Abstract
BACKGROUND AND OBJECTIVE:
The impact of maternal antiretrovirals (ARVs) during pregnancy, labor, and postpartum on infant outcomes is unclear.
METHODS:
Infants born to HIV-infected mothers in ARV studies were followed for 18 months.
RESULTS:
Between June 2006 and December 2008, 236 infants enrolled from Africa (n = 36), India (n = 47), Thailand (n = 152), and Brazil (n = 1). Exposure to ARVs in pregnancy included ≥3 ARVs (10%), zidovudine/intrapartum ARV (81%), and intrapartum ARV (9%). There were 4 infant infections (1 in utero, 3 late postpartum) and 4 deaths with 1.8% mortality (95% confidence interval [CI], 0.1%–3.5%) and 96.4% HIV-1–free survival (95% CI, 94.0%–98.9%). Birth weight was ≥2.5 kg in 86%. In the first 6 months, Indian infants (nonbreastfed) had lowest median weights and lengths and smallest increases in growth. After 6 months, African infants had the lowest median weight and weight-for-age z scores. Infants exposed to highest maternal viral load had the lowest height and height-for-age z scores. Serious adverse events occurred in 38% of infants, did not differ by country, and correlated with less maternal ARV exposure. Clinical diagnoses were seen in 84% of Thai, 31% of African, and 9% of Indian infants. Congenital defects/inborn errors of metabolism were seen in 18 (7.6%) infants, of which 17 were Thai (11%: 95% CI, 6.7%–17.0%); none had first trimester ARV exposure.
CONCLUSIONS:
Infant follow-up in large international cohorts is feasible and provides important safety and HIV transmission data following maternal ARV exposure. Increased surveillance increases identification of congenital/inborn errors.
KEY WORDS: maternal ARV exposure, infant safety, ARV toxicities, A5190, P1054, MTCT, HIV
What's Known on This Subject:
Information on infant safety after exposure to maternal antiretroviral regimens during pregnancy in international clinical trials is lacking. As antiretroviral drugs are released to populations in resource-limited settings through clinical trials, it becomes critical to collect pediatric outcome data.
What This Study Adds:
The study demonstrates the feasibility of reporting infant outcomes following adult antiretroviral trials in developing countries, provides HIV-free infant survival and prospective growth data in association with maternal parameters, and details morbidity, mortality, and genetic defects following maternal antiretroviral exposure.
The use of antiretrovirals (ARVs) in pregnant HIV-1–infected women has been highly beneficial to the mother/infant pair.1–5 Depending on safety and toxicity profiles, ARVs can be adjusted before conception or during pregnancy to reduce potential adverse events. It is critical to collect data on their safety during pregnancy and to monitor potential toxicities in infants after exposure to maternal regimens. The AIDS Clinical Trials Group protocol A5190/International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1054 was a prospective, observational, cohort study of infants born to HIV-1–infected mothers enrolled in National Institutes of Health (NIH)-sponsored ARV trials in resource-limited settings. Infants were enrolled from birth and followed for 18 months. The primary objective was to assess the safety of infant exposure to maternal ARV during pregnancy and postpartum.
Methods
The study was a prospective, observational cohort assessing the safety of in utero, intrapartum, and breast milk exposures to maternal ARVs. The study population consisted of live-born infants of HIV-1–infected women enrolled in NIH-sponsored international trials. Three ARV treatment studies and 2 ARV prevention of mother-to-child transmission (PMTCT) study groups participated.
A5175 is a Phase IV randomized trial of once-daily protease inhibitor and once-daily nonnucleoside reverse transcriptase inhibitor–containing combinations for initial treatment of HIV-1–infected individuals in resource-limited settings (PEARLS).6 A5208 is a randomized comparison of combination therapy to optimize therapy after nevirapine (NVP) exposure (OCTANE).7 HPTN 052 is a randomized trial to evaluate ARV effectiveness to prevent sexual HIV transmission in serodiscordant couples.8 A5207 is a randomized comparison of 3 ARV strategies given for 7 or 21 days to reduce NVP resistance after single-dose NVP.9 And P1032 is a randomized study of zidovudine (ZDV)/didanosine or ZDV/didanosine/lopinavir-ritonavir to reduce NVP resistance after single-dose NVP.10
Exposure of infants to ARVs was assessed via maternal ARVs in utero, via direct administration, and via breastfeeding. In utero ARV exposure is detailed in Table 1. The degree of exposure to maternal ARV varied by study and was categorized as exposure to ≥3 ARVs during pregnancy; ≥7 days of ZDV prophylaxis in utero plus intrapartum exposure to ARV, or intrapartum exposure to ARV. Direct exposure of infants to ARVs included single-dose NVP and single-dose NVP + ZDV. Maternal parameters included HIV-1 virus load and CD4 (cluster of differentiation 4 [T4 helper cell]) counts closest to delivery, medical history, and clinical status. Infants had 6 study visits over 18 months. Assessments included weight, height, head circumference, and laboratory evaluations (complete blood counts, liver function tests, serum chemistries, and urinalysis). HIV-1 RNA polymerase chain reaction (PCR) (Roche Amplicor Monitor Test, Version 1.5, Roche Molecular Diagnostics, Pleasanton, CA) was performed up to 12 months for breastfed infants and up to 3 months for nonbreastfed infants. HIV-1 antibody enzyme immunoassay was performed at 18 months. VDRL/rapid plasma regain testing was performed at the first visit. All participating sites were clinical research centers affiliated with NIH networks. All mothers provided written informed consent. The study was approved by foreign institutional review boards and collaborating US institutions.
