Abstract
OBJECTIVE:
Methylmalonic acidemia (MMA) is a metabolic disorder with a poorly defined long-term neurocognitive phenotype. We studied the neuropsychological outcomes of patients and examined clinical covariates that influenced cognition.
METHODS:
A diverse cohort with mut, cblA, or cblB subtypes of isolated MMA (N = 43), ages 2 to 32 years, were evaluated at a single center over a 6-year period. The influence of clinical, laboratory, and metabolic parameters on neuropsychological testing results was determined.
RESULTS:
Early-onset mut patients (n = 21) manifested the most severe neurocognitive impairments, with a mean ± SD full-scale IQ (FSIQ) of 71.1 ± 14.75. Late-onset mut patients (n = 6) had a mean FSIQ of 88.5 ± 27.62. cblA (n = 7), cblB (n = 6), and mut patients diagnosed prenatally or by newborn screening (n = 3) obtained mean FSIQs in the average range (100.7 ± 10.95, 96.6 ± 10.92, and 106.7 ± 6.66, respectively). Hyperammonemia at diagnosis and the presence of a seizure disorder were associated with a lower FSIQ (P = .001 and P = .041, respectively), but other clinical variables, including basal ganglia injury and mutation status, did not. FSIQ remained stable over longitudinal testing (n = 10). Decreased scores on processing speed, compared with all other intellectual domains, emerged as a specific neurocognitive manifestation.
CONCLUSIONS:
The neurocognitive outcomes seen in isolated MMA are highly variable. An earlier age of disease onset, the presence of hyperammonemia at diagnosis, and a history of seizures were associated with more severe impairment. In all patient subtypes, selective deficits in processing speed were present.
KEY WORDS: basal ganglia, cognition, FSIQ, hyperammonemia, IQ, methylmalonic acidemia, MMA, neuropsychology
What’s Known on This Subject:
Isolated methylmalonic acidemia, one of the most common inborn errors of organic acid metabolism, is known to be associated with variably impaired intellectual functioning and severe biochemical and clinical abnormalities. However, the neurocognitive outcomes have yet to be fully described.
What This Study Adds:
This research defines the neurocognitive phenotype of isolated methylmalonic acidemia and identifies processing speed as a specific impairment. Clinical, biochemical, and molecular genetic covariates were explored. A history of hyperammonemia at diagnosis was found to correlate with poorer cognitive outcomes.
The isolated methylmalonic acidemias (MMAs) are a heterogeneous group of autosomal recessive inborn errors of organic acid metabolism first described in 1967 and affect ∼1 in 50 000 to 1 in 80 000 births.1–3 Isolated MMA is frequently caused by deficient activity of methylmalonyl–coenzyme A mutase, resulting in partial (mut–) or complete (mut0) loss of enzymatic function. Other subtypes of MMA, cblA and cblB, involve disruption in the handling and synthesis of the methylmalonyl–coenzyme A cofactor 5′-deoxyadenosylcobalamin. All forms of MMA result in massive elevation of methylmalonic acid and metabolites derived from propionyl–coenzyme A in the body fluids of affected patients.4
Patients with MMA typically present within the first year of life, often in the neonatal period, with encephalopathy, hyperammonemia, and metabolic acidosis.5 The disorder is difficult to manage and has a highly variable outcome.6–12 Treatment options involve injections of vitamin B12 in responsive patients13; strict adherence to a low-protein diet; carnitine supplementation; and solid organ transplantation.14 Neurocognitive and neurologic deficits have been recognized to occur in patients with MMA,6,8,11 including global developmental delay and hyperkinetic movement disorders secondary to basal ganglia injury.15,16
Even though MMA is one of the most common organic acidemias and has been studied over the past 40 years by numerous investigators, the severity, high mortality, variable treatment regimens, and clinical heterogeneity of the disorder have made the natural history and long-term disease-related complications difficult to define.17 Consequently, many studies that have attempted to examine the neurocognitive outcomes of patients with MMA were retrospectively conducted,8,18 relied on surveys using historical data collected across multiple institutions,6,8,10,18,19 and/or used varied testing protocols to measure cognition.6,9,10
To investigate neurocognitive outcomes seen in the patient population, a cohort of 43 individuals with isolated MMA underwent a battery of clinical, laboratory, and psychological assessments to characterize the specific cognitive domains affected in MMA. The goal was to determine if cognitive deficits mirror clinical severity, to describe dependent neurologic covariates, and to explore cognitive phenotype–genotype enzymatic relationships. Our results define a disease-associated neuropsychological phenotype and identify clinical covariates associated with cognitive outcome.
Methods
Subjects
Patient studies were conducted through National Institutes of Health study 04-HG-0127 (“Clinical and Basic Investigations of Methylmalonic Acidemia and Related Disorders”; clinicaltrials.gov identifier: NCT00078078) in compliance with the Declaration of Helsinki. Patients were evaluated longitudinally between 2004 and 2010. The recruitment was not based on race, gender, nutritional status, or disease severity. Ongoing care and dietary management were provided by regional metabolic centers. Forty-three patients completed the neurocognitive battery and were included in this study.
Laboratory, Molecular Genetic, and Clinical Studies
In all cases, the diagnosis of MMA was confirmed with cellular biochemical studies and/or molecular genetic analysis. Because only 2 patients were classified enzymatically as mut–, they were combined with mut0 patients and in toto designated as the mut subtype, which was then separated into early- and late-onset groups for subsequent analyses. Age at onset was determined by using medical record review and/or parental interview. Patients were classified as early-onset when they presented on or before 30 days of life. Patients with a prenatal or newborn screening diagnosis (P/NBS) were grouped separately. Disease presentation was defined as the presence of symptoms and signs characteristic of MMA (tachypnea, recurrent vomiting, poor feeding, lethargy, encephalopathy, and failure to thrive). Hyperammonemia was defined as ammonia levels >100 µM at <1 month of age or >35 µM for those aged ≥1 month.
