Abstract
OBJECTIVE:
We sought to determine if a center’s approach to care of premature infants at the youngest gestational ages (22–24 weeks’ gestation) is associated with clinical outcomes among infants of older gestational ages (25–27 weeks’ gestation).
METHODS:
Inborn infants of 401 to 1000 g birth weight and 22 0/7 to 27 6/7 weeks’ gestation at birth from 2002 to 2008 were enrolled into a prospectively collected database at 20 centers participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Markers of an aggressive approach to care for 22- to 24-week infants included use of antenatal corticosteroids, cesarean delivery, and resuscitation. The primary outcome was death before postnatal day 120 for infants of 25 to 27 weeks’ gestation. Secondary outcomes were the combined outcomes of death or a number of morbidities associated with prematurity.
RESULTS:
Our study included 3631 infants 22 to 24 weeks’ gestation and 5227 infants 25 to 27 weeks’ gestation. Among the 22- to 24-week infants, use of antenatal corticosteroids ranged from 28% to 100%, cesarean delivery from 13% to 65%, and resuscitation from 30% to 100% by center. Centers with higher rates of antenatal corticosteroid use in 22- to 24-week infants had reduced rates of death, death or retinopathy of prematurity, death or late-onset sepsis, death or necrotizing enterocolitis, and death or neurodevelopmental impairment in 25- to 27-week infants.
CONCLUSIONS:
This study suggests that physicians’ willingness to provide care to extremely low gestation infants as measured by frequency of use of antenatal corticosteroids is associated with improved outcomes for more-mature infants.
KEY WORDS: low-birth weight infant, NICUs, treatment, patient outcome assessment
What’s Known on This Subject:
Although morbidity-free survival for preterm infants has remained constant in US NICUs when assessed collectively, morbidity-free survival differs among centers. Center-specific practices before, at, or after delivery might affect outcomes of the most premature infants.
What This Study Adds:
Our findings suggest that the approach taken to infants at the limits of viability is associated with outcomes of more-mature infants. Identifying centers with higher survival and lower morbidity might lead to identification of key practices to improve morbidity-free survival.
Recent studies have demonstrated little progress in reducing the mortality and morbidities associated with extremely preterm birth.1,2 Although morbidity-free survival for preterm infants has remained constant in US NICUs when assessed collectively, morbidity-free survival differs among centers, even after adjustment for maternal and infant demographic factors.3 Center-specific practices before, at, or after delivery might affect outcomes of the most premature infants.
Physicians and other health care providers in NICUs where resuscitation and provision of intensive care for infants at the border of viability (22–24 weeks’ gestation) is routine might develop center-specific practices, protocols, and expertise from treating the most immature infants that result in improved outcomes among more-mature infants (25–27 weeks’ gestation) for whom there is consensus for an aggressive intensive care approach. Whether center approach to caring for the smallest and least-mature infants translates into improved rates of survival and morbidity-free survival for more-mature infants is unknown. Markers of a more aggressive approach toward the care of 22- to 24-week infants include active interventions by obstetricians (antenatal corticosteroids and cesarean deliveries for fetal indications) and neonatologists (delivery room resuscitation).
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) Generic Database (GDB) provides a unique opportunity to better identify and characterize center practices that are associated with variations in survival and other common morbidities in extremely low gestation infants. In this analysis, we tried to answer the following question: In centers where physicians take an aggressive approach to the care of premature infants at the youngest gestational ages, are clinical outcomes for infants of older gestational ages better than in centers where a less aggressive approach is taken with younger gestation infants?4,5 We hypothesized that mortality and morbidities (severe retinopathy of prematurity [ROP], late-onset sepsis [LOS], severe intraventricular hemorrhage [IVH], periventricular leukomalacia [PVL], necrotizing enterocolitis [NEC], bronchopulmonary dysplasia [BPD], and neurodevelopmental impairment [NDI]) among infants at 25 to 27 weeks’ gestation at NRN centers would be lower in centers with the highest rates of antenatal corticosteroid use, cesarean delivery, or resuscitation among infants of 22 to 24 weeks’ gestation.