TABLE 1.
Fetal/Infant Exposure to Maternal ARVs In Utero, Intrapartum and Postpartum by Parent Protocol for Infants Enrolled in A5190/P1054
| Maternal ARV Treatment Studies | PMTCT Studies | ||||
|---|---|---|---|---|---|
| A5175 n = 8 | A5208 n = 3 | HPTN 052 n = 13 | A5207 n = 61 | P1032 n = 151 | |
| First trimester | ≥3 ARVs | ≥3 ARVs | ≥3 ARVs | None | None |
| Second trimester | ≥3 ARVs | ≥3 ARVs | ≥3 ARVs | None | None |
| Third trimester | ≥3 ARVs | ≥3 ARVs | ≥3 ARVs | ZDV | ZDV |
| Intrapartum | ≥3 ARVs | ≥3 ARVs | ≥3 ARVs | sd-NVP + 2 ARVs | sd-NVP + 2–3 ARVs |
| Postpartuma | ≥3 ARVs | ≥3 ARVs | ≥3 ARVsb | Possiblec | None |
ARVs, ≥3 or more maternal ARVs in combination; sd-NVP + 2–3, single-dose NVP and 2–3 ARVs.
Postpartum exposure via breastfeeding occurred only in African sites.
Women enrolled in HPTN 052 received combination ARV postpartum only if lower CD4 threshold.
Exposure to ≥2 ARVs within the first month of life only.
Statistical analysis was descriptive. A5190/P1054 was not powered to detect specific differences between rates of adverse events in ARV study arms. A 2-sided nominal P value <.05 was used to indicate comparisons for additional exploration. Comparisons between groups for continuous variables used Kruskal-Wallis tests while comparisons between categorical variables used Fisher’s exact test. Comparisons between groups for safety data used the log-rank test. Rates were estimated by using the Kaplan-Meier method with Greenwood’s confidence intervals (CIs).
Results
From June 2006 to December 2008, 236 infants were enrolled (Table 2), 74% within the first week of life. The majority of infants (n = 212) were enrolled through perinatal studies (P1032 = 151, A5207 = 61) in which ARV exposure occurred in the third trimester of pregnancy and/or intrapartum. Twenty-four infants were enrolled from adult treatment studies. Within the cohort, 29 (12%) infants were breastfed, all in African countries. Table 3 demonstrates the degree of exposure to maternal ARV and infant ARV exposure. Of 236 infants, 232 (98%) completed 6 months and 222 (94%) completed 18 months of follow-up. Four (1.8%) infants died and 10 (4.2%) were lost to follow-up. The last subject completed follow-up in May 2010.
TABLE 2.
Subject Enrollment Into A5190/P1054 per Parent Protocol and Participating Country
| Maternal Perinatal Studies for PMTCT, n | Maternal ARV Treatment Studies, n | |||||
|---|---|---|---|---|---|---|
| P1032 | A5207 | A5175 | A5208 | HPTN 052 | Total | |
| Thailand | 151 | — | 1 | — | — | 152 (64%) |
| India | — | 46 | 1 | — | — | 47 (20%) |
| Malawi | — | 15 | 2 | — | 8 | 25 (11%) |
| Zimbabwe | — | — | 3 | — | 3 | 6 (3%) |
| South Africa | — | — | 1 | 3 | 1 | 5 (2%) |
| Brazil | — | — | — | — | 1 | 1 (0.4%) |
—, no enrollments.
TABLE 3.
Fetal Exposure to Maternal ARVs and Direct Infant Exposure to ARV in A5190/P1054
| Maternal ARV | N = 236 | % | Direct Infant ARV Exposure | N = 236 | % |
|---|---|---|---|---|---|
| ≥3 ARVa | 24 | 10 | NVP only | 31 | 13 |
| ≥7 d ZDVb | 191 | 81 | ZDV/sd-NVP | 200 | 85 |
| Intrapartum only | 21 | 9 | ZDV only | 5 | 2 |
sd-NVP, single dose NVP.
Thirteen women continued triple ARV while breastfeeding.
Twelve women received triple ARVs in the first trimester of pregnancy.