Disease presentation and history of hyperammonemia, seizures, and dialysis were determined by using medical records and/or interview.
Neurocognitive Evaluation and Neurologic Examination
Age-appropriate versions of the Wechsler intelligence scales were administered (Wechsler Preschool and Primary Scale of Intelligence–Third Edition,20 Wechsler Intelligence Scale for Children–Fourth Edition,21 Wechsler Adult Intelligence Scale–Third Edition,22 or Wechsler Abbreviated Scale of Intelligence23). Verbal Comprehension, Perceptual Reasoning/Organization, Working Memory, and Processing Speed indices, as well as full-scale IQ (FSIQ), were calculated according to the Wechsler manuals. In a limited number of instances, we substituted a derived index with strong conceptual overlap21 (eg, Wechsler Preschool and Primary Scale of Intelligence–Third Edition Verbal IQ for Verbal Comprehension) for Wechsler scales that do not have 1 of the 4 aforementioned indices or if an index could not be computed for a particular subject. For visually or motorically impaired patients (n = 5), FSIQ was estimated by using verbal subtests from the Wechsler scales. For 2 additional early-onset mut patients, FSIQ was estimated by using available subtests because of cooperation considerations.
For patients aged <2 years 6 months (n = 2) or who evidenced marked sensory, motoric, or cognitive impairment (n = 6), the Vineland Adaptive Behavior Scales, Second Edition24 (Vineland-II), was independently analyzed. The Vineland-II Adaptive Behavior Composite comprises informant surveys of communication, daily living skills, socialization, and motor skills.
Fourteen patients were evaluated longitudinally (≥2 evaluations) by using either the Wechsler scale (n = 11) or Vineland-II (n = 3). Both measures have a mean ± SD population score of 100 ± 15. Scores <70 indicate impaired functioning.
Basal ganglia injury was defined as evidence of a globus pallidus lesion on MRI and/or a movement disorder diagnosed by a pediatric neurologist.
Statistical Analyses
One-way between-subject analysis of variance tests, unpaired t tests, and paired t tests were performed by using SPSS 19 (IBM Corporation, Armonk, NY). Given the exploratory nature of the study and limited samples, we favored type I error risk over type II error risk when thresholding for significance. An α level of .05 was used because a more conservative approach (eg, Bonferroni) would further reduce the power to detect meaningful effects. Because means and statistical comparisons can be misconstrued in small samples (due to the rarity of MMA), we also provided percentages and graphical presentation of the data. The results are presented as mean ± SD.
Results
Demographic, Enzymatic, and Clinical Characteristics
Characteristics of the 43 participants with isolated MMA (7 cblA, 6 cblB, 21 early-onset mut, 6 late-onset mut, and 3 P/NBS mut) who completed a Wechsler intelligence scale (n = 38) and/or a Vineland-II (n = 8) are presented in Table 1. The patient cohort was 62.8% male, primarily white, and genetically diverse, as reflected by the spectrum of mutations. Because the study was fully funded, including travel, lodging, and testing, patients representing a wide range of socioeconomic means were enrolled, including those who were impoverished. The age of diagnosis was highly variable and ranged from the prenatal period to 4 years. Twenty-six patients presented symptomatically as neonates, and 3 patients were diagnosed either prenatally or after newborn screening. The mean age at first neuropsychological testing was 12.5 ± 8.23 years (range: 2–32 years).
TABLE 1.
Demographic, Enzymatic, and Clinical Characteristics of 43 Patients With Isolated MMA
| Patient | Ages at Neurocognitive Evaluation (y) | Testing Battery | Age at Diagnosis | Gender | Subtype | Gene | Mutation 1 | Mutation 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Wechslera | 1 d | M | cblB | MMAB | p.R186W | p.R186Wb |
| 2 | 2 | Vineland-II | 7 d | F | mut, early-onset | MUT | p.R369H | p.R228X |
| 3 | 2, 3 | Vineland-II | 10 mo | M | cblA | MMAA | p. R145X | p.R359Q |
| 4 | 2 | Wechsler, Vineland-II | 2 y | M | cblA | MMAA | p.T198SfsX6 | p.Y129X |
| 5 | 3 | Wechsler | NBS | M | mut, P/NBS | MUT | p.G642R | p.L394X |
| 6 | 3, 4 | Wechsler, Vineland-II | 2 d | F | mut, early-onset | MUT | p.G203R | p.R228X |
| 7 | 4 | Vineland-II | 7 d | F | mut, early-onset | MUT | p.R403X | p.G670R |
| 8 | 4, 6 | Vineland-II | 4 d | M | mut, early-onset | MUT | p.E224X | p.R228X |
| 9 | 5 | Wechsler | NBS | F | mut, P/NBS | MUT | p.G642R | p.G642Rb |
