Abstract
BACKGROUND AND OBJECTIVE:
Infant formula is supplemented with long-chain polyunsaturated fatty acids (LCPUFAs) because they are hypothesized to improve cognition. Several randomized controlled clinical trials have examined the effect of LCPUFA supplementation of infant formula on cognitive development. We conducted this meta-analysis to examine the efficacy of LCPUFA supplementation of infant formula on early cognitive development.
METHODS:
Two authors searched PubMed, PsychInfo, and Scopus for randomized controlled clinical trials assessing the efficacy of LCPUFA supplementation of infant formulas on cognition. Our analysis was restricted to randomized controlled clinical trials that examined the effect of LCPUFA supplementation on infant cognition using Bayley Scales of Infant Development. Our primary outcome was the weighted mean difference in Bayley Scales of Infant Development score between infants fed formula supplemented with LCPUFA compared with unsupplemented formula. We conducted secondary subgroup analyses and meta-regression to examine the effects of study sample, LCPUFA dose, and trial methodologic quality on measured efficacy of supplementation.
RESULTS:
Twelve trials involving 1802 infants met our inclusion criteria. Our meta-analysis demonstrated no significant effect of LCPUFA supplementation of formula on infant cognition. There was no significant heterogeneity or publication bias between trials. Secondary analysis failed to show any significant effect of LCPUFA dosing or prematurity status on supplementation efficacy.
CONCLUSIONS:
LCPUFA supplementation of infant formulas failed to show any significant effect on improving early infant cognition. Further research is needed to determine if LCPUFA supplementation of infant formula has benefits for later cognitive development or other measures of neurodevelopment.
KEY WORDS: infant formula, unsaturated fatty acids, infant cognition, long-chain polyunsaturated fatty acids, meta-analysis, Bayley Scales of Infant Development
Infant formula is the main source of energy and nutritional requirements for many infants during the first 12 months of life. The Food and Drug administration estimates that 40% of infants are formula-fed at 3 months, 50% at 6 months, and >75% at 1 year.1 Many studies have suggested that breastfed children have higher intelligence quotient scores than their formula-fed counterparts.2 It is currently debatable whether these differences are due to the actual nutritional differences between breast milk and formula or confounding by other factors. Breastfeeding mothers have been demonstrated, on average, to have higher intelligence and larger incomes and spend more time with their infants than formula-feeding mothers.3–5 Any of these factors are potential confounders of the association between breastfeeding and improved cognition in children.
Long-chain polyunsaturated fatty acids (LCPUFAs) have been hypothesized as a difference between breast milk and infant formula that could be responsible for the differences in cognitive development observed between groups. LCPUFAs are vital for the integrity of cell membranes of different body tissues. Two main LCPUFAs, docosahexaenoic acid (DHA) and arachidonic acid (AA), play a critical role in development and growth during pregnancy and early childhood.6 DHA and AA are derived mainly from their precursors, α-linolinic acid (ALA), an omega-3 fatty acid, and linoleic acid (LA), an omega-6 fatty acid, respectively. Both of the latter fatty acids cannot be synthesized by the body de novo owing to a lack of Δ-12 and Δ-15 desaturases,7 and they are required in the diet. Early in pregnancy, LCPUFAs such as DHA and AA are able to cross the placenta to the fetus. Although human fetuses can synthesize DHA and AA from their precursors after 26 weeks’ gestation, the amount of these LCPUFAs synthesized ranges widely between infants, approaching 0 in some.8–10 Postnatally, these fatty acids are supplied in breast milk, which contains both LCPUFA and its metabolites; however, the breast milk of different mothers contains different amounts of LCPUFA that varies widely.11,12 Infants fed formula deficient in LCPUFAs have significantly lower levels of DHA and AA in plasma or red blood cells compared with those who were breastfed or who were fed formula supplemented with LCPUFA.13–16 Furthermore, a clear dose-response relationship between level of DHA and AA supplementation in infant formula and DHA and AA levels in erythrocyte membrane phospholipids has been established.17 An estimated 30-fold increase in the amount of DHA and AA in the infant forebrain occurs between the last trimester of pregnancy and the first 2 years of life.18,19
A series of randomized, double-blinded, placebo-controlled trials have been conducted in recent years to determine whether infants fed formula supplemented with LCPUFAs would have improved performance on measures of cognition compared with infants fed typical formula. Results of these trials have varied widely, with some trials demonstrating improved cognitive abilities in infants fed formula supplemented with LCPUFAs14,20–23 and other trials demonstrating no effect.24–29 We conducted a meta-analysis with a goal to establish whether supplementing infants with formula enriched with LCPUFA would improve cognitive outcomes at ∼1 year of age compared with infants fed nonenriched formula. We also used meta-regression to examine the effect of doses of LCPUFA in supplements, duration of supplementation, and study quality on cognitive outcomes.