Methods
Study Population
The NICHD NRN is a consortium of tertiary NICUs. The network’s GDB prospectively collects data on all extremely low birth weight (ELBW; 401–1000 g) infants born at participating centers or transferred to participating centers in the first 14 postnatal days. Trained research personnel collect maternal demographic, pregnancy, and delivery data until death, discharge, or postnatal day 120. Inborn infants entered into the database between January 1, 2002, and December 31, 2008, who were 22 0/7 to 27 6/7 weeks’ gestation and 401 to 1000 g at birth were included in the analysis. Infants with major congenital or chromosomal anomalies were excluded.
Clinical Methods
Definitions of terms and diagnoses were guided by the NICHD NRN GDB manual of operations. Gestational age was determined as the best obstetric estimate based on ultrasound and/or date of the last menstrual period, or, if the obstetric estimate was unavailable, as the best neonatologist estimate. Resuscitation was defined as intubation in the delivery room or survival >24 hours. Only severe ROP (requiring laser surgery) was considered in this analysis. LOS was defined by a positive blood culture obtained after postnatal day 3 in the presence of clinical signs compatible with septicemia and ≥5 days of antibiotic treatment.6 Blood cultures positive for organisms generally considered contaminants, including Corynebacterium (or “diphtheroids”), Propionibacterium, and Penicillium, were excluded. IVH was graded by using the method of Papile et al,7 with severe IVH defined as grade 3 or 4 IVH for this analysis. PVL was defined as cystic PVL. NEC was defined as ≥stage 2 according to the modified Bell criteria.8 BPD was defined by the use of supplemental oxygen at 36 weeks’ postmenstrual age or at the time of discharge home. NDI was defined as impairment by the Bayley Scales of Infant Development II (2004–2006) and included neurologic impairment (moderate-to-severe cerebral palsy with gross motor function classification system [GMFCS] level ≥2), developmental impairment (mental developmental index <70 or psychomotor developmental index <70), vision impairment (<20–200 in both eyes), and/or hearing impairment (bilateral amplification for permanent hearing loss). Impairment by Bayley Scales of Infant Development III (2006–2008) included neurologic impairment (moderate-to-severe cerebral palsy with GMFCS impairment ≥ level 2), developmental impairment (cognitive score <70 or GMFCS ≥ level 2), vision impairment (<20–200 in both eyes), and/or hearing impairment (permanent hearing loss that does not permit the child to understand the directions of the examiner and communicate despite amplification). NDI was assessed during a comprehensive neurodevelopmental evaluation at 18 to 22 months’ adjusted age.
Statistical Analysis
The primary outcome for this study was death before postnatal day 120 for infants of 25 to 27 weeks’ gestation. Secondary outcomes were death or ROP, death or LOS, death or severe IVH, death or PVL, death or BPD, and death or NDI for infants of 25 to 27 weeks’ gestation. We summarized demographic variables and outcomes in the 2 gestational age cohorts.
We used random effects multiple logistic regression to examine the relationship between center rates of antenatal corticosteroid use, cesarean delivery, and resuscitation for 22- to 24-week infants and clinical outcomes in 25- to 27-week infants.9,10 Separate models were used for each of the 3 predicting factors. Individual-level risk factors in the model included sex, birth weight, gestational age, race, maternal education level, and type of insurance, as well as antenatal corticosteroid use for 25- to 27-week infants. Because the variability of interventions among 23-week infants was greater than that observed in the 22- and 24-week infants, we conducted a secondary analysis limiting the predictor variables to rates of interventions (antenatal corticosteroid use, cesarean deliveries, and resuscitation) among 23-week infants controlling for the same variables.
We conducted sensitivity analyses for our results by repeating the analysis after removing the centers with the highest and lowest rates of intervention. Because of the concern that aggressive measures in 22- to 24-week infants would lead to poor outcomes in this group, we repeated the analysis to examine the effect of interventions among 22- to 24-week infants on mortality in this population. In this analysis, we controlled for infant sex, birth weight, gestational age, maternal education level, insurance type, race, and antenatal corticosteroid exposure at the individual level. Infants lost to follow-up at 18 to 22 months’ adjusted age were treated as missing in the final models.
All data were analyzed by using SAS version 9.2 (SAS Inc, Cary, NC) at RTI International, the data coordinating center for the NRN. The institutional review board at each NRN center and at RTI approved the inclusion of data in the GDB.
Results
The study cohort included 8858 ELBW infants born at 20 centers of the NICHD NRN; 3631 (41%) infants were born at 22 to 24 weeks’ gestation, and 5227 (59%) infants were at 25 to 27 weeks’ gestation (Table 1).