| 10 | 6 | Wechsler | 2.5 d | M | cblA | MMAA | p. R145X | Not determined |
| 11 | 6 | Wechsler | 6 d | M | mut, early-onset | MUT | p.R581X | p.splice |
| 12 | 6 | Wechslerc | 8 d | F | mut, early-onset | MUT | p.W309X | p.L328P |
| 13 | 7, 10 | Wechsler | Prenatal | F | mut, P/NBS | MUT | p.R369H | p.M186V |
| 14 | 7 | Vineland-II | 5 d | M | mut, early-onset | MUT | p.R369H | p.S594RfsX11 |
| 15 | 7 | Wechsler | 8 mo | M | mut, late-onset | MUT | p.G623R | p.G94V |
| 16 | 7 | Wechsler | 9 mo | M | mut, early-onset | MUT | p.R727X | p.R727Xd |
| 17 | 8 | Wechsler | 11 mo | F | cblB | MMAB | p.R186W | p.R186Wb |
| 18 | 9, 13 | Wechsler | 2 d | F | mut, early-onset | MUT | p.V553GfsX17 | p.L11TfsX38 |
| 19 | 9 | Wechsler | 24 d | M | mut, early-onset | MUT | p.R369C | p.R403X |
| 20 | 10 | Wechslerc | 3 d | F | mut, early-onset | MUT | p.A191E | Not determined |
| 21 | 10 | Wechsler | 6 d | M | mut, early-onset | MUT | p.R228X | p.splice |
| 22 | 10 | Wechsler | <1 mo | F | cblA | MMAA | p. R145X | p.P151AfsX19 |
| 23 | 10 | Wechslerc | 1 y | M | mut, late-onset | MUT | p.R108C | p.R108Cd |
| 24 | 11, 14 | Wechsler | 4 d | M | mut, early-onset | MUT | p.R369H | p.R369Hd |
| 25 | 11 | Wechsler | 4 d | M | mut, early-onset | MUT | p.R228X | p.R228Xb |
| 26 | 11 | Wechslerc | 5 d | F | mut, early-onset | MUT | p.G623R | p.R108H |
| 27 | 13 | Wechsler | 1.5 y | M | cblA | MMAA | p. R145X | p. R145Xb |
| 28 | 16, 18 | Wechsler | 10 mo | M | mut, late-onset | MUT | p.A191E | p.N219Y |
| 29 | 17, 22 | Wechsler | 0.5 y | M | cblA | MMAA | p.P151AfsX19 | p. E147G |
| 30 | 18 | Wechsler | 8 mo | F | cblB | MMAB | p.R186W | p.R186Wb |
| 31 | 20, 21, 22, 23, 25 | Wechsler | 2 d | F | mut, early-onset | MUT | p.R369H | p.R369Hb |
| 32 | 20 | Wechsler | 9 mo | F | mut, late-onset | MUT | p.685insL | p.685insLd |
| 33 | 21 | Wechsler | 3 d | M | cblB | MMAB | p.Q234X | Unknown |
| 34 | 21, 24, 24 | Wechsler, Vineland-II | 5 d | M | mut, early-onset | MUT | p.A191E | p.R228X |
| 35 | 21, 22, 24 | Wechsler | 2.5 mo | M | mut, early-onset | MUT | p.R581X | p.R581Xd |
| 36 | 22 | Wechsler | 4 y | M | mut, late-onset | MUT | p.R369H | p.R108C |
| 37 | 23 | Wechsler | 9 mo | M | cblB | MMAB | p.R186W | p.Q234X |
| 38 | 23, 25 | Wechsler | 14 mo | M | mut, late-onset | MUT | p.Y587S | p.G717V |
| 39 | 24 | Wechslerc | 10 d | M | mut, early-onset | MUT | p.N219Y | p.H350Y |
| 40 | 24 | Wechsler | 2.3 y | M | cblB | MMAB | p.R186W | p.Q234X |
| 41 | 25 | Wechsler | 7 d | F | mut, early-onset | MUT | Not determined | Not determined |
| 42 | 27, 28, 29, 30, 31 | Wechslerc | 2 mo | F | mut, early-onset | MUT | p.R369H | p.E276X |
| 43 | 32, 35 | Wechslerc | 3 mo | M | cblA | MMAA | p. R145X | p. R145Xd |
Only completed some subtests; no FSIQ.
No known consanguinity.
Only completed some subtests; FSIQ was estimated from available subtests.
Known consanguinity.
Selected Clinical and Laboratory Manifestations
A summary of the clinical presentation, symptoms, and laboratory findings in the study population is presented in Table 2. Fourteen patients presented with lethargy, 7 patients with failure to thrive, and 11 patients with recurrent vomiting. Twenty-five patients presented with hyperammonemia at diagnosis; 11 had laboratory documentation of ammonia levels at diagnosis (Supplemental Table 4) and 14 were reported to have hyperammonemia (Supplemental Table 5) according to parental interview.
TABLE 2.
Selected Clinical Findings in Patients in Each Subtype of Isolated MMA
| Type of MMA | n | Clinical/Laboratory Manifestations at Diagnosis (n [%]) | Selected Long-term Neurologic Sequelae (n [%]) | |||||
|---|---|---|---|---|---|---|---|---|
| Lethargy | Failure to Thrive | Recurrent Vomiting | Hyperammonemia | Neuro-ophthalmologic Disease | Basal Ganglia Injury | Seizure Disorder | ||
| Early-onset mut | 21 | 9 (43) | 5 (24) | 4 (19) | 17 (81) | 3 (14) | 6 (29) | 5 (24) |
| Late-onset mut | 6 | 2 (33) | 0 (0) | 4 (67) | 1 (17) | 1 (17) | 3 (50) | 0 (0) |
| P/NBS mut | 3 | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) |
| cblA | 7 | 2 (29) | 1 (14) | 2 (29) | 3 (43) | 0 (0) | 3 (43) | 1 (14) |
| cblB | 6 | 1 (17) | 1 (17) | 1 (17) | 3 (50) | 0 (0) | 3 (50) | 1 (17) |
Four patients manifested severe neuro-ophthalmalogic disease, and 15 patients had evidence of basal ganglia injury either on MRI or by using a neurologic evaluation. All patients with imaging findings had a movement disorder. Seven patients had a history of a seizure disorder; no patient had seizures at the time of study. Three patients (2 early-onset mut and 1 late-onset mut) had microcephaly.