Methods
Search Strategy
All meta-analytic methods and sensitivity analyses were specified before conducting the meta-analysis but were not registered online. PubMed (1965–June 2011), PsychInfo, and Scopus were searched by 2 reviewers (A.Q., A.L.W.) for relevant citations using the search terms “(Infant Nutrition [Mesh] OR Infant Formula [Mesh]) AND Fatty acids, Unsaturated [Mesh]” in PubMed, “Omega-3 and Infant Formula” in Scopus, and “Fatty Acids and Infant Formula” in PsychInfo. Our search in PubMed was limited further by using the randomized controlled trial and meta-analysis filters. The bibliographies of related review articles and included articles also were searched for additional eligible citations. Authors of some articles were contacted for missing information when necessary. There were no limitations based on the language of publication.
Inclusion Criteria
The inclusion criteria of our meta-analysis were (1) randomized controlled trials assessing infant formulas with LCPUFAs compared with unsupplemented formula, (2) trials assessing infant cognition by using a version of the Bayley Scales of Infant Development (BSID),30,31 (3) supplementation starting within 1 month after birth, and (4) studies that were published in a peer-reviewed journal. Identification of peer-reviewed journals was through the global serials directory ULRICHSWEB.32 Randomized controlled trials were considered to be so if the investigator defined them as such in the methods section of the article.
Meta-analysis Procedure
To extract data from included articles, we used Excel spreadsheets (Microsoft Corporation, Redmond, WA). Data extracted included start and duration of supplementation, dose and source of LCPUFA, ratings of trials using the Jadad scale,33 sample size, and infant cognition as assessed by the BSID. Our main outcome was BSID total score. When trials reported cognitive assessments for >1 time point, only the assessment closest to 12 months was used. The closest BSID assessments for trials ranged from 8 to 16 months. We made this decision because a preponderance of trials assessed cognition at ∼12 months and also to avoid overweighting samples that were assessed at multiple time points. As secondary analyses, we also examined the improvement on the Mental Development Index (MDI) and Psychomotor Development Index (PDI) subscales of the BSID separately.
Publication bias was assessed by plotting the effect size against sample size for each trial (funnel plot).34 We also assessed publication bias by conducting a meta-regression in which the association between sample size and effect size was tested. Heterogeneity of treatment response was assessed visually from the forest plot of weighted mean differences (WMDs) and relative risk of individual studies. Statistical estimates of heterogeneity also were assessed using the I-square heterogeneity statistic in Review Manager (RevMan 5.1; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). We conducted a sensitivity analysis to examine our decision to use a random-effects rather than a fixed-effects model for meta-analysis.
For secondary analyses, we performed several subgroup analyses and meta-regression. Stratified subgroup analysis in RevMan was used to assess the effects of (1) term versus preterm status, (2) methodologic quality of trials (Jadad score ≥4 versus Jadad score <4), and (3) method of analysis (completers versus intention-to-treat). We used the test for subgroup differences in RevMan to determine whether subgroups reduced overall heterogeneity.35
Meta-regression was performed in SPSS 19.0 (SPSS Inc, Chicago, IL) by using linear regression. To examine the association between LCPUFAs and continuous variables such as (1) dose of LCPUFA, (2) trial duration, and (3) sample size, we used a meta-regression technique. For meta-regression, mean difference of BSID score improvement with supplementation was the dependent variable, and our variable of interest was the independent variable. Studies were weighted by using the generic inverse-variance technique. Doses examined included DHA, AA, and eicosapentaenoic acid (EPA). We also examined ratio of AA:DHA in supplementation. Our P value of significance threshold was selected to be <.05 for the primary analysis and for all subgroup analyses and meta-regression. Any significant findings in secondary analysis should be regarded as exploratory because we did not adjust for inflation of false-positive error from our 10 secondary analyses.
Results
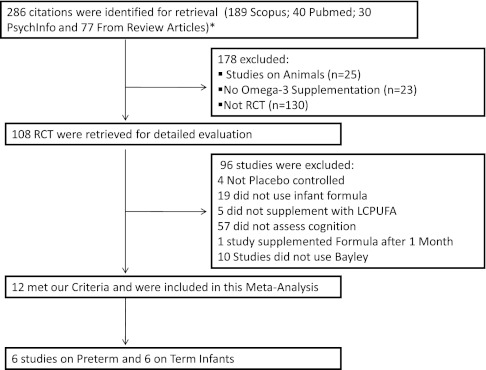
We identified 286 articles, 12 of which met the inclusion criteria for our review. Figure 1 is a flow diagram depicting the reasons for exclusion of the identified studies. The characteristics of included trials are shown in Table 1. We identified 12 trials involving 1802 participants. Two of 12 studies36,37 reported a significant benefit of supplementation with LCPUFAs on cognition. One study23,38 showed a positive benefit of supplementation on some but not all subscales of the BSID. Nine of 12 trials14,22,25,26,28,39–41 showed no effect of supplementation on cognition.