TABLE 1.
Neonatal Characteristics by Gestational Age Group (n, %)
| Characteristic | 22–24 wk (n = 3631) | 25–27 wk (n = 5227) |
|---|---|---|
| Birth weight, g | ||
| 401–500 | 634 (18) | 183 (4) |
| 501–750 | 2666 (73) | 1757 (34) |
| 751–1000 | 331 (9) | 3287 (63) |
| Female sex | 1658 (46) | 2614 (50) |
| SGA at birth | 162 (4) | 564 (11) |
| Race | ||
| White | 1884 (52) | 2796 (54) |
| African American | 1527 (42) | 2160 (41) |
| Other | 178 (5) | 235 (5) |
| Antenatal corticosteroids | 2263 (62) | 4551 (87) |
SGA, small for gestational age.
Overall, 3191 (36%) infants died. As expected, death was much more common among infants at 22 to 24 weeks (61%) than among those at 25 to 27 weeks (19%) (Table 2). The combined outcomes of death or morbidity for each of ROP, LOS, severe IVH, PVL, NEC, and BPD were higher among 22- to 24-week infants compared with 25- to 27-week infants (Table 2).
TABLE 2.
Unadjusted Neonatal Outcomes by Gestational Age Group (n, %)
| Outcome | 22–24 wk (n = 3631) | 25–27 wk (n = 5227) |
|---|---|---|
| Death | 2219 (61) | 972 (19) |
| ROP | 337 (9) | 306 (6) |
| LOS | 1147 (32) | 1839 (35) |
| IVH | 686 (19) | 740 (14) |
| PVL | 141 (4) | 243 (5) |
| NEC | 328 (9) | 578 (11) |
| BPD | 990 (27) | 2038 (39) |
| NDI | 500 (42) | 878 (26) |
| Death/ROP | 2549 (70) | 1272 (24) |
| Death/LOS | 3014 (83) | 2520 (48) |
| Death/IVH | 2515 (69) | 1446 (28) |
| Death/PVL | 2311 (64) | 1163 (22) |
| Death/NEC | 2389 (66) | 1359 (26) |
| Death/BPD | 3188 (88) | 2956 (57) |
| Death/NDI | 2806 (77) | 1999 (38) |
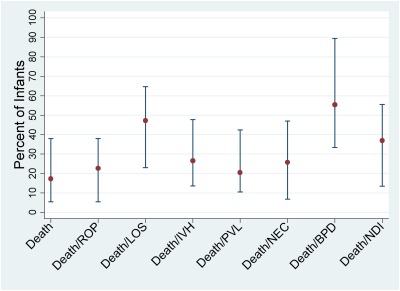
Outcomes for 25- to 27-week infants varied by center (Fig 1). Only 5% of 25- to 27-week infants died at the center with the lowest mortality compared with 38% at the center with the highest mortality. Death or NEC ranged from 7% at the lowest center to 47% at the highest center, and, similarly, death or BPD ranged from 33% to 89%. By using Pearson correlation, we found a statistically significant association between outcomes for 22- to 24-week and 25- to 27-week infants within centers for both death and each of the combined outcomes of death and ROP, LOS, severe IVH, PVL, NEC, and BPD (P < .001).
FIGURE 1.
Center variation in outcomes for 25- to 27-week infants (median, range).