Intelligence Was Stable in Patients With Isolated MMA Who Were Retested
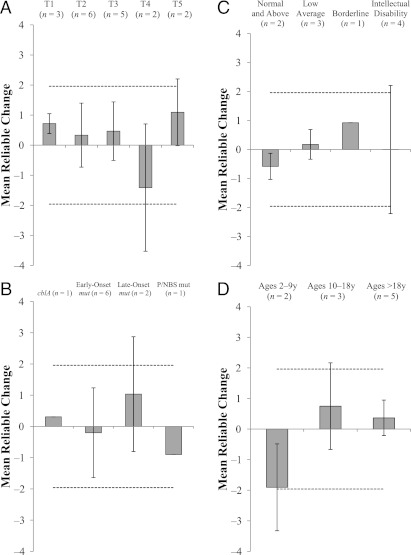
To determine whether neurocognitive outcomes were stable in our cohort, standard scores for the Wechsler indices were examined using the Reliable Change Index,25 a psychometric method for determining if changes in scores over time are due to cognitive differences. On average, patients did not show any change in FSIQ (n = 10) between first and last assessment (Reliable Change Index: 0.06; Fig 1) or across years since first assessment (Fig 2A). In addition, different subtypes of MMA (Fig 2B), different groups of patients according to intelligence score at baseline (Fig 2C), and different ages at first testing (Fig 2D) did not show any longitudinal change in FSIQ. These patients did not exhibit any consistent decline or improvement in Verbal Comprehension (n = 10), Perceptual Reasoning/Organization (n = 9), Working Memory (n = 7), Processing Speed (n = 6), or Adaptive Behavior Composite (n = 3) (data not shown). Because as a group there were no significant changes, mean index scores were calculated for each patient and used as a marker of abilities for subsequent analyses.
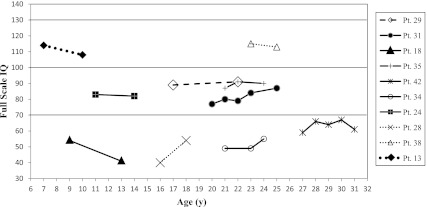
FIGURE 1.
FSIQ follow-up data plotted across age. Solid lines indicate early-onset mut patients; lines composed of small dots indicate late-onset mut; line composed of large dots indicates P/NBS patient; and dashed line indicates cblA patient. Due to missing data, multiple FSIQs could not be calculated for 1 patient who was evaluated serially.
FIGURE 2.
Reliable Change Index of FSIQ. The mean FSIQ at initial assessment (range of ages at first assessment: 7–27 years) was 76.7 ± 26.03, and at the most recent assessment it was 78.2 ± 24.26. A, Mean reliable change at each year after patients’ initial assessment. B, Across subtype of MMA. C, Across level of intellectual functioning at the initial assessment. D, Across years at initial evaluation. Dashed lines indicate cutoffs for Reliable Change significance. Error bars are SD.
Intelligence Was Influenced by MMA Subtype and Age at Onset in mut Patients
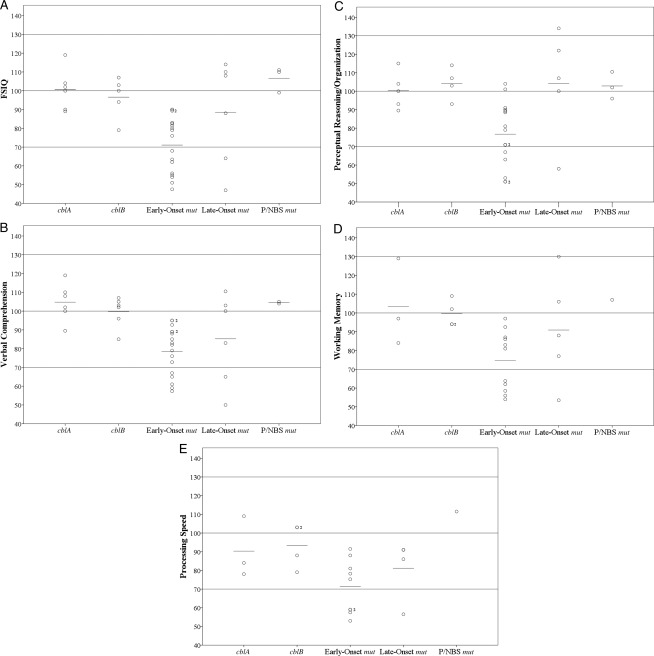
The mean FSIQ of all isolated MMA patients (n = 37) was 85.0 ± 20.68, which is in the low average range (80 ≤ IQ ≤89). There was variation between subtypes. Figure 3 illustrates each patient’s IQ and index scores, and Table 3 presents the percentage of patients (according to subtype) who were impaired (FSIQ <70).
FIGURE 3.
Plots of all patients’ neurocognitive test data points and mean scores for each index, including FSIQ, across subtypes of isolated MMA. A, FSIQ. B, Verbal Comprehension. C, Perceptual Reasoning/Organization. D, Working Memory. E, Processing Speed.
TABLE 3.