Regarding maternal parameters near the time of delivery, most mothers had high CD4 cell counts; 100 (42%) had CD4 cell counts >500 cells/mm3, 77 (33%) had CD4 counts between 350 and 500 cells/mm3, and 59 (25%) had counts between 200 and 350 cells/mm3 (range, 218–1233 cells/mm3). Maternal virus load near delivery was <400 copies/mL of HIV RNA in 28% of mothers, <1000 copies/mL in 39%, between 1000 and 10 000 copies/mL in 36%, between 10 000 and 100 000 copies/mL in 23%, and >100 000 copies/mL in 2%. Fifty-four percent of women with CD4 cell counts ≥500 had viral load <1000; conversely, 39% of women in the 200 to 349 cells/mm3 CD4 cell stratum had viral loads ≥10 000 copies/mL. Ninety-two percent of women (22 of 24) on regimens with ≥3 ARVs had viral loads <1000 copies/mL, compared with 36% of women (68 of 191) who received antepartum ZDV and intrapartum ARV and 10% (2 of 21) of women who only received intrapartum ARV.
There were 4 transmission events, with 4 infants identified as HIV infected during the 18-month period: 2 in Thailand, 2 in Africa (HIV-1 transmission rate: 1.8%; 95% CI, 0.048%–3.6%). All transmissions occurred in infants born to women who received antepartum ZDV throughout pregnancy and/or intrapartum ARV. One infant in Thailand had documented in utero transmission with a positive HIV-1 DNA PCR in the first day of life. Another Thai infant had sequentially negative HIV-1 DNA PCR results until 12 weeks but a positive antibody result at 12 months with retrospective RNA PCR testing showing transmission between 12 and 24 weeks of age. A third infant had late postnatal transmission with first positive HIV-1 RNA PCR at 9 months. One breastfed infant had positive antibody results at 19 months of age despite negative HIV-1 RNA PCR results to 12 months. Three of 4 infants were infected in the late postnatal period when mothers were no longer receiving ARVs through a study intervention when transmission occurred. Ninety-four percent of infants (n = 222) were not HIV-1 infected. Of 14 infants who died or were lost to follow-up, HIV-1 infection was indeterminate in 7. Of 6 breastfed infants lost to follow-up before 18 months, none had laboratory evidence of HIV-1 infection at the last visit. HIV-1–free survival was 98.7% (95% CI, 97.3%–99.9%) at 6 months and 96.4% (95% CI, 94.0%–98.9%) at 18 months.
Eighty-six percent of infants (n = 202) had a birth weight >2.5 kg and 10% (n = 24) were between 1.5 and 2.5 kg. Birth weight was not recorded in 4%. Seventy-seven percent of infants were born at term (≥37 weeks’ gestation), 8% had a gestational age between 32 and 37 weeks, and for 15% gestational age was not recorded. No statistically significant differences in birth weight or gestational age were observed based on maternal ARV regimen, viral load, CD4 cell count, infant’s ARV regimen, or place of birth (P > .5). In utero development was adequate in 76% of infants, categorized as appropriate for gestational age. Ten infants were classified as small for gestational age, and 10 as large for gestational age (4% each), with classification not performed in 14%. Three (1%) additional infants were classified as having intrauterine growth retardation. No statistically significant associations were observed between intrauterine growth parameters and maternal ARV, infant’s ARV, or maternal viral load or CD4 cell count. All infants with intrauterine growth retardation classification were from India, and all infants in the large for gestational age category were Thai. Apgar scores were ≥7 at 1 minute in 94% and in all infants by 5 minutes. No statistically significant associations were observed between Apgar scores and maternal parameters (P > .20).
A high proportion of surgical deliveries occurred: 53% of infants were born by spontaneous vaginal delivery, 45% by cesarean section, and 2% by forceps/vacuum extraction. Cesarean sections were performed for delivery of all Indian infants (n = 47), 38% of Thai infants (n = 58), and in 1 (3%) of 36 African deliveries. This reflects a difference in approach to mode of delivery by setting and was statistically significant (P < .001). No statistically significant associations were observed between mode of delivery and maternal viral load, CD4 cell count, or ARV use. The vast majority of deliveries occurred in hospital settings (97%), and all 8 nonhospital deliveries occurred in Africa. Twenty-six percent of deliveries in Malawi and Zimbabwe were in nonhospital settings.
In the first 6 months, 23 infants had at least 1 hospitalization, and 3 had ≥2 admissions (11% hospitalization). By 18 months, 36 infants were hospitalized once, and 11 had ≥2 admissions (20% hospitalization). The median duration of hospitalization was 5 days (range, 2–51 days). The majority occurred in uninfected infants for common childhood illnesses, (respiratory/gastrointestinal infections). Hospitalizations did not vary by geographic area: 11% in Africa, 17% in India, and 23% in Thailand (P = .25).