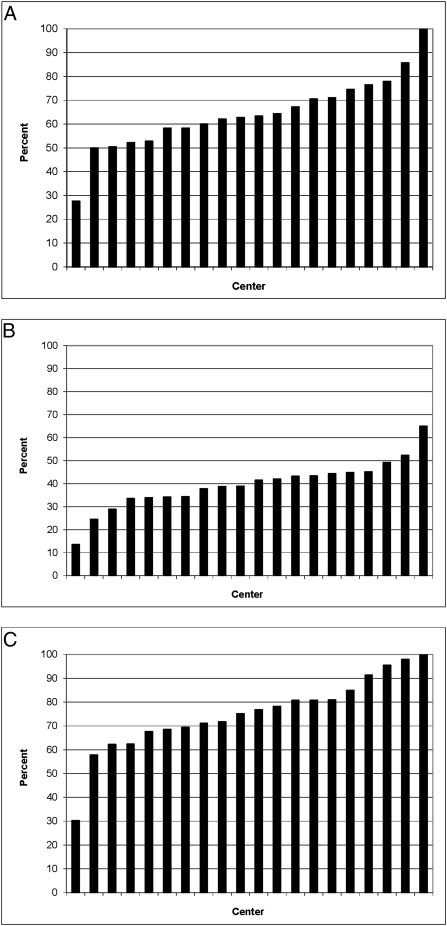
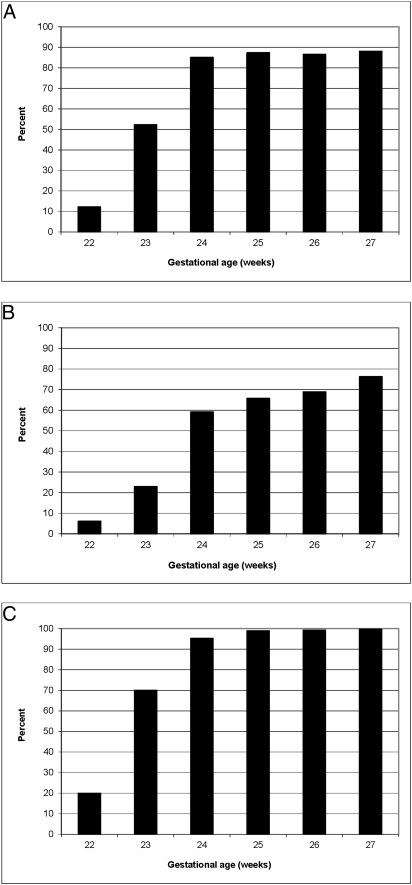
There were wide center variations in obstetrical and neonatal interventions among infants at 22 to 24 weeks (Fig 2 A–C). Use of antenatal corticosteroids ranged from 28% to 100%, cesarean deliveries from 13% to 65%, and resuscitation from 30% to 100%. Compared with infants born at 22 to 24 weeks’ gestation, infants born at 25 to 27 weeks’ gestation were more likely to have received antenatal corticosteroids (87% vs 62%), be delivered by cesarean delivery (70% vs 39%), and be resuscitated at birth (99% vs 75%). Infants born at 24 weeks had active interventions nearly as often as those born at 25 to 27 weeks (Fig 3 A–C). Few infants born at 22 weeks received antenatal corticosteroids (12%), were delivered via cesarean delivery (7%), or were resuscitated at birth (20%). By using Pearson correlation, we observed statistically significant associations between aggressive center practices (use of antenatal corticosteroids, cesarean deliveries, and resuscitation) within a center for 22- to 24-week infants (all correlation coefficients >0.74 and P < .001), as well as significant associations between aggressive center practices for 22- to 24-week infants and 25- to 27-week infants within centers.
FIGURE 2.
Interventions in 22- to 24-week infants by center. A, Antenatal corticosteroid use by center; B, cesarean delivery rate by center; C, resuscitation rate by center.
FIGURE 3.
Interventions by gestational age. A, Antenatal corticosteroid use by gestational age; B, cesarean delivery rate by gestational age; C, resuscitation rate by gestational age.
Adjusted random effects logistic regression modeling demonstrated that, for every 10% increase in center rate of antenatal corticosteroid use in 22- to 24-week infants, outcomes improved in 25- to 27-week infants for death, death or ROP, death or LOS, death or NEC, and death or NDI (Table 3). Specifically, a 10% increase in a center’s antenatal corticosteroid usage among 22- to 24-week infants was associated with a 15% reduction in the odds of death by 120 days among 25- to 27-week infants. None of the outcomes in 25- to 27-week infants were statistically different based on rate of cesarean delivery and resuscitation in 22- to 24-week infants; however, for centers with higher rates of cesarean delivery and resuscitation among infants at 22 to 24 weeks, 14 (88%) of 16 of the outcomes for 25- to 27-week infants had point estimates with improved outcomes, although these did not reach statistical significance. Limiting the analysis to interventions among infants born at 23 weeks’ gestational age, where there was most variation in use of antenatal corticosteroids, cesarean deliveries, and resuscitation, we observed similar results; an increase in center use of antenatal corticosteroids in the 22- to 24-week infants was associated with decreased risk of death, death or ROP, death or LOS, death or NEC, and death or NDI among 25- to 27-week infants.
TABLE 3.