Proportion (%) of Patients in the Impaired Range (FSIQ <70) According to Subtype and IQ Index
| Type of MMA | FSIQ | VC | PR/PO | WM | PS |
|---|---|---|---|---|---|
| Early-onset mut | 8/17 (47) | 5/17 (29) | 4/15 (27) | 5/11 (45) | 4/9 (44) |
| Late-onset mut | 2/6 (33) | 2/6 (33) | 1/5 (20) | 1/5 (20) | 1/4 (25) |
| P/NBS mut | 0/3 (0) | 0/2 (0) | 0/3 (0) | 0/1 (0) | 0/1 (0) |
| cblA | 0/6 (0) | 0/6 (0) | 0/5 (0) | 0/3 (0) | 0/3 (0) |
| cblB | 0/5 (0) | 0/6 (0) | 0/4 (0) | 0/4 (0) | 0/4 (0) |
PS, Processing Speed; PR/PO, Perceptual Reasoning/Organization; VC = Verbal Comprehension; WM = Working Memory.
Patients with cblA or cblB and P/NBS mut patients displayed mean FSIQs of 100.7 ± 10.95, 96.6 ± 10.92, and 106.7 ± 6.66, respectively, which were in the average range (90 ≤ IQ ≤109). Late-onset mut patients manifested a mean FSIQ of 88.5 ± 27.62, which is in the low average range. Early-onset mut patients had a mean FSIQ of 71.1 ± 14.75, which is in the borderline range (70 ≤ IQ ≤79). The early-onset mut patients had a larger percentage of FSIQ scores in the impaired range than the cblA, cblB, and P/NBS mut patients (Table 3).
We examined intergroup differences for each index of intelligence (Fig 3 B–E). cblA, cblB, and P/NBS mut patients obtained scores in the average range for all indices. No cblA, cblB, or P/NBS mut patients obtained scores in the impaired range for any index (Table 3). Late-onset mut patients showed mean Verbal Comprehension and Processing Speed scores in the low average range and Perceptual Reasoning/Organization and Working Memory scores in the average range. Early-onset mut patients manifested mean scores for all indices of intelligence in the borderline intellectual functioning range. A large proportion (27%–44%) of early-onset mut patients obtained scores in the impaired range on the indices. Patients with isolated MMA who were administered a Vineland-II (n = 8) had a mean Adaptive Behavior Composite of 64.8 ±18.14, which is >2 SDs below the mean for the general population and is significantly decreased, using a one-sample t test (P = .001). Supplemental Figure 7 shows the distribution of Vineland-II Adaptive Behavior Composite and Wechsler FSIQ scores superimposed over the normal curve of score distributions for the general population.
Patients With Isolated MMA Showed Deficits in Processing Speed Compared With Other Indices of Intelligence
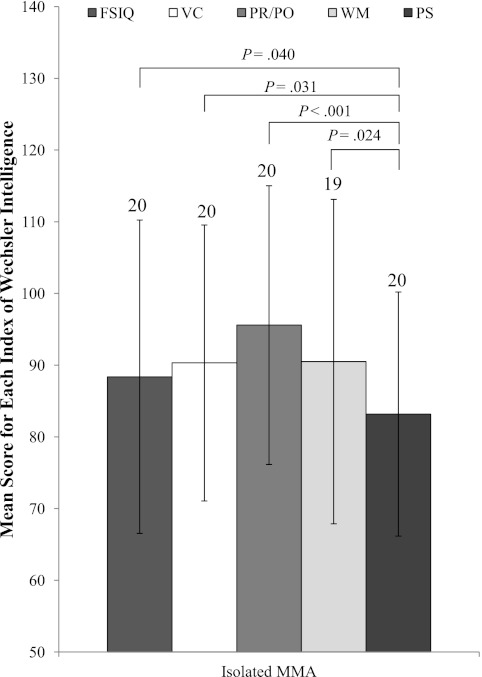
Patients with isolated MMA, excluding those with neuro-ophthalmologic disease (n = 4), showed significant deficits in Processing Speed when compared with FSIQ (n = 20; P = .040), Verbal Comprehension (n = 20; P = .031), Perceptual Reasoning/Organization (n = 20; P < .001), and Working Memory (n = 19; P = .024) (Fig 4). When those with a movement disorder were excluded, the remaining patients still manifested deficits in Processing Speed compared with Perceptual Reasoning/Organization (n = 13; P = .002), FSIQ (n = 13; P = .080), and Verbal Comprehension (n = 13; P = .080).
FIGURE 4.
Mean score for neurocognitive indices for isolated MMA patients as a group. Numbers at the top of each bar indicate the number of patients able to complete the intelligence measure. PR/PO, Perceptual Reasoning/Organization; PS, Processing Speed; VC, Verbal Comprehension; WM, Working Memory. Error bars indicate SD.
A Lower FSIQ Was Associated With Hyperammonemia at Diagnosis and a History of Seizures
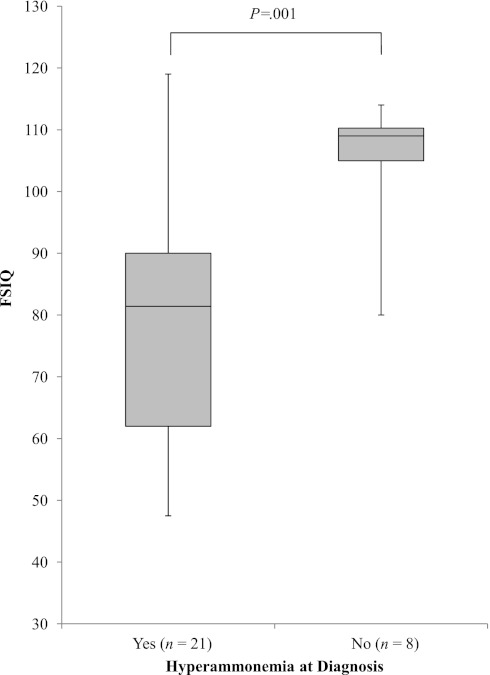
To determine the effect of hyperammonemia at diagnosis on cognition, Wechsler scores from patients with laboratory documentation (n = 11) and/or clinical history of hyperammonemia (n = 14) at the time of diagnosis were compared with those without hyperammonemia. Patients without a clinical or laboratory history of hyperammonemia at diagnosis (n = 8) had a mean FSIQ of 104.9 ±10.96, whereas those with hyperammonemia (n = 21) had a mean FSIQ of 78.8 ±19.29 (P = .001) (Fig 5). Verbal Comprehension (P = .007) and Perceptual Reasoning/Organization (P = .008) were also significantly decreased in patients with hyperammonemia (mean Verbal Comprehension: 84.0 ± 15.33, n = 21; mean Perceptual Reasoning/Organization: 84.6 ± 20.63, n = 18) compared with those without hyperammonemia (mean Verbal Comprehension: 101.8 ± 8.04, n = 7; mean Perceptual Reasoning/Organization: 108.3 ± 16.14, n = 8). Working Memory and Processing Speed were also lower in patients with hyperammonemia (mean Working Memory: 84.1 ± 20.44, n = 15; mean Processing Speed: 81.4 ± 16.94, n = 13) compared with those without hyperammonemia (mean Working Memory: 111.3 ± 12.69, n = 4; mean Processing Speed: 97.9 ± 11.55, n = 4); however, statistical tests were not reported due to the small sample size. An identical analysis conducted with the mut subtype only showed the same results (data not shown).
