomic status
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was funded by the National Heart, Lung, and Blood Institute; U01 HL 072438, G. Fritz and G. Canino, PIs, and Eunice Kennedy Shriver Institute of Children’s Health and Human Development, K24 HD 058794, E. McQuaid, PI. Funded by the National Institutes of Health (NIH).
References
- 1.Centers for Disease Control and Prevention. Asthma Prevalence, Health Care Use, and Mortality, 2002. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2004 [Google Scholar]
- 2.Bender B, Wamboldt FS, O'Connor SL, Rand C, Szefler S, Milgrom H. Measurements of children's asthma medication by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85(5):416–481 [DOI] [PubMed] [Google Scholar]
- 3.McNally KA, Rohan J, Schluchter M, et al. Adherence to combined montelukast and fluticasone treatment in economically disadvantaged African American youth with asthma. J Asthma. 2009;46(9):921–927 [DOI] [PubMed] [Google Scholar]
- 4.Burgess SW, Sly PD, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology. 2008;13(4):559–563 [DOI] [PubMed] [Google Scholar]
- 5.Ortega AN, Belanger KD, Paltiel AD, Horwitz SM, Bracken MB, Leaderer BP. Use of health services by insurance status among children with asthma. Med Care. 2001;39(10):1065–1074 [DOI] [PubMed] [Google Scholar]
- 6.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117(1):43–53 [DOI] [PubMed] [Google Scholar]
- 7.Canino G, McQuaid EL, Rand CS. Addressing asthma health disparities: a multilevel challenge. J Allergy Clin Immunol. 2009;123(6):1209–1217, quiz 1218–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vila D, Rand CS, Cabana MD, et al. Disparities in asthma medication dispensing patterns: the case of pediatric asthma in Puerto Rico. J Asthma. 2010;47(10):1136–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inkelas M, Garro N, McQuaid EL, Ortega AN. Race/ethnicity, language, and asthma care: findings from a 4-state survey. Ann Allergy Asthma Immunol. 2008;100(2):120–127 [DOI] [PubMed] [Google Scholar]
- 10.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28(5):323–333 [DOI] [PubMed] [Google Scholar]
- 11.McQuaid EL, Garro A, Seifer R, et al. Clinician, parent and child ratings of asthma severity and pulmonary function variability. Paper presented at: International Society for the Advancement of Respiratory Psychophysiology (ISARP), 16th Annual Meeting and 28th Symposium on Respiratory Psychophysiology; October 25–27, 2009; Berlin, Germany [Google Scholar]
- 12.Koinis-Mitchell D, McQuaid EL, Friedman D, et al. Latino caregivers’ beliefs about asthma: causes, symptoms, and practices. J Asthma. 2008;45(3):205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le TT, Bilderback A, Bender B, et al. Do asthma medication beliefs mediate the relationship between minority status and adherence to therapy? J Asthma. 2008;45(1):33–37 [DOI] [PubMed] [Google Scholar]
- 14.Eggleston PA, Malveaux FJ, Butz AM, et al. Medications used by children with asthma living in the inner city. Pediatrics. 1998;101(3 pt 1):349–354 [DOI] [PubMed] [Google Scholar]
- 15.Smith LA, Bokhour B, Hohman KH, et al. Modifiable risk factors for suboptimal control and controller medication underuse among children with asthma. Pediatrics. 2008;122(4):760–769 [DOI] [PubMed] [Google Scholar]
- 16.McQuaid EL, Vasquez J, Canino G, et al. Beliefs and barriers to medication use in parents of Latino children with asthma. Pediatr Pulmonol. 2009;44(9):892–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartlett SJ, Krishnan JA, Riekert KA, Butz AM, Malveaux FJ, Rand CS. Maternal depressive symptoms and adherence to therapy in inner-city children with asthma. Pediatrics. 2004;113(2):229–237 [DOI] [PubMed] [Google Scholar]
- 18.Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, Rand CS. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Arch Pediatr Adolesc Med. 2001;155(3):347–353 [DOI] [PubMed] [Google Scholar]
- 19.Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104(6):1274–1280 [DOI] [PubMed] [Google Scholar]
- 20.Fiese BH, Wamboldt FS, Anbar RD. Family asthma management routines: connections to medical adherence and quality of life. J Pediatr. 2005;146(2):171–176 [DOI] [PubMed] [Google Scholar]
- 21.Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it's not just black and white. J Clin Immunol. 2003;111(6):1219–1226 [DOI] [PubMed] [Google Scholar]
- 22.Canino G, McQuaid EL, Alvarez M, et al. Issues and methods in disparities research: the Rhode Island-Puerto Rico asthma center. Pediatr Pulmonol. 2009;44(9):899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esteban CA, Klein RB, McQuaid EL, et al. Conundrums in childhood asthma severity, control, and health care use: Puerto Rico versus Rhode Island. J Allergy Clin Immunol 2009;124(2):238-244, 244.e1-5. [DOI] [PMC free article] [PubMed]
- 24.Fritz GK, McQuaid EL, Kopel SJ, et al. Ethnic differences in perception of lung function: a factor in pediatric asthma disparities? Am J Respir Crit Care Med. 2010;182(1):12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services The 2005 HHS Poverty Guidelines. Washington, DC: US Department of Health and Human Services; 2005 [Google Scholar]
- 26.Resnick MD, Bearman PS, Blum RW, et al. Findings from the National Longitudinal Study on Adolescent Health Protecting adolescents from harm. JAMA. 1997;278(10):823–832 [DOI] [PubMed] [Google Scholar]
- 27.McQuaid EL, Walders N, Kopel SJ, Fritz GK, Klinnert MD. Pediatric asthma management in the family context: the family asthma management system scale. J Pediatr Psychol. 2005;30(6):492–502 [DOI] [PubMed] [Google Scholar]
- 28.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567 [DOI] [PubMed] [Google Scholar]
- 29.Eisen SV, Gerena M, Ranganathan G, Esch D, Idiculla T. Reliability and validity of the BASIS-24 Mental Health Survey for Whites, African-Americans, and Latinos. J Behav Health Serv Res. 2006;33(3):304–323 [DOI] [PubMed] [Google Scholar]
- 30.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;(AC-19):716–722 [Google Scholar]
- 31.Rhode Island Kids Count. 2010 Rhode Island Kids Count Fact Book RI Kids Count: Providence, RI; 2010 [Google Scholar]
- 32.George M. The challenge of culturally competent health care: applications for asthma. Heart Lung. 2001;30(5):392–400 [DOI] [PubMed] [Google Scholar]
- 33.Canino G, Vila D, Normand SL, et al. Reducing asthma health disparities in poor Puerto Rican children: the effectiveness of a culturally tailored family intervention. J Allergy Clin Immunol. 2008;121(3):665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Celano MP, Linzer JF, Demi A, et al. Treatment adherence among low-income, African American children with persistent asthma. J Asthma. 2010;47(3):317–322 [DOI] [PubMed] [Google Scholar]