FIGURE 1.
Selection of eligible trials. This flow diagram depicts reasons for exclusion of identified citations. There were 50 citations that were identified through multiple sources. Each of these citations is counted only once in the flow diagram for clarity. aThe number of articles was 336; duplicated articles were omitted. RCTs, randomized controlled clinical trials.
TABLE 1.
Characteristics of Included Trials
| Author | Year | N | Premature Versus Full-Term Infant | Start of Supplementation (After Birth) | Duration of Supplementation, mo | DHA, 22:6, %a | EPA, 20:5, % | AA, 20:4, % | ALA, 18:3 n-3, % | LA, 18:2 n-6, % | Jadad Scale | Contribution | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total BSID | MDI | PDI | |||||||||||||
| Scott | 1998 | 134 | Term | Within a wk | 12 | DHA | 0.23 | 0.07 | 0.0 | 1.90 | 20.70 | 5 | Y | Y | Y |
| DHA + AA | 0.12 | 0 | 0.43 | 1.90 | 21.70 | ||||||||||
| Lucas | 1999 | 309 | Term | Within a wk | 6 | 0.32 | 0.01 | 0.30 | 1.40 | 15.90 | 5 | Y | Y | Y | |
| Birch | 2000 | 56 | Term | Within 2 d | 4 | DHA | 0.35 | 0 | 0.02 | 1.54 | 15.10 | 5 | Y | Y | Y |
| DHA + AA | 0.36 | 0 | 0.72 | 1.53 | 14.90 | ||||||||||
| Makrides | 2000 | 68 | Term | Within 1 wk | 12 | 0.35 | 0.10 | 0.34 | 1.10 | 16.70 | 3 | Y | Y | Y | |
| Auestad | 2001 | 404 | Term | Within 9 d | 12 | Egg, DHA | 0.14 | 0 | 0.45 | 2.50 | 22.40 | 5 | Y | Y | Y |
| Fish & Fungi DHA | 0.13 | 0.04 | 0.46 | 2.40 | 21 | ||||||||||
| O'Connor | 2001 | 427 | Preterm | Within 3 d | 14 | Fish & Fungi DHA | 0.27 | 0.08 | 0.43 | 2.60 | 16.80 | 4 | Y | Y | Y |
| Egg & Fish DHA | 0.24 | 0 | 0.41 | 2.50 | 17.50 | ||||||||||
| Fewtrell | 2002 | 195 | Preterm | 5 d | 1 | 0.17 | 0.04 | 0.31 | 0.60 | 12.00 | 5 | Y | Y | Y | |
| Van Wezel | 2002 | 42 | Preterm | 2–3 wk | 8 | 0.34 | NR | 0.70 | NR | NR | 5 | Y | Y | Y | |
| Fewtrell | 2004 | 238 | Preterm | Discharge or wt 2 kg | 9 | 0.50 | 0.10 | 0.04 | 1.50 | 12.30 | 5 | Y | Y | Y | |
| Bouwstra | 2005 | 250 | Term | After delivery | 2 | 0.30 | NR | 0.45 | NR | NR | 5 | Y | Y | Y | |
| Clandinin | 2005 | 361 | Preterm | Within 10 d | 14.5 | Algae DH | 0.33 | 0.10 | 0.65 | 2.16 | 18.40 | 4 | Y | Y | Y |
| Fish DHA | 0.33 | 0 | 0.65 | 2.16 | 18.40 | ||||||||||
| Fang | 2005 | 28 | Preterm | 35 weeks PMA | 6 | 0.05 | NR | 0.10 | NR | NR | 5 | Y | Y | Y | |
ALA, α-linolinic acid; LA, linoleic acid; MDI, Mental Development Index; NR, not reported; PDI, Psychomotor Development Index.
Doses are defined in g/100 g of fatty acids.