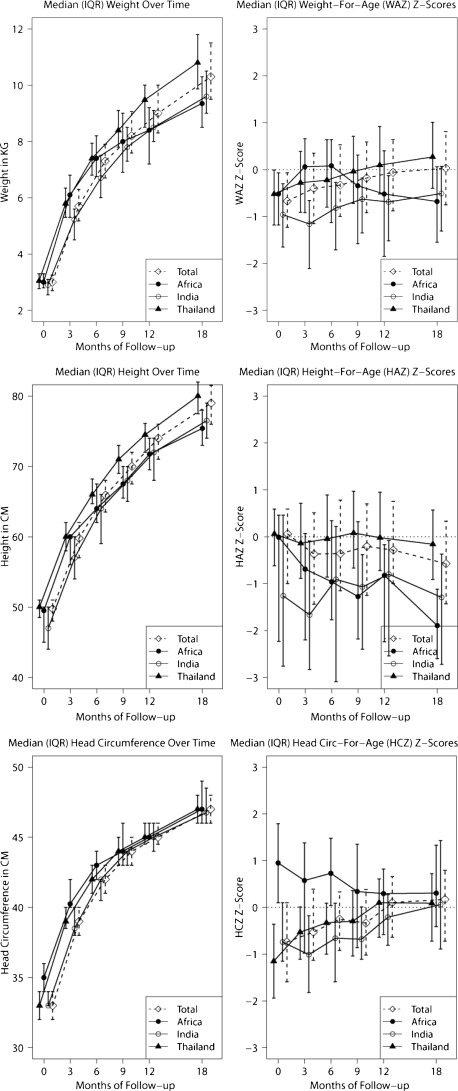
Median weight, height, head circumference and corresponding z scores are detailed in Fig 1. There were significant differences by geographic region for weight, weight-for-age z scores (WAZ), height, and height-for-age z scores (HAZ) at all time points, as well as head circumference up to 9 months of age and head-circumference-for-age z scores up to 6 months of age. Infants in India (nonbreastfed) had the lowest or second lowest median weights at all time points. Nonbreastfed infants in Africa had the largest median weight. Weight differences were observed at later time points (ages 9, 12, and 18 months). Specifically, African infants had lower median weights and WAZ after the first 6 months of life, and most were exposed in utero to ≥3 ARV drugs. Statistically significant differences by maternal viral load were observed at 3 months in infant length and HAZ (but not weight or WAZ) (P = .014, P = .007, respectively), 6 months for HAZ (P = .044), and 18 months for both parameters (P = .032, P = .02). The 5 infants born to mothers with ≥100 000 copies/mL RNA had the shortest stature and HAZ within the cohort.
FIGURE 1.
Median weight, height, and head circumference and WAZ, HAZ, and head-circumference-for-age z scores are plotted.
Throughout the first 18 months of life, 89 (38%) infants had ≥1 grade 3 or 4 serious adverse event (SAE) (Table 4). The most common laboratory abnormality was hematologic, primarily anemia (13%). Abnormal chemistries were primarily due to high potassium levels in infancy (8%), without clinical repercussions. Abnormal liver function findings were primarily due to hyperbilirubinemia in the neonatal period (4%) or transaminase elevations (5%). By maternal ARV exposure, serious adverse event (grades 3–5) rates were 38% in triple ARV–exposed infants (9 of 24), 35% in ZDV-exposed infants (67 of 191), and 62% in intrapartum-only ARV-exposed infants (13 of 21) (P = .01). No statistically significant differences in grades 3 to 5 adverse events by geography, maternal CD4 cell count, or maternal viral load were observed.
TABLE 4.