FIGURE 5.
FSIQ according to history of hyperammonemia at diagnosis by laboratory record or parental recall for all patients.
Patients with no history of seizure disorder (n = 31 [29 with FSIQ]) manifested a mean FSIQ of 87.4 ± 19.31, whereas those with a history of seizure disorder (n = 7 [6 with FSIQ]) attained a mean FSIQ of 68.5 ± 22.72 (Fig 6). Patients with a history of seizure disorder had lower FSIQ (P = .041) and Perceptual Reasoning/Organization (P = .030) scores than those without.
FIGURE 6.
FSIQ according to presence of a seizure disorder.
We next examined the effect of clinical and laboratory manifestations of isolated MMA on cognitive domains. Diagnosis within 10 days of disease onset, basal ganglia injury, number of nonsense mutations, and age at initial neurocognitive evaluation at the National Institutes of Health did not correlate with a difference in FSIQ or other indices of intelligence (Supplemental Figs 8–11). In addition, clinical manifestations at diagnosis such as gender, lethargy, failure to thrive, and recurrent vomiting did not show an effect on any neurocognitive parameter.
Discussion
The near-universal implementation of expanded newborn screening using tandem mass spectrometry has afforded the early detection of patients with a wide spectrum of disorders,26 such as MMA, where treatment regimens and long-term outcomes require clinical delineation. In contrast to disorders such as phenylketonuria and maple syrup urine disease, which were shown to have improved neurocognitive outcomes with early dietary and treatment interventions,27–29 isolated MMA has yet to receive similar study, perhaps because of the high lethality of the condition in childhood. Reports6,30 have suggested that isolated patients with MMA have experienced improvements in mortality in recent decades and have provided impetus to define the clinical phenotypes, such as intellectual disabilities, associated with increased longevity.
The goals of our study were first to delineate the neurocognitive outcome seen in a cohort of carefully phenotyped patients and then to query relationships between clinical variables and cognitive function. The use of a standardized clinical research protocol and single-center design that enrolled a group of diverse patients has allowed our studies to overcome limitations faced by others, particularly the precise biochemical, enzymatic, and molecular characterization of the affected individuals and the reliance on surveys to gather primary neurocognitive data.6,8,10,18,19 Consistent with previous observations,6,8,18 we documented that cblA, cblB, P/NBS mut, and, to a lesser degree, late-onset mut patients can have normal cognitive outcomes, whereas early-onset mut patients showed lower and more variable cognitive functioning (Fig 3).
The range of outcomes prompted an investigation of selected clinical parameters and their associations with FSIQ in this cohort. We found that a history of hyperammonemia at diagnosis was related to lower measures for FSIQ, Verbal Comprehension, and Perceptual Reasoning/Organization, as well as a trend toward lower Working Memory and Processing Speed. Hyperammonemia has been well recognized as an important determinant of cognitive impairment for infants with urea cycle disorders,31 indicating that this, too, may play a role in the outcome of infants with MMA. In addition, the finding that a history of a seizure disorder is associated with lower IQ is consistent with evidence linking seizures, particularly generalized tonic-clonic seizures, to impaired cognitive functioning.32 Whether this is a primary or secondary manifestation of another process in MMA is unclear.
Other clinical parameters were assessed to determine correlations with cognitive outcome. Homozygosity for nonsense mutations was not shown to have an association with indices of intelligence in any subtype (Supplemental Fig 10). Because many of the mutations in the patient cohort are “private,” specific genotype–phenotype correlations could not be ascertained. In addition, neurocognitive improvement in patients treated early after disease onset was not observed, indicating that once symptoms are present, an impact on cognition in the disease course is likely.
Detailed cognitive testing afforded exploration of an area of intelligence in which some patients had disproportionate impairment: Processing Speed (Fig 4). Patients with idiopathic mild to moderate intellectual disability are expected to show higher Processing Speed scores compared with other indices.21 In light of this expectation, the fact that patients with MMA, who are often diagnosed with intellectual disability, show impaired Processing Speed is particularly striking. This observation is consistent with the known vulnerability of the basal ganglia in MMA and the consequences of basal ganglia injury,33 and indicates a possible differential impact of toxic metabolites on cognition. Further evaluation using more precise Processing Speed measurements, such as reaction time tasks and measures of inspection time not necessitating motor response,34 may therefore provide a means to prospectively monitor disease manifestations in patients. Whether Processing Speed may be affected by white matter disease in the MMA patient population deserves further study, especially because several studies have established that whereas children with early-treated phenylketonuria exhibit a global pattern of impairment, a particular deficit in Processing Speed is present.35,36 Future investigations that concurrently and prospectively examine brain MRI and magnetic resonance spectroscopy findings in parallel with neuropsychological testing in patients with MMA should help define which central nervous system domains and structures clinically contribute to the observed neurocognitive phenotype.