The Efficacy of LCPUFAs on Infant Cognition
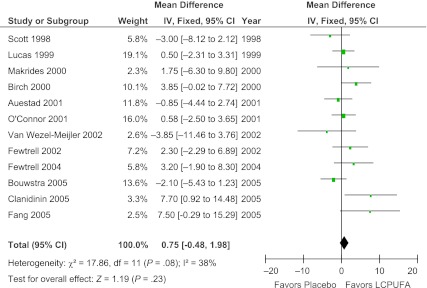
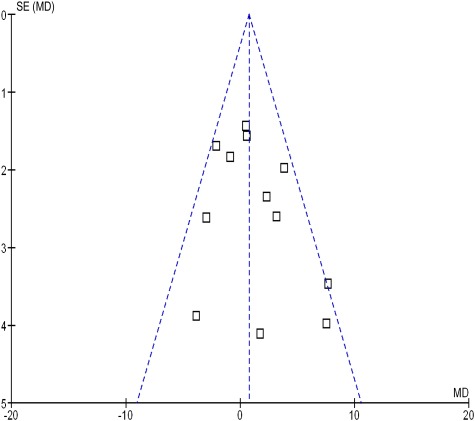
Meta-analysis of 12 trials involving 1802 participants demonstrated no significant effect of LCPUFA supplementation of formula on infant cognition (WMD = 0.75; [95% confidence interval (CI): −0.48 to 1.98]; z = 1.19, P = .23). Figure 2 depicts a forest plot comparing the difference between BSID scores between the LCPUFA group and the control group. There was modest heterogeneity between trials that did not reach statistical significance (χ2 = 17.86, df = 11 [P = .08]; I2 = 38%). There was no evidence of publication bias as depicted on a funnel plot (Fig 3). Meta-regression also demonstrated no significant association between measured study effect and sample size (β = −.005 [95% CI: −0.019 to 0.010], t = −0.7, P = .51). Using a random-effects model (WMD = 0.97 [95% CI: −0.70 to 2.63], z = 1.14, P = .25) as opposed to a fixed-effects model did not affect meta-analysis results appreciably.
FIGURE 2.
Effect of LCPUFA supplementation on infant cognition. This forest plot compares the difference between BSID scores of infants who were fed formula with LCPUFA supplementation and infants fed unsupplemented formula. There was no significant effect of LCPUFA supplementation on BSID score. IV, inverse-variance method.
FIGURE 3.
Funnel plot evaluating publication bias. This funnel plot graphs trial variance (SE) versus effect size (mean difference [MD]), which is designed to evaluate the presence of publication bias among trials included in this meta-analysis. The funnel plot appears symmetrical, indicating no evidence of publication bias.
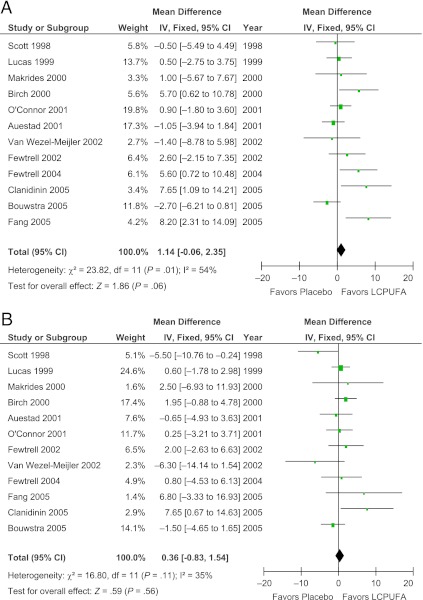
LCPUFA supplementation of formula also did not show any significant effect on the Mental Development Index (WMD = 1.14 [95% CI: −0.06 to 2.35], z = 1.86, P = .06) and Psychomotor Development Index (WMD = 0.36 [95% CI: −0.83 to 1.54], z = 0.59, P = .56) subscales of the BSID in secondary analysis. Figure 4 depicts the forest plot of LCPUFA supplementation on Mental Development Index and Psychomotor Development Index subscale scores.
FIGURE 4.
Effect of LCPUFA supplementation on Mental and Psychomotor Development Index of the BSID. Forest plots comparing the difference in the (A) Mental Development Index and (B) Psychomotor Development Index subscales of the BSID between infants who were fed formula supplemented with LCPUFAs and infants who were fed unsupplemented formula. IV, inverse-variance method.
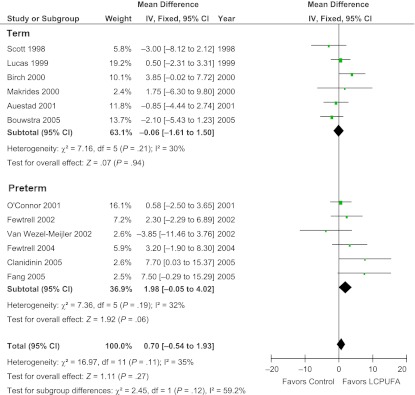
Effect of Supplementation on Preterm Versus Term Infants
Stratified subgroup analysis demonstrated no significant effect of preterm compared to term infant status on effect of LCPUFA supplementation on cognition (test for subgroup differences: χ2 = 2.45, df = 1, P = .12, I2 = 59.2%). LCPUFA supplementation in formula did not demonstrate significant benefit in either term or preterm infants. Figure 5 depicts a forest plot of the effects of LCPUFA supplementation of formula in these subgroups.