Clinical Outcomes for 25- to 27-Week Infants from Multivariable Analysis Based on a 10% Increase in Intervention Rates for 22- to 24-Week Infants
| Outcome | Antenatal Corticosteroids OR (95% CI) | Cesarean Delivery OR (95% CI) | Resuscitation OR (95% CI) |
|---|---|---|---|
| Death | 0.85 (0.74, 0.97)a | 0.84 (0.68, 1.05) | 0.89 (0.78, 1.02) |
| Death or ROP | 0.82 (0.71, 0.95)a | 0.84 (0.67, 1.06) | 0.88 (0.76, 1.01) |
| Death or LOS | 0.81 (0.73, 0.90)a | 0.83 (0.69, 1.00) | 0.89 (0.79, 1.01) |
| Death or severe IVH | 0.94 (0.81, 1.08) | 0.93 (0.76, 1.09) | 0.96 (0.84, 1.10) |
| Death or PVL | 0.91 (0.81, 1.04) | 0.91 (0.76, 1.09) | 0.94 (0.84, 1.06) |
| Death or NEC | 0.83 (0.73, 0.96)a | 0.83 (0.67, 1.03) | 0.89 (0.78, 1.02) |
| Death or BPD | 0.99 (0.75, 1.29) | 1.16 (0.80, 1.69) | 1.00 (0.78, 1.29) |
| Death or NDI | 0.88 (0.78, 0.98)a | 0.91 (0.76, 1.09) | 0.95 (0.85, 1.07) |
P < .05.
A sensitivity analysis performed by removing the centers with the highest and lowest rates of intervention produced similar effects for center rates of antenatal steroid use, cesarean delivery, and resuscitation on death (odds ratio [OR] = 0.89 [95% confidence interval (CI): 0.73, 1.09]; OR = 1.00 [0.74, 1.35]; OR = 0.93 [0.77, 1.12], respectively). Repeating the analysis to examine the effect of interventions among 22- to 24-week infants on mortality in this population, we found that increased center rates of antenatal corticosteroid use, cesarean deliveries, and resuscitation among 22- to 24-week infants were associated with lower mortality among 22- to 24-week infants (OR = 0.72 [95% CI: 0.60, 0.87]; OR = 0.67 [0.50, 0.89]; and OR = 0.77 [0.64, 0.92], respectively). To account for potential differences in the outcomes of the 25- to 27-week group associated with cesarean delivery use in this population, we repeated the analysis with the addition of a covariable for cesarean delivery in individual 25- to 27-week infants. The association between center use of antenatal corticosteroids and mortality in the 22- to 24-week infants was still statistically significant (OR = 0.85 [0.74, 0.97]).
Discussion
Center differences are an important factor for outcomes in premature infants. Although recent multicenter cohorts demonstrated static outcomes for premature infants, there were striking differences in mortality and morbidity among individual centers.11,12 These differences were present after accounting for center differences in confounding factors known to affect clinical outcomes, such as birth weight, gestational age at birth, antenatal corticosteroid use, and sex.11 A review of infants at 22 to 28 weeks’ gestation and <1500 g birth weight from 2003 to 2007 found that morbidity-free survival ranged from 7% to 50% across NICHD NRN centers.2 Multivariable modeling of GDB data found the ratio of observed to expected rate of adverse outcomes ranged from 0.60 to 1.38 for death and 0.75 to 1.23 for death or profound NDI by center (www.nichd.nih.gov/neonatalestimates).1 In a previous study, we compared multiple logistic regression and neural network models for predicting death for ELBW infants at 5 time points with cumulative data sets.13 Center was highly significant and important in all models in which it was entered. The magnitude of this center variation in outcome is much larger than the effect sizes of nearly all proven interventions, such as surfactant, continuous positive airway pressure, or vitamin A.14–16
Our study sought to determine whether aggressive obstetrical and delivery room practices in caring for the most premature infants (22–24 weeks’ gestation) were associated with improved outcomes for more-mature infants (25–27 weeks’ gestation). We limited the outcomes analyzed to death and the combined outcomes of death and common morbidities of prematurity. This was done to avoid the pitfall of comparing rates of individual outcomes without accounting for death as a competing outcome. NICUs in which health care providers routinely administer antenatal corticosteroids, perform a cesarean delivery if indicated, and resuscitate infants at the edge of viability might develop center-specific practices and protocols for these infants that, when implemented in more-mature infants, lead to improved outcomes in the more-mature cohort. In addition, physicians and other personnel at these centers might develop more seamless teamwork and skill at early management of extremely premature infants because of experience resuscitating and stabilizing these infants. Our study was limited, as it is a retrospective cohort study. Although our study was limited by the uncertainties inherent in determining gestational age, the estimates used in our analyses are the same as those used by clinicians discussing potential outcomes of preterm infants with families.17–19