SAEs Grade 3 or 4 (Requiring Medical Attention), Most Common Clinical Events in the Neonatal Period and Beyond, and Congenital Defects/Inborn Errors of Metabolism
| SAEs Grade 3 or 4 | ||
|---|---|---|
| No. of Subjects | % of Subjects (N = 236) | |
| Laboratory: hematologic SAEs | 38 | 16 |
| Laboratory: blood chemistry SAEs | 24 | 10 |
| Laboratory: liver/hepatic SAEs | 14 | 6 |
| Clinical: gastrointestinal SAEs | 9 | 4 |
| Clinical: respiratory SAEs | 9 | 4 |
| Total subjects with SAEsa | 89 | 38 |
| Neonatal conditions | ||
| Hematologic disordersb | 23 | 10 |
| Infections/infestations | 17 | 7 |
| Pregnancy/puerperium/perinatal disorders | 11 | 5 |
| Respiratory, thoracic mediastinal disorders | 8 | 3 |
| Eye disorders | 8 | 3 |
| Metabolism/nutrition disorders | 6 | 3 |
| Skin/soft tissue disorders | 5 | 2 |
| Total neonatal conditions | 70 | 30 |
| Conditions beyond neonatal periodc | ||
| Respiratory infections | 107 | 45 |
| Gastrointestinal infections | 52 | 22 |
| Hematologic disorders | 42 | 18 |
| Systemic viral infections | 24 | 10 |
| Allergic/autoimmune diseases | 22 | 9 |
| Skin and tissue infections | 20 | 8 |
| Congenital abnormalities/inborn errors metabolism (18 subjects) | ||
| Urinary/genital tract malformations | 8 | 3 |
| Hydrocele | 5 | — |
| Cryptorchism | 1 | — |
| Inguinal hernia | 1 | — |
| Pelvic-ureteric obstruction | 1 | — |
| Congenital heart defects | 8 | 3 |
| Ventricular septal defect | 3 | — |
| Patent ductus arteriosus | 1 | — |
| Congenital pulmonary artery stenosis | 1 | — |
| Congenital pulmonary hypertension | 1 | — |
| Complex congenital heart disease | 1 | — |
| Tetralogy of Fallot | 1 | — |
| Congenital hypothyroidism | 2 | 1 |
| Congenital lacrimal gland anomaly | 1 | 1 |
| Hypertelorism of orbit | 1 | 1 |
| Glucose-6-phosphate hydrogenase deficiency | 2 | 1 |
| Inborn error of bilirubin metabolism | 1 | 1 |
| Trisomy 21 | 1 | 1 |
| Total number of conditions | 24 | |
| Total number of events | 398 | |
| Total number of subjects with events | 144 | |
—, percent figures not given for subcategories.
Subjects may have >1 SAE.
Anemia in 22 subjects.
Conditions with <3% frequency are not listed.
Neonatal medical conditions were present in 30% of infants (n = 70), as shown in Table 4, (39% of Thai, 17% of Indian, and 6% of African infants). Beyond the neonatal period, clinical conditions were frequently diagnosed (Table 4). Sixty-seven percent of Thai infants had ≥1 event in the newborn period and 84% had events beyond this period. Thai infants constituted 64% of the patient cohort, and all were in the ZDV-in-pregnancy-arm receiving ZDV/single-dose NVP in the neonatal period. Reporting of ≥1 clinical diagnosis varied geographically: 84% of infants in Thailand, 31% in Africa, and 9% in India.
Congenital abnormalities/inborn errors of metabolism (n = 24) were reported in 18 (7.6%) children as shown in Table 4. Except for 1 case of hydrocele, all were Thai infants (11%; 95% CI, 6.7%–17%). None of the infants with congenital defects had first-trimester exposure to maternal ARV. No significant associations with maternal CD4 or viral load were observed.
Four deaths occurred (1.8%; 95% CI, 0.05%–3.49%): 3 infants in Africa from gastroenteritis, pneumonia, and pneumonia/malnutrition. The remaining death was in Thailand, in an 8-week-old infant with trisomy 21 and cardiac defects with aspiration pneumonia. All infants who died had negative HIV-1 DNA PCRs.
Discussion
A5190/P1054 was designed to monitor pregnancy and infant outcomes in subjects participating in NIH-sponsored international trials that used ARV for maternal treatment or PMTCT purposes. It accompanied the debut of a large international initiative by the NIH with the release of multiple ARV trials in the early to mid-2000s. The present analysis focused on infant outcomes in a protocol endorsed by the ACTG, HPTN, and IMPAACT networks. A5190/P1054 demonstrated the feasibility of extended monitoring of HIV-exposed infants up to 18 months of age in settings where clinical trials infrastructure is in place and trained medical staff are available for recognition of clinical events. Although a relatively high percentage of eligible infants was enrolled while parent studies and the current study were enrolling simultaneously (∼85%, data not shown), the absolute number of subjects participating in the current study was relatively small. Enrollment of mother/infant pairs in observational studies, particularly internationally, can pose a challenge, especially when participating sites are not inherently affiliated with pediatric networks. Synchronization of maternal and infant visits can be difficult, as clinics might have different locations or schedules, complicating transportation and logistics. Enrollment from adult sites was initially slower, but the challenge was overcome once pediatric providers were incorporated by sites for performance of study visits. In a time of competing priorities and budget restraints, observational safety studies with long-term follow-up are not as competitive for funding as intervention studies. Nevertheless, it was shown through this endeavor that clinical trial sites in diverse settings are capable of effectively managing children of women enrolled in research protocols. Careful clinical monitoring demonstrated that in HIV-exposed, uninfected infants, conditions requiring medical attention were not infrequent, but long-lasting clinical repercussions were rare.
The vast majority of subjects (95%) did not have first-trimester exposure to maternal ARV; therefore, the study did not assess the safety of triple ARV during embryogenesis. However, no significant association was observed between later maternal exposure to ARV, particularly ZDV and intrapartum single-dose NVP plus 2 or 3 other ARVs, and SAEs. Postpartum ARV exposure was rare as only 12% of infants were breastfed. Findings were reflective of the natural history of HIV exposure in infants who received maternal ARV in later months of intrauterine life.