FIGURE 5.
Effect of LCPUFA supplementation on infant cognition stratified by prematurity. This forest plot compares BSID scores between infants who were fed formula supplemented with LCPUFAs versus those who were fed unsupplemented formula stratified by whether trials enrolled premature or full-term infants. LCPUFA supplementation of infant formula did not significantly improve cognition in either subgroup. IV, inverse-variance method.
Dose of LCPUFA in Supplemented Infant Formula
Meta-regression demonstrated no significant effect of dose of DHA (β = 8.9 [95% CI: −5.7 to 23.4], t = 1.3, P = .21), AA (β = −.3 [95% CI: −7.9 to 7.3], t = −0.8, P = .94), or EPA (β = 9.4 [95% CI: −34.2 to 53.1], t = 0.5, P = .65) on efficacy of LCPUFA supplementation. There was also no significant association of AA:DHA ratio (β = −.64 [95% CI: −2.02 to 0.73], t = −1.0, P = .34) on efficacy of LCPUFA supplementation.
Methodologic Quality of Trials
Stratified subgroup analysis did not demonstrate any significant difference in the reported efficacy of LCPUFA supplementation based on trial methodologic quality (test for subgroup differences: χ2 = 1.53, df = 1, P = .22, I2 = 34.5%). Lower-quality trials (Jadad score <4, WMD = −1.63 [95% CI: −5.95 to 2.69], z = 0.74, P = .46) did not show a greater benefit of LCPUFAs than higher-quality trials (Jadad score = 4 or 5; WMD = 1.32 [95% CI: −0.49 to 3.13], z = 1.43, P = .15).
Method of Analysis
Stratified subgroup analysis did not demonstrate any significant difference in the reported efficacy of LCPUFA supplementation based on whether intention-to-treat or completers’ analyses were used (test for subgroup differences: χ2 = 0.21, df = 1, P = .65, I2 = 0%). LCPUFA supplementation did not show significant benefit in improving infant cognition in either the trials that used an intention-to-treat (WMD = 1.55 [95% CI: −0.65 to 3.74], z = 1.38, P = .17) or completers’ analyses (WMD = 0.78 [95% CI: −1.69 to 3.25], z = 0.62, P = .54).
Discussion
Our meta-analysis involving 12 trials and >1800 infants demonstrated no significant association between LCPUFA supplementation of infant formula and cognitive development at ∼1 year of age. Based on the precision of estimated CIs, our meta-analysis provided strong evidence to suggest that LCPUFA supplementation does not have more than a minimal effect on infant cognition at the age of ∼1 year. The results of our meta-analysis are similar to other recent meta-analyses that showed no significant benefit of LCPUFA supplementation of formula for the cognitive development of premature infants.42–44 Although our meta-analysis demonstrates no significant benefit of LCPUFA supplementation of formula on infant cognition, other important aspects of development, such as visual acuity and immune function, may be influenced by LCPUFA supplementation. There also remains the possibility that LCPUFA could impact later cognitive development or more specific aspects of cognitive development such as attention, information processing, mood, or behavior.
Doses of DHA and AA and the AA:DHA ratio varied widely among included trials. Current recommendations for LCPUFA supplementation of infant formula is to provide the formula with at least 0.2% of DHA and a similar or higher dose of AA, maintaining a ratio of at least 1:1.6,45,46 Meta-regression of the dose of DHA, AA, and EPA as well as examining the AA:DHA ratio failed to demonstrate any significant association with measured efficacy of LCPUFA supplementation of infant formula. We cannot rule out the possibility, however, that higher-order, more subtle interaction of dosing between individual LCPUFA could be responsible for the observed heterogeneity between some trials. A meta-analysis involving individual patient data would be much more powerful to examine potential moderators of treatment effects but is not feasible based on the information currently available from trials. Another potential moderator that would be worth examining using meta-analysis of individual patient data is the effect of the gene cluster FADS1 FADS2 that encodes for the 2 enzymes Δ-5 and Δ-6 desaturases. These enzymes are responsible for the elongation and desaturation of shorter fatty acids into LCPUFAs.47 Carriers of minor alleles in this gene cluster have higher levels of α-linolinic acid and linoleic acid and decreased levels of LCPUFAs, including AA and DHA.48
Limitations of our meta-analysis included the combining of trials that used multiple different versions of the BSID. There may be some systematic differences when assessing cognition with different versions of the scale.42,49 Although the BSID is the most widely used to assess infant cognition, the BSID’s main use is to detect delays in neurodevelopment, and it may be less sensitive to detecting more subtle differences in cognitive ability.49 Researchers have argued that other scales that measure specific areas of cognition, such as attention and information processing, may be more sensitive to the effects of LCPUFA supplementation.50 Several cognitive tests were used in excluded trials to test cognition. These tests included the Fagan test of infant intelligence that assesses information processing,23,51,52 MacArthur communicative Development Inventories,23and problem-solving assessment.53 Including these trials that used these scales in the meta-analysis would have been inappropriate because they would substantially increase the likely heterogeneity between trials (their outcome would have measured different areas of cognition), and the results would have been harder to interpret and less clinically meaningful.