Antenatal corticosteroid use is known to improve clinical outcomes in infants ≥26 weeks’ gestation at birth.20 In a recent analysis of NICHD NRN data, investigators observed a similar association between exposure to antenatal corticosteroid use among 22- to 25-week infants on the combined outcome of death or NDI (OR = 0.58 [95% CI: 0.42, 0.80])21; however, these results may be biased by the selective use of corticosteroids in pregnancies with other favorable prognostic factors. Current guidelines advise administering antenatal corticosteroids to women at high risk of delivery between 24 and 34 weeks’ gestation22; further study is needed to evaluate administration of antenatal corticosteroids to infants <24 weeks’ gestation. We repeated our analysis to examine the effect of interventions among 22- to 24-week infants on mortality in 22- to 24-week infants to ensure that aggressive measures were not associated with poor outcomes in this group. In our cohort, increased center rates of antenatal corticosteroid use, cesarean delivery, and resuscitation among 22- to 24-week infants were associated with lower mortality among 22- to 24-week infants.
Despite evidence that delivery via cesarean delivery imparts a survival benefit for infants at 22 to 25 weeks’ gestation,23 some obstetricians do not routinely use electronic fetal monitoring or consider cesarean delivery for fetal indications for infants at <24 weeks’ gestation.24 The beneficial impact of cesarean delivery on survival must be weighed against the risk of long-term morbidities in the surviving infant and the potential effects of cesarean delivery on maternal health.25
The decision to resuscitate infants at 22 to 24 weeks’ gestation is controversial and varies greatly by neonatologist and by center.9 We noted that for each of the interventions, 1 center was strikingly less aggressive than the others. In our sensitivity analysis, performed by removing the centers with the highest and lowest rates of intervention, the association between antenatal steroid use and survival was no longer statistically significant, but the point estimate was nearly identical (0.89 vs 0.84), suggesting that the loss of statistical significance was caused by the reduced sample size available for the sensitivity analysis. Among NICHD NRN centers, intensive care is routinely administered to infants ≥25 weeks’ gestation. Decisions to intervene at earlier gestational ages vary among centers but are typically made after discussions between the parents and medical staff based on evidence that does not take into account the wide variations in center outcomes.26
Our findings suggest that aggressive clinical management of pregnancies and infants at the limits of viability, as measured by use of antenatal steroids, is associated with improved outcomes in more-mature infants, for whom consensus supports universal use of an aggressive intensive care approach. We did not observe a relationship between rates of cesarean delivery and resuscitation of the most premature infants and outcomes of more-mature infants.
Identifying centers with consistently higher survival and less morbidity among the lowest gestational age infants might lead to identification of key practices that improve the chance of morbidity-free survival. These practices might form the basis for intervention trials to identify the extent to which the practices translate into better outcomes in other centers. Increased center use of antenatal corticosteroids among 22- to 24-week infants was associated with improved outcomes for 25- to 27-week infants, including lower rates of death or NDI. Expertise developed and intangible lessons learned from caring for the most premature infants might translate into improved outcomes for more-mature infants. The challenge is to systematically determine the center-specific practices accounting for the variations in outcomes among the extremely low gestational age infants and consider these results when making programmatic decisions about obstetrical and neonatal interventions at the lowest gestational ages.