One critical finding was the excellent HIV-free survival observed at 18 months of age. Intrapartum HIV-1 transmission was not noted, as 1 infant had diagnosed in utero infection, and the remaining 3 infants had late postpartum HIV acquisition, long after PMTCT interventions were completed. Although women with lower CD4 cell counts are at higher risk of HIV-1 mother-to-child transmission, transmitting mothers had higher cell numbers and came from perinatal studies evaluating tail-end ARV regimens for prevention of NVP resistance. None of the 24 mothers receiving multiple ARV for treatment purposes transmitted HIV-1 (92% had viral loads <1000 copies/mL). Mode of delivery reflected country practices with a high cesarean section rate of 45% which could also explain no intrapartum HIV transmission. Most deliveries were hospital based, including 74% of deliveries in Malawi and Zimbabwe. This likely reflected the participation of clinical trial sites with greater resources, increased surveillance, and greater patient access to care. Infant mortality was not associated with HIV status.
Birth and neonatal parameters were within normal limits and varied by geographic region. Indian infants had the lowest growth parameters in the first months of life, while Thai infants had significantly higher WAZ and HAZ in the first months, as did African infants. However, at later time points, infants born in Africa had the lowest growth parameters (negative WAZ and HAZ), consistent with weaning practices in the second or third semesters of life, a phenomenon observed in breastfeeding studies.11–14 One interesting finding was the association between higher maternal viral load and shorter infant stature at 3, 6, and 18 months. Studies have demonstrated a positive association between maternal health and infant outcomes as late as 12 months of age.15–17 The potential association between maternal viremia and infant stunting deserves further investigation.
The relatively large number of reported adverse events was likely due to increased study surveillance. Likewise, a much larger proportion of Thai infants had clinical diagnoses or adverse events (84%) as opposed to Indian or African infants, and they also had higher hospitalization rates. The reason why Thai infants had relatively high rates of congenital malformations or inborn errors of metabolism is unclear. Thai infants were exposed to ZDV prophylaxis only in later months of pregnancy. Congenital malformation rates are reported at ∼3% to 6% in HIV-exposed infant cohorts18,19 but longer periods of observation tend to uncover higher rates of conditions unidentified at birth.20 One advantage of prospectively managing HIV-exposed infants is the opportunity for multiple diagnostic evaluations, which enable detection of clinical conditions, something that cannot be ascertained through antiretroviral pregnancy registries, as these do not include follow-up for infants. There were a number of SAEs reported in this study that would require additional follow-up time for evaluation of clinical significance and/or potential resolution as there is a paucity of data on long-term pediatric outcomes. Another plausible explanation for the high rate of congenital defects is increased diagnostic capacity at selected sites, which potentially could explain the higher rate of findings observed in Thailand. This underscores the need for longitudinal data from HIV-exposed pediatric populations, since comparative population data are not available. Had ARV exposures occurred within the first trimester of pregnancy in the current study, the higher rate of congenital malformations might have been erroneously associated with maternal ARV use. Longer periods of infant follow-up are also necessary for identification of potential mitochondrial dysfunction or neurologic disorders.
A5190/P1054 did not uncover an association between adverse events in HIV-exposed infants and moderate maternal ARV exposure (95% women had no first-trimester ARV exposure and 90% had no triple-ARV exposure), although a larger cohort would be needed to evaluate associations with lower frequency rates. Data from this study, nevertheless, are reassuring as they suggest that participation of pregnant patients in ARV clinical trials renders benefits to women and their infants. Continued monitoring of infant safety, however, is paramount as unexpected outcomes after in utero exposure to drugs might not be easily recognizable.21 Infant safety data should be systematically collected as our findings document that this approach is feasible and acceptable within the network infrastructure. This approach should be continued as novel ARVs are made available through international research initiatives.