The validity of the meta-analysis is also always limited by the quality of the included trials. The trials included in this meta-analysis were, on the whole, of good quality, although some trials did not properly account for dropout and may not have described adequate safeguards to ensure concealment of allocation and blinding. The use of the generic inverse-variance methods to weight trials in this meta-analysis also tended to apply more weight to trials involving term infants rather than preterm infants, because they have less variance in cognitive development. For instance, a trial that examined the effects of LCPUFA in a group of 361 low birth weight and physically ill children contributed almost the same weight as a trial of 42 healthy, term infants because of large differences in the variance of cognitive development outcomes.37,41
Research suggests that breastfed infants score 3 to 5 points higher on measures of cognitive development than infants fed formula. The differences in cognitive development between breastfed and formula-fed infants were a substantial motivating factor in adding LCPUFA to infant formulas. Our results suggest that LCPUFA differences between breast milk and infant formulas may not be a significantly contributing factor. Other potential differences between breast milk and formula, such as the antimicrobial, anti-inflammatory, and immunomodulatory properties of breast milk, should be investigated.54 The difference in cognitive development seen between breast- and formula-fed infants may not be caused merely by composition differences between breast and formula milk, per se. Breast feeding also may promote maternal-infant bonding. Research has shown that breast-fed infants not only had better neurobehavioral profiles as opposed to their formula-fed peers but also were more alert during social interactions, and their mothers provided more affectionate touch.3 Neuroimaging studies also have demonstrated that breastfeeding mothers have greater activation in response to their babies’ cry in brain regions associated with maternal-infant bonding than formula-feeding mothers.55
Glossary
- AA
arachidonic acid
- ALA
a-linolinic acid
- BSID
Bayley Scales of Infant Development
- CI
confidence interval
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- LA
linoleic acid
- LCPUFA
long-chain polyunsaturated fatty acid
- WMD
weighted mean difference
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The authors acknowledge support from the National Institute of Mental Health for the Yale Child Study Center Research Training Program (Dr Bloch); the National Institutes of Health (NIH) grant 1K23MH091240-01 (Dr Bloch), grant T32MH018268-26 (Dr Leckman), and grant R25 MH077823 (Dr Leckman); the American Psychiatric Institute of Research Education/Eli Lilly Psychiatric Research Fellowship (Dr Bloch); the American Academy of Child and Adolescent Psychiatry/Eli Lilly Junior Investigator Award (Dr Bloch); the Trichotillomania Learning Center (Dr Bloch); NARSAD (Dr Bloch); and grant UL1 RR024139 from the National Center for Research Resources, a component of the NIH, and NIH roadmap for Medical Research (Dr Bloch). Funded by the National Institutes of Health (NIH).
COMPANION PAPERS: Companions to this article can be found on pages 1134 and 1166, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2011-3121 and www.pediatrics.org/cgi/doi/10.1542/peds.2012-0934.