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chairs: Alan H. Jobe, MD, PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2007). Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904): William Oh, MD; Angelita M. Hensman, RN, BSN. Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364, M01 RR80): Avroy A. Fanaroff, MD; Nancy S. Newman, RN. Cincinnati Children's Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084): Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Marcia Worley Mersmann, RN, CCRC; Holly L. Mincey, RN, BSN; Jody Hessling, RN. Duke University School of Medicine, Duke University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30): Kathy J. Auten, MSHS; Melody B. Lohmeyer, RN, MSN. Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, M01 RR39): David P. Carlton, MD; Ellen C. Hale, RN, BS, CCRC; Ann M. Blackwelder, RNC, BS, MS. Eunice Kennedy Shriver National Institute of Child Health and Human Development: Stephanie Wilson Archer, MA. Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750): Brenda B. Poindexter, MD, MS; James A. Lemons, MD; Dianne E. Herron, RN; Lucy C. Miller, RN, BSN, CCRC; Leslie Dawn Wilson, BSN, CCRC. RTI International (U10 HD36790): W. Kenneth Poole, PhD; Betty K. Hastings; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN, BSN. Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children's Hospital (U10 HD27880, M01 RR70): Krisa P. Van Meurs, MD; David K. Stevenson, MD; Marian M. Adams, MD; M. Bethany Ball, BS, CCRC; Melinda S. Proud, RCP; Andrew W. Palmquist, RN. Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54): Ivan D. Frantz III, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN, BSN. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32): Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN. University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461): Neil N. Finer, MD; Paul R. Wozniak, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Chris Henderson, RCP, CRTT; Wade Rich, BSHS, RRT. University of Iowa Children's Hospital (U10 HD53109, M01 RR59): John A. Widness, MD; Karen J. Johnson, RN, BSN; Nancy J. Krutzfield, RN, MA. University of Miami, Holtz Children's Hospital (U10 HD21397, M01 RR16587): Shahnaz Duara, MD; Ruth Everett-Thomas, RN, MSN. University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997): Kristi L. Watterberg, MD; Lu-Ann Papile, MD; Conra Backstrom Lacy, RN. University of Rochester, Golisano Children's Hospital (U10 HD40521, M01 RR44, UL1 RR24160): Dale L. Phelps, MD; Linda J. Reubens, RN, CCRC; Mary Rowan, RN; Erica Burnell, RN. University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633): Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Gaynelle Hensley, RN; Melissa H. Leps, RN; Nancy A. Miller, RN; Alicia Guzman; Susie Madison, RN. University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373): Kathleen A. Kennedy, MD, MPH; Jon E. Tyson, MD, MPH; Esther G. Akpa, RN, BSN; Patty A. Cluff, RN; Beverly Foley Harris, RN, BSN; Claudia I. Franco, RNC, MSN; Anna E. Lis, RN, BSN; Sarah Martin, RN, BSN; Georgia E. McDavid, RN; Maegan C. Simmons, RN; Patti Pierce Tate, RCP. University of Utah, University Hospital, LDS Hospital, and Primary Children's Medical Center (U10 HD53124, UL1 RR25764, M01 RR64): Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN, BSN, CCRC; Jennifer J. Jensen, RN, BSN; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN, BSN. Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (U10 HD40498, M01 RR7122): T. Michael O’Shea, MD, MPH; Nancy J. Peters, RN, CCRP. Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385): Rebecca Bara, RN, BSN; Geraldine Muran, RN, BSN. Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, MO1 RR125, M01 RR6022): Richard A. Ehrenkranz, MD; Harris C. Jacobs, MD; Patricia Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Janet Taft, RN, BSN.
Glossary
- BPD
bronchopulmonary dysplasia
- CI
confidence interval
- ELBW
extremely low birth weight
- GDB
Generic Database
- GMFCS
gross motor function classification system
- IVH
intraventricular hemorrhage
- LOS
late-onset sepsis
- NDI
neurodevelopmental impairment
- NEC
necrotizing enterocolitis
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- OR
odds ratio
- PVL
periventricular leukomalacia
- ROP
retinopathy of prematurity
Footnotes
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Data collected at participating sites of the National Institute of Child Health and Human Development Neonatal Research Network were transmitted to RTI International, the data coordinating center for the network, which stored, managed, and analyzed the data for this study. On behalf of the Neonatal Research Network, Drs Das (data coordinating center principal investigator) and Li (data coordinating center statistician) had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. All authors contributed to the study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; administrative, technical, or material support; and study supervision. Dr Smith was also responsible for drafting of the manuscript; Drs Smith, Das, and Li were also responsible for statistical analysis; and Drs Ambalavanan, Cotten, Laughon, Walsh, Das, Bell, Carlo, Stoll, Shankaran, Laptook, and Goldberg obtained funding for the study.
The funding source for this manuscript did not play a role in the study design; the collection, analysis, and interpretation of the data; the writing of the report; or the decision to submit the article for publication.