The stability of the intelligence test scores (Figs 1 and 2) documented in our study cohort indicates that cognitive decline may not be intrinsic to isolated MMA. This observation bolsters a previous case report12 demonstrating exceptional abilities in a patient with biochemically severe MMA. Although numerous cell studies have claimed that methylmalonic acid itself is neurotoxic,37,38 the patient data indicate that sequelae of neurodegeneration, if present, may exhibit a very slow rate of progression and/or manifest only in a select group of nuclei that control processing speed.
Our study had limitations. We did not systematically characterize the socioeconomic status of the patients or assess the ascertainment bias in our study, but because we have determined the enzymatic and mutation spectrum of the participants, direct comparisons with other cohorts should be easily accomplished through molecular genetics and enzymology. Although we were unable to define a critical ammonia level or duration of hyperammonemia, a relationship between hyperammonemia and long-term cognitive outcome suggested by our analysis mandates further study.
Conclusions
The data presented in this report represent the largest effort to date to define the neurocognitive phenotype seen in isolated MMA and establish a valuable reference set and framework for future investigations in cohorts of patients, particularly those diagnosed through newborn screening. Important clinical variables that influence cognitive outcomes, such as hyperammonemia, were identified, and new metrics for monitoring intellectual functioning in isolated MMA have emerged. The identification of impaired Processing Speed and its potential relationship to neurocognitive function should provide a prospective marker to monitor central nervous system manifestations of MMA.
Supplementary Material
Acknowledgments
The authors thank David Luckenbaugh and Dr Deanna Greenstein for their assistance with statistical analyses and Julia Fekecs and Alexander Liu for their preparation of the figures.
Glossary
- MMA
methylmalonic acidemia
- FSIQ
full-scale IQ
- P/NBS
prenatal or newborn screening diagnosis
Footnotes
Mr O’Shea collated data, analyzed results, and wrote and edited the manuscript; Dr Sloan recruited patient subjects, provided clinical care, analyzed results, collected clinical and laboratory data, and assisted in writing and editing of the manuscript; Dr Wiggs performed neuropsychological evaluations and assisted in editing of the manuscript; Dr Pao assisted in writing and editing of the manuscript; Dr Gropman performed neurologic examination on subjects, participated in discussions about the manuscript, and assisted in editing of the manuscript; Dr Baker performed and analyzed brain MRIs and assisted in editing of the manuscript; Dr Manoli provided clinical care, participated in data analysis, collected clinical and laboratory data, and assisted in writing and editing of the manuscript; Dr Venditti directed the clinical research protocol, provided clinical care, supervised the study, participated in data analysis, and assisted in writing and editing the manuscript; and Dr Snow performed neuropsychological evaluations, participated in data analysis, supervised the study, and assisted in writing and editing of the manuscript.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00078078).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Drs Sloan, Gropman, Manoli, and Venditti were supported, in part, by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health (NIH). Mr O’Shea, Dr Pao, and Dr Snow were supported, in part, by the Intramural Research Program of the National Institute of Mental Health, NIH. Dr Wiggs was supported, in part, by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH. Dr Baker was supported, in part, by the National Institutes of Health Clinical Center. Funded by the National Institutes of Health (NIH).
References
- 1.Oberholzer VG, Levin B, Burgess EA, Young WF. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child. 1967;42(225):492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stokke O, Eldjarn L, Norum KR, Steen-Johnsen J, Halvorsen S. Methylmalonic acidemia: a new inborn error of metabolism which may cause fatal acidosis in the neonatal period. Scand J Clin Lab Invest. 1967;20(4):313–328 [Google Scholar]
- 3.Sniderman LC, Lambert M, Giguère R, et al. Outcome of individuals with low-moderate methylmalonic aciduria detected through a neonatal screening program. J Pediatr. 1999;134(6):675–680 [DOI] [PubMed] [Google Scholar]
- 4.Fenton WA, Gravel RA, Rosenblatt DS. Disorders of propionate and methylmalonate metabolism. In: Scriver C, et al, eds. Metabolic and Molecular Bases of Inherited Disease – OMMBID. New York, NY: McGraw-Hill; 2001: chapter 94 [Google Scholar]
- 5.Manoli I, Venditti CP. Methylmalonic acidemia. GeneReviews at GeneTests: Medical Genetics Information Resource (database online): Copyright, University of Washington, Seattle, WA; 1997–2011, updated 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK1231/ Accessed June 6, 2011
- 6.Hörster F, Baumgartner MR, Viardot C, et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB). Pediatr Res. 2007;62(2):225–230 [DOI] [PubMed] [Google Scholar]
- 7.Ledley FD, Levy HL, Shih VE, Benjamin R, Mahoney MJ. Benign methylmalonic aciduria. N Engl J Med. 1984;311(16):1015–1018 [DOI] [PubMed] [Google Scholar]