References
- 1.Grummer-Strawn LM, Scanlon KS, Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics. 2008;122(suppl 2):S36–S42 [DOI] [PubMed] [Google Scholar]
- 2.Kramer MS, Aboud F, Mironova E, et al. Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65(5):578–584 [DOI] [PubMed] [Google Scholar]
- 3.Feldman R, Eidelman AI. Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Dev Psychobiol. 2003;43(2):109–119 [DOI] [PubMed] [Google Scholar]
- 4.Britton JR, Britton HL, Gronwaldt V. Breastfeeding, sensitivity, and attachment. Pediatrics. 2006;118(5). Available at: www.pediatrics.org/cgi/content/full/118/5/e1436. [DOI] [PubMed] [Google Scholar]
- 5.Furman L, Minich NM, Hack M. Breastfeeding of very low birth weight infants. J Hum Lact. 1998;14(1):29–34 [DOI] [PubMed] [Google Scholar]
- 6.Koletzko B, Lien E, Agostoni C, et al. World Association of Perinatal Medicine Dietary Guidelines Working Group The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36(1):5–14 [DOI] [PubMed] [Google Scholar]
- 7.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43 [DOI] [PubMed] [Google Scholar]
- 8.Cetin I, Koletzko B. Long-chain omega-3 fatty acid supply in pregnancy and lactation. Curr Opin Clin Nutr Metab Care. 2008;11(3):297–302 [DOI] [PubMed] [Google Scholar]
- 9.Uauy R, Mena P, Wegher B, Nieto S, Salem N., Jr Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res. 2000;47(1):127–135 [DOI] [PubMed] [Google Scholar]
- 10.Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2-3):151–158 [DOI] [PubMed] [Google Scholar]
- 11.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85(6):1457–1464 [DOI] [PubMed] [Google Scholar]
- 12.Jensen CL, Lapillonne A. Docosahexaenoic acid and lactation. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2-3):175–178 [DOI] [PubMed] [Google Scholar]
- 13.Makrides M, Neumann M, Simmer K, Pater J, Gibson R. Are long-chain polyunsaturated fatty acids essential nutrients in infancy? Lancet 1995 Jun 10;345(8963):1463–1468 [DOI] [PubMed] [Google Scholar]
- 14.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42(3):174–181 [DOI] [PubMed] [Google Scholar]
- 15.Auestad N, Montalto MB, Hall RT, et al. Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas with long chain polyunsaturated fatty acids for one year. Ross Pediatric Lipid Study. Pediatr Res. 1997;41(1):1–10 [DOI] [PubMed] [Google Scholar]
- 16.Innis SM, Lupton BA, Nelson CM. Biochemical and functional approaches to study of fatty acid requirements for very premature infants. Nutrition. 1994;10(1):72–76 [PubMed] [Google Scholar]
- 17.Clandinin MT, Van Aerde JE, Parrott A, Field CJ, Euler AR, Lien EL. Assessment of the efficacious dose of arachidonic and docosahexaenoic acids in preterm infant formulas: fatty acid composition of erythrocyte membrane lipids. Pediatr Res. 1997;42(6):819–825 [DOI] [PubMed] [Google Scholar]
- 18.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120(4 pt 2):S129–S138 [DOI] [PubMed] [Google Scholar]
- 19.Lauritzen L, Jorgensen MH, Olsen SF, Staarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast-fed infants. Reprod Nutr Dev 2005;45(5):535–547 [DOI] [PubMed] [Google Scholar]
- 20.Agostoni C, Trojan S, Bellù R, Riva E, Giovannini M. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: the role of long-chain polyunsaturated fatty acids. Pediatr Res. 1995;38(2):262–266 [DOI] [PubMed] [Google Scholar]
- 21.Jensen CL, Voigt RG, Prager TC, et al. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J Clin Nutr 2005;82(1):125–132 [DOI] [PubMed] [Google Scholar]
- 22.Bouwstra H, Dijck-Brouwer DA, Boehm G, Boersma ER, Muskiet FA, Hadders-Algra M. Long-chain polyunsaturated fatty acids and neurological developmental outcome at 18 months in healthy term infants. Acta Paediatr. 2005;94(1):26–32 [DOI] [PubMed] [Google Scholar]
- 23.O’Connor DL, Hall R, Adamkin D, et al. Ross Preterm Lipid Study Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108(2):359–371 [DOI] [PubMed] [Google Scholar]
- 24.Singhal A, Morley R, Cole TJ, et al. Infant nutrition and stereoacuity at age 4-6 y. Am J Clin Nutr 2007;85(1):152–159 [DOI] [PubMed] [Google Scholar]
- 25.Lucas A, Stafford M, Morley R, et al. Efficacy and safety of long-chain polyunsaturated fatty acid supplementation of infant-formula milk: a randomised trial Lancet 1999;354(9194):1948–1954 [DOI] [PubMed] [Google Scholar]
- 26.Fewtrell MS, Morley R, Abbott RA, et al. Double-blind, randomized trial of long-chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics. 2002;110(1 pt 1):73–82 [DOI] [PubMed] [Google Scholar]
- 27.Helland IB, Saugstad OD, Smith L, et al. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women Pediatrics 2001;108(5). Available at: www.pediatrics.org/cgi/content/full/108/5/e82 [DOI] [PubMed] [Google Scholar]