Acknowledgments
The authors thank the A5190 study participants who volunteered their time and efforts. The authors acknowledge the contributions of the following A5190 investigators from the ACTG, IMPAACT, and HPTN networks: Kimberly Hudgens, BS, ACTG Operations Center, Social and Scientific Systems; Edward Acosta, PharmD, University of Alabama at Birmingham; Susan Eshleman, MD, PhD, Johns Hopkins Medical Institution; Susan Fiscus, PhD, University of North Carolina School of Medicine; Ana Martinez, RPh, DAIDS, NIAID, NIH; Lynne Kidd-Freeman; Apsara Nair, MS; Laura M. Stevens, MB, FSTRF; Ann Walawander, MA, FSTRF; Joan Gormley, MBN, The Miriam Hospital; Carol A. Vincent, CRNP, MSN, Children's Hospital of Philadelphia; David L. Shugarts, MA, University of Colorado Health Sciences Center; Marlene Cooper, MS, FSTRF; Suzanne Siminski, MS, MBA, FSTRF; Barbara Brizz (in memoriam); Kulkanya Chokephaibulkit, MD, and Nirun Vanprapar, MD, Siriraj Hospital Mahidol University; Nagalingeshwaran Kumarasamy, MBMB, PhD, and Dr S. Poongulali, YRG CARE Medical Center VHS Chennai CRS; Dr Prapaisri Layangool and Dr Jutarat Mekmullica, Bhumibol Adulyadej Hospital CRS; Virat Sirisanthana, MD, and Thanyawee Puthanakit, MD, Chiang Mai University Pediatrics-Obstetrics CRS; Chaiwat Ngampiyasakul, MD, and Ms Wanna Chamjamrat, Prapokklao Hospital CRS; Suchat Hongsiriwon, MD, and Ms Donyapattra Ekkomonrat, Chonburi Hospital CRS; Newton Kumwenda, MPH, PhD, College of Medical JHU CRS; Pornchai Techakunakorn, MD, and Mrs Chutima Ruklao Phayao, Provincial Hospital CRS; Portia Kamthunzi and Tionge Kamvaunamwali, University of North Carolina Lilongwe CRS; Dr Wadzanai Samaneka and Dr Nehemiah Nhando, Parirenyatwa CRS, UZ-UCSF CTU, Harare Zimbabwe CRS; Prudence Ive, FCP, Wits HIV CRS; Chulapong Chanta, MD, and Dr Kanchana Preedisripipat, Chiang Rai Regional Hospital CRS; Esau C Joao, MD, and Maria Letícia S, Cruz, Ped MD, Hospital dos Servidores Rio de Janeiro NICHD CRS.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- ARV
antiretroviral
- CD4
cluster of differentiation 4 (T4 helper cell)
- CI
confidence interval
- HAZ
height-for-age z score
- NIH
National Institutes of Health
- NVP
nevirapine
- PCR
polymerase chain reaction
- PMTCT
prevention of mother-to-child transmission
- SAE
significant adverse event
- WAZ
weight-for-age z score
- ZDV
zidovudine
Footnotes
All authors fully contributed to the preparation of this manuscript and are responsible for the reported research; and all authors have participated in the concept and design, analysis, and interpretation of data and in drafting or revising the manuscript and have approved the manuscript as submitted.
Preliminary findings were presented at the annual meeting of the Pediatric Academic Societies; May 1–4, 2010; Vancouver, Canada [Abstract 2870.577].
This trial has been registered with A5190/p1054 Clinical Trials.gov: NCT00084136.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The project described was supported by award U01 AI068636 from the National Institute of Allergy and Infectious Diseases (NIAID) and supported by the National Institute of Mental Health (NIMH) and the National Institute of Dental and Craniofacial Research (NIDCR). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the NIAID (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the NIMH (AI068632). This work was supported by the Statistical and Data Analysis Center at the Harvard School of Public Health, under NIAID cooperative agreement 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and 1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract N01-DK-9-01/HHSN267200800001C). This study was also supported by the HIV Prevention Trials Network (HPTN) and sponsored by the NIAID, NICHD, National Institute on Drug Abuse, NIMH, and Office of AIDS Research, of the National Institutes of Health, US Department of Health and Human Services, under award U01 AI046749. The UCLA HPTU was supported through U01 AI047986, NIH/NIAID, and the IMPAACT CTU through U01 AI069401, NIH/NIAID. The University of Colorado CTU was supported through award 5 U01 AI069450, NIH/NIAID. Additional support was provided by grants AI68634 (Statistical and Data Management Center of the AIDS Clinical Trials Group [ACTG]); U01AI069512 (Siriraj Hospital Mahidol University, Site 8251); U01 AI069432 (YRG CARE Medical Center VHS Chennai CRS, Site 11701); U01 AI069429 (Bhumibol Adulyadej Hospital CRS, Site 8355); 5 U01 AI069399-05 (Chiang Mai University Pediatrics-Obstetrics CRS, Site 20101); U01 AI069429 (Prapokklao Hospital CRS, Site 8354); U01 AI069429 (Chonburi Hospital CRS, Site 8356); 1 U01 AI069518 (College of Medical JHU CRS, Site 30301); U01 AI069429 (Provincial Hospital CRS, Site 8353); 1 U01 AI069518 (University of North Carolina Lilongwe CRS, Site 12001); 5 U01 AI069436-02 (Parirenyatwa CRS, UZ-UCSF CTU, Harare Zimbabwe CRS, Site 30313); 1 U01 AI069463-01 (Wits HIV CRS, Site 11101); U01 AI069429 (Chiang Rai Regional Hospital CRS, Site 8352); NICHD contract NO1-HD-3-3345 (Hospital dos Servidores Rio de Janeiro NICHD CRS, Site 5072). Funded by the National Institutes of Health (NIH).