- 8.Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983;308(15):857–861 [DOI] [PubMed] [Google Scholar]
- 9.Nicolaides P, Leonard J, Surtees R. Neurological outcome of methylmalonic acidaemia. Arch Dis Child. 1998;78(6):508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shevell MI, Matiaszuk N, Ledley FD, Rosenblatt DS. Varying neurological phenotypes among muto and mut- patients with methylmalonylCoA mutase deficiency. Am J Med Genet. 1993;45(5):619–624 [DOI] [PubMed] [Google Scholar]
- 11.van der Meer SB, Poggi F, Spada M, et al. Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J Pediatr. 1994;125(6 pt 1):903–908 [DOI] [PubMed] [Google Scholar]
- 12.Varvogli L, Repetto GM, Waisbren SE, Levy HL. High cognitive outcome in an adolescent with mut- methylmalonic acidemia. Am J Med Genet. 2000;96(2):192–195 [PubMed] [Google Scholar]
- 13.Morrow G, III, Barness LA, Cardinale GJ, Abeles RH, Flaks JG. Congenital methylmalonic acidemia: enzymatic evidence for two forms of the disease. Proc Natl Acad Sci U S A. 1969;63(1):191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van’t Hoff WG, Dixon M, Taylor J, et al. Combined liver-kidney transplantation in methylmalonic acidemia. J Pediatr. 1998;132(6):1043–1044 [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich R, Natowicz M, Hainline BE, et al. Acute extrapyramidal syndrome in methylmalonic acidemia: “metabolic stroke” involving the globus pallidus. J Pediatr. 1988;113(6):1022–1027 [DOI] [PubMed] [Google Scholar]
- 16.Korf B, Wallman JK, Levy HL. Bilateral lucency of the globus pallidus complicating methylmalonic acidemia. Ann Neurol. 1986;20(3):364–366 [DOI] [PubMed] [Google Scholar]
- 17.Zwickler T, Lindner M, Aydin HI, et al. Diagnostic work-up and management of patients with isolated methylmalonic acidurias in European metabolic centres. J Inherit Metab Dis. 2008;31(3):361–367 [DOI] [PubMed] [Google Scholar]
- 18.Baumgarter ER, Viardot C. Long-term follow-up of 77 patients with isolated methylmalonic acidaemia. J Inherit Metab Dis. 1995;18(2):138–142 [DOI] [PubMed] [Google Scholar]
- 19.Hörster F, Garbade SF, Zwickler T, et al. Prediction of outcome in isolated methylmalonic acidurias: combined use of clinical and biochemical parameters. J Inherit Metab Dis. 2009;32(5):630–639 [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. San Antonio, TX: The Psychological Corporation; 2002 [Google Scholar]
- 21.Wechsler D. The Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: The Psychological Corporation; 2004 [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997 [Google Scholar]
- 23.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999 [Google Scholar]
- 24.Sparrow S, Chicchetti D, Balla D. Vineland Adaptive Behavior Scales. 2nd ed. Minneapolis, MN: Pearson Assessment; 2002 [Google Scholar]
- 25.Speer DC. Clinically significant change: Jacobson and Truax (1991) revisited. J Consult Clin Psychol. 1992;60(3):402–408 [DOI] [PubMed] [Google Scholar]
- 26.Dionisi-Vici C, Deodato F, Röschinger W, Rhead W, Wilcken B. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inherit Metab Dis. 2006;29(2–3):383–389 [DOI] [PubMed] [Google Scholar]
- 27.Dobson J, Koch R, Williamson M, et al. Cognitive development and dietary therapy in phenylketonuric children. N Engl J Med. 1968;278(21):1142–1144 [DOI] [PubMed] [Google Scholar]
- 28.Kaplan P, Mazur A, Field M, et al. Intellectual outcome in children with maple syrup urine disease. J Pediatr. 1991;119(1 pt 1):46–50 [DOI] [PubMed] [Google Scholar]
- 29.Nord A, van Doorninck WJ, Greene C. Developmental profile of patients with maple syrup urine disease. J Inherit Metab Dis. 1991;14(6):881–889 [DOI] [PubMed] [Google Scholar]
- 30.Touati G, Valayannopoulos V, Mention K, et al. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis. 2006;29(2–3):288–298 [DOI] [PubMed] [Google Scholar]
- 31.Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;310(23):1500–1505 [DOI] [PubMed] [Google Scholar]
- 32.Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(suppl 1):S21–S24 [DOI] [PubMed] [Google Scholar]
- 33.Leyden J, Kleinig T. The role of the basal ganglia in data processing. Med Hypotheses. 2008;71(1):61–64 [DOI] [PubMed] [Google Scholar]
- 34.Vickers D, Nettelbeck T, Willson RJ. Perceptual indices of performance: the measurement of ‘inspection time’ and ‘noise’ in the visual system. Perception. 1972;1(3):263–295 [DOI] [PubMed] [Google Scholar]
- 35.Anderson PJ, Wood SJ, Francis DE, et al. Neuropsychological functioning in children with early-treated phenylketonuria: impact of white matter abnormalities. Dev Med Child Neurol. 2004;46(4):230–238 [DOI] [PubMed] [Google Scholar]
- 36.Anderson PJ, Wood SJ, Francis DE, Coleman L, Anderson V, Boneh A. Are neuropsychological impairments in children with early-treated phenylketonuria (PKU) related to white matter abnormalities or elevated phenylalanine levels? Dev Neuropsychol. 2007;32(2):645–668 [DOI] [PubMed] [Google Scholar]
- 37.Okun JG, Hörster F, Farkas LM, et al. Neurodegeneration in methylmalonic aciduria involves inhibition of complex II and the tricarboxylic acid cycle, and synergistically acting excitotoxicity. J Biol Chem. 2002;277(17):14674–14680 [DOI] [PubMed] [Google Scholar]
- 38.Morath MA, Okun JG, Müller IB, et al. Neurodegeneration and chronic renal failure in methylmalonic aciduria—a pathophysiological approach. J Inherit Metab Dis. 2008;31(1):35–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.