FINANCIAL DISCLOSURE: Dr Laughon is a consultant for Nycomed, Inc, Astellas, and Pfizer; Dr Higgins is a federal employee; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development provided grant support for the Neonatal Research Network’s Generic Database Study. Dr Smith received support from National Institute of Child Health and Human Development 1K23HD060040-01 and DHHS-1R18AE000028-01. Dr Cotten received support from National Institute of Child Health and Human Development 5U10 HD040492-10 and SBIR 2RRHD057713-02. Funded by the National Institutes of Health (NIH).
References
- 1.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD, National Institute of Child Health and Human Development Neonatal Research Network Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358(16):1672–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Dusick AM, et al. Neonatal Research Network Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113(4):781–789 [DOI] [PubMed] [Google Scholar]
- 4.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520 [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–2127 [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 pt 1):285–291 [DOI] [PubMed] [Google Scholar]
- 7.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 8.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh J, Fanaroff J, Andrews B, et al. Resuscitation in the “gray zone” of viability: determining physician preferences and predicting infant outcomes. Pediatrics. 2007;120(3):519–526 [DOI] [PubMed] [Google Scholar]
- 10.Lantos JD, Meadow W. Variation in the treatment of infants born at the borderline of viability. Pediatrics. 2009;123(6):1588–1590 [DOI] [PubMed] [Google Scholar]
- 11.Tyson JE, Younes N, Verter J, Wright LL, National Institute of Child Health and Human Development Neonatal Research Network Viability, morbidity, and resource use among newborns of 501- to 800-g birth weight. JAMA. 1996;276(20):1645–1651 [PubMed] [Google Scholar]
- 12.Walsh M, Laptook A, Kazzi SN, et al. National Institute of Child Health and Human Development Neonatal Research Network A cluster-randomized trial of benchmarking and multimodal quality improvement to improve rates of survival free of bronchopulmonary dysplasia for infants with birth weights of less than 1250 grams. Pediatrics. 2007;119(5):876–890 [DOI] [PubMed] [Google Scholar]
- 13.Ambalavanan N, Carlo WA, Bobashev G, et al. National Institute of Child Health and Human Development Neonatal Research Network Prediction of death for extremely low birth weight neonates. Pediatrics. 2005;116(6):1367–1373 [DOI] [PubMed] [Google Scholar]
- 14.Ho JJ, Subramaniam P, Henderson-Smart DJ, Davis PG. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2002;(2):CD002271. [DOI] [PubMed] [Google Scholar]
- 15.Soll RF. Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2000;(2):CD001079. [DOI] [PubMed] [Google Scholar]
- 16.Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database Syst Rev. 2002;(4):CD000501. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MS, McLean FH, Boyd ME, Usher RH. The validity of gestational age estimation by menstrual dating in term, preterm, and postterm gestations. JAMA. 1988;260(22):3306–3308 [PubMed] [Google Scholar]
- 18.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol. 2007;21(suppl 2):86–96 [DOI] [PubMed] [Google Scholar]
- 19.Donovan EF, Tyson JE, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network Inaccuracy of Ballard scores before 28 weeks’ gestation. J Pediatr. 1999;135(2 pt 1):147–152 [DOI] [PubMed] [Google Scholar]
- 20.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. [DOI] [PubMed] [Google Scholar]
- 21.Carlo WA, McDonald SA, Fanaroff AA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306(21):2348–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, Saling E, Coordinators of World Association of Perinatal Medicine Prematurity Working Group Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med. 2008;36(3):191–196 [DOI] [PubMed] [Google Scholar]
- 23.Malloy MH. Impact of cesarean section on neonatal mortality rates among very preterm infants in the United States, 2000–2003. Pediatrics. 2008;122(2):285–292 [DOI] [PubMed] [Google Scholar]
- 24.McElrath TF, Norwitz ER, Nour N, Robinson JN. Contemporary trends in the management of delivery at 23 weeks’ gestation. Am J Perinatol. 2002;19(1):9–15 [DOI] [PubMed] [Google Scholar]
- 25.Kollée LA, Cuttini M, Delmas D, et al. MOSAIC Research group Obstetric interventions for babies born before 28 weeks of gestation in Europe: results of the MOSAIC study. BJOG. 2009;116(11):1481–1491 [DOI] [PubMed] [Google Scholar]
- 26.Higgins RD, Delivoria-Papadopoulos M, Raju TN. Executive summary of the workshop on the border of viability. Pediatrics. 2005;115(5):1392–1396 [DOI] [PubMed] [Google Scholar]