References
- 1.World Health Organization. Rapid advice: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. November, 2009. Available at: www.who.int/hiv/pub/mtct/advice/en/ Accessed May 29, 2011
- 2.Dorenbaum A, Cunningham CK, Gelber RD, et al. International PACTG 316 Team Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA. 2002;288(2):189–198 [DOI] [PubMed] [Google Scholar]
- 3.Thomas TK, Masaba R, Borkowf CB, et al. KiBS Study Team Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding—the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8(3):e1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lallemant M, Jourdain G, Le Coeur S, et al. Perinatal HIV Prevention Trial (Thailand) Investigators Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351(3):217–228 [DOI] [PubMed] [Google Scholar]
- 5.Marazzi MC, Nielsen-Saines K, Buonomo E, et al. Increased infant human immunodeficiency virus-type one free survival at one year of age in sub-Saharan Africa with maternal use of highly active antiretroviral therapy during breastfeeding. Pediatr Infect Dis J. 2009;28(6):483–487 [DOI] [PubMed] [Google Scholar]
- 6.Campbell T, Smeaton I, Kumnarasmay N, et al. Efficacy and safety of EFV with either co-formulated 3tC/ZDV or FTC/TDF for initial treatment of HIV-1 infected men and women in diverse multinational settings: ACTG PEARLS Study. In: Proceedings of the 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA; February 27-March 2, 2011. Abstract 149LB [Google Scholar]
- 7.Lockman S, Hughes MD, McIntyre J, et al. OCTANE A5208 Study Team Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363(16):1499–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YC, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy: Results from HPTN 052. N Engl J Med. 2011;365(6):493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon D, Noel F, Zheng L, et al. Suppression of nevirapine resistance with 7- vs. 21-day antiretroviral regimens after single dose nevirapine: results of A5207. In: Proceedings of the 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA; February 27-March 2, 2011. Abstract T-135 [Google Scholar]
- 10.Van Dyke R, Jourdain G, Shapiro D, et al. A Phase II study of the incidence of nevirapine (NVP) resistance mutations in HIV-infected Thai women receiving a single intrapartum dose of NVP followed by a postpartum tail of ZDV/ddI or ZDV/ddI/LPV/r. In: Proceedings from the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada; February 8-11, 2009. Abstract T-1003 [Google Scholar]
- 11.Kuhn L, Sinkala M, Semrau K, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50(3):437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espo M, Kulmala T, Maleta K, Cullinan T, Salin ML, Ashorn P. Determinants of linear growth and predictors of severe stunting during infancy in rural Malawi. Acta Paediatr. 2002;91(12):1364–1370 [DOI] [PubMed] [Google Scholar]
- 13.Fawzy A, Arpadi S, Kankasa C, et al. Early weaning increases diarrhea morbidity and mortality among uninfected children born to HIV-infected mothers in Zambia. J Infect Dis. 2011;203(9):1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palombi L, Buonomo E, Scarcella P, et al. Provision of highly active antiretroviral therapy (HAART) to mothers while breastfeeding and nutritional supplementation during the weaning process favorably impacts infant growth and survival in African HIV-exposed infants. In: Proceedings of the 5TH IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa; July 19-22, 2009. Abstract TUPEC044 [Google Scholar]
- 15.Chilongozi D, Wang L, Brown L, et al. HIVNET 024 Study Team Morbidity and mortality among a cohort of human immunodeficiency virus type 1-infected and uninfected pregnant women and their infants from Malawi, Zambia, and Tanzania. Pediatr Infect Dis J. 2008;27(9):808–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marinda E, Humphrey JH, Iliff PJ, et al. ZVITAMBO Study Group Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26(6):519–526 [DOI] [PubMed] [Google Scholar]
- 17.Marazzi MC, Liotta G, Nielsen-Saines K, et al. Extended antenatal antiretroviral use correlates with improved infant outcomes throughout the first year of life. [Epub ahead of print] AIDS. 2010;24(18):2819–2826 [DOI] [PubMed] [Google Scholar]
- 18.Calvet GA, João E, Nielsen-Saines K, et al. Trends in characteristics and management of HIV-infected pregnant women over a nine-year period: a prospective cohort study in Rio de Janeiro, Brazil. Revista Brasileira de Epidemiologia. 2007;10(3):323–337 [Google Scholar]
- 19.Joao EC, Calvet GA, Krauss MR, et al. NISDI Perinatal Study Group Maternal antiretroviral use during pregnancy and infant congenital anomalies: the NISDI perinatal study. J Acquir Immune Defic Syndr. 2010;53(2):176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346(24):1863–1870 [DOI] [PubMed] [Google Scholar]
- 21.Goodman A, Schorge J, Greene MF. The long-term effects of in utero exposures: the DES story. N Engl J Med. 2011;364(22):2083–2084 [DOI] [PubMed] [Google Scholar]