- 28.Auestad N, Halter R, Hall RT, et al. Growth and development in term infants fed long-chain polyunsaturated fatty acids: a double-masked, randomized, parallel, prospective, multivariate study. Pediatrics. 2001;108(2):372–381 [DOI] [PubMed] [Google Scholar]
- 29.Makrides M, Neumann MA, Simmer K, Gibson RA. A critical appraisal of the role of dietary long-chain polyunsaturated fatty acids on neural indices of term infants: a randomized, controlled trial. Pediatrics. 2000;105(1 pt 1):32–38 [DOI] [PubMed] [Google Scholar]
- 30.Bayley N. Bayley Scales of Infant Development. San Antonio, TX: Psychological Corporation.; 1993 [Google Scholar]
- 31.Bayley N. Bayley Scales of Infant Development. New York, NY: Psychological Corp.; 1969 [Google Scholar]
- 32.ULRICHSWEB. Available at: http://ulrichsweb.serialssolutions.com/ December, 2011
- 33.Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73 [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeks J, Higgins J, Altman D. Cochrane Reviewers' Handbook 4.2.1. Chichester, UK: John Wiley & Sons, Ltd; 2003 [Google Scholar]
- 36.Fang PC, Kuo HK, Huang CB, Ko TY, Chen CC, Chung MY. The effect of supplementation of docosahexaenoic acid and arachidonic acid on visual acuity and neurodevelopment in larger preterm infants. Chang Gung Med J. 2005;28(10):708–715 [PubMed] [Google Scholar]
- 37.Clandinin MT, Van Aerde JE, Merkel KL, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr. 2005;146(4):461–468 [DOI] [PubMed] [Google Scholar]
- 38.Carlson SE. Lipid requirements of very low-birth weight infants for optimal growth and development. In: Lipids, Learning and the Brain: Fats in Infant Formulas. Columbus, OH: Ross Laboratories; 1993:188–207 [Google Scholar]
- 39.Scott DT, Janowsky JS, Carroll RE, Taylor JA, Auestad N, Montalto MB. Formula supplementation with long-chain polyunsaturated fatty acids: are there developmental benefits? Pediatrics. 1998;102(5). Available at: www.pediatrics.org/cgi/content/full/102/5/e59. [DOI] [PubMed] [Google Scholar]
- 40.Fewtrell MS, Abbott RA, Kennedy K, et al. Randomized, double-blind trial of long-chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. J Pediatr. 2004;144(4):471–479 [DOI] [PubMed] [Google Scholar]
- 41.van Wezel-Meijler G, van der Knaap MS, Huisman J, Jonkman EJ, Valk J, Lafeber HN. Dietary supplementation of long-chain polyunsaturated fatty acids in preterm infants: effects on cerebral maturation. Acta Paediatr. 2002;91(9):942–950 [DOI] [PubMed] [Google Scholar]
- 42.Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: a systematic review of randomized controlled trials. Am J Clin Nutr. 2008;87(4):912–920 [DOI] [PubMed] [Google Scholar]
- 43.Schulzke SM, Patole SK, Simmer K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev. 2011;(2):CD000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmer K, Patole SK, Rao SC. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev. 2008;(1):CD000376. [DOI] [PubMed] [Google Scholar]
- 45.Guesnet P, Alessandri JM. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS) - Implications for dietary recommendations. Biochimie. 2011;93(1):7–12 [DOI] [PubMed] [Google Scholar]
- 46.Kris-Etherton PM, Innis S, Ammerican Dietetic Assocition; Dietitians of Canada Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107(9):1599–1611 [PubMed] [Google Scholar]
- 47.Glaser C, Lattka E, Rzehak P, Steer C, Koletzko B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern Child Nutr. 2011;7(suppl 2):27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rzehak P, Thijs C, Standl M, et al. KOALA study group. LISA study group Variants of the FADS1 FADS2 gene cluster, blood levels of polyunsaturated fatty acids and eczema in children within the first 2 years of life. PLoS ONE. 2010;5(10):e13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szajewska H. The role of meta-analysis in the evaluation of the effects of early nutrition on mental and motor development in children. Am J Clin Nutr 2011;94(suppl 6):1889S–1895S [DOI] [PubMed]
- 50.Cheatham CL, Colombo J, Carlson SE. N-3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. Am J Clin Nutr. 2006;83(suppl 6):1458S–1466S [DOI] [PubMed] [Google Scholar]
- 51.Carlson SE, Werkman SH. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until two months. Lipids. 1996;31(1):85–90 [DOI] [PubMed] [Google Scholar]
- 52.Werkman SH, Carlson SE. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until nine months. Lipids. 1996;31(1):91–97 [DOI] [PubMed] [Google Scholar]
- 53.Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352(9129):688–691 [DOI] [PubMed] [Google Scholar]
- 54.Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J. 1993;12(8):664–671 [DOI] [PubMed] [Google Scholar]
- 55.Kim P, Feldman R, Mayes LC, et al. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry 2011;52(8):907–915 [DOI] [PMC free article] [PubMed]